Abstract
Background
Reduced growth is common in children with sickle cell anemia, but few data exist on associations with long-term clinical course. Our objective was to determine the prevalence of malnutrition at enrolment into a hospital-based cohort and whether poor nutritional status predicted morbidity and mortality within an urban cohort of Tanzanian sickle cell anemia patients.
Design and Methods
Anthropometry was conducted at enrolment into the sickle cell anemia cohort (n=1,618; ages 0.5–48 years) and in controls who attended screening (siblings, walk-ins and referrals) but who were found not to have sickle cell anemia (n=717; ages 0.5–64 years). Prospective surveillance recorded hospitalization at Muhimbili National Hospital and mortality between March 2004 and September 2009.
Results
Sickle cell anemia was associated with stunting (OR=1.92, P<0.001, 36.2%) and wasting (OR=1.66, P=0.002, 18.4%). The greatest growth deficits were observed in adolescents and in boys. Independent of age and sex, lower hemoglobin concentration was associated with increased odds of malnutrition in sickle cell patients. Of the 1,041 sickle cell anemia patients with a body mass index z-score at enrolment, 92% were followed up until September 2009 (n=908) or death (n=50). Body mass index and weight-for-age z-score predicted hospitalization (hazard ratio [HZR]=0.90, P=0.04 and HZR=0.88, P=0.02) but height-for-age z-score did not (HZR=0.93, NS). The mortality rate of 2.5 per 100 person-years was not associated with any of the anthropometric measures.
Conclusions
In this non-birth-cohort of sickle cell anemia with significant associated undernutrition, wasting predicted an increased risk of hospital admission. Targeted nutritional interventions should prioritize treatment and prevention of wasting.
Keywords: sickle cell anemia, nutrition, growth, anthropometry, Africa, mortality, morbidity
Introduction
Sickle cell anemia (SCA) is one of the most common single gene disorders in the world, designated by the World Health Organization as a public health priority.1 Knowledge of the natural history and improved management of SCA has been gained from systematic studies, largely in the USA and Jamaica. However, SCA is predominantly an African disease, with the majority of affected births (homozygous inheritance of hemoglobin S–[HbSS]) occurring in Africa.2 Although SCA is a single gene disorder, the clinical expression and severity of the disease are surprisingly varied, and are thought to result from epistatic interactions from other genes and/or environmental factors.3
Energy and nutrient supplies are likely to be limited or compromised in SCA by a combination of factors: (i) reduced intake4 potentially from the anorexic effects of co-morbidities;5 (ii) decreased absorption of nutrients; (iii) increased degradation and losses of nutrients;6 increased requirements from an elevated basal metabolic rate;7 and alterations in metabolic pathways.8,9 Poor growth has been previously documented in SCA.10–12 However, the causes and clinical significance are not well understood.13
In Africa, where dietary intakes are sub-optimal for many populations, we hypothesized that nutrition is an important modifiable risk factor for SCA morbidity and that poor nutritional status is associated with increased mortality and morbidity. Here we describe anthropometric measures of nutritional status in a large cohort of Tanzanian SCA patients at enrolment. Factors associated with poor nutritional status and its consequences on mortality and morbidity are analyzed.
Design and Methods
Ethics statement
The study received ethical approval from Muhimbili University of Health and Allied Sciences reference MU/RP/AEC/VOL XI/33) and the London School of Hygiene and Tropical Medicine (reference 5158).
Patients and clinical procedures
Screening is not currently available for the newborn in Tanzania. Patients were recruited from the SCA clinic at Muhimbili National Hospital, the national referral hospital in Dares-Salaam, Tanzania, for five years from March 2004. Informed consent was obtained from patients or guardians at screening and at enrolment into the cohort. Anthropometric status of SCA patients at enrolment was compared to individuals screened for SCA but found to be either HbAA or HbAS during this recruitment period. All SCA patients are seen at scheduled routine visits (every 3–6 months) at the Muhimbili National Hospital SCA clinic and receive daily folate supplementation (5 mg/day) and anti-malarial prophylaxis according to Tanzanian government guidelines. Those subjects with SCA receive free public health care. Those with acute illness were referred from district health facilities or came directly to Muhimbili National Hospital emergency department and were managed or hospitalized according to hospital protocols.
Procedures at enrolment
Out of 1,982 screened patients with HbSS, 1,748 (88%) returned for enrolment and were issued with a unique identity number. Physical examinations were performed and detailed histories were recorded, including history of dactlytis (painful inflammation of fingers or toes) and number of previous hospital admissions. Blood samples were collected for full blood counts (FBC), detection of malaria plus confirmation of HbSS status and fetal hemoglobin (HbF) fraction by high performance liquid chromatography (HPLC) and, for a period of time, a clinical chemistry panel were all carried out.
Procedures for detection of inpatient admissions to MNH, death and loss to follow up
SCA patients hospitalized at Muhimbili National Hospital were identified through daily ward surveillance. The decision to admit SCA patients was the responsibility of the attending clinician in the hospital casualty department, according to criteria of hospital protocols. SCA patients who did not attend scheduled clinic visits for 6–12 months or more were traced by telephone or home visits. SCA patients were followed up until September 2009.
Anthropometry
Height (nearest cm), weight (nearest 0.1 kg) were measured using standardized techniques. Z-scores for height-for-age (HAZ), weight-for-age (WAZ), and body-mass-index for age (BMIZ) were calculated for patients aged over 60 months against the UK1990 reference values using the zanthro programme14 (STATA 9; StataCorp, College Station, TX, USA). For children aged under 60 months, WHO 2006/2007 reference values15 were used using igrowup-stata (http://www.who.int/childgrowth/en/). Levels of malnutrition (stunting, underweight and wasting) were defined as Z-scores of less than -2 but more than -3 for moderate and less than -3 for severe. HAZ scores determined the severity of stunting, WAZ, underweight and BMIZ, wasting. Nutritional status centiles were plotted and internal z-scores for SCA generated using LMS chartmaker-Pro-v.2.3 (Tim Cole and Huiqi Pan. Copyright 1997–2006, Medical Research Council, UK).
Laboratory procedures
At screening or after an appropriate interval for patients who had recently had a blood transfusion, individuals were typed for HbS by alkaline Hb electrophoresis (Helena, Sunderland, Tyne and Wear, UK). At enrolment, hemoglobin fractions, including HbF, were quantified by HPLC using the β-thalassemia Short Programme on the Variant analyser (BioRad, Hercules, CA, USA) confirming the previous diagnosis. Full blood counts (FBC) were performed using an automated cell counter (Pentra 60, Horiba ABX, Kyoto, Japan). Biochemical tests were performed using an automated chemistry analyzer (Abbott Architect, NY, USA).
Statistical analysis
Data were analyzed using STATA ICv9. Internal z-scores were used to assess relative nutritional status within our SCA population (ensuring a normal distribution) with risk of mortality and risk of in-patient admission to Muhimbili National Hospital using Cox’s regression with the Cnaan and Ryan approach, modeling the effect of age at enrolment.16 Hospital admissions less than seven days apart were treated as a single event and the previous number of admissions was included as a co-factor when analyzing the risk of subsequent admissions. Adjustment for clustering within families (124 family groups [full or half siblings or mother/father child groupings]) was performed and repeated events within individuals accounted for using robust standard errors. The end date for observation was the date of last contact either at the clinic, through tracing activities or during a hospital admission, or date of death.
Results
Height, weight, age, sex and a valid BMI were available for 1,041 SCA cases at enrolment (49% males) and for 717 controls (HbAS or HbAA). Cases were significantly younger than controls (mean 10.1 vs. 13.8 years, P<0.001). Overall, sex distribution was similar with 49% male cases vs. 48% in controls, but the proportion of males decreased significantly with age in both groups (P=0.001). Mean HbF% in SCA was 5.5 (SD=4.2; N=965 [>5y]) and mean hemoglobin 7.5g/dl (SD=1.4) vs. 11.0g/dl (SD=2.6) (P<0.001).
Nutritional status in Tanzanian SCA
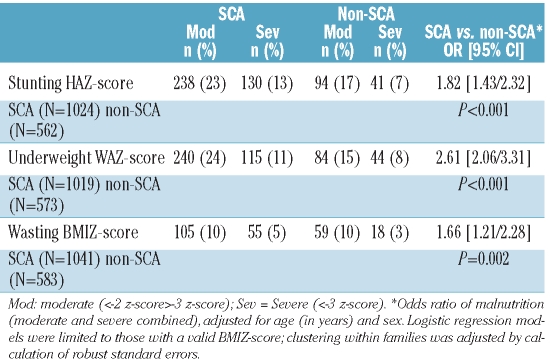
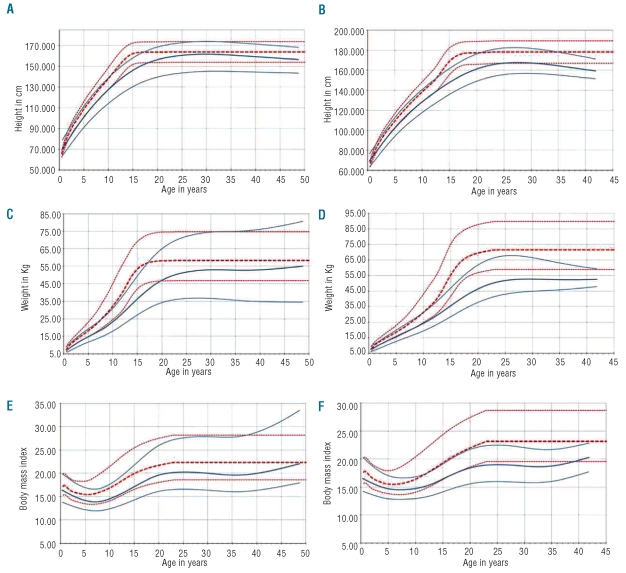
Table 1 demonstrates a significantly increased prevalence of malnutrition (<−2 z-scores for HAZ, WAZ, and BMIZ) in SCA versus non-SCA. Centile plots displaying the 5th to 95th percentile ranges of height, weight and BMI in SCA at enrolment by age and sex compared to UK reference data are shown in Figure 1.
Table 1.
Prevalence of malnutrition in SCA compared to non-SCA.
Figure 1.
Height, weight and BMI centile plots for SCA compared to UK reference data.14 (A, C) and (E) are for females and (B, D) and (F) for males. Red dotted lines are the 95th, median and 5th centile curves for UK reference data14 (1990); solid blue lines represent the 95th, median and 5th centile curves for the Tanzanian SCA population. UK reference curves were extrapolated at the same level after the maximum age available of 23 years.
Stunting
The centile plots in Figure 1 demonstrate the greatest deficits in height in SCA during adolescence, but also an extended growth period and potential for catch-up growth, more pronounced for females (Figure 1), resulting in males being more likely to be stunted as adults (>18y, OR=2.48 [1.19/5.17] P=0.015).
Underweight
Adult males were seven times more likely to be underweight than females (OR=7.01 [3.21/15.50] P<0.001), whilst in the 5–10 years age group, males were less likely to be underweight (OR=0.52 [0.31/0.86] P=0.011). Figure 1 clearly demonstrates low weight in adolescence, most pronounced in males, and the large variation in weight for females.
Wasting
Wasting was most prevalent in adolescents and adults. However, the mean BMI in adults was 20.0 kg/m2 (95%CI 19.5–20.5) and was thus considered to be within the healthy range (17.5–25 kg/m2). Similar to underweight status, adult males had an increased risk of wasting (OR=4.15 [1.95/8.82] P<0.001) but males aged 5–10 years had a significantly decreased risk (OR=0.50 [0.25/0.99] P=0.048).
Factors associated with increased odds of malnutrition at enrolment
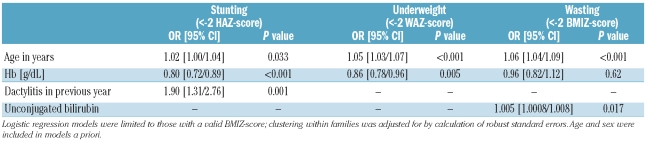
When sex and age were included in multivariable models a priori, there was no effect of HbF%, generally reported to be a strong modulator of SCA disease severity, and associated with age and sex.17 In multivariable models, the only significant variables were age, history of dactylitis, unconjugated bilirubin and Hb concentrations (Table 2).
Table 2.
Multivariable models of factors associated with malnutrition in SCA at enrolment into the cohort.
Hemolysis, anemia and malnutrition in SCA
Unconjugated bilirubin and lactate dehydrogenase are considered markers of hemolysis and are reported to be associated with disease severity.18 Raised levels of these markers were significantly associated with decreased hemoglobin concentrations (data not shown). However, none were significantly associated with nutritional status when age and sex (but not hemoglobin) were included in models, except for a positive association between unconjugated bilirubin concentrations and odds of wasting (P=0.037).
Nutritional status at enrolment and risk of mortality
Internal z-scores were used to assess how relative nutritional status of individuals was associated with mortality. Of the 1,041 SCA patients with a BMI z-score at enrolment, 958 (92%) were followed up until September 2009 or until death (n=50). Median follow up was 2.1 (range 0.02–5.4) years. The incidence rate of death was 2.5 per 100 person years observation (PYO) (95%CI 1.9–3.3), similar to the full cohort at 2.0 per 100 PYO.19 Controlling for age at enrolment, and stratified by age group, there was no evidence of an association between nutritional status at enrolment and risk of mortality, for example: BMI-internal z-score (BMIiZ score) hazard ratio [HZR]=1.02 [95% CI 0.76–1.37; P=0.91] and no difference according to sex. However, as for the whole cohort,19 hemoglobin concentrations were significantly negatively associated with mortality, with a 30% decrease in risk per g/dl increase in hemoglobin (HZR=0.69, [95% CI 0.56–0.85], P=0.001) when limited to this population with anthropometric data.
Nutritional status at enrolment and risk of hospitalization
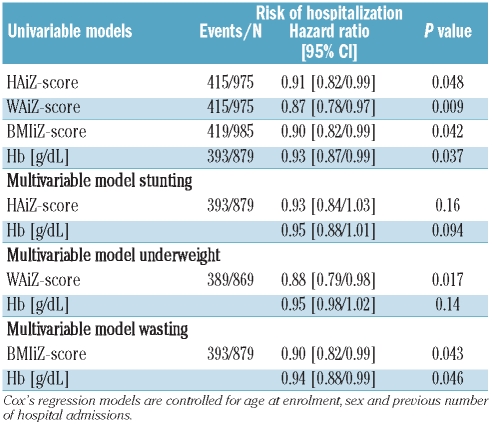
Of the 1,041 with BMIZ scores at enrolment, 985 returned for at least one routine follow-up visit and were included in this analysis. There were 419 admissions in 385 (36%) subjects; 238 of these contributing one event only (maximum number of events per person was 11) and a total of 2,378 PYO. There were 17.6 (95%CI 16.0–19.4) admissions per 100 PYO. Independent of age at enrolment, poorer nutritional status was significantly associated with increased risk of hospitalization (Table 3), with the greatest effect size for relative weight-for-age (greater WAiZ score, HZR=0.87, P=0.009), equivalent to a 13% reduction in risk for each increase in WAiZ score of one SD. Baseline hemoglobin concentration was also negatively associated (HZR=0.93 per 0.1g/dl, P=0.037). Baseline hemoglobin and BMIiZ score were independently associated with risk of admission (Table 3). Sex had no effect on hospitalization (HZR=0.95, P=0.50).
Table 3.
Relative nutritional status within SCA and risk of hospital admission.
Discussion
Prevalence of malnutrition in SCA by age and sex
This is one of the largest analyses of nutritional status of SCA patients at a single site, and certainly within Africa. Our data suggest a similar or lower prevalence of malnutrition in SCA compared to other African studies.20–22 Unsurprisingly, compared to patients enrolled in comprehensive care programs in the USA23,24 or the UK,25 Tanzanian SCA patients experience a greater prevalence of malnutrition compared to local controls. For example, in 178 American SCA children (0–18 years), the proportion who were ever less than the US National Center Health Statistics (NCHS) 5th percentile for height, weight or BMI during four years of follow up was 22–26%,12 compared to 36–38% less than the 2.5th percentile (<−2 SD) for height and weight in our cohort at baseline. However, only 18% of our cohort were less than the 2.5th percentile for BMI, suggesting adaptation to chronic energy/nutrient deficiency, perhaps from slowed rates of growth and stunting. Alternatively, it may represent a survivor cohort effect.
Differences in nutritional status according to sex within SCA have been reported previously. In most cases, similar to our study, nutritional status was worse in males compared to females11,26–28 although one study reported the contrary.29 However, in the one study that assessed disease severity in relation to growth, effect was limited to females.12 Disparity between the age groups in these studies and differences in effects of SCA on pubertal delay between males and females may underlie these discrepancies. We observed that the most growth retardation was experienced in adolescents. This is likely, at least in part, an artifact from delayed and extended puberty, particularly in females which allows for a substantial degree of catch-up growth. This has already been seen in Jamaican patients, for whom longitudinal centile growth curves were plotted and reference data generated.11 The reasons why females should experience more catch-up growth than males is not clear.
Clinical impact of nutritional status in SCA
We believe that this is the first report that has attempted to assess the relationship between nutritional status and mortality within SCA. In the UK East London Cohort with a very high rate of survival,25 malnutrition is uncommon with only 6.5% of 2–15 year olds having a z-score of less than −2 for weight-for-age and 4.2% for height-for-age or BMI13 suggesting that improved nutritional status may have increased survival or that increased care has resulted in milder disease and improved nutritional status. Supporting the latter possibility, hydroxyurea therapy has been associated with a decrease in resting energy expenditure, correlated with increased HbF expression, and decreased wasting.5 The lack of an association between nutritional status and mortality in our cohort is, therefore, surprising. There is strong evidence to show that poor nutritional status is a contributory factor for mortality in children in general.30–32 Furthermore, we observed strong associations between hemoglobin and nutritional status and between hemoglobin and mortality; in addition poor nutritional status at enrolment was associated with risk of hospitalization, expected to be a good indicator of disease severity. It is possible that an effect of malnutrition on mortality in our SCA population is limited to young children who have the highest mortality among SCA patients, due particularly to infections,25,33–35 including in our cohort.19 Malnutrition may have the greatest effect on mortality when mediated through an interaction with infections, as suggested in a Kenyan study in which the majority of deaths in young children attributable to malnutrition were ascribed to infection as the immediate cause of death.36 In the absence of newborn screening, this survivor cohort, with a small number of patients in the youngest, most vulnerable age group, may have resulted in a lack of power to detect a true association. Reduced growth may also be an adaptation to a chronic undersupply of energy and nutrients, reducing total energy requirements, and allowing available energy and nutrients to be directed towards maintaining essential metabolic defenses against disease processes. Smaller body size will also ameliorate the effects of chronic anemia on cardiac load. The lower prevalence of wasting compared to stunting and underweight may support this hypothesis. Good nutritional status was not found to predict a benign clinical course in Jamaican SCA patients, also indicating an element of protective adaptation.37 Finally, although wasting and underweight were risk factors for hospitalization, stunting was not. Therefore, wasting may represent a failure of the adaptive processes or alternatively be precipitated by events that lead to the hospital admission. Unfortunately, the cause of death remains unknown for the majority of cases in our cohort, due to the high number of deaths that occurred in the home.19
Conclusions
In our urban setting, sickle cell anemia is associated with a high prevalence of malnutrition and growth retardation in patients of all ages, but particularly in adolescents. Contrary to expectations, there was no evidence of an association between malnutrition and mortality during the 5.5 year period of follow up, although there was an association with symptomatic disease as captured by data regarding hospitalization. A birth cohort and analysis of growth rates with hospitalization and other indicators of disease severity in different age groups is required to determine relationships between malnutrition and SCA disease outcome.
The relationships between hemoglobin concentration, nutritional status and severity of disease, and the potential effects and extent of delayed pubertal development warrant further longitudinal investigation. Nutritional interventions may play an important role in enhancing healthy survival rates in SCA patients, but need to be appropriately tested before widespread implementation can be recommended.
Acknowledgments
we warmly thank the patients and staff of MNH and MUHAS, Dar-es-Salaam, Tanzania who made this work possible. We also thank Josephine Mgaya and Harvest Mariki for the sickle diagnoses and work in generating the laboratory data, and Fenella Kirkham for commenting on the manuscript.
Footnotes
Funding: we thank the Wellcome Trust for funding this research: (SC) project grant 080025; (JM) 072064; strategic award 084538 and the Kenya Medical Research Institute (KEMRI) -Centre for Geographic Medicine Research (Coast).
Authorship and Disclosures
The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
References
- 1.Weatherall DJ, Clegg JB. Inherited hemoglobin disorders: an increasing global health problem. Bull World Health Organ. 2001;79(8):704–12. [PMC free article] [PubMed] [Google Scholar]
- 2.Weatherall DJ. The global problem of genetic disease. Ann Hum Biol. 2005;32(2):117–22. doi: 10.1080/03014460500075480. [DOI] [PubMed] [Google Scholar]
- 3.Nagel RL. Pleiotropic and epistatic effects in sickle cell anemia. Curr Opin Hematol. 2001;8(2):105–10. doi: 10.1097/00062752-200103000-00008. [DOI] [PubMed] [Google Scholar]
- 4.Kawchak DA, Schall JI, Zemel BS, Ohene-Frempong K, Stallings VA. Adequacy of dietary intake declines with age in children with sickle cell disease. J Am Diet Assoc. 2007;107(5):843–8. doi: 10.1016/j.jada.2007.02.015. [DOI] [PubMed] [Google Scholar]
- 5.Fung EB, Malinauskas BM, Kawchak DA, Koh BY, Zemel BS, Gropper SS, et al. Energy expenditure and intake in children with sickle cell disease during acute illness. Clin Nutr. 2001;20(2):131–8. doi: 10.1054/clnu.2000.0367. [DOI] [PubMed] [Google Scholar]
- 6.Westerman MP, Zhang Y, McConnell JP, Chezick PA, Neelam R, Freels S, et al. Ascorbate levels in red blood cells and urine in patients with sickle cell anemia. Am J Hematol. 2000;65(2):174–5. doi: 10.1002/1096-8652(200010)65:2<174::aid-ajh15>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 7.Barden EM, Zemel BS, Kawchak DA, Goran MI, Ohene-Frempong K, Stallings VA. Total and resting energy expenditure in children with sickle cell disease. J Pediatr. 2000;136(1):73–9. doi: 10.1016/s0022-3476(00)90053-2. [DOI] [PubMed] [Google Scholar]
- 8.Borel MJ, Buchowski MS, Turner EA, Goldstein RE, Flakoll PJ. Protein turnover and energy expenditure increase during exogenous nutrient availability in sickle cell disease. Am J Clin Nutr. 1998;68(3):607–14. doi: 10.1093/ajcn/68.3.607. [DOI] [PubMed] [Google Scholar]
- 9.Borel MJ, Buchowski MS, Turner EA, Peeler BB, Goldstein RE, Flakoll PJ. Alterations in basal nutrient metabolism increase resting energy expenditure in sickle cell disease. Am J Physiol. 1998;274(2 Pt 1):E357–64. doi: 10.1152/ajpendo.1998.274.2.E357. [DOI] [PubMed] [Google Scholar]
- 10.Platt OS, Rosenstock W, Espeland MA. Influence of sickle hemoglobinopathies on growth and development. N Engl J Med. 1984;311(1):7–12. doi: 10.1056/NEJM198407053110102. [DOI] [PubMed] [Google Scholar]
- 11.Thomas PW, Singhal A, Hemmings-Kelly M, Serjeant GR. Height and weight reference curves for homozygous sickle cell disease. Arch Dis Child. 2000;82(3):204–8. doi: 10.1136/adc.82.3.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zemel BS, Kawchak DA, Ohene-Frempong K, Schall JI, Stallings VA. Effects of delayed pubertal development, nutritional status, and disease severity on longitudinal patterns of growth failure in children with sickle cell disease. Pediatr Res. 2007;61(5 Pt 1):607–13. doi: 10.1203/pdr.0b013e318045bdca. [DOI] [PubMed] [Google Scholar]
- 13.Al-Saqladi AW, Cipolotti R, Fijnvandraat K, Brabin BJ. Growth and nutritional status of children with homozygous sickle cell disease. Ann Trop Paediatr. 2008;28(3):165–89. doi: 10.1179/146532808X335624. [DOI] [PubMed] [Google Scholar]
- 14.http://www.stata-journal.com/software/sj4-1.
- 15.http://www.who.int/childgrowth/software/en/
- 16.Cnaan A, Ryan L. Survival analysis in natural history studies of disease. Stat Med. 1989;8(10):1255–68. doi: 10.1002/sim.4780081009. [DOI] [PubMed] [Google Scholar]
- 17.Chang YC, Smith KD, Moore RD, Serjeant GR, Dover GJ. An analysis of fetal hemoglobin variation in sickle cell disease: the relative contributions of the X-linked factor, β-globin haplotypes, alpha-globin gene number, gender, and age. Blood. 1995;85(4):1111–7. [PubMed] [Google Scholar]
- 18.Kato GJ, Gladwin MT, Steinberg MH. Deconstructing sickle cell disease: reappraisal of the role of hemolysis in the development of clinical subphenotypes. Blood Rev. 2007;21(1):37–47. doi: 10.1016/j.blre.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Makani J, Cox SE, Soka D, Komba AN, Oruo J, Mwamtemi H, et al. Mortality in sickle cell anemia in Africa: a prospective cohort study in Tanzania. PLoS ONE. 2011;6(2):e14699. doi: 10.1371/journal.pone.0014699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ebomoyi E, Adedoyin MA, Ogunlesi FO. A comparative study of the growth status of children with and without SS disease at Ilorin, Kwara State, Nigeria. Afr J Med Med Sci. 1989;18(1):69–74. [PubMed] [Google Scholar]
- 21.Athale UH, Chintu C. The effect of sickle cell anemia on adolescents and their growth and development--lessons from the sickle cell anemia clinic. J Trop Pediatr. 1994;40(4):246–52. doi: 10.1093/tropej/40.4.246. [DOI] [PubMed] [Google Scholar]
- 22.Sadarangani M, Makani J, Komba AN, Ajala-Agbo T, Newton CR, Marsh K, et al. An observational study of children with sickle cell disease in Kilifi, Kenya. Br J Haematol. 2009;146(6):675–82. doi: 10.1111/j.1365-2141.2009.07771.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Henderson RA, Saavedra JM, Dover GJ. Prevalence of impaired growth in children with homozygous sickle cell anemia. Am J Med Sci. 1994;307(6):405–7. doi: 10.1097/00000441-199406000-00004. [DOI] [PubMed] [Google Scholar]
- 24.Williams R, George EO, Wang W. Nutrition assessment in children with sickle cell disease. J Assoc Acad Minor Phys. 1997;8(3):44–8. [PubMed] [Google Scholar]
- 25.Telfer P, Coen P, Chakravorty S, Wilkey O, Evans J, Newell H, et al. Clinical outcomes in children with sickle cell disease living in England: a neonatal cohort in East London. Haematologica. 2007;92(7):905–12. doi: 10.3324/haematol.10937. [DOI] [PubMed] [Google Scholar]
- 26.Phebus CK, Gloninger MF, Maciak BJ. Growth patterns by age and sex in children with sickle cell disease. J Pediatr. 1984;105 (1):28–33. doi: 10.1016/s0022-3476(84)80351-0. [DOI] [PubMed] [Google Scholar]
- 27.Silva CM, Viana MB. Growth deficits in children with sickle cell disease. Arch Med Res. 2002;33(3):308–12. doi: 10.1016/s0188-4409(01)00360-5. [DOI] [PubMed] [Google Scholar]
- 28.Modebe O, Ifenu SA. Growth retardation in homozygous sickle cell disease: role of calorie intake and possible gender-related differences. Am J Hematol. 1993;44(3):149–54. doi: 10.1002/ajh.2830440302. [DOI] [PubMed] [Google Scholar]
- 29.Stevens MC, Maude GH, Cupidore L, Jackson H, Hayes RJ, Serjeant GR. Prepubertal growth and skeletal maturation in children with sickle cell disease. Pediatrics. 1986;78(1):124–32. [PubMed] [Google Scholar]
- 30.Pelletier DL, Frongillo EA, Jr, Habicht JP. Epidemiologic evidence for a potentiating effect of malnutrition on child mortality. Am J Public Health. 1993;83(8):1130–3. doi: 10.2105/ajph.83.8.1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pelletier DL, Frongillo EA, Jr, Schroeder DG, Habicht JP. The effects of malnutrition on child mortality in developing countries. Bull World Health Organ. 1995;73(4):443–8. [PMC free article] [PubMed] [Google Scholar]
- 32.Black RE, Allen LH, Bhutta ZA, Caulfield LE, de Onis M, Ezzati M, et al. Maternal and child undernutrition: global and regional exposures and health consequences. Lancet. 2008;371(9608):243–60. doi: 10.1016/S0140-6736(07)61690-0. [DOI] [PubMed] [Google Scholar]
- 33.Leikin SL, Gallagher D, Kinney TR, Sloane D, Klug P, Rida W. Mortality in children and adolescents with sickle cell disease. Cooperative Study of Sickle Cell Disease. Pediatrics. 1989;84(3):500–8. [PubMed] [Google Scholar]
- 34.Platt OS, Brambilla DJ, Rosse WF, Milner PF, Castro O, Steinberg M, et al. Mortality in sickle cell disease. Life expectancy and risk factors for early death. New England Journal of Medicine. 1994;330(23):1639–44. doi: 10.1056/NEJM199406093302303. [DOI] [PubMed] [Google Scholar]
- 35.Quinn CT, Rogers ZR, Buchanan GR. Survival of children with sickle cell disease. Blood. 2004;103(11):4023–7. doi: 10.1182/blood-2003-11-3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bejon P, Mohammed S, Mwangi I, Atkinson SH, Osier F, Peshu N, et al. Fraction of all hospital admissions and deaths attributable to malnutrition among children in rural Kenya. Am J Clin Nutr. 2008;88(6):1626–31. doi: 10.3945/ajcn.2008.26510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thomas PW, Higgs DR, Serjeant GR. Benign clinical course in homozygous sickle cell disease: a search for predictors. J Clin Epidemiol. 1997;50(2):121–6. doi: 10.1016/s0895-4356(96)00320-4. [DOI] [PubMed] [Google Scholar]