Abstract
Background
Patients with chromosome 5 abnormalities and high-risk myelodysplastic syndromes or acute myeloid leukemia have a poor outcome. We hypothesized that increasing doses of lenalidomide may benefit this group of patients by inhibiting the tumor clone, as assessed by fluorescence in situ hybridization for del(5q31).
Design and Methods
Twenty-eight patients at diagnosis or with relapsed disease and not eligible for standard therapy (16 with acute myeloid leukemia, 12 with intermediate-risk 2 or high-risk myelodysplastic syndrome) were enrolled in this prospective phase II multicenter trial and treated with lenalidomide up to 30 mg daily for 16 weeks. Three patients had isolated del(5q), six had del(5q) plus one additional aberration, 14 had del(5q) and a complex karyotype, four had monosomy 5, and one had del(5q) identified by fluorescence in situ hybridization only.
Results
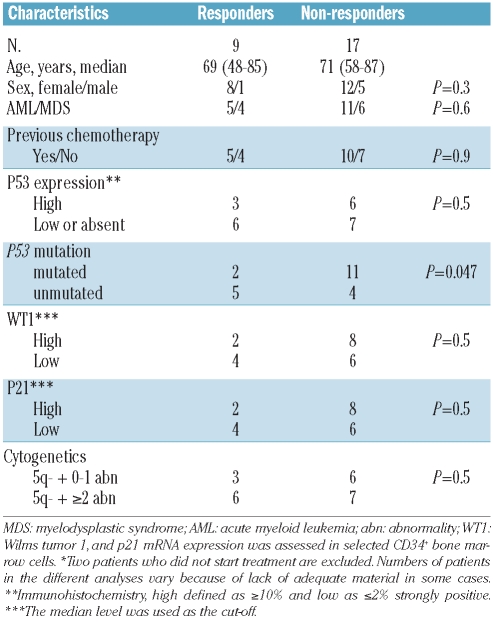
Major and minor cytogenetic responses, assessed by fluorescence in situ hybridization, were achieved in 5/26 (19%) and 2/26 (8%) patients, respectively, who received one or more dose of lenalidomide, while two patients achieved only a bone marrow response. Nine of all 26 patients (35%) and nine of the ten who completed the 16 weeks of trial responded to treatment. Using the International Working Group criteria for acute myeloid leukemia and myelodysplastic syndrome the overall response rate in treated patients with acute myeloid leukemia was 20% (3/15), while that for patients with myelodysplastic syndrome was 36% (4/11). Seven patients stopped therapy due to progressive disease and nine because of complications, most of which were disease-related. Response rates were similar in patients with isolated del(5q) and in those with additional aberrations. Interestingly, patients with TP53 mutations responded less well than those without mutations (2/13 versus 5/9, respectively; P=0.047). No responses were observed among 11 cases with deleterious TP53 mutations.
Conclusions
Our data support a role for higher doses of lenalidomide in poor prognosis patients with myelodysplastic syndrome and acute myeloid leukemia with deletion 5q. (Clinicaltrials.gov identifier NCT00761449).
Keywords: lenalidomide, myelodysplastic syndrome, acute myeloid leukemia, P53 mutation
Introduction
Azacytidine was approved for use in Europe in 2009, and has since then been used as first-line treatment for patients with high-risk myelodysplastic syndrome (MDS) and the former MDS category refractory anemia with excess of blasts. The overall response rate to azacytidine in the pivotal multicenter trial was 29%.1 However, there is limited information about response rates to azacytidine in patients with previously treated MDS, in patients with acute myeloid leukemia (AML) that has relapsed after chemotherapy, and in patients with specific molecular features.2
The presence of del(5q) or monosomy 5 in patients with higher-risk MDS and AML is associated with a poor outcome, regarless of whether the patients are untreated or managed with chemotherapy and stem cell transplantation.3–5 The response rate in AML patients with adverse cytogenetics including del(5q) or monosomy 5 is around 35% with conventional induction therapy.6 AML patients with chromosome 5 abnormalities who are not eligible for standard treatment or have refractory or relapsed disease have a dismal prognosis.7
Lenalidomide induces a cytogenetic response in two-thirds of patients with low and intermediate-risk 1 MDS with del(5q).8 A recent study evaluated the effect of lenalidomide 10 mg/day for 21/28 days in patients with intermediate-risk 2 and high-risk MDS and del(5q).3,9 Thirteen of 47 patients achieved a hematologic response, including seven who had cytogenetic responses. Most responses were seen in patients with isolated del(5q), and none of the 27 patients with a complex karyotype responded to treatment. In two recent studies of patients with AML, complete remission was achieved in 16–30% of patients treated with lenalidomide at doses up to 50 mg/day; however, none of the responders had chromosome 5 abnormalities.10,11
In vitro studies have shown that lenalidomide selectively inhibits growth of del(5q) progenitors while sparing normal progenitors.12,13 These studies also suggested that lenalidomide activates certain genes, such as SPARC, Cdc25C and PP2Acα, located within the commonly deleted region on 5q31, which could potentially explain its therapeutic effect in the 5q- syndrome.
Chromosome 5 abnormalities frequently occur within a complex karyotype and TP53 mutations are common in this group of patients.14–16 TP53 mutant clones in low-risk 5q- MDS may be resistant to lenalidomide, and there are studies suggesting that histone modifications may activate p21 transcription independently of P53.17,18 The P53 pathway is, therefore, of specific interest both in MDS and in relation to lenalidomide treatment.
We hypothesized that higher doses of lenalidomide may induce cytogenetic and clinical responses in patients with high-risk MDS and AML and chromosome 5 abnormalities and enrolled 28 patients not eligible for any type of standard treatment in a phase II trial assessing lenalidomide as monotherapy. A documented anti-tumor effect would constitute a basis for subsequent combination trials.
Design and Methods
Study group
Eligible patients for this prospective clinical phase II multicenter trial fulfilled the following inclusion criteria: (i) age 18 years or more; (ii) International Prognostic Scoring System intermediate-risk 2 or high-risk MDS, or AML with a karyotype including del(5q) or monosomy 5 confirmed by fluorescence in situ hybridization (FISH) for del(5q31); (iii) considered, by the treating physician, in accordance with Nordic Guidelines (a) not eligible for primary treatment with induction chemotherapy or stem cell transplantation (MDS, AML in the very elderly), (b) refractory to induction therapy, (c) not suitable for re-induction chemotherapy at relapse after induction chemotherapy, (d) not suitable for conventional relapse therapy at relapse after stem cell transplantation; (iv) signed informed consent; and (v) negative pregnancy test for women of childbearing potential. Prior therapy with lenalidomide was an exclusion criterion. The trial was approved by the National Ethical committees of Sweden, Denmark, and Norway, conducted in accordance with the Declaration of Helsinki and registered at www.clinicaltrials.gov: NCT00761449. The Nordic MDS Group was the sponsor of this trial, for which Celgene provided the study drug and a research grant.
Study design
The main objective of this prospective non-randomized multicenter phase II trial was to study the efficacy of lenalidomide at inhibiting the myeloid tumor clone containing del(5q) or monosomy 5. For this reason major cytogenetic response (assessed by FISH) after 16 weeks of treatment was chosen as the primary endpoint. Secondary objectives were to study the safety of increasing doses of lenalidomide and to assess hematologic response and the predictive value of a series of biomarkers for response to treatment. The total study period was 16 weeks. Studies of patients with lower-risk MDS with del(5q) have shown that there is a significant inter-patient variability regarding therapeutic sensitivity and drug-induced bone marrow suppression.8 The intended cohort of patients was also expected to show a marked variation in terms of age, bone marrow cellularity, previous treatment and biological sensitivity to lenalidomide. We, therefore, chose an intra-patient dose-escalation procedure, allowing for dose titration of individual patients, instead of a phase I cohort design.19 Oral lenalidomide was given at a dose of 10 mg/day in weeks 1 to 5. The dose was increased to 20 mg/day in weeks 6 to 9, and to 30 mg/day in weeks 10 to 16, if toxicity was acceptable, based on data from List et al.20 Toxicity, including cytopenias, was graded according to National Cancer Institute toxicity criteria (CTC for Adverse Events, version 3.0). In the case of suspected drug-related toxicity the dose could be lowered to 5 mg/day. It was expected that several patients would have severe pancytopenia at the start of treatment, and dose modifications due to asymptomatic hematologic toxicity were not, therefore, performed. In the case of worsening cytopenia and/or suspected drug-related adverse events, the dose could be modified according to the clinical judgment of the investigator. Doses were reduced in the case of grade 3 or 4 non-hematologic toxicity.
Morphology and cytogenetics
Metaphase cytogenetic analysis performed within 6 months of inclusion was used to establish the karyotype and confirm the presence of del(5q) or monosomy 5. During the screening phase the loss of the chromosome region containing the LSI EGR1/D5S23,D5S721 probe, del(5q) was verified by FISH. FISH on bone marrow smears was centrally and blindly analyzed at inclusion, week 8 and week 16. Blood and bone marrow morphology, including a biopsy for assessment of cellularity, was assessed at inclusion and weeks 4, 8, 12 and 16, and centrally reviewed at inclusion and week 16, according to the standard procedures of the Nordic MDS Group.21,22 Ten patients were analyzed after 16 weeks of treatment. Bone marrow aspirates at inclusion and at the end of study were subject to lymphoprep separation, extraction of DNA from mononuclear cells, further CD34+ separation by a magnetic activated cell sorting (MACS) system and isolation of RNA, as previously described.12
Immunohistochemical studies for p53 protein
Paraffin sections (4 μm) on Super Frost microscopic slides were pre-treated at 60°C for at least 60 min. The monoclonal antibody, DO-1 (Santa Cruz, Biotechnology, Inc.), which recognizes both wild-type and mutant p53 proteins, was used as the primary antibody to detect p53 protein expression (Ventana, BenchMark XT iVIEW DAB v3). The staining of the p53 signals was evaluated at high magnification (100x objective) in randomly selected fields and scored as negative, weak (p53+/light brown), or strong (p53++/dark brown) nuclear staining according to Iwasaki et al.23 At least 300 cells were counted. Immunohistochemical studies for p53 were also performed on 20 normal bone marrow samples (control group).
Assessment of genetic markers
Poor prognostic factors in MDS and AML following MDS include quantitative assessment of the WT1 transcript.24,25 In addition, we assessed P21 expression in relation to P53 mutational status. Real-time quantitative polymerase chain reaction (PCR) analysis was used to evaluate the expression levels of the WT1 and CDKN1A (p21) genes in CD34+ bone marrow cells. Pre-developed TaqMan Assays were used (Assays-on-Demand; Applied Biosystems, Foster City, CA, USA), and reactions were run on a LightCycler 480 real-time PCR system (Roche Diagnostics, Lewes, UK). The expression level of the GAPDH gene was used to normalize for differences in input cDNA.
The coding sequences and splice sites of exons 5–9 of the TP53 gene were scanned for mutations by PCR in combination with denaturing gradient gel electrophoresis, as previously described.26 Abnormal electrophoretic bands were excised from the gels, the DNA was eluted in water, then re-amplified, and automatically sequenced.
Response criteria
FISH analysis (percentage of positive bone marrow cells) was used to establish the baseline percentage of del(5q) cells, against which the response was calculated. The primary endpoint of the study was major cytogenetic response after 16 weeks of treatment, as evaluated by FISH. The International Working Group cytogenetic response criteria for MDS are based on conventional cytogenetic karyotyping but allow FISH for specific cytogenetic aberrations. We aimed for a differentiated response scale (3 levels) and, therefore, used the following criteria for cytogenetic response: complete response: less than 5% (detection level for FISH) del5(q) or monosomy 5 FISH-positive cells; major response, 50% or greater reduction in the percentage of del5(q) or monosomy 5 FISH-positive cells; minor response, 25% or greater reduction in the percentage of del5(q) or monosomy 5 FISH-positive cells. Secondary FISH endpoints encompassed minor and complete cytogenetic responses after 16 weeks and any cytogenetic response after 8 weeks. Hematologic response was assessed and reported using International Working Group response criteria for MDS and AML.27,28 Safety was evaluated by monitoring and recording adverse events.
Study evaluation and statistics
This was a single-arm, open-label, phase II clinical trial. The expected number of patients for this study was calculated according to a Simon optimal two-stage design. The safety population consisted of all patients, and the evaluable population of patients was defined as subjects who received at least one dose of lenalidomide. The predicted cytogenetic response rate (major + minor cytogenetic responses) without treatment was considered to be zero. If two or more cytogenetic responses were observed in the first 15 evaluable patients, 15 additional patients were to be recruited; otherwise, the study was to be terminated. If five or more cytogenetic responses were observed in 30 evaluable patients (response rate ≥ 15%), the regimen was considered sufficiently active to be further evaluated in combination studies.
Continuous variables were summarized using the mean or median (range) value depending on the distribution of data, and frequency tables were used to summarize categorical variables. A correlation between TP53 mutational status and response was investigated using univariate analysis with a T-test for independent samples, as the size of the cohort, according to plan, did not allow for multivariate analysis. χ2 tests were used to compare responders and non-responders. A two-sided α level of 0.05 was used to determine statistical significance.
Results
Patients’ characteristics
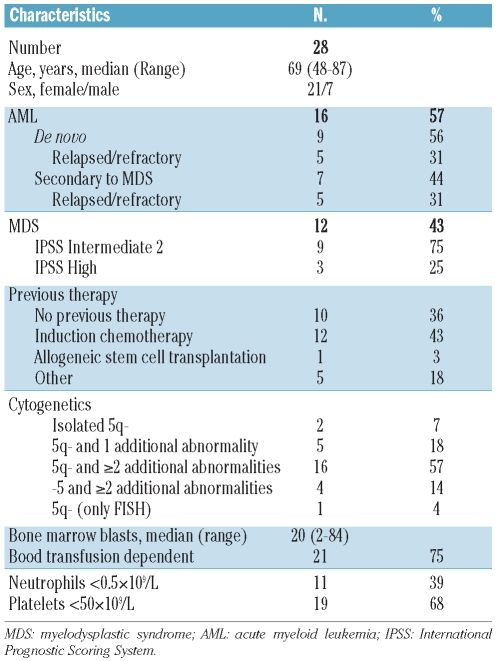
Twenty-eight patients (7 men and 21 women) from ten centers were enrolled between October 2007 and December 2009; the median age of these patients was 69 years (range, 48–87 years) (Table 1). Sixteen patients had AML and 12 had MDS (9 intermediate-risk 2 and 3 high-risk), 21 patients were transfusion-dependent and 11 and 19 had grade 4 neutropenia and thrombocytopenia, respectively, prior to the start of treatment. The median bone marrow blast percentage was 20% (range, 2–84%). Three patients had isolated del(5q), six had del(5q) plus one additional aberration, and 14 had del(5q) plus two or more additional abnormalities. Four patients had monosomy 5, and in one patient with severe marrow fibrosis, del(5q31) was confirmed by FISH only. In 25 patients both cytogenetics and FISH showed del(5q) and monosomy 5. In two patients the cytogenetics showed monosomy 5 but the FISH showed del(5q). Seven patients had chromosome 17 abnormalities but none had a 17p deletion. Thirteen patients had received induction chemotherapy prior to inclusion in the study. Three patients were treated after failure of azacytidine therapy. One patient had therapy-related AML. A detailed characterization of all 28 patients is given in Online Supplementary Table S1.
Table 1.
Patients’ demographics and clinical characteristics.
Treatment results
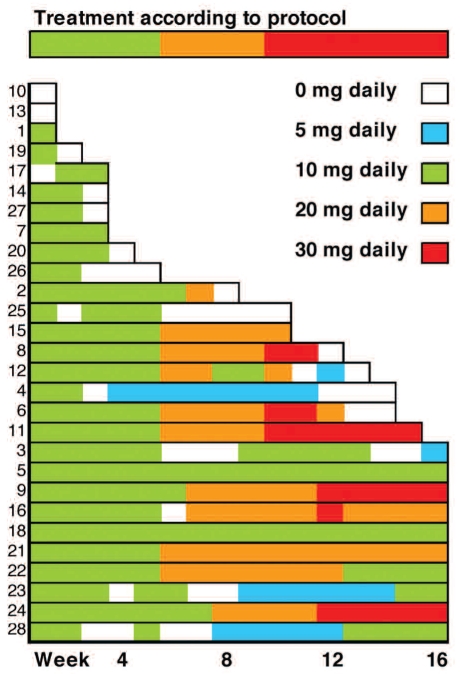
Two patients died due to rapid deterioration during the screening phase and received no lenalidomide. More than two of the first 15 evaluable patients achieved a cytogenetic response. The study was closed after a total of 28 patients had been enrolled after the approval of azacytidine by the EMEA and introduction of this drug as first-line treatment for high-risk MDS. Of the 26 evaluable patients, ten and eight completed 16 and 8 or more weeks of treatment, respectively (Figure 1). Eight patients withdrew before the first follow-up at 8 weeks. Of the 26 evaluable patients, five achieved a major cytogenetic response (19%), two achieved a minor cytogenetic response (8%), and another two had a bone marrow response (8%) resulting in an overall response rate of 35% (CI:19–50%). The total cytogenetic response rate was 27% (CI: 13–41%) (Table 2). Four of the patients with a major cytogenetic response and one with a minor cytogenetic response also had a bone marrow response and/or hematologic response. One patient had both bone marrow and hematologic responses, but no cytogenetic response. All responders completed 16 weeks of treatment. Using the International Working Group criteria for AML and MDS, the overall response rate in treated patients with AML was 20% (3/15), while that in patients with MDS was 36% (4/11).
Figure 1.
Summary of dosages and duration of lenalidomide treatment in all 28 patients.
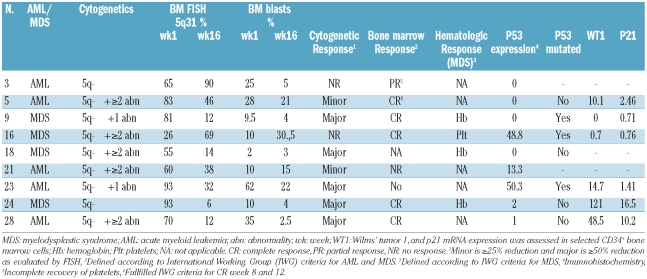
Table 2.
Characteristics of the nine responders in the study.
In 11 patients (39%) the lenalidomide dose was increased to 20 mg/day, and in six (21%) a further increase to 30 mg/day was possible (Figure 1). Seven patients who reached week 6 were not subject to further dose escalation due to neutropenic fever (n=4), neutropenia (n=1), rash (n=1) and an allergic reaction (n=1). In four patients who received 20 mg/day the dose could not be increased to 30 mg/day because of progressive disease (n=1), abdominal pain (n=1), rash (n=1) and hematologic toxicity (n=1). Among the nine responding patients the maximum dose of lenalidomide was 10 mg/day (n=5), 20 mg/day (n=1) and 30 mg/day (n=3). Three of the five patients with a major cytogenetic response achieved this response at week 16 and two at week 8. Of the three patients with a major cytogenetic response at week 16, one received lenalidomide 30 mg/day according to protocol, while two did not tolerate dose escalation. Four of the five patients with a major cytogenetic response were previous untreated and one had received induction therapy. Two of the nine responders had a baseline neutrophil count less than 0.5x109/L and five had a platelet count less than 50x109/L. None of the patients in whom azacytidine treatment failed responded to therapy with lenalidomide.
Overall survival and follow-up of responding patients
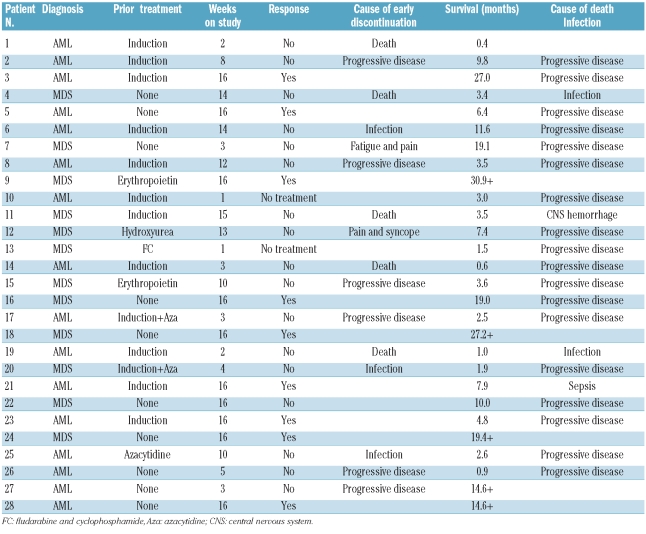
Patients responding at 16 weeks were, according to protocol, eligible for compassionate use of lenalidomide treatment. Four responders continued lenalidomide with response durations of 6, 19+, 15+ and 27+ months. Four patients did not continue lenalidomide and had response durations of 1, 1, 3 and 8 months. In one patient the duration of the cytogenetic response could not be assessed because no further bone marrow samples were taken during the follow up. The median overall survival time for the whole cohort was 5.6 months (range, 0.4–30.9+ months), while it was 19.0 months (range, 4.8–30.9+ months) in responding patients (Table 4 and Online Supplementary Figure S1).
Table 4.
Time on study and survival.
p53 protein expression by immunohistochemistry
Paraffin blocks were available for 24 patients at inclusion, and in ten of these also at week 16. Cells with strong nuclear p53 expression (p53++) were seen in 15/24 patients before the start of treatment (Online Supplementary Table S1). In the control subjects (n=20) p53++ cells were detected in two bone marrow samples at levels less than 1%, while 18 samples stained negative. The percentage of p53++ cells was 1–2% in five patients, and ranged between 13–65% in the other ten patients. A cut-off of 10% was, therefore, used when comparing immunohistochemical results and TP53 mutational status. In six of the nine patients who responded to treatment, p53 expression was absent or low. In the remaining three patients, the percentage of p53-expressing cells decreased in one patient and increased slightly in the other two patients.
TP53 mutational status
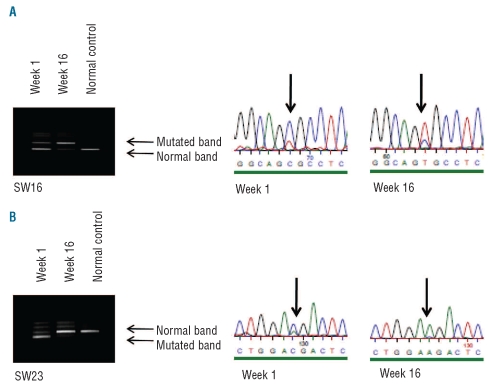
Material to assess TP53 mutational status was available from 24 patients. Seventeen different mutations were observed in 15 patients (Online Supplementary Table S1). Two patients with confirmed mutations died before starting treatment and are not included in Table 3. The majority of mutations were missense mutations (13/18), while frame shift mutations were observed in three patients and a splice mutation in one patient. Post-treatment samples were available for two patients with TP53 mutations. In patient SW16 (Figure 2) the relative amount of TP53 mutant cells (R175H) increased after treatment. This patient had a complete bone marrow response but no cytogenetic response, indicating that the 5q- clone was resistant to lenalidomide. In patient SW23 the mutant clone (E258D) was reduced by lenalidomide. This patient had a major cytogenetic response and a reduction of bone marrow blasts from 62% to 22%. However, E258D is a conservative substitution that may not have a dramatic influence on p53 function. The results of immunohistochemical staining for p53 correlated well with TP53 mutational status in that all patients with more than 10% positively stained cells had a TP53 mutation. However, only 12/15 patients with a mutation had more than 10% p53++ cells, according to the immmunohistochemical staining. Two of the patients with a TP53 mutation whose cells stained negative for p53 had a frame shift mutation leading to a premature stop codon, and one had a G266V mutation.
Table 3.
Characteristics of responders and non-responders in 26* lenalidomide-treated patients.
Figure 2.
Denaturating gradient cell electrophoresis and sequencing analysis of DNA from bone marrow cells isolated before (week 1) and after treatment with lenalidomide (week 16). In patient SW16 (A) the relative amount of TP53 mutant cells (R175H) increased after treatment, while in patient SW23 (B) the mutant clone (E258D) was reduced by lenalidomide.
p21 and WT1 expression
CD34+ cell separation yielded sufficient cells for extraction of RNA in 22/28 patients at the start of treatment (Online Supplementary Table S1, Online Supplementary Figure S2). The levels of expression of p21 and WT1 mRNA in patients’ CD34+ cells were compared to those of normal CD34+ cells from two healthy donors. Both p21 expression (19/22, P=0.001) and WT1 expression (18/22, P=0.004) were significantly higher in patients than in controls (Online Supplementary Figure S2).
Markers for a response to treatment
There were no significant differences in clinical and morphological inclusion variables between responders and non-responders (Table 3). However, no patient with monosomy 5 responded. The response rates in patients with del(5q) alone, del(5q) plus one abnormality, and del(5q) plus two or more abnormalities were 67%, 17% and 46%, respectively. Two of 13 patients with a TP53 mutation compared to five of nine without a mutation responded (P=0.047). Importantly, no patients with deleterious mutations responded to treatment. p53++ expression, categorized as absent or low (≤2%) versus high (>10%), was not associated with a response to treatment, and expression of WT1 and P21 genes did not differ significantly between the groups.
Safety evaluation
Sixteen patients terminated therapy early due to progressive disease (n=7), infection (n=6), central nervous system hemorrhage (n=1), fatigue and pain (n=1) and pain and syncope (n=1). Five of these patients died due to infection (n=3), progressive disease (n=1) and central nervous system hemorrhage (n=1) (Table 4). Five AML patients progressed between weeks 3–12, and three MDS patients progressed to AML at weeks 1, 4 and 10.
Six patients developed grade 3–4 neutropenia and seven developed grade 3–4 thrombocytopenia. Thirteen patients experienced grade 3 or 4 infections or febrile neutropenia. Other grade 3 or 4 toxicities included skin rash (n=3, whereof one blistering), intracerebral hemorrhage (n=2), abdominal pain (n=2), syncope (n=1), thromboembolism (n=1), bleeding (n=1), arthritis (n=1) and mucositis (n=1). Details of the adverse events are presented in Online Supplementary Table S2).
Twenty-six serious adverse events were reported in 18 of the patients. The main criterion for a serious adverse event was admission to hospital as an in-patient (92%). In five patients the outcome of the serious adverse event was death. The cause of death was the underlying disease or complications of the underlying disease. For these patients the causality/relationship with lenalidomide was judged as not suspected or not related.
Discussion
This phase II hypothesis-generating study shows for the first time that monotherapy with higher doses of lenalidomide than conventionally used for low-risk del(5q) MDS was able to inhibit the del(5q)-containing tumor clone in a cohort of patients with extremely advanced MDS or AML and a karyotype involving a deletion of 5q. In fact, five of ten patients who completed 16 weeks of lenalidomide treatment had a major cytogenetic response. One of the patients subsequently underwent allogeneic stem cell transplantation and remains in remission after 2 years. This shows that recent in vitro findings demonstrating a selective inhibitory effect of progenitors containing del(5q) may be translated into a therapeutic response in vivo.12,13 There was no difference in response between patients with isolated del(5q) and those with complex cytogenetic patterns, indicating that lenalidomide may have an anti-tumor effect also in the latter group of patients. A recent study evaluating an average dose of 7.5 mg daily in patients with high-risk MDS showed limited effects in patients with del(5q) and additional chromosomal abnormalities, hence the higher doses may have been important in our study.9 It is, however, likely that the patients who completed 16 weeks of trial and reached the highest lenalidomide dose may constitute a selection of patients with more favorable features, and that the high response rate in this cohort should not be extrapolated to the population of high-risk del(5q) MDS and AML without further studies. The complete remission rate in AML patients who started treatment in our study was 13%. In two recent AML studies with lenalidomide none of the patients with chromosome 5 abnormalities had a response.10,11
The enrolled cohort was characterized by multiple risk factors and patients generally had very advanced disease. Of the 26 patients who started treatment, 15 had AML and 15 were refractory to or relapsing after chemotherapy, and all patients treated de novo were elderly. However, neither previous treatment nor age showed any association with response to treatment. WT1 expression, which has been associated with poor prognosis in MDS and AML evolving from MDS, as well as P21 expression, which is associated with P53 function and is frequently dysregulated in myeloid malignancies, were also similar in responding and non-responding patients.29
As expected, we identified a high frequency of TP53 mutated cases (15/24; 62.5%) in the cohort, all but one of which were most likely associated with disruption of TP53 function. Interestingly, the presence of a TP53 mutation was significantly associated with failure of lenalidomide treatment and only two cases with mutations responded to this treatment. In case 23 (E258D) a major response was observed with a reduction of the mutant clone, but in case 16, with a hot spot mutation R175H, the mutant clone increased in spite of morphological and hematologic responses (Figure 2). It is well known that alterations of different TP53 codons are associated with different phenotypes. The R175H is a deleterious change which has been shown to abrogate both protein conformation and DNA binding.30 The E258D mutation has less frequently been involved in cancer. The glutamine to aspartate change is a conservative amino acid substitution and is located in the very last codon of the zinc binding domain L3 and may thus be less important for p53 function. All together these observations indicate that clones with deleterious TP53 mutations are insensitive to lenalidomide.
In a recently published study by our group we identified small subclones of mutated TP53 in ten of 55 patients with low- and intermediate-1 risk MDS with del(5q) using deep sequencing by Roche 454 technology.31 Importantly, mutations were associated with a significantly higher risk of leukemic transformation, and also a poorer survival in patients with intermediate-risk 1 MDS. There was no relation between mutational status and other risk factors such as isolated versus other karyotypes, or percentage of blasts. In one patient treated with lenalidomide there was a clear reduction in FISH-positive cells, but no effect on the size of the mutated clone, indicating that the TP53 mutation rendered the subclone resistant to the drug. A similar negative effect of TP53 mutations on the probability of response is also suggested by the present study, in which treatment had no effect on the majority of mutated cases. A recent phase I study did, however, indicate that the combination of lenalidomide and azacytidine may have an effect that is superior to that of each drug alone.32
Overall there was a good correlation between TP53 mutational status and protein expression. Importantly, all patients with more than 10% cells stained positively by immunohistochemistry had TP53 mutations, suggesting that immunohistochemistry could be used as an easy and cost-effective screening method. Lack of immunohistochemical expression does not, however, preclude a mutation so sequencing techniques may be indicated in negatively stained cases. All but one of the missense mutations were associated with greater than 10% p53 expression, and immunohistochemistry was negative in the two cases which only had frame shift mutations leading to a premature termination codon. Since p21 acts downstream of p53 in the regulation of cell cycling and apoptosis it has been suggested that p21 up-regulation by lenalidomide may compensate for p53 deficiency.17,29 Surprisingly, p21 expression levels at diagnosis were not particularly low in the TP53 mutant cases, and the importance of p21 expression levels in this cohort remains unclear.
Lenalidomide treatment was associated with a relatively high rate of adverse events, and neutropenic infections, in particular, were frequent. Many of these were probably related to the underlying severe disease and patients with major responses tolerated the treatment well. We conclude, however, that increased doses of lenalidomide should by viewed as a relatively toxic treatment in high-risk MDS and AML with del(5q).
The primary aim of this study of patients with advanced disease was to evaluate the effect of lenalidomide on the del(5q) tumor clone and hence to develop a basis for subsequent trials using lenalidomide in combination with other treatments. Importantly seven of the ten patients who completed the trial achieved a cytogenetic response. Six of these had a median response duration of 9.5 months (range, 1–20 months), and one underwent stem cell transplantation. It is, therefore, conceivable that lenalidomide given upfront together with azacytidine or induction chemotherapy may actually lead to higher response rates and potentially reduce the risk of relapse after stem cell transplantation. Based on these promising results, we propose that subsequent studies should evaluate the addition of lenalidomide in the primary treatment of del(5q) high-risk MDS and AML.
Footnotes
Funding: Celgene provided the drug and a research grant
The online version of this article has a Supplementary Appendix.
Authorship and Disclosures
The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
References
- 1.Fenaux P, Mufti GJ, Hellstrom-Lindberg E, Santini V, Finelli C, Giagounidis A, et al. Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: a randomised, open-label, phase III study. Lancet Oncol. 2009;10(3):223–32. doi: 10.1016/S1470-2045(09)70003-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Borthakur G, Huang X, Kantarjian H, Faderl S, Ravandi F, Ferrajoli A, et al. Report of a phase 1/2 study of a combination of azacitidine and cytarabine in acute myelogenous leukemia and high-risk myelodysplastic syndromes. Leuk Lymphoma. 2010;51(1):73–8. doi: 10.3109/10428190903318329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Greenberg P, Cox C, LeBeau MM, Fenaux P, Morel P, Sanz G, et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood. 1997;89(6):2079–88. [PubMed] [Google Scholar]
- 4.Grimwade D, Hills RK, Moorman AV, Walker H, Chatters S, Goldstone AH, et al. Refinement of cytogenetic classification in acute myeloid leukemia: determination of prognostic significance of rare recurring chromosomal abnormalities amongst 5,876 younger adult patients treated in the UK Medical Research Council trials. Blood. 2010;116(3):354–65. doi: 10.1182/blood-2009-11-254441. [DOI] [PubMed] [Google Scholar]
- 5.Haase D, Germing U, Schanz J, Pfeilstocker M, Nosslinger T, Hildebrandt B, et al. New insights into the prognostic impact of the karyotype in MDS and correlation with subtypes: evidence from a core dataset of 2124 patients. Blood. 2007;110(13):4385–95. doi: 10.1182/blood-2007-03-082404. [DOI] [PubMed] [Google Scholar]
- 6.Grimwade D, Walker H, Harrison G, Oliver F, Chatters S, Harrison CJ, et al. The predictive value of hierarchical cytogenetic classification in older adults with acute myeloid leukemia (AML): analysis of 1065 patients entered into the United Kingdom Medical Research Council AML11 trial. Blood. 2001;98(5):1312–20. doi: 10.1182/blood.v98.5.1312. [DOI] [PubMed] [Google Scholar]
- 7.Ferrara F, Palmieri S, Mele G. Prognostic factors and therapeutic options for relapsed or refractory acute myeloid leukemia. Haematologica. 2004;89(8):998–1008. [PubMed] [Google Scholar]
- 8.List A, Dewald G, Bennett J, Giagounidis A, Raza A, Feldman E, et al. Lenalidomide in the myelodysplastic syndrome with chromosome 5q deletion. N Engl J Med. 2006;355(14):1456–65. doi: 10.1056/NEJMoa061292. [DOI] [PubMed] [Google Scholar]
- 9.Ades L, Boehrer S, Prebet T, Beyne-Rauzy O, Legros L, Ravoet C, et al. Efficacy and safety of lenalidomide in intermediate-2 or high-risk myelodysplastic syndromes with 5q deletion: results of a phase 2 study. Blood. 2009;113(17):3947–52. doi: 10.1182/blood-2008-08-175778. [DOI] [PubMed] [Google Scholar]
- 10.Blum W, Klisovic RB, Becker H, Yang X, Rozewski DM, Phelps MA, et al. Dose escalation of lenalidomide in relapsed or refractory acute leukemias. J Clin Oncol. 2010;28(33):4919–25. doi: 10.1200/JCO.2010.30.3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fehniger TA, Uy GL, Trinkaus K, Nelson AD, Demland J, Abboud CN, et al. A phase II study of high dose lenalidomide as initial therapy for older patients with acute myeloid leukemia. Blood. 2011;117(6):1828–33. doi: 10.1182/blood-2010-07-297143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pellagatti A, Jadersten M, Forsblom AM, Cattan H, Christensson B, Emanuelsson EK, et al. Lenalidomide inhibits the malignant clone and up-regulates the SPARC gene mapping to the commonly deleted region in 5q- syndrome patients. Proc Natl Acad Sci USA. 2007;104(27):11406–11. doi: 10.1073/pnas.0610477104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wei S, Chen X, Rocha K, Epling-Burnette PK, Djeu JY, Liu Q, et al. A critical role for phosphatase haplodeficiency in the selective suppression of deletion 5q MDS by lenalidomide. Proc Natl Acad Sci USA. 2009;206(31):12974–9. doi: 10.1073/pnas.0811267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Christiansen DH, Andersen MK, Pedersen-Bjergaard J. Mutations with loss of heterozygosity of p53 are common in therapy-related myelodysplasia and acute myeloid leukemia after exposure to alkylating agents and significantly associated with deletion or loss of 5q, a complex karyotype, and a poor prognosis. J Clin Oncol. 2001;19(5):1405–13. doi: 10.1200/JCO.2001.19.5.1405. [DOI] [PubMed] [Google Scholar]
- 15.Merlat A, Lai JL, Sterkers Y, Demory JL, Bauters F, Preudhomme C, et al. Therapy-related myelodysplastic syndrome and acute myeloid leukemia with 17p deletion. A report on 25 cases. Leukemia. 1999;13(2):250–7. doi: 10.1038/sj.leu.2401298. [DOI] [PubMed] [Google Scholar]
- 16.Pedersen-Bjergaard J, Andersen MK, Andersen MT, Christiansen DH. Genetics of therapy-related myelodysplasia and acute myeloid leukemia. Leukemia. 2008;22(2):240–8. doi: 10.1038/sj.leu.2405078. [DOI] [PubMed] [Google Scholar]
- 17.Escoubet-Lozach L, Lin IL, Jensen-Pergakes K, Brady HA, Gandhi AK, Schafer PH, et al. Pomalidomide and lenalidomide induce p21 WAF-1 expression in both lymphoma and multiple myeloma through a LSD1-mediated epigenetic mechanism. Cancer Res. 2009;69(18):7347–56. doi: 10.1158/0008-5472.CAN-08-4898. [DOI] [PubMed] [Google Scholar]
- 18.Jadersten M, Saft L, Pellagatti A, Gohring G, Wainscoat JS, Boultwood J, et al. Clonal heterogeneity in the 5q- syndrome: p53 expressing progenitors prevail during lenalidomide treatment and expand at disease progression. Haematologica. 2009;94 (12):1762–6. doi: 10.3324/haematol.2009.011528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Amato RJ, Harris P, Dalton M, Khan M, Alter R, Zhai Q, et al. A phase II trial of intra-patient dose-escalated sorafenib in patients (pts) with metastatic renal cell cancer (MRCC) J Clin Oncol (Meeting Abstracts) 2007;25(18 suppl):5026. [Google Scholar]
- 20.List A, Kurtin S, Roe DJ, Buresh A, Mahadevan D, Fuchs D, et al. Efficacy of lenalidomide in myelodysplastic syndromes. N Engl J Med. 2005;352(6):549–57. doi: 10.1056/NEJMoa041668. [DOI] [PubMed] [Google Scholar]
- 21.Jadersten M, Malcovati L, Dybedal I, Della Porta MG, Invernizzi R, Montgomery SM, et al. Erythropoietin and granulocyte-colony stimulating factor treatment associated with improved survival in myelodysplastic syndrome. J Clin Oncol. 2008;26 (21):3607–13. doi: 10.1200/JCO.2007.15.4906. [DOI] [PubMed] [Google Scholar]
- 22.Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, et al. WHO classification of tumours of haematopoietic and lymphoid tissues. IARC Press; Lyon: 2008. [Google Scholar]
- 23.Iwasaki T, Murakami M, Sugisaki C, Sobue S, Ohashi H, Asano H, et al. Characterization of myelodysplastic syndrome and aplastic anemia by immunostaining of p53 and hemoglobin F and karyotype analysis: differential diagnosis between refractory anemia and aplastic anemia. Pathol Int. 2008;58(6):353–60. doi: 10.1111/j.1440-1827.2008.02236.x. [DOI] [PubMed] [Google Scholar]
- 24.Cilloni D, Gottardi E, Messa F, Fava M, Scaravaglio P, Bertini M, et al. Significant correlation between the degree of WT1 expression and the International Prognostic Scoring System Score in patients with myelodysplastic syndromes. J Clin Oncol. 2003;21(10):1988–95. doi: 10.1200/JCO.2003.10.503. [DOI] [PubMed] [Google Scholar]
- 25.Tamura H, Dan K, Yokose N, Iwakiri R, Ohta M, Sakamaki H, et al. Prognostic significance of WT1 mRNA and anti-WT1 antibody levels in peripheral blood in patients with myelodysplastic syndromes. Leuk Res. 2010;34(8):986–90. doi: 10.1016/j.leukres.2009.11.029. [DOI] [PubMed] [Google Scholar]
- 26.Guldberg P, Nedergaard T, Nielsen HJ, Olsen AC, Ahrenkiel V, Zeuthen J. Singlestep DGGE-based mutation scanning of the p53 gene: application to genetic diagnosis of colorectal cancer. Hum Mutat. 1997;9(4):348–55. doi: 10.1002/(SICI)1098-1004(1997)9:4<348::AID-HUMU8>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 27.Cheson BD, Bennett JM, Kopecky KJ, Buchner T, Willman CL, Estey EH, et al. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J Clin Oncol. 2003;21(24):4642–9. doi: 10.1200/JCO.2003.04.036. [DOI] [PubMed] [Google Scholar]
- 28.Cheson BD, Greenberg PL, Bennett JM, Lowenberg B, Wijermans PW, Nimer SD, et al. Clinical application and proposal for modification of the International Working Group (IWG) response criteria in myelodysplasia. Blood. 2006;108(2):419–25. doi: 10.1182/blood-2005-10-4149. [DOI] [PubMed] [Google Scholar]
- 29.Abbas T, Dutta A. p21 in cancer: intricate networks and multiple activities. Nat Rev Cancer. 2009;9(6):400–14. doi: 10.1038/nrc2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zambetti GP, Levine AJ. A comparison of the biological activities of wild-type and mutant p53. FASEB J. 1993;7(10):855–65. doi: 10.1096/fasebj.7.10.8344485. [DOI] [PubMed] [Google Scholar]
- 31.Jädersten M, Saft L, Smith A, Kulasekararaj A, Pomplun S, Hedlund A, et al. TP53 mutations in low-risk 1 myelodysplastic syndromes with del(5q) predict disease progression. J Clin Oncol. 2010;29(15):1971–9. doi: 10.1200/JCO.2010.31.8576. [DOI] [PubMed] [Google Scholar]
- 32.Sekeres MA, List AF, Cuthbertson D, Paquette R, Ganetzky R, Latham D, et al. Phase I combination trial of lenalidomide and azacitidine in patients with higher-risk myelodysplastic syndromes. J Clin Oncol. 2010;28(13):2253–8. doi: 10.1200/JCO.2009.26.0745. [DOI] [PMC free article] [PubMed] [Google Scholar]