Abstract
Background
Renal involvement is uncommon in diffuse large B-cell lymphoma. Recent data suggest that it is an independent risk factor for central nervous system relapse. We reviewed the clinical features, risk of central nervous system involvement, and survival of patients with diffuse large B-cell lymphoma with involvement of the kidney at diagnosis.
Design and Methods
All patients with diffuse large B-cell lymphoma and renal involvement diagnosed from January 1, 1982 to December 31, 2008 at the British Columbia Cancer Agency were retrospectively identified in the Lymphoid Cancer Database. Patients were included if they were 16 years old or over, had advanced stage disease [stage III/IV, or stage I/II with B symptoms or bulky mass (>10 cm)] and were treated with curative intent. Central nervous system involvement was diagnosed by cerebrospinal fluid cytology, radiology or clinically.
Results
We identified 55/2656 (2%) patients with diffuse large B-cell lymphoma and renal involvement at diagnosis. The male to female ratio was 2:1. The patients’ median age was 58 years. Bilateral renal involvement was present in 24 (44%) and stage IV disease in 50 (91%). The International Prognostic Index score was 3, 4 or 5 in 52 (95%), the glomerular filtration rate was less than 30 mL/min/m2 in 9 (16%) and elevated lactate dehydrogenase was recorded in 46 (84%). Twenty-five (46%) patients received CHOP plus rituximab and 30 (54%) received CHOP-like regimens without rituximab. In total, 20 (36%) patients had central nervous system involvement: four at the time of diagnosis and 16 at relapse. The median time to central nervous system relapse was 5.6 months (range, 1.2 months–4.6 years), and was not affected by the addition of rituximab (P=0.547). The 5-year overall and progression-free survival rates for the whole cohort were 29% and 25%, respectively. In patients who received rituximab, there were trends towards improved 5-year overall survival (43% versus 18%, P=0.071) and progression-free survival (40% versus 13%, P=0.057).
Conclusions
There is an exceptionally high incidence of central nervous system relapse in patients with diffuse large B-cell lymphoma and kidney involvement at diagnosis. The addition of rituximab may improve overall survival in this poor-risk population, likely through improved control of systemic disease.
Keywords: rituximab, aggressive non-Hodgkin’s lymphoma, kidney, central nervous system
Introduction
Renal parenchymal involvement is uncommon in diffuse large B-cell lymphoma (DLBCL). In almost all cases, renal involvement appears to be a secondary process, either by direct extension from a retroperitoneal mass or via hematogenous spread in the setting of disseminated disease.1,2 Primary renal non-Hodgkin’s lymphoma is even more uncommon, and only a few cases have been reported in the literature.3–8
There are limited data describing the clinical course and outcomes of patients with DLBCL and renal involvement and many prior studies were based on older lymphoma classification systems.9–12 Retrospective cohort studies reveal that these patients tend to present with widespread disease and have a poor outcome in part due to a high rate of central nervous system (CNS) relapse.13,14
A recently published report from the British Columbia Cancer Agency (BCCA) showed that kidney involvement is an independent risk factor for CNS relapse in patients with DLBCL in both the pre- and post-rituximab treatment eras.14 However, to date, there are no published studies directly evaluating the risk of CNS relapse exclusively in patients with renal involvement, especially in those treated with rituximab.
The purpose of this study was two-fold: (i) to evaluate the incidence of CNS relapse in an expanded cohort of patients with DLBCL and kidney involvement, and (ii) to determine the impact of rituximab on outcome and CNS relapse in this population of patients.
Design and Methods
Patients
All patients with biopsy-proven DLBCL, according to the current World Health Organization classification, and extranodal renal involvement, diagnosed between 1 January 1982 and 31 December 2008 were identified in the BCCA Lymphoid Cancer database. Patients were included if they were 16 years old or over and had advanced stage disease [stage III/IV, or stage I/II with B symptoms or bulky tumor (>10cm)]. Human immunodeficiency virus-positive individuals, and those with insufficient follow-up information were excluded. All cases were reviewed by a hematopathologist from the BCCA. The CNS was evaluated at diagnosis by imaging or cerebrospinal fluid analysis only if clinically indicated.
Extranodal renal involvement was diagnosed either by biopsy (surgical or core needle biopsy) or imaging (ultrasound, computed tomography, or magnetic resonance imaging). Patients with hydronephrosis secondary to ureteric obstruction by a retroperitoneal mass without obvious renal parenchymal involvement were excluded. Pre-treatment glomerular filtration rate was estimated using the Modification of Diet in Renal Disease study equation, which incorporates information on age, race, gender, and serum creatinine.15–17 Severe renal failure was defined as a glomerular filtration rate less than 30 mL/min/1.73 m2, in accordance with the National Kidney Foundation’s most recent clinical practice guidelines.18
Treatment and follow-up
All patients received at least one cycle of cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) chemotherapy or similar regimen with curative intent. After March 1, 2001, all patients received CHOP in combination with rituximab (R-CHOP). Intrathecal prophylaxis, consisting of alternating methotrexate (12 mg) and cytarabine (50 mg) for six doses, was administered upon completion of chemotherapy to patients with involvement of the bone marrow, peripheral blood, epidural space, testicles, or paranasal sinuses. After September 2002, only patients with paranasal sinus involvement received intrathecal prophylaxis in accordance with a change in institutional policy.
Baseline clinical, laboratory, pathology, and imaging information for each patient were obtained from their paper and/or electronic medical records. Follow-up information, including details on relapse and death, was obtained primarily through the Cancer Agency Information System. For those patients who relapsed, the sites of relapse were documented. When necessary, local physicians and hospitals were contacted to complete the follow-up data. This study was approved by the University of British Columbia BCCA Research Ethics Board.
Statistical analysis
The primary study endpoint was the time to CNS relapse, defined as new involvement of the CNS after chemotherapy, in isolation or as part of progressive disease involving other sites. CNS involvement was diagnosed by cerebrospinal fluid cytology, brain imaging (computed tomography or magnetic resonance imaging), or objective neurological examination findings. The time to CNS relapse was calculated from the initial date of pathological diagnosis to the date of CNS relapse. Patients with CNS involvement at diagnosis were not included in the time to CNS relapse analyses.
Overall survival was calculated from the date of initial diagnosis [or from the date of CNS relapse where applicable (overall survival after CNS relapse)] to the date of last follow-up or death from any cause. Progression-free survival was calculated from the date of diagnosis to the date of first disease progression or relapse following treatment, or death from any cause. Patients alive and without progressive disease were censored on the date of their last follow-up visit. Survival analyses and time to CNS relapse were estimated using the Kaplan-Meier method and compared using the log-rank test.19
Patients’ characteristics at diagnosis and relapse were compared using Fisher’s exact test for discrete variables, and Wilcoxon’s rank sum test for continuous variables. Data were analyzed using the Statistical Package for the Social Sciences (SPSS version 14.0 for Windows; SPSS Inc, Chicago, IL, USA).
Results
Baseline characteristics
A total of 2656 patients with DLBCL diagnosed between 1982 and 2008 were initially identified. Sixty-two had renal involvement at diagnosis. Seven were excluded: two patients with misclassified diagnoses (T-cell immunoblastic lymphoma and renal cell carcinoma), and five patients with incomplete/unobtainable follow-up information. All cases were considered DLBCL not otherwise specified according to the 2008 World Health Organization classification, including cases diagnosed before 2001, which were updated accordingly. There were no cases of T-cell-rich B-cell lymphoma, primary mediastinal B-cell lymphoma, transformed or discordant lymphoma. The final cohort consisted of 55 (2%) patients with a median follow-up of 5.7 years (range, 8 months – 18 years) for living patients.
Renal involvement was diagnosed by histopathological studies in 19 patients (core needle biopsy n=8, laparotomy/open biopsy n=9, and nephrectomy n=2) and by imaging in 36 patients (computed tomography and/or ultrasound), which required demonstration of a solid metastasis to the kidney.
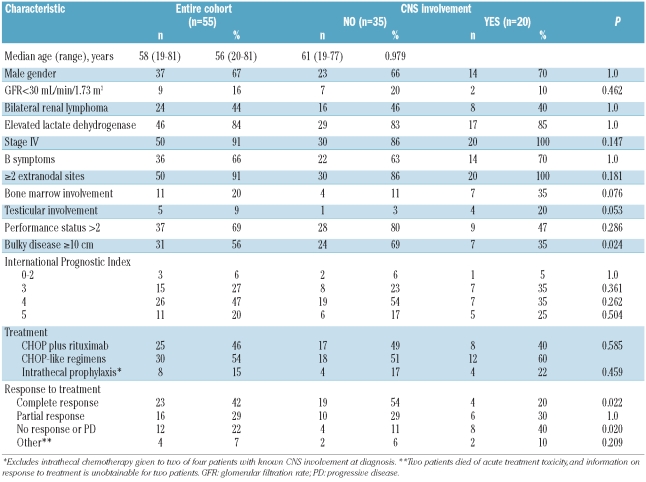
The patients’ baseline characteristics are displayed in Table 1. The median age at diagnosis was 58 years, and most patients were male (67%). The majority of patients had poor prognostic features, including elevated lactate dehydrogenase (84%), stage IV disease (91%), performance status of 2 or more (69%), high or high intermediate International Prognostic Index score (>3) (67%), involvement of other extranodal sites (91%), and bulky disease (56%). Severe renal failure was present in 16% of patients, and 44% had bilateral renal involvement.
Table 1.
Patients’ characteristics.
In total, 30 (54%) patients were treated with CHOP-like chemotherapy and 25 (46%) with R-CHOP (Table 1). Intrathecal prophylaxis was given to eight of 51 (15%) patients without known CNS involvement at diagnosis. Four patients had CNS disease at diagnosis: three were treated with CHOP (with intrathecal chemotherapy plus whole brain irradiation in one patient, whole brain irradiation in another patient, corticosteroids in the third patient), and one was treated with R-CHOP plus intrathecal chemotherapy.
Central nervous system involvement
Twenty (36%) patients had CNS involvement at some point in their disease course: 4 (7%) at diagnosis and 16 (31%) suffered a relapse in the CNS following primary systemic therapy. CNS relapses occurred shortly after diagnosis and treatment, with a median time to CNS relapse of 5.6 months (range, 1.2 months – 4.6 years). The actuarial risk of CNS relapse at 5 years for patients without CNS involvement at diagnosis was 41%. Thirteen patients (81%) experienced their CNS relapse within the first year after diagnosis.
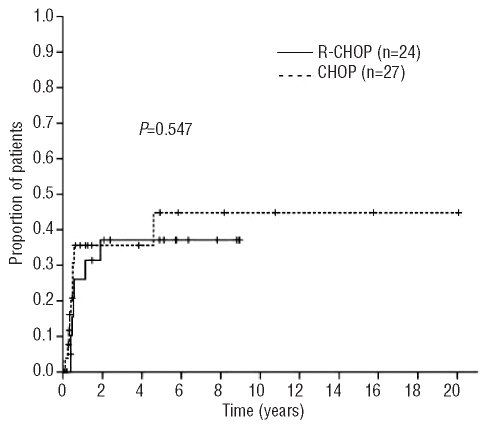
The risk of CNS relapse was similar in patients who received R-CHOP chemotherapy and CHOP alone (median time to CNS relapse 6.3 versus 4.8 months, respectively; P=0.547) (Figure 3). No relapses were noted beyond 2 years after diagnosis in patients treated with rituximab. One patient treated with CHOP and intrathecal prophylaxis had a late relapse involving the leptomeninges 4.6 years after diagnosis.
Figure 3.
Time to CNS relapse according to initial therapy (n=51).
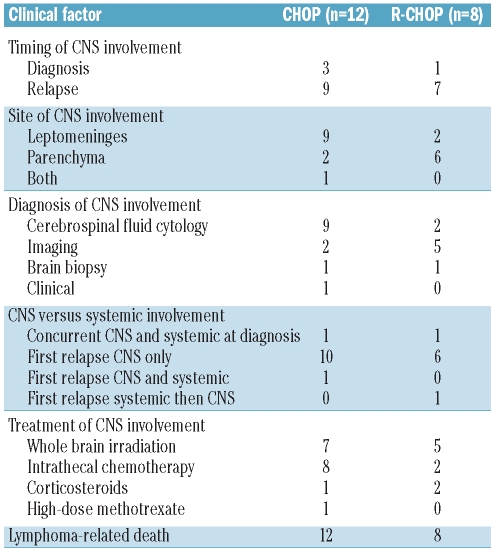
All four patients with CNS involvement at diagnosis developed progressive disease despite treatment. Among the other 51 patients, 34 suffered a relapse of their lymphoma: in 18 (53%) cases the relapse was systemic, in 13 cases it involved only the CNS (38%), and in three cases it involved both (Table 2). The majority of CNS events were diagnosed by cerebrospinal fluid cytology (n=11) or CNS imaging (n=7). About half of the CNS events were leptomeningeal (n=11) and the other half were parenchymal (n=8); only one patient had involvement of both compartments. Almost all patients received at least one form of treatment for CNS involvement: whole brain radiation and intrathecal chemotherapy were the most commonly used therapies. None of the patients received high-dose chemotherapy and autologous stem cell transplantation.
Table 2.
Characteristics of patients with CNS involvement (n=20).
Risk factors for central nervous system relapse
There were no significant differences in most baseline characteristics between patients who had CNS involvement and those who did not, with the exception of a higher proportion of bulky disease in those without a CNS event (69% versus 35%; P=0.021); conversely, there was a trend towards a higher risk of CNS disease in patients with bone marrow (P=0.076) and testicular involvement (P=0.053) at diagnosis (Table 1).
Patients who suffered a CNS relapse were less likely to have achieved a complete remission following primary systemic therapy than those who did not have a CNS relapse (20% versus 54%, respectively; P=0.022). Similarly, those who suffered a CNS relapse were more likely to have stable or progressive disease after chemotherapy (40% versus 11%, respectively; P=0.020). Among the 16 patients who achieved a complete remission, the addition of rituximab did not influence the time to CNS relapse (2-year CNS relapse rate in CHOP and R-CHOP recipients: 21% versus 17%, respectively; P=0.692).
Patients’ outcomes
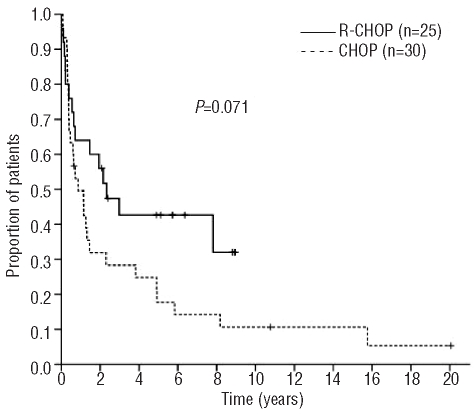
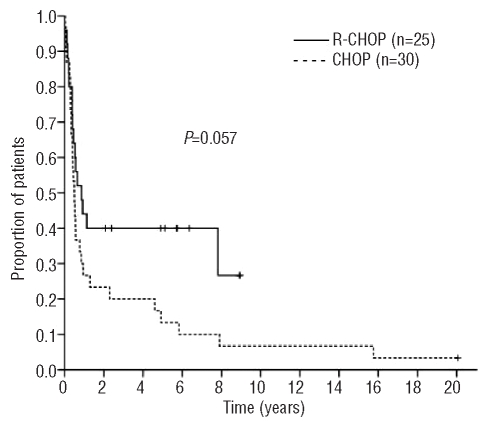
The 5-year overall and progression-free survival rates for the whole cohort were 29% and 25%, respectively, reflecting an aggressive course. There was trends towards improved 5-year overall survival (43% versus 18%; P=0.071) and progression-free survival (40% versus 13%; P=0.057) in patients treated with R-CHOP, despite their poor prognostic characteristics, as shown in Figures 1 and 2. Patients with severe renal failure did not experience worse outcomes (overall survival, P=0.220; progression-free survival P=0.480).
Figure 1.
Overall survival according to initial therapy (n=55).
Figure 2.
Progression-free survival according to initial therapy (n=55).
Outcomes were equally poor regardless of whether patients had CNS involvement or systemic relapse, with a median overall survival slightly less than 1 year. Following CNS relapse, all patients died of progressive disease, with a very short median survival of 2.2 months (range, 2 weeks – 2.6 years).
Discussion
In keeping with other studies using modern imaging techniques, we observed a low incidence of kidney involvement at the time of diagnosis in patients with DLBCL (2%).9–14 This may underestimate the true involvement, however, as older autopsy series reveal that the incidence may be as high as 50% in patients who ultimately succumb to their disease.9,20–23 Renal parenchymal involvement does not necessarily result in impaired organ function, suggesting that a number of patients may have microscopic, subclinical kidney involvement at diagnosis.1,6,11
Consistent with prior reports, we observed that renal involvement occurred in the setting of extensive nodal11 and extranodal9 disease. Indeed, 90% of our patients had at least two extranodal sites involved at presentation. The presence of more than one involved extranodal site has been consistently identified as an independent risk factor for CNS relapse in several studies.13,24–28 CNS relapse occurred early after completion of primary systemic therapy and CNS involvement was also found in four patients at the time of diagnosis. Since staging lumbar puncture and brain imaging were not routinely performed on all patients, it is possible that a proportion may have had occult CNS disease at diagnosis. Given the low sensitivity of cerebrospinal fluid cytology studies, a negative test is probably insufficient to rule out subclinical CNS involvement in such high-risk patients; in these cases, other techniques such as flow cytometry should be employed to increase detection rates.29–31 The detection of subclinical CNS involvement may help to guide treatment recommendations and define the prognosis of such patients better.
Although the mechanism of lymphoma dissemination into the CNS remains unclear, the role of adhesion molecules, chemokines, and matrix metalloproteinases in tumor invasion and metastasis has become increasingly recognized.32,33 The expression of certain genes34,35 and adhesion molecules on the surface of non-Hodgkin’s lymphoma cells36,37 has been associated with a tendency towards extranodal involvement, tumor aggressiveness, and worse outcomes. These observations provide a biological hypothesis accounting for the high rates of CNS involvement seen at diagnosis and relapse in our cohort of patients.
The poor outcomes reported in the current study are similar to those in prior studies of patients with non-Hodgkin’s lymphoma involving the kidneys who were treated with combination chemotherapy in the pre-rituximab era. Such studies showed rapid improvement in renal function and tumor size, but treatment failure was common.9,11,12 The addition of rituximab appears to improve both progression-free and overall survival rates. However, it does not appear to reduce rates of CNS relapse, although this conclusion is limited by our small sample size. A recent post-hoc analysis of 1222 patients with aggressive B-cell non-Hodgkin’s lymphoma treated in the RICOVER-60 study showed that those treated with R-CHOP had a lower incidence of CNS relapse (2-year CNS relapse rates of 4.1% versus 6.9%, RR 0.58; P=0.046); however, the absolute difference was marginal in this low-risk population.27 Furthermore, our previously published results of 435 patients suggest that rituximab may have a greater impact on reducing the risk of CNS relapse in patients who have achieved a complete remission, perhaps through better systemic disease control.14 Given that most relapses in the current study occurred early, subclinical disease at diagnosis may have been underappreciated and rituximab may not have been sufficient to eradicate CNS disease in this group due to poor penetration.
Current strategies to prevent CNS relapse in patients with aggressive histology lymphoma in the rituximab era, including intrathecal chemotherapy, are generally ineffective.14,27,28 A recent retrospective study of 65 selected patients with high-risk DLBCL receiving primary chemoimmunotherapy concurrently with intravenous high-dose methotrexate and leucovorin rescue suggests that this strategy may be associated with a lower risk of CNS relapse,38 but prospective evaluation is necessary.
In summary, patients with DLBCL and kidney involvement at diagnosis have a poor prognosis in part due to the high incidence of CNS relapses which occur early. The addition of rituximab appears to improve overall survival modestly in this poor-risk population, likely due to enhanced systemic disease control as the risk of CNS relapse is unaltered. Clinicians should consider evaluating these patients for CNS involvement at the time of diagnosis and staging, even in the absence of neurological signs and symptoms. R-CHOP is not sufficient therapy in patients with renal involvement with or without CNS disease and improved diagnostic and treatment modalities are, therefore, necessary for this population.
Footnotes
A preliminary analysis of the results reported in this paper was presented at the 10th International Conference on Malignant Lymphoma 4–7 June, 2008: Lugano, Switzerland
Authorship and Disclosures
The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
References
- 1.Heiken JP, Gold RP, Schnur MJ, King DL, Bashist B, Glazer HS. Computed tomography of renal lymphoma with ultrasound correlation. J Comput Assist Tomogr. 1983;7(2):245–50. doi: 10.1097/00004728-198304000-00007. [DOI] [PubMed] [Google Scholar]
- 2.Sheth S, Ali S, Fishman E. Imaging of renal lymphoma: patterns of disease with pathologic correlation. Radiographics. 2006;26 (4):1151–68. doi: 10.1148/rg.264055125. [DOI] [PubMed] [Google Scholar]
- 3.Kandel LB, McCullough DL, Harrison LH, Woodruff RD, Ahl ET, Jr, Munitz HA. Primary renal lymphoma. Does it exist? Cancer. 1987;60(3):386–91. doi: 10.1002/1097-0142(19870801)60:3<386::aid-cncr2820600317>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 4.Stallone G, Infante B, Manno C, Campobasso N, Pannarale G, Schena FP. Primary renal lymphoma does exist: case report and review of the literature. J Nephrol. 2000;13(5):367–72. [PubMed] [Google Scholar]
- 5.Okuno SH, Hoyer JD, Ristow K, Witzig TE. Primary renal non-Hodgkin’s lymphoma. An unusual extranodal site. Cancer. 1995;75(9):2258–61. doi: 10.1002/1097-0142(19950501)75:9<2258::aid-cncr2820750911>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 6.Da’as N, Polliack A, Cohen Y, Amir G, Darmon D, Kleinman Y, et al. Kidney involvement and renal manifestations in non-Hodgkin’s lymphoma and lymphocytic leukemia: a retrospective study in 700 patients. Eur J Haematol. 2001;67(3):158–64. doi: 10.1034/j.1600-0609.2001.5790493.x. [DOI] [PubMed] [Google Scholar]
- 7.Vandermolen LA, Dillman RO. Primary lymphoma of the kidney: complete remission after systemic chemotherapy. J Natl Cancer Inst. 1993;85(6):505–6. doi: 10.1093/jnci/85.6.505. [DOI] [PubMed] [Google Scholar]
- 8.Harris GJ, Lager DJ. Primary renal lymphoma. J Surg Oncol. 1991;46(4):273–7. doi: 10.1002/jso.2930460413. [DOI] [PubMed] [Google Scholar]
- 9.Richards MA, Mootoosamy I, Reznek RH, Webb JA, Lister TA. Renal involvement in patients with non-Hodgkin’s lymphoma: clinical and pathological features in 23 cases. Hematol Oncol. 1990;8(2):105–10. doi: 10.1002/hon.2900080206. [DOI] [PubMed] [Google Scholar]
- 10.Straus DJ, Filippa DA, Lieberman PH, Koziner B, Thaler HT, Clarkson BD. The non-Hodgkin’s lymphomas. I. A retrospective clinical and pathologic analysis of 499 cases diagnosed between 1958 and 1969. Cancer. 1983;51(1):101–9. doi: 10.1002/1097-0142(19830101)51:1<101::aid-cncr2820510121>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 11.Geffen DB, Fisher RI, Longo DL, Young RC, DeVita VT., Jr Renal involvement in diffuse aggressive lymphomas: results of treatment with combination chemotherapy. J Clin Oncol. 1985;3(5):646–53. doi: 10.1200/JCO.1985.3.5.646. [DOI] [PubMed] [Google Scholar]
- 12.Morel P, Dupriez B, Herbrecht R, Bastion Y, Tilly H, Delannoy A, et al. Aggressive lymphomas with renal involvement: a study of 48 patients treated with the LNH-84 and LNH-87 regimens. Groupe d’Etude des Lymphomes de l’Adulte. Br J Cancer. 1994;70(1):154–9. doi: 10.1038/bjc.1994.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haioun C, Besson C, Lepage E, Thieblemont C, Simon D, Rose C, et al. Incidence and risk factors of central nervous system relapse in histologically aggressive non-Hodgkin’s lymphoma uniformly treated and receiving intrathecal central nervous system prophylaxis: a GELA study on 974 patients. Groupe d’Etudes des Lymphomes de l’Adulte. Ann Oncol. 2000;11(6):685–90. doi: 10.1023/a:1008394827806. [DOI] [PubMed] [Google Scholar]
- 14.Villa D, Connors JM, Shenkier TN, Gascoyne RD, Sehn LH, Savage KJ. Incidence and risk factors for central nervous system relapse in patients with diffuse large B-cell lymphoma: the impact of the addition of rituximab to CHOP chemotherapy. Ann Oncol. 2010;21(5):1046–52. doi: 10.1093/annonc/mdp432. [DOI] [PubMed] [Google Scholar]
- 15.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130 (6):461–70. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 16.Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145(4):247–54. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 17.Stevens LA, Coresh J, Feldman HI, Greene T, Lash JP, Nelson RG, et al. Evaluation of the modification of diet in renal disease study equation in a large diverse population. J Am Soc Nephrol. 2007;18(10):2749–57. doi: 10.1681/ASN.2007020199. [DOI] [PubMed] [Google Scholar]
- 18.National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39(2 Suppl 1):S1–266. [PubMed] [Google Scholar]
- 19.Kaplan E, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Ass. 1958;53:457–81. [Google Scholar]
- 20.Martinez-Maldonado M, Ramirez de Arellano GA. Renal involvement in malignant lymphomas: a survey of 49 cases. J Urol. 1966;95(4):485–8. doi: 10.1016/S0022-5347(17)63482-7. [DOI] [PubMed] [Google Scholar]
- 21.Richmond J, Sherman RS, Diamond HD, Craver LF. Renal lesions associated with malignant lymphomas. Am J Med. 1962;32:184–207. doi: 10.1016/0002-9343(62)90289-9. [DOI] [PubMed] [Google Scholar]
- 22.Risdall R, Hoppe RT, Warnke R. Non-Hodgkin’s lymphoma: a study of the evolution of the disease based upon 92 autopsied cases. Cancer. 1979;44(2):529–42. doi: 10.1002/1097-0142(197908)44:2<529::aid-cncr2820440222>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 23.Wentzell RA, Berkheiser SW. Malignant lymphomatosis of the kidneys. J Urol. 1955;74(2):177–85. doi: 10.1016/S0022-5347(17)67262-8. [DOI] [PubMed] [Google Scholar]
- 24.Boehme V, Zeynalova S, Kloess M, Loeffler M, Kaiser U, Pfreundschuh M, et al. Incidence and risk factors of central nervous system recurrence in aggressive lymphoma--a survey of 1693 patients treated in protocols of the German High-Grade Non-Hodgkin’s Lymphoma Study Group (DSHNHL) Ann Oncol. 2007;18(1):149–57. doi: 10.1093/annonc/mdl327. [DOI] [PubMed] [Google Scholar]
- 25.van Besien K, Ha CS, Murphy S, McLaughlin P, Rodriguez A, Amin K, et al. Risk factors, treatment, and outcome of central nervous system recurrence in adults with intermediate-grade and immunoblastic lymphoma. Blood. 1998;91(4):1178–84. [PubMed] [Google Scholar]
- 26.Hollender A, Kvaloy S, Nome O, Skovlund E, Lote K, Holte H. Central nervous system involvement following diagnosis of non-Hodgkin’s lymphoma: a risk model. Ann Oncol. 2002;13(7):1099–107. doi: 10.1093/annonc/mdf175. [DOI] [PubMed] [Google Scholar]
- 27.Boehme V, Schmitz N, Zeynalova S, Loeffler M, Pfreundschuh M. CNS events in elderly patients with aggressive lymphoma treated with modern chemotherapy (CHOP-14) with or without rituximab: an analysis of patients treated in the RICOVER-60 trial of the German High-Grade Non-Hodgkin Lymphoma Study Group (DSHNHL) Blood. 2009;113(17):3896–902. doi: 10.1182/blood-2008-10-182253. [DOI] [PubMed] [Google Scholar]
- 28.Bernstein SH, Unger JM, Leblanc M, Friedberg J, Miller TP, Fisher RI. Natural history of CNS relapse in patients with aggressive non-Hodgkin’s lymphoma: a 20-year follow-up analysis of SWOG 8516 -- the Southwest Oncology Group. J Clin Oncol. 2009;27(1):114–9. doi: 10.1200/JCO.2008.16.8021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hegde U, Filie A, Little RF, Janik JE, Grant N, Steinberg SM, et al. High incidence of occult leptomeningeal disease detected by flow cytometry in newly diagnosed aggressive B-cell lymphomas at risk for central nervous system involvement: the role of flow cytometry versus cytology. Blood. 2005;105(2):496–502. doi: 10.1182/blood-2004-05-1982. [DOI] [PubMed] [Google Scholar]
- 30.French CA, Dorfman DM, Shaheen G, Cibas ES. Diagnosing lymphoproliferative disorders involving the cerebrospinal fluid: increased sensitivity using flow cytometric analysis. Diagn Cytopathol. 2000;23(6):369–74. doi: 10.1002/1097-0339(200012)23:6<369::aid-dc1>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 31.Ward MS. The use of flow cytometry in the diagnosis and monitoring of malignant hematological disorders. Pathology. 1999;31(4):382–92. doi: 10.1080/003130299104774. [DOI] [PubMed] [Google Scholar]
- 32.Bashir R, Coakham H, Hochberg F. Expression of LFA-1/ICAM-1 in CNS lymphomas: possible mechanism for lymphoma homing into the brain. J Neurooncol. 1992;12(2):103–10. doi: 10.1007/BF00172658. [DOI] [PubMed] [Google Scholar]
- 33.Kinoshita M, Izumoto S, Hashimoto N, Kishima H, Kagawa N, Hashiba T, et al. Immunohistochemical analysis of adhesion molecules and matrix metalloproteinases in malignant CNS lymphomas: a study comparing primary CNS malignant and CNS intravascular lymphomas. Brain Tumor Pathol. 2008;25(2):73–8. doi: 10.1007/s10014-008-0232-x. [DOI] [PubMed] [Google Scholar]
- 34.Jehan Z, Siraj AK, Abubaker J, Ruiz C, Simon R, Sultana M, et al. Distinct gene expression profiles: nodal versus extranodal diffuse large B-cell lymphoma. Oncology. 2008;75(1–2):71–80. doi: 10.1159/000155144. [DOI] [PubMed] [Google Scholar]
- 35.Pals ST, de Gorter DJ, Spaargaren M. Lymphoma dissemination: the other face of lymphocyte homing. Blood. 2007;110(9):3102–11. doi: 10.1182/blood-2007-05-075176. [DOI] [PubMed] [Google Scholar]
- 36.Drillenburg P, Pals ST. Cell adhesion receptors in lymphoma dissemination. Blood. 2000;95(6):1900–10. [PubMed] [Google Scholar]
- 37.Horst E, Meijer CJ, Radaszkiewicz T, Ossekoppele GJ, Van Krieken JH, Pals ST. Adhesion molecules in the prognosis of diffuse large-cell lymphoma: expression of a lymphocyte homing receptor (CD44), LFA- 1 (CD11a/18), and ICAM-1 (CD54) Leukemia. 1990;4(8):595–9. [PubMed] [Google Scholar]
- 38.Abramson JS, Hellmann M, Barnes JA, Hammerman P, Toomey C, Takvorian T, et al. Intravenous methotrexate as central nervous system (CNS) prophylaxis is associated with a low risk of CNS recurrence in high-risk patients with diffuse large B-cell lymphoma. Cancer. 2010;116(18):4283–90. doi: 10.1002/cncr.25278. [DOI] [PubMed] [Google Scholar]