Abstract
Background
Bortezomib belongs to a new class of anti-cancer agents, the proteasome inhibitors, and has documented activity in multiple myeloma and mantle cell lymphoma. Preclinical studies suggest that bortezomib has synergistic activity with rituximab, which provides a rationale for the exploration of treatment combinations.
Design and Methods
The activity and safety of bortezomib in combination with rituximab and dexamethasone were investigated in patients with relapsed or chemotherapy-refractory mantle cell lymphoma. A treatment cycle consisted of bortezomib (1.3 mg/m2 on days 1, 4, 8, and 11; six 21-day cycles), rituximab (375 mg/m2, day 1) and dexamethasone (40 mg orally, days 1 to 4). Responding patients received four consolidating doses of rituximab. Sixteen patients with progressive mantle cell lymphoma after a median of three prior lines of therapy were enrolled.
Results
The overall response rate was 81.3% (13 patients), with seven patients achieving a complete response (43.8%). Six of these patients were also negative for disease activity by positron emission tomography scanning. The median progression-free survival and overall survival were 12.1 and 38.6 months, respectively. In patients achieving a complete response, the median progression-free survival and overall survival have not yet been reached. Adverse events (greater than grade II) included thrombocytopenia (37.5%), fatigue (18.8%) and peripheral neuropathy (12.5%). Two patients discontinued bortezomib because of grade III neuropathy.
Conclusions
Bortezomib combined with rituximab and dexamethasone has promising activity and manageable toxicity in patients with heavily pretreated mantle cell lymphoma. Achievement of complete response emerged as an important factor for sustained disease control.
This trial was registered at www.clinicaltrials.gov as #NCT00261612.
Keywords: bortezomib, rituximab, mantle cell lymphoma, relapse
Introduction
Mantle cell lymphoma (MCL) is an aggressive and difficult to treat subtype of B-cell non-Hodgkin’s lymphoma which accounts for approximately 6% of all cases of non-Hodgkin’s lymphoma.1 Historically, MCL had a poor prognosis with a median survival of about 3 years when treated with conventional dose chemotherapy. The administration of the anti-CD20 monoclonal antibody rituximab in combination with conventional chemotherapy was shown to increase responses but it had only a minor impact on survival.2,3
Intensified treatment approaches including the hyper-CVAD regimen and high-dose chemotherapy with autologous stem cell support result in an improvement of progression-free and overall survival.4–6 However, this therapeutic approach is not feasible for the large proportion of patients with MCL presenting at an advanced age or with co-morbidities. Moreover, after first relapse, the prognosis of MCL patients is considered to be very poor with patients having a median survival of approximately 1 to 2 years,7 which underscores the urgent need for novel therapeutic interventions.
In recent years, inhibition of the proteasome by its specific inhibitor bortezomib has become an emerging antitumor strategy approved by the US Food and Drug Administration first for relapsed multiple myeloma and later for the treatment of relapsed MCL. The proteasome is responsible for the degradation of intracellular proteins, including several proteins involved in cell cycle control and the regulation of apoptosis.8 Preclinical studies have shown that the proteasome inhibitor bortezomib decreases proliferation, induces apoptosis, enhances the activity of chemotherapy and radiation, and reverses chemoresistance in a variety of in vitro and in vivo models of hematologic and solid malignancies.9,10 Proteasome inhibition with bortezomib has specifically promoted apoptosis of tumor cells through the stabilization of p53, p21, p27, Bax, and IκB α, resulting in nuclear factor κB (NF-κB) inhibition. There is strong experimental evidence that the transcription factor NF-κB is active in promoting chemoresistance, cytokine-mediated proliferation, tumor metastasis, and angiogenesis. By blocking proteasomal degradation of IκB, a negative regulator of NF-κB, bortezomib diminishes NF-κB activity, thereby enhancing treatment responses and reversing chemoresistance. For example, bortezomib was approximately two times more potent in inhibiting the growth of chemoresistant multiple myeloma cells compared with chemosensitive cells, in direct correlation with NF-κB activity.11
NF-κB is constitutively activated in MCL cell lines and in biopsy specimens from patients with MCL.12 Bortezomib produced cell cycle arrest in G1 of the MCL cells and induced apoptosis. Cell death was associated with down-regulation of the anti-apoptotic factors Bcl-xL and bfl/A1 and activation of caspase-3, leading to mitochondrial cytochrome c release. Cell cycle arrest was associated with reduced expression of cyclin D1, which is a molecular genetic marker of MCL. These preclinical data provided the basis for the evaluation of bortezomib in phase II clinical trials among patients with relapsed MCL. Five phase II trials have now documented the activity of bortezomib, as a single agent, in relapsed MCL, with response rates ranging between 30% and 50%: some patients had a complete response.13–18
Rituximab has been tested as a single agent for the treatment of previously untreated and relapsed MCL and was shown to induce partial remissions in 27% to 38% of patients.19–21 In various preclinical studies, evidence was obtained for additive and possibly synergistic tumor cell killing of various combinations of bortezomib, dexamethasone, and rituximab.22–24 This provided the basis for our investigation to explore bortezomib, rituximab, and dexamethasone (BORID) in patients with relapsed and chemotherapy-refractory MCL.
Design and Methods
Selection of patients
Patients were required to have histologically confirmed, CD20-positive MCL according to the WHO/Revised European-American Lymphoma classification. Patients had to meet the following eligibility requirements for enrollment into the study: have measurable disease (defined as > 1cm by computed tomography scanning); have received at least one prior line of conventional cytotoxic therapy including CHOP (or a CHOP-like regimen); be 19 years of age or older; have a life expectancy of at least 3 months; and have a Karnofsky performance status of more than 60%. Patients were eligible only if they had grade 1 or less sensory neuropathy at baseline. Additional inclusion criteria included a hemoglobin concentration of more than 8.0 g/dL (without transfusion support within 7 days prior to the assessment), a neutrophil count more than 1.0x109/L (>0.5x109/L in the case of bone marrow involvement), a platelet count more than 50x109/L (without transfusion support within 7 days prior to the assessment), and a creatinine clearance of more than 30 mL/min.
Patients were excluded if signs of severe congestive heart failure (New York Heart Failure Guidelines Class III/IV) or active infection were present. Patients were also excluded if there was evidence that the lymphoma had involved the central nervous system, if they were positive for human immunodeficiency virus, had any psychiatric illness that could limit compliance with study requirements, had another primary malignancy (other than squamous or basal cell skin cancer or in situ cervical cancer) diagnosed within 5 years, or were pregnant or breast feeding.
Patients were required to give written informed consent.
Study design
This was a single-center, phase II study of bortezomib combined with rituximab and dexamethasone in patients with relapsed/refractory MCL. The primary endpoint was efficacy of the regimen defined by the response rate. Secondary objectives were tolerability and efficacy defined as progression-free survival and overall survival. The study was approved by the institutional review board of the Medical University of Vienna. A summary of this study was registered at www.clinicaltrials.gov as #NCT00261612.
Treatment
Bortezomib was administered at a dose of 1.3 mg/m2 as an intravenous bolus push over 3 to 5 seconds twice weekly for 2 weeks (days 1, 4, 8 and 11) followed by a 10-day rest period (days 12–21) of a 3-week cycle. Doses were repeated if the absolute neutrophil count was 1.0x109/L or higher and the platelet count was 30x109/L or higher. The protocol included prophylactic intravenous fluids in the form of 500 to 1000 mL of saline, administered before each dose of bortezomib. Rituximab was administered at the dose of 375 mg/m2 on day 1 of each cycle with a standardized infusion protocol. Premedication included acetaminophen and diphenhydramine. Pulsed dexamethasone was given orally at a daily dose of 40 mg on days 1–4 of each treatment cycle.
Treatment was repeated every 3 weeks and administered for six cycles unless there was progressive disease, unacceptable toxicity, or the patient wished to discontinue therapy. Support with granulocyte colony-stimulating factor and/or erythropoietin was permitted. In responding patients, rituximab was given as consolidation therapy at a dose of 375 mg/m2 every 8 weeks for four doses.
Treatment with bortezomib was withheld in patients with grade 3 or higher non-hematologic toxicity or grade 4 hematologic toxicity until the side effects diminished to at least grade 1. After resolution, treatment was resumed at a lower dose (stepwise dose reduction from 1.3 to 1.0 or 0.7 mg/m2).
Evaluation of response and toxicity
Baseline evaluations included clinical examination, bone marrow aspirate and biopsy, and computed tomography of the chest, abdomen, and pelvis, and head and neck, if indicated. Patients were restaged every three cycles and then every 3 months afterwards until progression. Positron emission tomography was repeated in patients with positive nuclear imaging studies at baseline.
Response criteria for patients enrolled into the study were defined as previously described.25 All responses were classified as either complete remission, partial remission, stable disease or progressive disease. Patients with measurable gastrointestinal involvement by endoscopy were considered to have a complete remission if all lesions were resolved and multiple random biopsies were negative.
Progression-free survival was calculated from the date of starting BORID treatment until the date of progression or death. Overall survival was calculated from the time of initiation of therapy to death from any cause or last follow-up. Survival curves were plotted using the Kaplan-Meier method.
Toxicity was recorded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE), Version 3.0.
Results
Patients
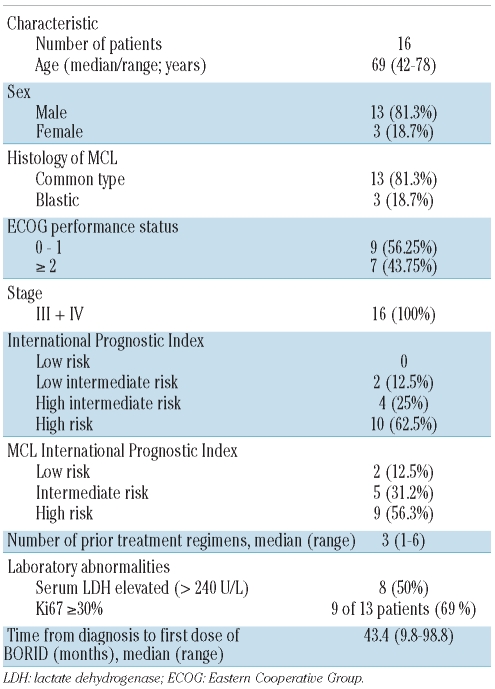
Sixteen patients (13 males, 3 females) with relapsed/refractory MCL were enrolled in this phase II study. Patient had received a median of three prior lines of therapy (range, 1–6) including CHOP-polychemotherapy (100%), rituximab (88%), high-dose chemotherapy followed by autologous stem cell transplantation (31%), a fludarabine-containing regimen (31%), and a thalidomide-combination (50%; combined with either rituximab26 or rituximab plus chemotherapy). Three patients were refractory to their last line of therapy preceding the study treatment. Additional patients’ characteristics and disease-related features are summarized in Table 1. According to International Prognostic Index (IPI) risk group,27 ten patients (62.5%) were at high-risk. Based on the MCL International Prognostic Index (MIPI),28 14 patients (87.5%) were at intermediate or high risk.
Table 1.
Patients’ characteristics at study entry.
Response and survival
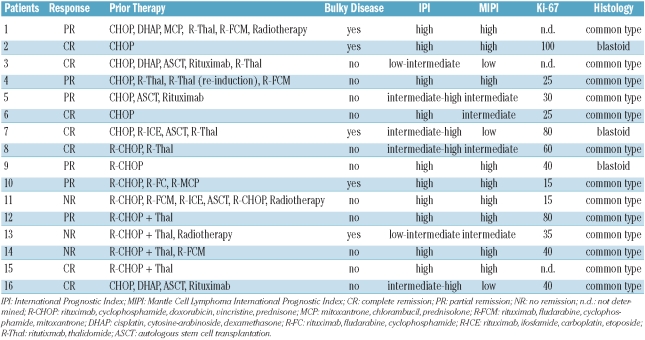
A median of 4.4 treatment cycles (range, 2–6) was administered. Applying standard response criteria,25 we observed an objective response to the BORID treatment combination in 13 of the 16 patients (overall response rate of 81.3%) including complete remission in seven patients (43.8%) and partial remission in six patients (37.5%). Fluorodeoxyglucose positron emission tomography scanning was performed in six of the seven patients who achieved a complete remission and confirmed the remission status by absence of metabolic activity in all six patients. The Ki-67 growth fraction was assessed in only 13 patients, but it appeared that achievement of complete remission was independent of proliferative activity (4 complete remissions among 9 patients with Ki-67 ≥30%; 1 complete remission among 4 patients with Ki-67 <30%). The patients’ characteristics, prior therapies, and responses are detailed in Table 2.
Table 2.
Response, prior therapy, and disease characteristics.
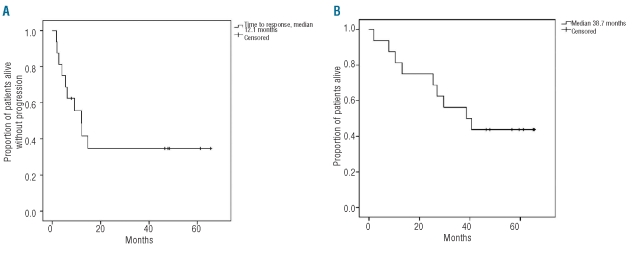
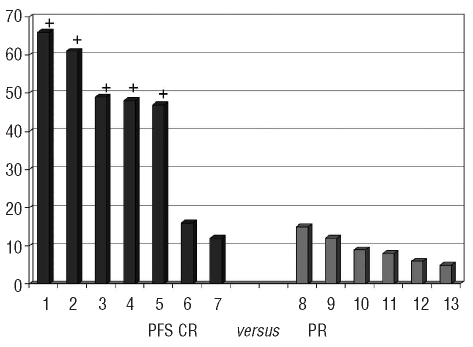
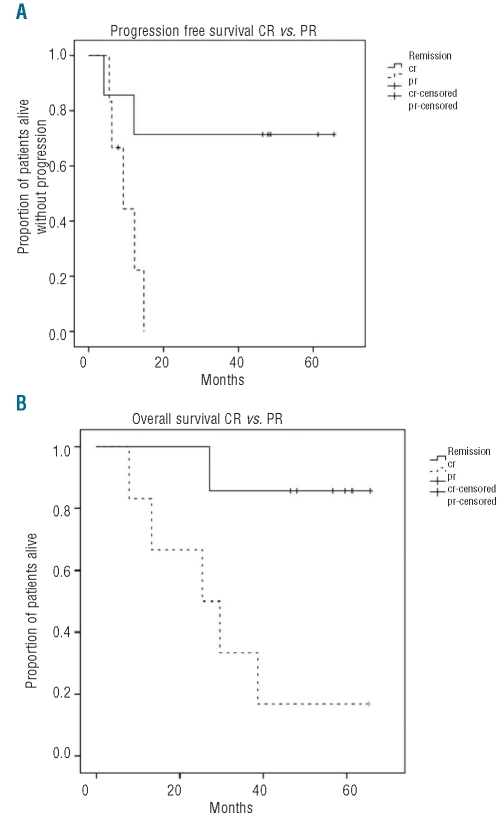
The median progression-free survival and overall survival were 12.1 months and 38.7 months, respectively (Figure 1). The association between quality of response and progression-free survival is illustrated in Figure 2. Patients who achieved a complete remission had significantly longer progression-free survival (with 5 patients still being in complete remission beyond 48 months) compared to patients who only reached a partial remission. The median progression-free survival and overall survival of patients who achieved a complete remission have not yet been reached, which is significantly different from the outcome in patients reaching only a partial remission (Figure 3).
Figure 1.
(A) Progression-free survival (median, 12.1 months) and (B) overall survival (median, 38.6 months) of the 16 trial patients.
Figure 2.
Progression-free survival (months, y-axis) in patients achieving a complete remission (black bars, 1 to 7) compared to patients in partial remission (gray bars, 8 to 13). A + denotes patients in ongoing complete remission.
Figure 3.
(A) Progression-free survival for patients in complete remission (solid line) versus patients in partial remission (dotted line), log rank: P=0.037; (B) Overall survival for patients in complete remission (solid line) versus patients in partial remission (dotted line), log rank: P=0.013.
Toxicity
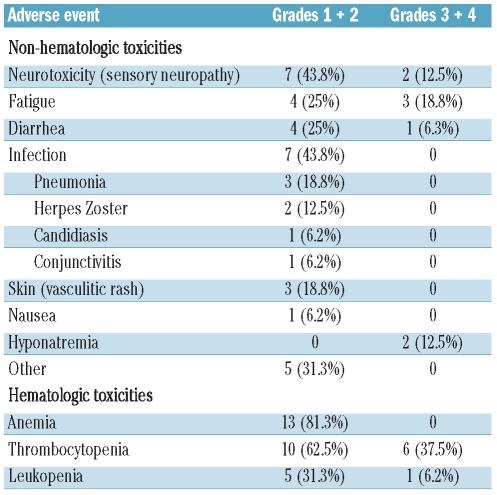
A summary of adverse events is given in Table 2. Most of the toxicities were grades 1 and 2. Among hematologic toxicities, the most frequent adverse event was thrombocytopenia following the typical cyclic pattern of bortezomib-induced thrombocytopenia29 (grade 3 thrombocytopenia in 6 patients, corresponding to 37.5% of patients). No bleeding events were recorded. Frequent grade 3 and 4 non-hematologic toxicities were fatigue (18.8%), peripheral neuropathy (12.5%), and hyponatremia (12.5%). Bortezomib treatment was discontinued because of toxicity in two patients: both patients developed grade 3 peripheral neuropathy, and bortezomib was discontinued after cycles 3 and 5.
Grade 1 and 2 toxicities observed at high frequencies included infections (7 patients, 43.8%), peripheral neuropathy (43.8%), fatigue (25%), diarrhea (25%), and skin toxicity (18.8%). These cutaneous reactions were consistent with the vasculitic rash known to be associated with bortezomib;30 biopsies performed in two patients confirmed the presence of T-cell infiltrates consistent with a drug reaction. Interestingly, all three patients with a rash had a response to the BORID treatment (2 complete remissions, 1 partial remission).
Discussion
This phase II study evaluated the combination of bortezomib, rituximab, and dexamethasone in the setting of relapsed/refractory MCL and demonstrated promising activity of the regimen with respect to response rate (overall response rate 81.3%; complete remission 43.8%), progression-free survival (median, 12.1 months) and overall survival (median, 38.7 months). It needs to be pointed out that these treatment results were obtained in a predominantly heavily pretreated population of patients with features of advanced disease and poor prognosis (Table 1). This is reflected by a median of three prior lines of therapy, risk scores (IPI, MIPI)28 suggesting intermediate and high risk in the majority of patients, as well as a high proliferative activity31 (Ki-67 index >30% in 9 of the 13 patients tested). The observed efficacy of this treatment combination clearly exceeds the activity of each single agent in the BORID regimen. Bortezomib by itself may induce a remission in 29%–45% of previously treated MCL patients as reported in five phase II studies.13–18
Table 3.
Adverse events
A complete remission following bortezomib treatment may be achieved in up to 8% of patients.16,18 In these trials with bortezomib used as a single agent, the median progression-free survival was reported to range between 5.3 and 6.7 months. The remission rate among patients with MCL treated with single-agent rituximab is about 30–35%, and virtually all responses are partial remissions.19–21 It is difficult to assess the contribution of pulsed dexamethasone to the activity of the BORID regimen. Based on observations in multiple myeloma, we can assume that dexamethasone may enhance the activity of bortezomib in inducing apoptosis in malignant cells.23,32,33
The achievement of durable responses among patients who reached a complete remission after treatment with the BORID regimen is of particular interest. With five out of seven patients who had a complete response still in complete remission beyond 48 months, the median progression-free survival and overall survival have not yet been reached in complete remission patients. These clinical results are in agreement with recent data demonstrating the importance of good quality remissions in MCL as a crucial factor for sustained disease control.34 A favorable outcome for patients in complete remission after treatment with single-agent bortezomib was also reported in the PINNACLE-trial.16,18 Consolidation treatment with rituximab may have contributed to these favorable results, although we did not observe a clinically detectable improvement in the remission status during rituximab consolidation. The toxicity of the BORID regimen was limited in the series of patients in this study, and adverse events were manageable with routine clinical care. Notably, it was safe to combine bortezomib with rituximab and dexamethasone, and the side effects of bortezomib were similar to those reported in MCL13–18 and multiple myeloma.35,36 Besides hematologic side effects, including thrombocytopenia, peripheral neuropathy needs to be carefully monitored during treatment with bortezomib. We observed bortezomib-induced grade 3 sensory neuropathy in two patients with MCL and this side effect eventually resulted in treatment discontinuation. This frequency of grade 3 or more neuropathy appears to be in the range that was also reported in previous trials with bortezomib using a twice-weekly schedule of administration. A recent trial of bortezomib with rituximab in relapsed follicular lymphoma and MCL, however, reported grade 3 neurotoxicity in 13 of 25 patients (52%) limiting the use of this treatment combination.37 This high rate of neurotoxicity may be related to the dose of bortezomib (1.5 mg/m2 twice weekly) used in the trial by Baiocchi et al.; the bortezomib dose used in our study was 1.3 mg/m2. Neuropathy also developed frequently in a recent trial of bortezomib in MALT lymphoma, in which the dose of bortezomib was 1.5 mg/m2 administered twice weekly.38 Besides the early dose modification of bortezomib, alternative doses and schedules of bortezomib should be considered in lymphoma patients,39 in particular if bortezomib is used after prior administration of neurotoxic agents.
Thus far, several bortezomib combinations have been evaluated in patients with relapsed/refractory MCL. Bortezomib may not only be combined with rituximab,37,39 but also with cytarabine,40 fludarabine,41 and even polychemotherapy.42 Although the actual numbers of MCL patients in trials of such combinations are very small, the combinations do appear to have greater efficacy compared to bortezomib alone. We believe that our trial adds important information to these reports because of the long-term follow-up demonstrating a durable benefit, particularly for those patients achieving a complete remission.
Footnotes
Funding: supported in part by the Austrian Federal Ministry for Education, Science and Culture (GZ 200.062/2-VI/1/2002) and a grant from the Lymphoma Research Foundation
Authorship and Disclosures
The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
References
- 1.Barista I, Romaguera JE, Cabanillas F. Mantle cell lymphoma. Lancet Oncol. 2001;2(3):141–8. doi: 10.1016/S1470-2045(00)00255-2. Erratum in: Lancet Oncol. 2001;2(4):198. Lancet Oncol. 2002;3(7):396. [DOI] [PubMed] [Google Scholar]
- 2.Howard OM, Gribben JG, Neuberg D, Grossbard M, Poor C, Janicek MJ, et al. Rituximab and CHOP induction therapy for newly diagnosed mantle cell lymphoma: molecular complete responses are not predictive of progression-free survival. J Clin Oncol. 2002;20(5):1288–94. doi: 10.1200/JCO.2002.20.5.1288. [DOI] [PubMed] [Google Scholar]
- 3.Lenz G, Dreyling M, Hoster E, Wormann B, Duhrsen U, Metzner B, et al. Immunochemotherapy with rituximab and cyclophosphamide, doxorubicin, vincristine, and prednisone significantly improves response and time to treatment failure, but not long-term outcme in patients with previously untreated mantle cell lymphoma: results of a prospective randomized trial of the German Low Grade Lymphoma Study Group (GLSG) J Clin Oncol. 2005;23(9):1984–92. doi: 10.1200/JCO.2005.08.133. [DOI] [PubMed] [Google Scholar]
- 4.Romaguera JE, Fayad L, Rodriguez MA, Broglio KR, Hagemeister FB, Pro B, et al. High rate of durable remissions after treatment of newly diagnosed aggressive mantle cell lymphoma with rituximab plus hyper-CVAD alternating with rituximab plus high-dose methotrexate and cytarabine. J Clin Oncol. 2005;23(28):7013–23. doi: 10.1200/JCO.2005.01.1825. Erratum in J Clin Oncol. 2006;24(4):724. [DOI] [PubMed] [Google Scholar]
- 5.Dreyling M, Lenz G, Hoster E, Van Hoof A, Gisselbrecht C, Schmits R, et al. Early consolidation by myeloablative radiochemo-therapy followed by autologous stem cell transplantation in first remission significantly prolongs progression-free survival in mantle cell lymphoma: results of a prospective randomized trial of the European MCL Network. Blood. 2005;105 (7):2677–84. doi: 10.1182/blood-2004-10-3883. [DOI] [PubMed] [Google Scholar]
- 6.Geisler CH, Elonen E, Kolstad A, Laurell A, Andersen N, Pedersen LB, et al. Mantle cell lymphoma can be cured by intensive immunochemotherapy with in-vivo purged stem-cell support; final report of the Nordic Lymphoma Group MCL2 study. Blood. 2007;110:Abstract LB1. [Google Scholar]
- 7.Zucca E, Roggero E, Pinotti G, Pedrinis E, Capella C, Venco A, et al. Patterns of survival in mantle cell lymphoma. Ann Oncol. 1995;6(3):257–62. doi: 10.1093/oxfordjournals.annonc.a059155. [DOI] [PubMed] [Google Scholar]
- 8.Adams J, Palombella VJ, Sausville EA, Johnson J, Destree A, Lazarus DD, et al. Proteasome inhibitors: a novel class of potent and effective anti-tumor agents. Cancer Res. 1999;59(11):2615–22. [PubMed] [Google Scholar]
- 9.Mitsiades N, Mitsiades CS, Poulaki V, Chauhan D, Fanourakis G, Gu X, et al. Molecular sequelae of proteasome inhibition in human multiple myeloma cells. Proc Natl Acad Sci USA. 2002;99(22):14374–9. doi: 10.1073/pnas.202445099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rajkumar SV, Richardson PG, Hideshima T, Anderson KC. Proteasome inhibition as a novel therapeutic target in human cancer. J Clin Oncol. 2005;23(3):630–9. doi: 10.1200/JCO.2005.11.030. [DOI] [PubMed] [Google Scholar]
- 11.Hideshima T, Mitsiades C, Akiyama M, Hayashi T, Chauhan D, Richardson P, et al. Molecular mechanisms mediating anti-myeloma activity of proteasome inhibitor PS-341. Blood. 2003;101(4):1530–4. doi: 10.1182/blood-2002-08-2543. [DOI] [PubMed] [Google Scholar]
- 12.Pham LV, Tamyo AT, Yoshimura LC, Lo P, Ford RJ. Inhibition of constitutive NF-kB activation in mantle cell lymphoma B-cells leads to induction of cell cycle arrest and apoptosis. J Immunol. 2003;171(1):88–95. doi: 10.4049/jimmunol.171.1.88. [DOI] [PubMed] [Google Scholar]
- 13.O’Connor OA, Wright J, Moskowitz C, Muzzy J, MacGregor-Cortelli B, Stubble-field M, et al. Phase II clinical experience with the novel proteasome inhibitor bortezomib in patients with indolent non-Hodgkin’s lymphoma and mantle cell lymphoma. J Clin Oncol. 2005;23(4):676–84. doi: 10.1200/JCO.2005.02.050. [DOI] [PubMed] [Google Scholar]
- 14.Goy A, Younes A, McLaughlin P, Pro B, Romaguera JE, Hagemeister F, et al. Phase II study of proteasome inhibitor bortezomib in relapsed or refractory B-cell non-Hodgkin’s lymphoma. J Clin Oncol. 2005;23(4):667–75. doi: 10.1200/JCO.2005.03.108. [DOI] [PubMed] [Google Scholar]
- 15.Strauss SJ, Maharaj L, Hoare S, Johnson PW, Radford JA, Vinnecombe S, et al. Bortezomib therapy in patients with relapsed or refractory lymphoma: potential correlation of in vitro sensitivity and tumor necrosis factor alpha response with clinical activity. J Clin Oncol. 2006;24(13):2105–12. doi: 10.1200/JCO.2005.04.6789. [DOI] [PubMed] [Google Scholar]
- 16.Fisher RI, Bernstein SH, Kahl BS, Djulbegovic B, Robertson MJ, de Vos S, et al. Multicenter phase II study of bortezomib in patients with relapsed or refractory mantle cell lymphoma. J Clin Oncol. 2006;24(30):4867–74. doi: 10.1200/JCO.2006.07.9665. [DOI] [PubMed] [Google Scholar]
- 17.Belch A, Kouroukis CT, Crump M, Sehn L, Gascoyne RD, Klasa R, et al. A phase II study of bortezomib in mantle cell lymphoma: the National Cancer Institute of Canada Clinical Trials Group trial IND.150. Ann Oncol. 2007;18(1):116–21. doi: 10.1093/annonc/mdl316. [DOI] [PubMed] [Google Scholar]
- 18.Goy A, Bernstein SH, Kahl BS, Djulbegovic B, Robertson BJ, et al. Bortezomib in patients with relapsed or refractory mantle cell lymphoma: updated time-to-event analyses of the multicenter phase 2 PINNACLE study. Ann Oncol. 2009;20:520–5. doi: 10.1093/annonc/mdn656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ghielmini M, Schmitz SF, Cogliatti S, Bertoni F, Waltzer U, Fey MF, et al. Effect of single-agent rituximab given at the standard schedule or as prolonged treatment in patients with mantle cell lymphoma: a study of the Swiss Group for Clinical Cancer Research (SAKK) J Clin Oncol. 2005;23(4):705–11. doi: 10.1200/JCO.2005.04.164. [DOI] [PubMed] [Google Scholar]
- 20.Coiffier B, Haioun C, Ketterer N, Engert A, Tilly H, Ma D, et al. Rituximab (anti-CD20 monoclonal antibody) for the treatment of patients with relapsing or refractory aggressive lymphoma: a multicenter phase II study. Blood. 1998;92(6):1927–32. [PubMed] [Google Scholar]
- 21.Foran JM, Rohatiner AZ, Cunningham D, Popescu RA, Solal-Celigny P, Ghielmini M, et al. European phase I study of rituximab (chimeric anti-CD20 monoclonal antibody) for patients with newly diagnosed mantle-cell lymphoma and previously treated mantle cell lymphoma, immunocytoma, and small B-cell lymphocytic lymphoma. J Clin Oncol. 2000;18(2):317–24. doi: 10.1200/JCO.2000.18.2.317. Erratum in J Clin Oncol. 2000;18(9):2006. [DOI] [PubMed] [Google Scholar]
- 22.Wang M, Han XH, Zhang L, Yang J, Qian JF, Si YK, et al. Bortezomib is synergistic with rituximab and cyclophosphamide in inducing apoptosis of mantle cell lymphoma cells in vitro and in vivo. Leukemia. 2008;22(1):179–85. doi: 10.1038/sj.leu.2404959. [DOI] [PubMed] [Google Scholar]
- 23.Hideshima T, Richardson P, Chauhan D, Palombella VJ, Elliott PJ, Adams J, Anderson KC. The proteasome inhibitor PS-341 inhibits growth, induces apoptosis, and overcomes drug resistance in human multiple myeloma cells. Cancer Res. 2001;61(7):3071–6. [PubMed] [Google Scholar]
- 24.Rose AL, Smith BE, Maloney DG. Glucocorticoids and rituximab in vitro: synergistic direct antiproliferative and apoptotic effects. Blood. 2002;100(5):1765–73. [PubMed] [Google Scholar]
- 25.Cheson BD, Horning SJ, Coiffier B, Shipp MA, Fisher RI, Connors JM, et al. Report of an international workshop to standardize response criteria for non-Hodgkin’s lymphoma. NCI Sponsored International Working Group. J Clin Oncol. 1999;17(4):1244–53. doi: 10.1200/JCO.1999.17.4.1244. Erratum in: J Clin Oncol. 2000;18(11):2351. [DOI] [PubMed] [Google Scholar]
- 26.Kaufmann H, Raderer M, Wöhrer S, Püspöck A, Bankier A, Zielinski C, et al. Antitumor activity of rituximab plus thalidomide in patients with relapsed/refractory mantle cell lymphoma. Blood. 2004;104(8):2269–71. doi: 10.1182/blood-2004-03-1091. [DOI] [PubMed] [Google Scholar]
- 27.Witzig TE. Current treatment approaches for mantle-cell lymphoma. J Clin Oncol. 2005;23(26):6409–14. doi: 10.1200/JCO.2005.55.017. [DOI] [PubMed] [Google Scholar]
- 28.Hoster E, Dreyling M, Klapper W, Gisselbrecht C, van Hoof A, Kluin-Nelemans HC, et al. German Low Grade Lymphoma Study Group (GLSG); European Mantle Cell Lymphoma NetworkBlood. A new prognostic index (MIPI) for patients with advanced-stage mantle cell lymphoma. Blood. 2008;111(2):558–65. doi: 10.1182/blood-2007-06-095331. [DOI] [PubMed] [Google Scholar]
- 29.Lonial S, Waller EK, Richardson PG, Jagannath S, Orlowski RZ, et al. Risk factors and kinetics of thrombocytopenia associated with bortezomib for relapsed, refractory multiple myeloma. Blood. 2005;106(12):3777–84. doi: 10.1182/blood-2005-03-1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gerecitano J, Goy A, Wright J, MacGregor-Cortelli B, Neylon E, et al. Drug-induced cutaneous vasculitis in patients with non-Hodgkin lymphoma treated with the novel proteasome inhibitor bortezomib: a possible surrogate marker of response? Br J Haematol. 2006;134(4):391–8. doi: 10.1111/j.1365-2141.2006.06201.x. [DOI] [PubMed] [Google Scholar]
- 31.Determann O, Hoster E, Ott G, Wolfram Bernd H, Loddenkemper C, et al. Ki-67 predicts outcome in advanced stage mantle cell lymphoma patients treated with anti-CD20 immunochemotherapy: results from randomized trials of the European MCL Network and the German Low Grade Lymphoma Study Group. Blood. 2008;111(4):2385–7. doi: 10.1182/blood-2007-10-117010. [DOI] [PubMed] [Google Scholar]
- 32.Mitsiades N, Mitsiades CS, Richardson PG, Poulaki V, Tai YT, Chauhan D, et al. The proteasome inhibitor PS-341 markedly enhances sensitivity of multiple myeloma cells to conventional chemotherapy agents: therapeutic applications. Blood. 2003;101(6):2377–80. doi: 10.1182/blood-2002-06-1768. [DOI] [PubMed] [Google Scholar]
- 33.Jagannath S, Richardson P, Barlogie B, Berenson JR, Singhal S, Irwin D, et al. Bortezomib in combination with dexamethasone for the treatment of patients with relapsed and/or refractory multiple myeloma with less than optimal response to bortezomib alone. Haematologica. 2006;91(7):929–34. [PubMed] [Google Scholar]
- 34.Pott C, Schrader C, Gesk S, Harder L, Tiemann M, Raff T, et al. Quantitative assessment of molecular remission after high-dose therapy with autologous stem cell transplantation predicts long-term remission in mantle cell lymphoma. Blood. 2006;107(6):2271–8. doi: 10.1182/blood-2005-07-2845. [DOI] [PubMed] [Google Scholar]
- 35.Richardson PG, Sonneveld P, Schuster MW, Irwin D, Stadtmauer EA, Facon T, et al. Assessment of Proteasome inhibition for Extending remissions (APEX) Investigators. Bortezomib or high-dose dexamethasone for relapsed multiple myeloma. N Engl J Med. 2005;352(24):2487–98. doi: 10.1056/NEJMoa043445. [DOI] [PubMed] [Google Scholar]
- 36.San Miguel JF, Schlag R, Khuageva NK, Dimopoulos MA, Shpilberg O, Kropff M, et al. Bortezomib plus melphalan and prednisone for initial treatment of multiple myeloma. N Engl J Med. 2008;359(9):906–17. doi: 10.1056/NEJMoa0801479. [DOI] [PubMed] [Google Scholar]
- 37.Baiocchi RA, Alinari L, Lustberg ME, Lin TS, Porcu P, Li X, et al. Phase 2 trial of rituximab and bortezomib in patients with relapsed or refractory mantle cell lymphoma. Cancer. 2011 doi: 10.1002/cncr.25792. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Troch M, Jonak C, Müllauer L, Püspök A, Formanek M, Hauff W, et al. A phase II study of bortezomib in patients with MALT lymphoma. Haematologica. 2009;94 (5):738–42. doi: 10.3324/haematol.2008.001537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Agathocleous A, Rohatiner A, Rule S, Hunter H, Kerr JP, Neeson SM, et al. Weekly versus twice weekly bortezomib given in conjunction with rituximab, in patients with recurrent follicular lymphoma, mantle cell lymphoma and Waldenström macroglobulinemia. Br J Haematol. 2010;151(4):346–51. doi: 10.1111/j.1365-2141.2010.08340.x. [DOI] [PubMed] [Google Scholar]
- 40.Weigert O, Weidmann E, Mueck R, Bentz M, von Schilling C, Rohrberg R, et al. A novel regimen combining high dose cytarabine and bortezomib has activity in multiply relapsed and refractory mantle cell lymphoma - long-term results of a multicenter observation study. Leuk Lymphoma. 2009;50(5):716–22. doi: 10.1080/10428190902856790. [DOI] [PubMed] [Google Scholar]
- 41.Barr PM, Fu P, Lazarus HM, Horvath N, Gerson SL, Koc ON, et al. Phase I trial of fludarabine, bortezomib and rituximab for relapsed and refractory indolent and mantle cell non-Hodgkin lymphoma. Br J Haematol. 2009;147(1):89–96. doi: 10.1111/j.1365-2141.2009.07836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Furman RR, Martin P, Ruan J, Cheung YK, Vose JM, Lacasce AS, et al. Phase 1 trial of bortezomib plus R-CHOP in previously untreated patients with aggressive non-Hodgkin lymphoma. Cancer. 2010;116(23):5432–9. doi: 10.1002/cncr.25509. [DOI] [PubMed] [Google Scholar]