Abstract
Background
Inherited deficiency of antithrombin, protein C and protein S, three important, naturally occurring coagulation inhibitors, might play a major role in the occurrence of venous thromboembolism in Chinese. The establishment of age- and gender-related normal ranges of these inhibitors is crucial for an accurate diagnosis of these deficiencies.
Design and Methods
We designed a prospective cross-sectional study recruiting healthy adults from four university–affiliated hospitals in China. Antithrombin, protein C and protein S were studied by measuring their activity. Gene analysis was performed when natural anticoagulant deficiency was suspected. Polymorphisms of the factor V gene were searched for among subjects who were positive for activated protein C resistance.
Results
In 3493 healthy Chinese adults (1734 men, 1759 women; age 17–83 years), we found higher age-adjusted activities for protein C and protein S in men than in women but no sex difference for antithrombin. In women, mean protein C and protein S activities increased with age. In men, mean protein C levels increased with age up to the age of 49 but decreased after 50 years old; mean protein S levels decreased after 50 years of age. Antithrombin levels remained stable over time in women but decreased significantly after 50 years of age in men. Reference values according to age and sex allowed the identification of 15 genetic variants (protein C: 10, antithrombin: 3, protein S: 2) in subjects with protein activity below the 1st percentile.
Conclusions
This is the largest survey ever conducted in the healthy general Chinese population. These normal ranges provide the essential basis for the diagnosis and treatment of thrombosis in Chinese.
Keywords: antithrombin, protein C, protein S, normal ranges, genetics, Chinese
Introduction
Venous thromboembolism is one of the leading causes of mortality and morbidity in the world. In the past, venous thromboembolism was thought to be rare in Asia as compared to in Western countries,1 although according to recent studies, this is not true.2–6 It is, however, becoming increasingly clear that there are differences in risk factors for thrombosis between Eastern and Western countries.7 Factor V (FV) Leiden (FV R506Q) associated with activated protein C resistance (APC-R)8,9 and factor II G20210A polymorphism,10 the two most common genetic risk factors for venous thromboembolism in Western populations, are absent or very rare in Eastern populations.11–13 By contrast, genetic deficiencies of the three main natural anticoagulants, antithrombin (AT), protein C (PC) and protein S (PS) are rare in Western countries but considered important risk factors for venous thromboembolism in Asian countries.14–20 The diagnosis of AT, PC and PS deficiencies in Asians is, therefore, of substantial clinical interest.
The identification of people with low levels of these anticoagulants is based on the knowledge of reference ranges of AT, PC and PS plasma activities in the corresponding general population. Some publications have reported age- and gender-related normal ranges of AT, PC and PS activities in large series of healthy Western individuals.21–29 However, such reference ranges have only been reported in small series of Asians, except in Japanese.20,30–32 As variations can be expected between populations of different ethnic backgrounds, the results published for Caucasians should not be extrapolated to the Chinese and, likewise, results from Japanese series may differ from those for Chinese people.
In this prospective multicenter cross-sectional study, we explored the distribution of AT, PC and PS levels in a large series of healthy Chinese individuals in order to determine the normal activity ranges according to gender and age and to identify the underlying genetic variants.
Design and Methods
Population
The Chinese Haemostasis Investigation on Natural Anticoagulants (C.H.I.N.A) Study I recruited consecutive Han Chinese individuals over 17 years old among adult blood donors and healthy individuals evaluated for routine check-up in four university-affiliated hospitals in China in a period of 4 consecutive months. Of the potentially eligible subjects, 95% agreed to participate in the study and gave written informed consent. Subjects with abnormal renal function (serum creatinine levels >133 μmol/L) or liver function (serum transaminases >2 times the upper limit of normal) or previous thrombosis, individuals receiving anticoagulant therapy, oral contraceptives or hormonal replacement therapy, and pregnant women were excluded. The study was coordinated by Peking Union Medical College Hospital and University, Beijing, China, and approved by the Ethics Committee of this Center.
Collection of blood samples
Venous blood (5.4 mL) was drawn directly by venipuncture into vacutainer tubes (Becton-Dickinson) containing 0.105 mol/L trisodium citrate (9:1, v/v) from each fasting participant in the morning. Each sample was immediately centrifuged at 2500g for 15 min; the supernatant platelet-poor plasma was separated into aliquots which were frozen quickly and stored at −70°C until activity and antigen assays. Leukocytes were also isolated after centrifugation and stored at −70°C for DNA extraction. Hemolysed or clotted samples were discarded.
Plasma activity of antithrombin, protein C, and protein S, and activated protein C resistance assays
AT, PC, PS activities and APC- R were measured on a STA-R Instrument (Diagnostica Stago, Asnières, France) using the same batches of reagents (Diagnostica Stago). The activities of AT and PC were assayed using a chromogenic substrate method (STA-Stachrom ATIII and STA-Stachrom Protein C, respectively). PS and APC-R were assayed by a clotting method (STA-Staclot Protein S and Staclot APC-R). The STA-Staclot APC-R test is based on the activation of factor X by snake venom in the presence of activated protein C. The venom triggers the coagulation cascade downstream of factor X, thus eliminating the influence of all coagulation factors acting upstream. The results are given as clotting time in seconds. Activity levels below the 2.5th percentile were considered to be lower than normal. However, only subjects with activity levels below the 1st centile were strongly suspected of having an inherited deficiency and underwent further gene analysis.
Genetic analysis of antithrombin, protein C, and protein S and factor V polymorphisms
Genomic DNA was isolated from leukocytes by phenol extraction. All the primers for PS, PC and AT genetic analyses were selected as previously published.33–35 All the exons and 5′-and 3′-untranslated regions of the genes encoding for the three proteins were amplified. For each polymerase chain reaction (PCR), a total of 100 ng DNA template was amplified at 95°C (5 min), then 95°C (30 sec), 50ºC~68ºC (30 sec), and 72°C (30~60 sec) for 30 cycles, using 100 nmol primers, 1×PCR buffer (containing MgCl2), 0.25 mmol/L dNTP 2.5 μL, and 0.6 U Taq DNA polymerase in a 25 μL final volume. A final extension step was performed at 72°C for 10 min. The PCR products of AT, PC and PS were then digested by shrimp alkaline phosphatase (SAP, USB Corporation, Cleveland, OH, USA) and exonuclease I (EPICENTRE Biotechnologies, Madison, WI, USA) at 37°C for 60 min and then at 80°C for 15 min. Finally, the amplified products were directly sequenced (ABI Prism® 377 DNA Sequencer, Applied Biosystems, Warrington, UK). Mutations were numbered according to the HGVS recommendations for mutation nomenclature (http//www.hgvs.org/mutnomen/). The reference sequences were: NM_000312.2 for PROC, NM_000488.2 for SERPINC1 and NM_000313.3 for PROS1.
Samples positive for APC-R were screened for five single nucleotide polymorphisms in the FV gene (F5) predicting the following five substitutions in the FV protein: R306T or FV Hong Kong,36 R306G or FV Cambridge,37 R506Q, I359T, and H1299R. The single nucleotide polymorphisms were detected by restriction fragment length polymorphism analysis using five different restriction enzymes. The digestion products were then examined by agarose gel electrophoresis.
Antigen assays of antithrombin, protein C, and protein S
Antigen levels were assayed only for the individuals who underwent genetic analysis. All the reagents were from Diagnostica Stago. AT antigen was measured by latex immunoassay (Liatest ATIII) and PC antigen by enzyme-linked immunosorbent assay (ELISA, Asserachrom Protein C). Antigen levels of total PS and free PS were also measured with ELISA kits (Asserachrom Total Protein S and Asserachrom Free Protein S).
Statistical analysis
Statistical analyses were performed with SAS 9.1 (SAS Inc., Cary, North Carolina, USA) and STATA 10.0 (StataCorp, College Station, Texas, USA) for PC. Results are expressed as medians (ranges) for quantitative variables and frequencies (percentages) for qualitative variables. Age-specific reference intervals were estimated using the parametric method described by Royston and Wright.38 Details of the statistical analysis and equations used for estimating reference values are given in the Online Supplementary Appendix and Online Table S1, respectively.
Results
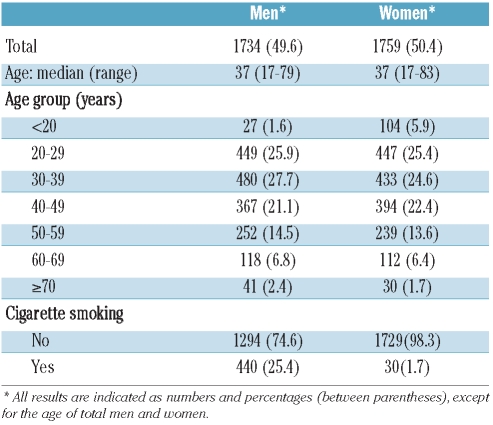
Among 3600 individuals who were asked to participate in the study and who signed the informed consent, 3493 were enrolled since 107 were excluded because of abnormal liver or renal function tests. The participants comprised 738 (21.1%) blood donors and 2755 (78.9%) adults who underwent voluntary health checkups. Detailed characteristics of the population are presented in Table 1. The median age of these participants was 37 years (range, 17–83 years).
Table 1.
Demographic data of the study population.
Plasma activity of antithrombin, protein C, and protein S, and activated protein C resistance assays
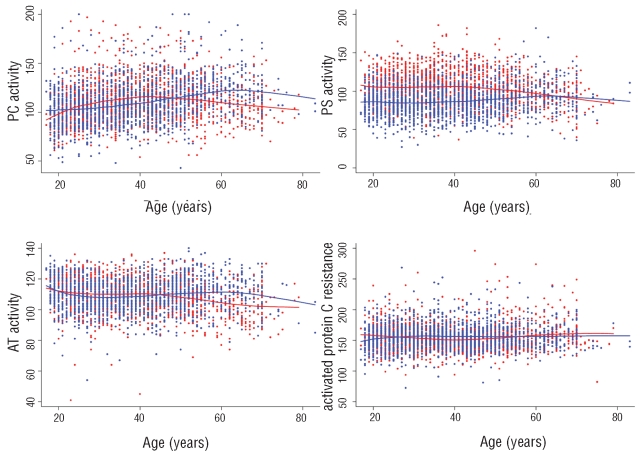
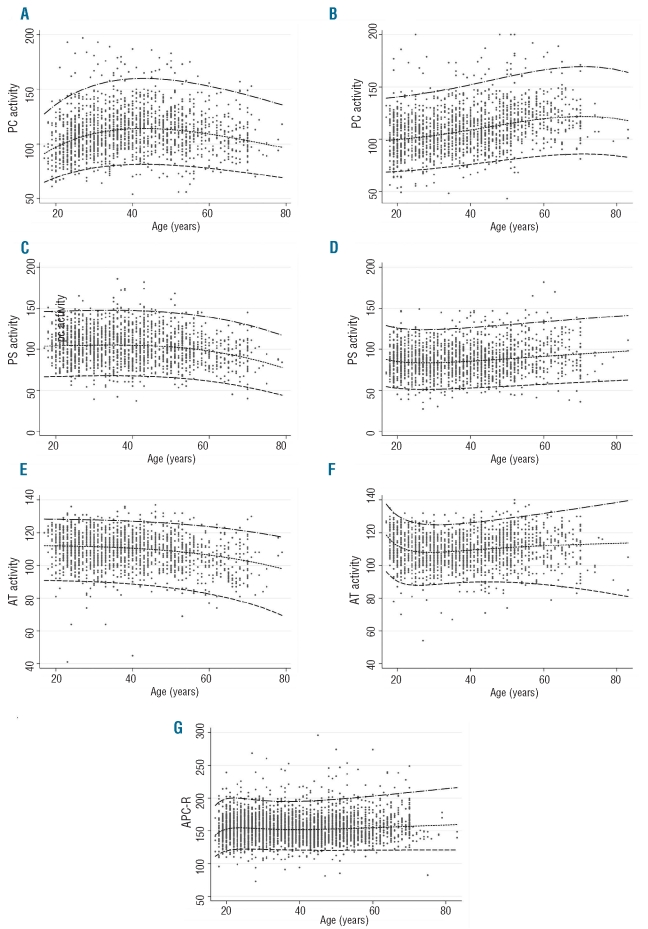
The distributions of AT, PC and PS activities and APC-R values according to age and sex are illustrated in Figure 1. The 2.5th–97.5th percentile reference values of AT, PC and PS activities according to sex and age are given in Online Supplementary Table S2. Sex had a significant effect, when adjusted for age, on PS and PC activities, with men showing higher mean levels than women in the first 50 years of life (Figure 1). However, in men a trend to a change in this pattern could be observed with smoothing curves for AT, PC and PS activity levels after 50 years old. The effect of sex was also significant for AT activity as women had higher mean levels than men from around 50 years of age. Conversely, sex did not have an effect on mean APC-R after adjustment for age. The effect of age on APC-R levels was insignificant in both men and women (P>0.05). Reference intervals (2.5th, 50th, 97.5th) for PC, PS, AT activities and APC-R values are shown in Figure 2, confirming the results of the Loess smoothing described above. No association between cigarette smoking and AT, PC and PS activities or APC-R values was identified. Interestingly, no subjects had combined low values of two or three anticoagulants.
Figure 1.
Loess smoothing curves of PC, PS, AT activity and APC-R depending on age (in years) and sex. The X axis indicates age (years) and the Y axis indicates activity values (% for AT, PC, PS; sec for APC-R). Red points and lines: men; blue points and lines: women,
Figure 2.
Reference intervals (2.5th, 50th, 97.5th) for PC activity (A for men and B for women), PS activity (C for men and D for women), AT activity (E for men and F for women) and APC-R (G for both).
Genetic analysis of antithrombin, protein C, and protein S and factor V polymorphisms
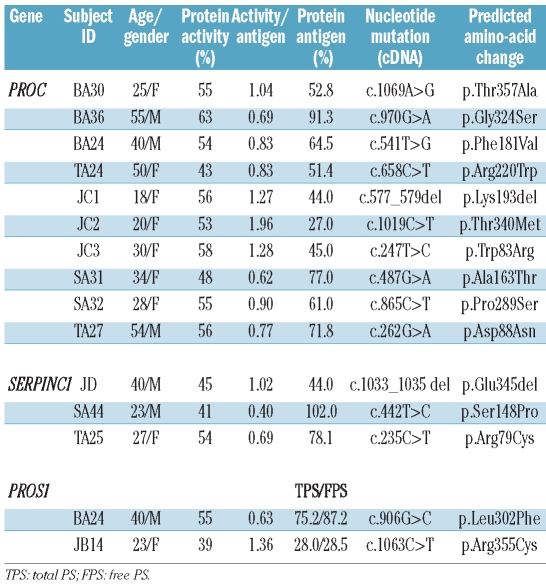
All the subjects (total=95) with either AT, PC or PS activity below the first percentile (AT: women=15, men=16; PC women=16, men=16; PS women=17, men=15) were strongly suspected of bearing mutations and underwent genetic analysis. In addition, all the subjects with AT activity between the 1st and the 2.5th percentile (women=19, men=25) could also undergo a genetic analysis. A genetic variant was found in 15 subjects with an anticoagulant activity level below the 1st percentile: three mutations were found in the SERPINC1 gene (2 missense substitutions and 1 small deletion), ten in the PROC gene (9 missense substitutions and 1 small deletion) and two missense substitutions in the PROS1 gene. The characteristics of these 15 individuals are shown in Table 2. Nine of them with an activity/antigen ratio between 0.77 and 1.36 might be suspected of having a type I deficiency. Five of the subjects, BA36, SA31 (PROC gene substitutions), SA44, TA25 (SERPINC1 gene substitutions) and BA24 (PROS1 gene substitution) with an activity/antigen ratio below 0.70 might be suspected of having a type II deficiency. Finally, JC2 had a high activity/antigen ratio, suggesting that the predicted mutated PC was not recognized fully by the antibody used for the ELISA. None of these subjects was symptomatic or had a strong family history of thrombosis. No recurrent genetic variants were found. Four substitutions in the PROC gene, c.247T>C, c.487G>A, c.865C>T, c.1069A>G, and two in the PROS1 gene, c.906G>C, and c.1063C>T, were not found in previous reports and databases. One subject, BA24, was heterozygous for two genetic variants, one in PC and another in PS. According to these genetic results, the percentages of subjects with genetically confirmed low AT, PC and PS levels were at least 0.08%, 0.29% and 0.057%, respectively. No FV polymorphism was detected in the 41 APC-R positive samples.
Table 2.
Main characteristics of 15 subjects with PC or PS or AT mutation.
Discussion
To our knowledge this study is the largest cross-sectional survey ever performed with the aim of establishing normal ranges of the three main natural anticoagulants, AT, PC and PS in a large series of healthy Chinese Han people and of identifying their genetic variants. The strength of this study lies not only in the large size of the population sample but also in the wide age range of the participants (17–83 years), the similar numbers of representatives of both genders, the calibrated assays for measuring the activity of the three major natural coagulation inhibitors with the same batches of reagents and controlled equipment, and the selection of unquestionable statistical methods. Using the method of Royston and Wright, one of the methods recommended by the World Health Organization,39 effects of age and gender on plasma activities of anticoagulant proteins and APC-R were identified. The main findings were that PS and PC activities were lower in women than in men (in particular, for PS), while AT levels remained constant over time in women but decreased significantly in men after 50 years. PC and PS increased constantly with age until old age in women but decreased after 50 years in men. The remarkable combined decline of all three inhibitors in men over 50 years old might favor an increased risk of thrombosis with age in Chinese males, as indeed has already been reported.40 A small effect of age on APC-R levels was found in both genders but the effect of sex on mean APC-R level was lost after adjustment for age. The cut-off value for the 2.5th percentile differed for each 5-year age group in both sexes. Interestingly, the variability of 2.5th percentiles for men (PC: 66 to 81%, PS: 46 to 66%, AT: 71 to 91%) and for women (PC: 69 to 86%, PS: 54 to 62%, AT: 81 to 95%) may reflect a difference in thrombotic risk between men and women.
Among the 15 genetic variants found in our series, nine had been reported previously and have been found to be associated with venous thromboembolism.41–44 Six novel genetic variants were identified in subjects without previous thrombotic episodes, which might be explained by the young age of these subjects and/or the absence of thrombogenic challenges. The ongoing follow-up and pedigree studies of these subjects will help to define the genotype-phenotype correlation and the significance of these genetic variants for thrombotic risk. No FV Leiden or any of the four other FV polymorphisms were detected in the 41 APC-R-positive subjects.
As the demographic composition of the population studied was very similar to that of the general Chinese Han population (about 92% of the total Chinese population), it is reasonable to believe that the results are accurately representative of the Han population.
The differences in protein activities between men and women, especially for PS, might indicate a role of sex hormones, most likely estrogens, in the regulation of anticoagulant factors levels under physiological conditions, as already reported,45,46 although other mechanisms have been proposed. Falkon et al.47 found no relationship between estradiol levels and total or free PS concentration and suggested that low PS levels in young women might result from a combined effect of diverse stimuli on PS synthesis and/or metabolism. Nevertheless, they measured PS antigen only and did not identify any precise molecular mechanism. Variations of other parameters such as triglycerides and cholesterol with sex and age might also play a role.27 Most of the genetic variants were missense substitutions, as listed in the databases of these inhibitors. Mechanisms by which the genetic variants may be deleterious will be presented in detail in another report. The absence of recurrent genetic variants suggests that there was no founder effect, but larger prospective studies are necessary to confirm this.
Compared with antigen assays, functional assays to determine the levels of AT, PC and, especially, PS are subject to the influence of other coagulation factors.48 For example, it is known that the presence of APC-R or FV Leiden can cause artifactually low functional PS levels. However, consistently with other studies in Chinese,12,15,18 no subjects with FV Leiden were identified in our population. Furthermore, there was no correlation between low PS levels and APC-R-positive results either in the whole study population or in the selected APC-R-positive individuals (data not shown). Of more importance, functional assays can identify all types of defects (quantitative and qualitative). APC-R-positive tests cannot be explained by either FV gene mutations or by low PS or PC levels because no FV gene mutation and no interference of low PS or PC activity was observed in these individuals. Interestingly, no interference of low PS level has been found with the Staclot APC-R assay.49 There might be other causes of APC-R, such as still unknown genetic polymorphisms (single nucleotide polymorphisms) especially in the Chinese population in which FV Hong Kong has been discovered.
A bibliographic search was made of PubMed for articles written in English and published between 1975 to 2009. The following key words were used: antithrombin, protein C protein S, Asia population, genetic populations, database, reference ranges. This search did yield some reports of normal ranges for small Asian or Caucasian groups but only a few reports concerned large series of healthy cohorts for Western21–29 or Eastern20,31,32 countries (Online Supplementary Table S3). The largest studies from Asia were conducted in Japan. Age- and sex-related tendencies were found to be similar in the Chinese population and three other populations from Scotland,28 Italy27 and Japan.20 These four studies and a number of other reports26,45–46 were concordant in showing that PS levels were lower in women than in men, with the values being lowest before the age of 45 years,50 as in our study. Interestingly, the findings of the Japanese studies were most closely similar to those in our study. However, no mutation found in the Chinese population was reported in the Japanese population. Our study was not designed to give information about the prevalence of AT, PC and PS deficiencies in the Chinese population. Nevertheless, if we consider people with a confirmed anticoagulant deficiency (low activity level and mutation), PC deficiency (n=10) was more frequent in Chinese subjects (0.29%) than in Japanese ones (0.13%), while AT (n=3) and PS (n=2) deficiencies were less common among the Chinese (0.08% and 0.056%, respectively) than among the Japanese (0.15% and 1.12%, respectively).
Our study has some limitations. First, the clinical relevance of the results should be interpreted cautiously because there is no consensus about the cut-off activity value of the three natural anticoagulants under which there is a true thrombotic risk. Although statistically significant differences were documented for PC and AT activities between the various sex and age groups, the absolute differences were relatively slight, as reported for the Scottish population.22 By contrast, if the same cut-off value was to be applied to make a diagnosis of PS deficiency whatever the sex, this might lead to misdiagnosis of PS deficiency in women. Secondly, since DNA analysis was only performed in subjects with a level of PC or PS activity below the 1st percentile, some true PC and PS hereditary deficiencies might have been missed because some subjects with a level of PC or PS activity between the 1st and 2.5th percentiles might have a genetic defect. A possible overlap of AT, PC or PS activity levels between heterozygotes and individuals without mutations should also be mentioned. In addition, some genetic variants, such as large deletions, might have been missed by direct sequencing. Finally, due to financial, logistic and local reasons, only one sample was obtained for each participant: however, given the large number of samples, one could argue that supposed over- and under-estimated results for some samples would balance each other out.
In conclusion, for the first time we have established sex and age-related normal ranges for levels of AT, PC and PS activity in a large series of 3493 healthy Chinese Han subjects. Fifteen subjects (4.3 per 1000) with a genetic variant were detected by direct sequencing. These reference ranges are crucial in daily clinical practice for the detection of Chinese people with deficiency of the naturally occurring anticoagulant proteins and for better prevention and treatment of venous thromboembolism in the Chinese population.
Acknowledgments
we would like to thank Olivier Morboeuf, Diagnostica Stago, for his help and intellectual input, and Diagnostica Stago for its financial support.
Appendix
Members of the Chinese Hemostasis Investigation on Natural Anticoagulants (C.H.I.N.A) Study I Group: TZ, BH, and YZ (Department of Hematology, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences, Beijing), RY, XW (Institute of Hematology, Peking Union Medical College, Tianjin), XW, QD, XW (Department of Hematology, Shanghai Ruijin Hospital, Shanghai) and CR, ZW, XB (Department of Hematology, Jiangsu Institute of Hematology, Suzhou), China.
Footnotes
Funding: this study was supported by a grant from Diagnostica Stago, Asnières, France.
A complete list of the members of the Chinese Hemostasis Investigation on Natural Anticoagulants Study I Group is given in the Appendix.
The online version of this article has a Supplementary Appendix.
Authorship and Disclosures
The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
References
- 1.Stein PD, Kayali F, Olson RE, Milford CE. Pulmonary thromboembolism in Asians/Pacific Islanders in the United States: analysis of data from the National Hospital Discharge Survey and the United States Bureau of the Census. Am J Med. 2004;116(7):435–42. doi: 10.1016/j.amjmed.2003.11.020. [DOI] [PubMed] [Google Scholar]
- 2.Lee LH, Gu KQ, Heng D. Deep vein thrombosis is not rare in Asia – the Singapore general hospital experience. Ann Acad Med Singapore. 2002;31(6):761–4. [PubMed] [Google Scholar]
- 3.Leizorovicz A, Turpie AGG, Cohen AT, Dhillon KS, Anychaisusksiri P, Wang CJ. Epidemiology of post-operative venous thromboembolism in Asian countries. Int J Angiol. 2004;13(3):101–8. [Google Scholar]
- 4.Leizorovicz A, Turpie AGG, Cohen AT, Wong L, Yoo MC, Dans A for the SMART Study group. Epidemiology of venous thromboembolism in Asian patients undergoing major orthopaedic surgery without thromboprophylaxis. The SMART Study. J Thromb Haemost. 2005;3(1):28–34. doi: 10.1111/j.1538-7836.2004.01094.x. [DOI] [PubMed] [Google Scholar]
- 5.Leizorovicz A SMART Venography Study Steering Committee. Epidemiology of postoperative venous thromboembolism in Asian patients. Results of the SMART venography study. Haematologica. 2007;92 (09):1194–200. doi: 10.3324/haematol.10819. [DOI] [PubMed] [Google Scholar]
- 6.Piovella F, Wang CJ, Lu H, Lee K, Lee LH, Lee WC, et al. Deep-vein thrombosis rates after major orthopedic surgery in Asia. An epidemiological study based on postoperative screening with centrally adjudicated bilateral venography. J Thromb Haemost. 2005;3(12):2664–70. doi: 10.1111/j.1538-7836.2005.01621.x. [DOI] [PubMed] [Google Scholar]
- 7.Manucci PM. Thrombosis and bleeding disorders outside Western countries. J Thromb Haemost. 2007;5(Suppl 1):68–72. doi: 10.1111/j.1538-7836.2007.02463.x. [DOI] [PubMed] [Google Scholar]
- 8.Bertina RM, Koeleman BP, Koster T, Rosendaal FR, Dirven RJ, de Ronde H, et al. Mutation in blood coagulation factor V associated with resistance to activated protein C. Nature. 1994;369(6475):64–7. doi: 10.1038/369064a0. [DOI] [PubMed] [Google Scholar]
- 9.Dahlbäck B, Villoutreix BO. Regulation of blood coagulation by the protein C anticoagulant pathway: novel insights into structure-function relationships and molecular recognition. Arterioscler Thromb Vasc Biol. 2005;25(7):1311–20. doi: 10.1161/01.ATV.0000168421.13467.82. [DOI] [PubMed] [Google Scholar]
- 10.Poort SR, Rosendaal FR, Reitsma PH, Bertina RM. A common genetic variation in the 3′-untranslated region of the prothrombin gene is associated with elevated plasma prothrombin levels and an increase in venous thrombosis. Blood. 1996;88(10):3698–703. [PubMed] [Google Scholar]
- 11.Chan DK, Hu G, Tao H, Owens D, Vun CM, Woo J, et al. A comparison of polymorphism in the 3′-untranslated region of the prothrombin gene between Chinese and Caucasians in Australia. Br J Haematol. 2000;111(4):1253–5. doi: 10.1046/j.1365-2141.2000.02477.x. [DOI] [PubMed] [Google Scholar]
- 12.Hu Y, Chen F, Xie Q, Jian Z, Wang G, Zuo X, et al. No association between thrombosis and factor V gene polymorphisms in Chinese Han population. Thromb Haemost. 2003;89(3):446–51. [PubMed] [Google Scholar]
- 13.Bauduer F, Lacombe D. Factor V Leiden, prothrombin 20210A, methylenetetrahydrofolate reductase 677T, and population genetics. Mol Genet Metab. 2005;86(1–2):91–9. doi: 10.1016/j.ymgme.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 14.Liu HW, Kwong YL, Bourke C, Lam CK, Lie AK, Wei D, et al. High incidence of thrombophilia detected in Chinese patients with venous thrombosis. Thromb Haemost. 1994;71(4):416–9. [PubMed] [Google Scholar]
- 15.Shen MC, Lin JS, Tsay W. High prevalence of antithrombin III, protein C and protein S deficiency, but no factor V Leiden mutation in venous thrombophilic Chinese patients in Taiwan. Thromb Res. 1997;87 (4):377–85. doi: 10.1016/s0049-3848(97)00141-2. [DOI] [PubMed] [Google Scholar]
- 16.Shen MC, Lin JS, Tsay W. Protein C and protein S deficiencies are the most important risk factors associated with thrombosis in Chinese Venous thormbophilic patients in Taiwan. Thromb Res. 2000;99(5):447–52. doi: 10.1016/s0049-3848(00)00265-6. [DOI] [PubMed] [Google Scholar]
- 17.Suehisa E, Nomura T, Kawasaki T, Kanakura Y. Frequency of natural coagulation inhibitor (antithrombin III, protein C and protein S) deficiencies in Japanese patients with spontaneous deep vein thrombosis. Blood Coagul Fibrinolysis. 2001;12(2):95–9. doi: 10.1097/00001721-200103000-00002. [DOI] [PubMed] [Google Scholar]
- 18.Zheng H, Tzeng CC, Butt C, Randell E, Xie YG. An extremely low prevalence of factor V Leiden, FIIG20210A and FXIIIV34L in Taiwan Chinese population. Thromb Haemost. 2002;87(6):1081–2. [PubMed] [Google Scholar]
- 19.Akkawat B, Rojnuckarin P. Protein S deficiency is common in a healthy Thai, population. J Med Assoc Thai. 2005;88(Suppl 4):S249–54. [PubMed] [Google Scholar]
- 20.Miyata T, Kimura R, Kokubo Y, Sakata T. Genetic risk factors for deep vein thrombosis among Japanese: importance of protein S K196E mutation. Int J Hematol. 2006;83(3):217–23. doi: 10.1532/IJH97.A20514. [DOI] [PubMed] [Google Scholar]
- 21.Miletic J, Sherman L, Broze G., Jr Absence of thrombosis in subjects with heterozygous protein C deficiency. N Engl J Med. 1987;317(16):991–6. doi: 10.1056/NEJM198710153171604. [DOI] [PubMed] [Google Scholar]
- 22.Tait RC, Walker ID, Islam SI, Mc Call F, Conkie JA, Mitchell R, et al. Influence of demographic factors in antithrombin III activity in a healthy population. Br J Haematol. 1993;84(3):476–80. doi: 10.1111/j.1365-2141.1993.tb03104.x. [DOI] [PubMed] [Google Scholar]
- 23.Tait RC, Walker ID, Perry DJ, Islam SI, Daly ME, McCall F, et al. Prevalence of antithrombin deficiency in the healthy population. Br J Haematol. 1994;87(1):106–12. doi: 10.1111/j.1365-2141.1994.tb04878.x. [DOI] [PubMed] [Google Scholar]
- 24.Dolan G, Neal K, Cooper P, Brown P, Preston FE. Protein C, antithrombin III and plasminogen: effect of age, sex and blood group. Br J Haematol. 1994;86(4):798–803. doi: 10.1111/j.1365-2141.1994.tb04832.x. [DOI] [PubMed] [Google Scholar]
- 25.Tait RC, Walker ID, Reitsma PH, Islam SI, McCall F, Poort SR, et al. Prevalence of protein C deficiency in the healthy population. Thromb Haemost. 1995;73(1):87–93. [PubMed] [Google Scholar]
- 26.Koster T, Rosendaal FR, Briët E, van der Meer FJ, Colly LP, Trienekens PH, et al. Protein C deficiency in a controlled series of unselected outpatients: an infrequent but clear risk factor for venous thrombosis (Leiden Thrombophilia Study) Blood. 1995;85(10):2756–61. [PubMed] [Google Scholar]
- 27.Rodeghiero F, Tosetto A. The VITA project: population-based distributions of protein C, antithrombin III, heparin-cofactor II and plasminogen-relationship with physiological variables and establishment of reference ranges. Thromb Haemost. 1996;76(2):226–33. [PubMed] [Google Scholar]
- 28.Lowe GD, Rumley A, Woodward M, Morrison CE, Philippou H, Lane DA, et al. Epidemiology of coagulation factors, inhibitors and activation markers: the third Glasgow MONICA Survey I. Illustrative reference ranges by age, sex and hormone use. Br J Haematol. 1997;97(4):775–84. doi: 10.1046/j.1365-2141.1997.1222936.x. [DOI] [PubMed] [Google Scholar]
- 29.Dykes AC, Walker ID, McMahon AD, Islam SI, Tait RC. A study of protein S antigen levels in 3788 healthy volunteers: influence of age, sex and hormone use, and estimate for prevalence of deficiency state. Br J Haematol. 2001;113(3):636–41. doi: 10.1046/j.1365-2141.2001.02813.x. [DOI] [PubMed] [Google Scholar]
- 30.Nomura T, Suehisa E, Kawasaki T, Okada A. Frequency of protein S deficiency in general Japanese population. Thromb Res. 2000;100(5):367–71. doi: 10.1016/s0049-3848(00)00337-6. [DOI] [PubMed] [Google Scholar]
- 31.Sakata T, Okamoto A, Mannami T, Tomoike H, Miyata T. Prevalence of protein S deficiency in the Japanese general population: the Suita study. J Thromb Haemost. 2004;2(6):1012–3. doi: 10.1111/j.1538-7836.2004.00742.x. [DOI] [PubMed] [Google Scholar]
- 32.Sakata T, Okamoto A, Mannami T, Matsuo H, Miyata T. Protein C and antithrombin deficiency are important risk factors for deep vein thrombosis in Japanese. J Thromb Haemost. 2004;2(3):528–30. doi: 10.1111/j.1538-7836.2004.00603.x. [DOI] [PubMed] [Google Scholar]
- 33.Reitsma PH, Ploos van Amstel HK, Bertina RM. Three novel mutations in five unrelated subjects with hereditary protein S deficiency type I. J Clin Invest. 1994;93(2):486–92. doi: 10.1172/JCI116997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lind B, van Solinge WW, Schwartz M, Thorsen S. Splice site mutation in the human protein C gene associated with venous thrombosis: demonstration of exon skipping by ectopic transcript analysis. Blood. 1993;82(8):2423–32. [PubMed] [Google Scholar]
- 35.Olds RJ, Lane DA, Chowhury V, De Stefano V, Leone G, Thein SL. Complete nucleotide sequence of the antithrombin gene: evidence for homologous recombination causing thrombophilia. Biochemistry. 1993;32(16):4216–24. doi: 10.1021/bi00067a008. [DOI] [PubMed] [Google Scholar]
- 36.Chan WP, Lee CK, Kwong YL, Lam CK, Liang R. A novel mutation of Arg306 of factor V gene in Hong Kong Chinese. Blood. 1998;91(4):1135–9. [PubMed] [Google Scholar]
- 37.Williamson D, Brown K, Luddington R, Baglin C, Baglin T. Factor V Cambridge: a new mutation (Arg306→Thr) associated with resistance to activated protein C. Blood. 1998;91(4):1140–4. [PubMed] [Google Scholar]
- 38.Royston P, Wright EM. A method for estimating age-specific reference intervals ('normal ranges') based on fractional polynomials and exponential transformation. Journal of the Royal Statistical Society: Series A (Statistics in Society) 1998;161(1):79–101. [Google Scholar]
- 39.Borghi E, de Onis M, Garza C, Van den Broeck J, Frongillo EA, Grummer-Strawn L, et al. WHO Multicentre Growth Reference Study Group. Construction of the World Health Organization child growth standards: selection of methods for attained growth curves. Stat Med. 2006;25(2):247–65. doi: 10.1002/sim.2227. [DOI] [PubMed] [Google Scholar]
- 40.Yeh CJ, Pan WH, Bai CH, You MS, Wang WC, Wang LY, et al. Curvilinear relations between age and hemostatic parameters in Chinese. Thromb Haemost. 1994;72(2):239–43. [PubMed] [Google Scholar]
- 41.Lane DA, Bayston T, Olds RJ, Fitches AC, Cooper DN, Millar DS, et al. Antithrombin III mutation database: 2nd (1997) update. For the Plasma Coagulation Inhibitors Subcommittee of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Thromb Haemost. 1997;77 (1):197–211. [PubMed] [Google Scholar]
- 42.Reitsma PH, Bernardi F, Doig RG, Gandrille S, Greengard JS, Ireland H, et al. Protein C deficiency: a database of mutations, 1995 update. On behalf of the Subcommittee on Plasma Coagulation Inhibitors of the Scientific and Standardization Committee of the ISTH. Thromb Haemost. 1995;73(5):876–89. [PubMed] [Google Scholar]
- 43.Gandrille S, Borgel D, Ireland H, Lane DA, Simmonds R, Reitsma PH, et al. Protein S deficiency: a database of mutations. For the Plasma Coagulation Inhibitors Subcommittee of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Thromb Haemost. 1997;77 (6):1201–14. [PubMed] [Google Scholar]
- 44.Gandrille S, Borgel D, Sala N, Espinosa-Parrilla Y, Simmonds R, Rezende S, et al. Plasma Coagulation Inhibitors Subcommittee of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Protein S deficiency: a database of mutations--summary of the first update. Thromb Haemost. 2000;84 (5):918. [PubMed] [Google Scholar]
- 45.Gari M, Falkon L, Urrutia T, Vallvé C, Borrell M, Fontcuberta J. The influence of low protein S plasma levels in young women, on the definition of normal range. Thromb Res. 1994;73(2):149–52. doi: 10.1016/0049-3848(94)90091-4. [DOI] [PubMed] [Google Scholar]
- 46.Henkens CM, Bom VJ, Van der Schaaf W, Pelsma PM, Sibinga CT, de Kam PJ, et al. Plasma levels of protein S, protein C, and factor X: effects of sex, hormonal state and age. Thromb Haemost. 1995;74(5):1271–5. [PubMed] [Google Scholar]
- 47.Falkon L, Gari M, Garcia Mora JL, Rodriguez Espinosa J, Oliver A, Fontcuberta J. The effect of endogenous oestradiol levels on protein S concentration during a menstrual cycle and after GnRH analogues and gonadotropin therapy. Br J Haematol. 1995;90(2):438–41. doi: 10.1111/j.1365-2141.1995.tb05171.x. [DOI] [PubMed] [Google Scholar]
- 48.Goodwin AJ, Rosendaal FR, Kottke-Marchant K, Bovill EG. A review of the technical, diagnostic, and epidemiologic considerations for protein S assays. Arch Pathol Lab Med. 2002;126(11):1349–66. doi: 10.5858/2002-126-1349-AROTTD. [DOI] [PubMed] [Google Scholar]
- 49.Quenhenberger P, Handler S, Mannhalter C, Pabinger-Fasching I, Speiser W. Evaluation of a highly specific functional test for the detection of factor V Leiden. Int J Clin Lab Res. 2000;30(3):113–7. doi: 10.1007/s005990070009. [DOI] [PubMed] [Google Scholar]
- 50.Falkon L, Garí M, Fontcuberta J. The accurate definition of protein S deficiency may avoid the misestimation of the frequency of this defect. Blood. 1996;87(6):2604. [PubMed] [Google Scholar]