Abstract
The field of hematopoietic oncology has traditionally focused on the study of hematopoietic cell autonomous genetic events in an effort to understand malignant transformation and develop therapeutics. Although highly rewarding in both aspects, this cell autonomous approach has failed to fully satisfy our need to understand tumor cell behavior and related clinical observations. In recent years, it has been increasingly recognized that the tumor microenvironment plays a pivotal role in cancer initiation and progression. This review will discuss recent experimental evidence in support of this view derived from investigations in both epithelial and hematopoietic systems. Based on this, conceptual views and therapeutic implications will be provided on the emerging role of the bone marrow microenvironment in leukemogenesis.
Keywords: leukemia, leukemogenesis, hematopoietic stem cell niche-microenvironment
Introduction
Hematopoietic cells in oncogenesis: a reductionist view
The ability to separate, with relative ease, individual hematopoietic cells from their surrounding tissue in the bone marrow has greatly facilitated insight into hematopoiesis and hematopoietic malignancy at the cellular and molecular level. It was the hematopoietic system that, almost 50 years ago, revealed the first experimental evidence of the existence of stem cells in Till and McCulloch’s seminal work.1 These observations were followed by the identification of hematopoietic stem cell markers which led to their prospective identification and isolation,1041 followed by the delineation of lineage progression in the hematopoietic system, emanating in the iconic scheme of cellular differentiation in hematopoiesis, known to all hematologists and biologists. These pioneering insights into the cellular hierarchy of blood cell formation, and the unique ability to interrogate well defined subsets of hematopoietic cells, fostered the identification of tumor-initiating cells3 and molecular insights into hematopoietic stem and progenitor cell (HSPC) behavior, including the genetic events leading to its malignant transformation. This knowledge has resulted in an increasingly detailed molecular categorization of leukemia and the dissection of genetic abnormalities in acquired functional capabilities that define hematopoietic neoplasm and other cancers,4 such as self-sufficiency in growth signals, evading apoptosis, limitless replicative potential, insensitivity to anti-growth signaling, sustained angiogenesis, and tissue invasion and metastasis. These insights led to the emergence of highly effective targeted therapies against subtypes of leukemia, e.g. imatinib in bcr/abl positive CML,5 and helped widen our understanding of tumor cell biology in other systems.
A role for the microenvironment in tumorigenesis
Although highly rewarding in these aspects, the reductionist, hematopoietic cell-centered approach to the understanding of hematopoiesis and its deregulation in hematopoietic cancer has its limitations. It has been increasingly recognized that cancer initiation and progression is not solely a cancer cell autonomous process.6 Primary tissue cells live in a complex mesenchymal environment, characterized by heterotypic signaling between ancillary cells and hematopoietic cells. This signaling is considered to play a role in the regulation of the behavior of HSPC, including their proliferation and differentiation. The early events driving the initiation and malignant evolution of pre-malignant states remain largely unknown but involvement of a permissive tissue microenvironment has been hypothesized.7,8 This view is fueled by several intriguing observations in hematopoietic neoplasms that have long been at odds with the notion of a predominantly hematopoietic cell autonomous genesis of malignant transformation. These include the well described phenomenon of donor cell-derived hematopoietic neoplasm, defined as oncogenic transformation of apparently normal donor hematopoietic cells in the transplant recipient (but not the donor).9 Also, the inability to propagate certain leukemias, derived from humans or mice, in immunodeficient mice10 appears to be incongruent with the view of hematopoietic cell autonomous acquisition of all cancer cell characteristics. Albeit infrequent, these observations defy the dogma that the events that initiate cancer, or its defining characteristics, including infinite proliferation and self-renewal capacity, are completely or predominantly cancer cell autonomous. Given the number of genetic alterations required to develop clinically overt malignancy, it has been proposed that in addition to the acquired characteristics cells should acquire “enabling” characteristics, promoting genomic instability.4 These ‘enabling’ characteristics, that allow a single cell to accumulate the number of genetic changes required to become malignant, are still not completely understood. In the hematopoietic system, the observation that neoplasm may have polyclonal origins11 is not easily reconciled with the conception that the accumulation of rare, stochastic genetic events leads to cancer. The observation suggests a more generalized susceptibility to malignant transformation. It then seems reasonable to consider the possibility that the tumor milieu or microenvironment is a determinant of the ‘enabling’ and ‘defining’ characteristics of cancer in the hematopoietic system.
Dissecting the regulatory environment in the hematopoietic system
Abnormalities in the tissue architecture in hematopoietic neoplasms have been noted historically but the contribution to disease pathogenesis, if any, has remained poorly explored. In order to clarify this we need an understanding of the regulatory role of the bone marrow microenvironment in hematopoiesis. Interestingly, it was observations in the hematopoietic system that first focused on the regulatory role of the microenvironment in tissue homeostasis. In 1978, Schofield predicted that there was a specific hematopoietic stem cell niche which ‘fixed’ the stem cells in place and prevented their differentiation, allowing the stem cell to proliferate and retain its stemness.12 Once the stem cell progeny left the stem cell niche they would then be able to differentiate. These predictions were based on his observations that hematopoietic stem cells (HSCs) needed to reside in the bone marrow to retain their ‘infinite’ potential, whereas those that homed to the spleen and formed colonies (CFU-S) were more restricted in their capacity to sustain hematopoiesis.
Since then, and in remarkable contrast to the growing understanding of hematopoietic cells, progress in defining the cellular and molecular constituents of this niche has been slow.
It is only over the last decade that substantial progress has been made in delineating the cellular components of this ancillary niche and the molecular communication that underlies its regulatory function.13 Cell types that, to date, have been implicated in this regulatory environment include osteolineage cells,14,15 endothelial cells,16,17 Cxcl12-expressing reticular cells,18 osteo(adipo)progenitor cells,19,20 osteoclasts,21 adipocytes,22 and Nestin-expressing mesenchymal cells.23 These cell types form a dynamic environment in which the number of niche cells can correlate with the number of HSCs, a characteristic that can be exploited for therapeutic purposes.24
Many questions remain, including the functional and hierarchical relationship between these identified subsets and the hematopoietic subsets that are under their control, but the identification of niche participants rapidly creates a framework in which the role of these cells can be investigated, not only in tissue homeostasis but also in disease pathogenesis. This review will address evolving concepts in the contribution of the microenvironment to the initiation, evolution and progression of hematopoietic neoplasm. It will discuss recent experimental evidence supporting these concepts including precedents from epithelial systems that may serve as models for understanding the role of the bone marrow environment in leukemogenesis. Finally, diagnostic and therapeutic perspectives emanating from these insights will be provided.
Emerging concepts on the role of the tissue microenvironment in carcinogenesis
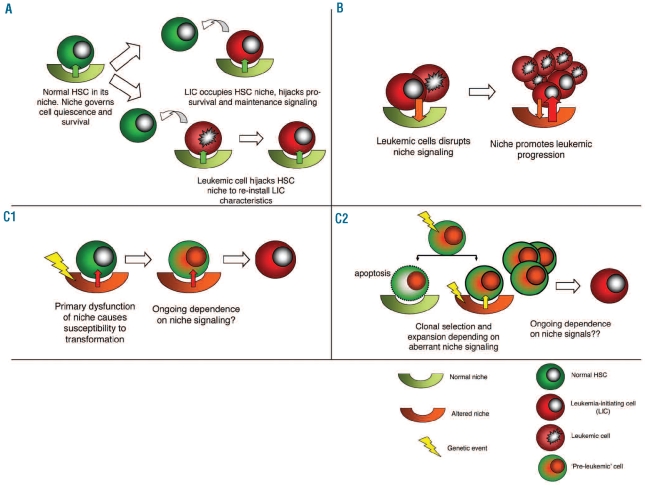
How, in principle, can ancillary tissue cells play a role in oncogenesis? Experimental data support several views (Figure 1). First, tumor cells can usurp existing HSC niches and take advantage of niche specific signaling for their maintenance and survival. This can be achieved through competition for niches with normal tissue residents, particularly stem and progenitor cells, or by actively repressing normal hematopoiesis or ‘hijacking’ these niches. Secondly, tumor cells may manipulate their environment in such a way that the environment becomes a ‘partner in crime’, promoting tumor progression and metastasis. In particular, data from diverse epithelial systems support this view. Thirdly, it is conceivable that disruption of heterotypic signaling between HSCs and their niches contributes to cancer initiation. Recent observations in stem cell niches and murine disease models have added credibility to this concept of niche driven oncogenesis. These models, the evidence to support this concept, and their relevance for hematopoietic neoplasm will be discussed.
Figure 1.
Concepts of niche contribution to leukemogenesis. (A) Competition for innate niches for LIC maintenance and survival. LICs compete with residual normal HSCs for the survival signalling conferred by the niche. Alternatively, leukemic cells occupy HSC niches to re-install LIC characteristics. Clinical consequences would include persistence of minimal residual disease, relapse and suppression of residual normal hematopoiesis. (B) Niche disruption to facilitate leukemia progression. Leukemic cells alter the microenvironment in such a way that it cooperates in leukemic progression. (C) Niche-driven oncogenesis. Primary dysfunction of the niche is required for oncogenesis. This can occur through promoting malignant transformation by increasing the target cell pool size or inducing genomic instability in normal HSPC (C1) Alternatively, an aberrant niche may be required for clonal expansion and further malignant transformation of genetically aberrant (preleukemic) cells (‘interclonal oncogenic cooperation’) (C2). For experimental data supporting these concepts: see text.
1. Competition for innate niches for leukemia maintenance and survival
The normal hematopoietic stem cell niche is the site where HSCs are nurtured to ensure their life-long contribution to hematopoiesis, principally by ensuring HSC survival and self-renewal. This is in part achieved by creating an immuno-privileged sanctuary governing cellular quiescence. The niche and its occupying HSC, however, do not represent a fixed entity but rather a fragile relationship. Recent studies in Drosophila have demonstrated that stem cell niches can be occupied by cells that outcompete normal stem cells for residence.25,26 Similarly, neoplastic cells can take advantage of these privileged sites to promote their own malignant agenda of evading apoptosis and acquiring self-renewal capacity (Figure 1A). Many, predominantly in vitro investigations have demonstrated the chemo-protective effect of hematopoietic-stromal cell interactions, a phenomenon often referred to as “cell adhesion mediated drug resistance” and implicated in the occurrence and persistence of minimal residual disease.27 Recent in vivo experimental support for this view in the hematopoietic system has come from human AML xeno-transplantation models using newborn non-obese diabetic/severe combined immunodeficient/interleukin (NOD/SCID/IL)2rγ null mice showing that leukemia-initiating cells (LICs) home to and engraft within the endosteal area of the bone marrow, where they are protected from chemotherapy-induced apoptosis.28 Subsequent studies demonstrated that cellular quiescence of human LICs at these sites governed resistance to cell cycle-dependent cytotoxic therapy, which could be abrogated by inducing these cells to enter the cell cycle by treatment with granulocyte colony-stimulating factor (G-CSF).29 In combination with cell cycle-dependent chemotherapy, G-CSF treatment significantly enhanced induction of apoptosis and elimination of human primary LICs in vivo, further corroborated by significantly increased survival of secondary recipients after transplantation of leukemia cells compared with chemotherapy alone.
The occupation of niches may take place at the expense of normal HSPC, a phenomenon likely reflected by the common observation of the suppression of normal hematopoiesis by hematopoietic neoplasm, even if this cannot readily be explained by ‘outcrowding’ in the bone marrow. It has been shown that this may occur by creating aberrant malignant niches that have the capacity to attract and impair normal HSPC30. Proliferation of pre-B acute lymphoblastic leukemia cells in the bone marrow of xenografted mice altered the stromal microenvironment, creating niches for leukemic cells that outcompeted native HSPC niches for CD34+ cell engraftment but failed to preserve the normal HSPC pool. Together these observations support the view that leukemic cells can usurp normal niches and that tumor induced alterations of the microenvironment may affect normal hematopoiesis.
Hijacking the niche to re-install cancer stem cell characteristics
A defining characteristic of stem cell niches is their capacity to endow stem cell characteristics on the cells that occupy them. Reversion of differentiated cells to cells with stem cell characteristics upon exposure to niche factors has been demonstrated in both Drosophila31,32 and mammals.33 The power of the bone marrow microenvironment to govern lineage progression and differentiation choices in the hematopoietic system is further documented by the ability of microenvironmental clues to direct lineage decisions of leukemic stem cells in a model of biphenotypic MLL-AF9 leukemia.34 Importantly for oncogenesis, recent evidence suggests that the dominance of environmental clues to endow and install stem cell characteristics may not only occur in normal stem cells but may also be relevant to tumor-initiating cells (Figure 1A). In adenocarcinomas, high activity of the Wnt pathway is observed preferentially in tumor cells located close to stromal myofibroblasts, indicating that Wnt activity and cancer stemness may be regulated by extrinsic cues. In agreement with this, differentiated cancer cells which had lost the capacity to form tumors and were no longer clonogenic, could be reprogrammed to express colonic stem cell markers and regain their tumorigenic capacity when stimulated with myofibroblast-derived factors, including hepatocyte growth factor (HGF).35 The data suggest that cancer stemness is not a rigid feature but can be modulated and even installed by the microenvironment. Although the relevance of these findings to hematopoietic neoplasms must still be defined, it is conceivable that the proposed scheme of linear hierarchy in leukemia has much more plasticity than previously recognized. This would raise daunting challenges to treatment; whereas, in a unidirectional hierarchy, eradication of cancer stem cells should theoretically be sufficient to cure disease, this is clearly not the case if more differentiated cells regain leukemia-initiating capacity after surviving treatment.
2. Niche disruption to facilitate cancer progression and metastasis
There is increasing evidence indicating that tumor-stromal cell interactions have a crucial role in tumor progression (Figure 1B). Most of this knowledge is derived from studies in epithelial tumors where the role of cancer associated fibroblasts (CAFs) has been extensively studied.7,8 The functions of normal fibroblasts in epithelial tissues include the deposition of extracellular matrix (ECM), regulation of epithelial differentiation and regulation of inflammation and wound healing.36,37 In tumorigenesis, these fibroblasts become activated through signaling induced by tumor cell or microenvironment-derived ligands.38 Activated fibroblasts secrete increased levels of ECM-degrading proteases (such as matrix metalloproteinases) and growth factors such as hepatocyte growth factor (HGF) and tumor growth factor (TGF-β1) which can induce proliferative signals within adjacent epithelial cells. In a xenograft model of breast cancer, CAFs were shown to play a central role in promoting the growth of tumor cells through their ability to secrete stromal cell-derived factor 1 (CXCL12/SDF1).39 SDF-1promoted angiogenesis by recruiting endothelial progenitor cells (EPCs) into carcinomas and directly stimulated tumor growth, acting through the cognate receptor, CXCR4, which is expressed by carcinoma cells. Further evidence to support a role for stromal cell types in breast cancer cell progression is provided by the demonstration that bone marrow derived human mesenchymal stem cells cause otherwise weakly metastatic human breast carcinoma cells to greatly increase their metastatic potency.40 In this xenograft model, the breast cancer cells stimulate de novo secretion of the chemokine CCL5 (RANTES) from mesenchymal stem cells, which then acts in a paracrine fashion on the cancer cells to enhance their motility, invasion and metastasis.
Recent studies have revealed additional molecular targets to inhibit the promotion of tumorigenesis by stromal cells, including a non-cell autonomous role for hedgehog (hh) signaling in the stromal microenvironment in the progression of epithelial tumors.41 Inhibition of ligand-dependent activation of the Hh pathway in the stromal microenvironment resulted in growth inhibition in xenograft tumor models. In another study, genetic inactivation of Pten and the resulting activation of Ets2 in stromal fibroblasts of mouse mammary glands accelerated the progression and malignant transformation of mammary epithelial tumors.42 This was associated with the massive remodeling of the extracellular matrix (ECM), innate immune cell infiltration and increased angiogenesis. Remarkably, Ets2 inactivation in Pten stroma-deleted tumors ameliorated disruption of the tumor microenvironment and was sufficient to reduce tumor growth and progression. It can be concluded that the stromal tumor microenvironment in epithelial tumors contributes to tumorigenesis and that the molecular players behind it can, in principle, be targeted for therapeutic purposes.
So what evidence is there that stromal cell types in the bone marrow promote progression or evolution of leukemic cells? Interestingly, osteoblastic cells have been described “as sophisticated fibroblasts”43 with nearly identical gene expression signatures in osteoblasts and fibroblasts. Many of the cytokines implicated in epithelial tumor progression are present in the bone marrow. Although these parallels predict a role for bone marrow stromal cells in leukemic progression, surprisingly little is known about the role of the microenvironment in this process. Our current knowledge is largely derived from co-culture studies of leukemic cells with different osteoblastic and mesenchymal cell subsets. Upon co-culture, stromal cells can alter their behavior, resulting in increased proliferation and altered cytokine profiles of leukemic cells in some studies.44,45 If and how ancillary cells contribute to disease progression in vivo has not been studied in depth and there have been no studies in which leukemic cells are co-transplanted with microenvironmental subsets. Challenges in long-term survival, propagation and differentiation of mesenchymal cells in the bone marrow, together with a lack of cell-type specific genes to genetically target subsets of cells in the environment, have hampered progress in this area.
3. Niche-driven oncogenesis
Given the increasing amount of data demonstrating a key regulatory role for the microenvironment in tissue homeostasis, it seems reasonable to propose that primary disruption of the microenvironment, in principle, has the capacity to result in disruption of steady state hematopoiesis, perhaps even hematopoietic disease. It has long been hypothesized that disruption of heterotypic niche signaling governing quiescence in stem cell niches may lead to increased proliferation of primitive cells allowing them to acquire genetic abnormalities and initiate tumorigenesis.6,46 Of interest, the activated state of fibroblasts, promoting tumorigenesis, can also be induced by stimuli such as sublethal irradiation, senescence and inflammation, providing an attractive working model for understanding the association between inflammation, aging and cancer.47–49 However, emerging data indicate that not all tissue stem cells in mammals are in a quiescent state and may switch between a deeply quiescent, reserve, pool and an activated, proliferating state upon increased demands of the tissue.50 Examples of the existence of stem cells with different proliferation rates include the intestinal and hematopoietic systems.50,51 Distinct activation stages of hematopoietic stem cells may be associated with distinct niches, although as yet there have been no experiements which have shown this. This indicates that HSC proliferation in itself does not have to be detrimental to the genomic integrity of the cell. At the same time, the concept of ‘activation-state switching’ opens the possibility that, in addition to loss of quiescence governing signaling in the ‘quiescent’ niche, disruption of the extrinsic clues regulating traffic between such niches, may lead to loss of anchorage of stem cells from their ‘quiescent’ niche (protecting genomic integrity) towards an ‘activation’ niche where proliferation may poise these long-lived cells for genetic transformation.
The first demonstration that primary alteration of ancillary cells can initiate tumorigenesis in an animal model was provided through targeted deletion of the TGF- β-receptor in fibroblasts using the fibroblast-specific protein, FSP1, as a promoter. This resulted in intraepithelial neoplasia in prostate and invasive squamous cell carcinoma of the forestomach in the mouse.52 Although accidental deletion of TGFBr in a subset of epithelial cells cannot be entirely excluded, activation of paracrine hepatocyte growth factor (HGF) signaling upon loss of TGF-β responsiveness was identified as one possible mechanism for stimulation of epithelial proliferation. The findings indicated that normal fibroblasts are required to maintain epithelial homeostasis, whereas ‘activated’ CAFs promote, and may in fact initiate, tumorigenic alterations in epithelial cells.
Recently, the first evidence was provided that primary alterations in the bone marrow microenvironment can drive oncogenesis in the hematopoietic system.19 In this study, dysfunction of a well-defined stromal subset of bone progenitor cells initiated a pre-leukemic tissue state, enabling the development of acute leukemia. In an effort to investigate the role of developmental stage-specific osteoblastic cells in the regulation of hematopoiesis, the authors faced the common conundrum of how to select genes to modulate and therefore chose a strategy to alter gene expression programs, rather than single genes, by targeting the ‘landscape-modifying’ miRNA-processing endonuclease Dicer1. Targeted deletion of this gene from osterix-expressing osteoprogenitor cells broadly disrupted the integrity of the hematopoietic system, affecting survival, proliferation and differentiation of heterotypic hematopoietic stem and progenitor cells in a manner recapitulating key features of the human leukemia-predisposition myelodysplastic syndrome (MDS). These changes were entirely dependent on the microenvironment and were not observed when Dicer1 was deleted in mature osteoblasts. Transplantation of hematopoietic cells from mutant mice displaying myelodysplasia into wild-type mice reversed their phenotype whereas transplantation of wild-type hematopoietic cells into mutant mice resulted in myelodysplasia, indicating that changes were entirely microenvironment dependent. The recapitulation of human MDS characteristics included the propensity to develop acute myelogenous leukemia, manifested by leukocytosis with myeloblasts, anemia, splenomegaly, and the development of myeloid sarcomas which harbored distinct cytogenetic abnormalities.
Dicer1 deleted osteoprogenitors expressed reduced levels of Sbds, the gene mutated in Shwachman-Bodian-Diamond Syndrome, the human congenital bone marrow failure and leukemia predisposition state and deletion of Sbds from osteoprogenitors largely phenocopied the myelodysplastic phenotype. The data demonstrate the central role individual cellular stromal elements can play in tissue homeostasis and reveal that primary dysfunction of such cells can initiate secondary neoplastic disease in the hematopoietic system. The study added to earlier work showing that deletion of the retinoic acid receptor γ (RARγ) from the bone marrow microenvironment in mice led to the development of a myeloproliferative syndrome (MPS), but not hematopoietic malignancy.53 This phenotype could partly be attributed to an increase in the expression of TNFα, as transplantation with TNFα −/−bone marrow in RARγ −/− mice led to a partial rescue of the MPS phenotype. Also noteworthy in this context is a recent study in which induction of VEGF in osteo-chon-droprogenitors and their progeny was shown to induce increased bone mass, and aberrant vascularization of the bone and hematopoietic abnormalities.54 These abnormalities include pronounced bone marrow fibrosis, altered megakaryocytes and enhanced mobilization of HPCs from the bone marrow into the circulation, findings remarkably similar to those in patients with primary myelofibrosis, another preleukemic bone marrow disorder. Induction of beta-catenin transcriptional activity in endothelial and osteoblastic cells, likely through modulation of glycogen synthase kinase 3-beta phosphorylation, was involved, coupling angiogenesis and osteogenesis in this model. Together, the studies illustrate the capacity of primary alterations in the bone marrow microenvironment to initiate hematopoietic abnormalities that resemble human disease. Interestingly, these diseases have in common a predisposition to the development of acute leukemia and the data point to the microenvironment as the site of the initiating event that leads to secondary genetic changes in other cells. It is, therefore, possible to imagine a niche-based model of oncogenesis whereby a change in a specific microenvironmental cell can serve as the primary moment in a multi-step process toward malignancy of a supported but distinct cell type. The question should then be raised: how does the niche act as a leukemogenesis-driving entity? Based on the data, several scenarios are conceivable (Figure 1C).
First, it may be that the niche acts as an initiator of oncogenesis (Figure 1C1). Increased proliferation and impaired, dysplastic differentiation of hematopoietic progenitor cells induced by the microenvironment may poise hematopoietic cells for genetic events initiating malignant transformation. In such a scenario, the niche acts through increasing the size of the target cell pool to increase probability of tumor initiation. Alternatively, genetic events could result from genomic instability induced by dysfunction of the microenvironment. Support for this view has been provided by the demonstration that the matrix met-alloproteinase MMP3 and hypoxia, both under regulatory control of the bone marrow microenvironment, can induce genomic instability in heterotypic cells.55–57 In mammary epithelial cells, MMP3 caused genomic alterations by activating genotoxic cellular metabolic pathways and subsequent generation of reactive oxygen species. Other mechanisms through which MMPs have been suggested to promote early tumorigenesis include disruption of cell-ECM adhesion resulting in loss of anchorage-dependent maintenance of genomic integrity or by compromising normal cytokinesis.56
Rather than directly initiating oncogenesis through the induction of genetic instability, the aberrant microenvironment may enable the expansion and subsequent malignant transformation of pre-existing, stochastically occurring, cytogenetic clones in the bone marrow (Figure 1C2). In such a scenario, random cytogenetic abnormalities occur in HSPC, but do not lead to clonal advantage over non-mutated competitors unless alterations of ancillary cells provide a selective environment for expansion. In this view, the microenvironment is a ‘facilitator’, required but not sufficient for malignant transformation. The sporadic occurrence of AML in mice with a genetically altered microenvironment19 may agree with such a view.
Recent support for this came from data in Drosophila demonstrating that genetic abnormalities in adjacent cells can cooperate in the process of carcinogenesis,58 referred to as “interclonal oncogenic cooperation”. In this study, Ras(V12) (an oncogenic form of the Drosophila Ras85D protein) and scrib(−) mutations in different adjacent epithelial cells caused tumors, whereas the tumorigenic capacity of cells with RAS(V12) mutations alone was low. Theoretically, these concepts for niche-driven oncogenesis are not mutually exclusive but may be co-operative. The current mouse models do not differentiate between these possibilities.
Clinical perspectives and future challenges
Despite the increasing recognition of the roles of ancillary cells in the development and regulation of hematopoiesis, the contribution of the bone marrow microenvironment to human hematopoietic disease has still not been adequately explored. Much of the knowledge about the microenvironmental contribution to oncogenesis has been derived from epithelial tumors. The emerging models suggest that the bone marrow microenvironment may be pivotal not only in causing disease maintenance and resistance but could also be critically implicated in disease initiation and progression. Emerging mouse models link primary alterations in the microenvironment to human pre-leukemic bone marrow conditions, such as congenital bone marrow failure syndromes, MDS, MPD and primary myelofibrosis.
Today, it must still be determined whether any ancillary cell abnormality plays a role in the pathogenesis of these disorders in humans, but several observations may be congruent with this view. In MDS, unlike some other clonal hematopoietic disorders, it has been very difficult to faithfully engraft and propagate human disease in murine models by transplanting hematopoietic cells from MDS patients into immunodeficient mice.10 This observation has sparked a long standing debate about a potential causative or facilitating role for the microenvironment in the pathogenesis of this disease.59 A sick environment with an altered cytokine milieu may work together with sick hematopoietic stem cells in the cause and evolution of these diseases but the relative contribution of these cellular compartments to disease development, the molecular crosstalk governing their evolution, and the specific identity of the cellular elements in the bone marrow microenvironment remain to be explored.
Intriguingly, aberrant localization of immature progenitors (ALIP) is a well documented histological finding in MDS and refers to the more central medullary localization of primitive hematopoietic cells instead of their normal localization near the trabecular endosteum.60 It may be worthwhile revisiting this phenomenon in the light of the expanding potential to identify cellular constituents of hematopoietic niches and the emerging understanding that distinct HSC niches, e.g. endosteal and endothelial niches, may govern discrete proliferation stages of HSPC.
An increasing number of studies indicate that stromal cells in human hematopoietic pre-leukemia have undergone both phenotypic and genetic abnormalities,61,62 although the heterogeneity of ex vivo expanded BMDSC and the experimental bias introduced by in vitro culture of plastic adherent cells have limited the conclusiveness of such investigations. Similarly, somatic genetic alterations within stromal cells have been demonstrated in epithelial tumors and attributed to clonal selection or co-evolution in a mutagenic cancer-environment although these findings remain controversial due to debates about the methodology used.63 In the light of emerging experimental data, including the recognition that genetic abnormalities in adjacent cells can co-operate in the process of carcinogenesis, it is now conceivable that these genetic abnormalities, if confirmed, are not only the result of co-evolution in the tumor environment but may in fact be at the basis of leukemia initiation and progression. The recognition that signals from the microenvironment may drive or select for subsequent transforming events, and that evolving leukemia may remain dependent on niche signaling, implies that such signals may represent candidate prognostic factors and therapeutic targets in both treatment and prevention strategies. In this light, it will also be interesting to revisit the effects of current immunomodulatory agents on this signaling64 in order to fully understand the heterogeneity in efficacy in some subtypes of MDS and myelofibrosis.
Looking ahead, major challenges remain. It will be important to further identify and characterize subsets of ancillary cells in the bone marrow, their functional relationships and roles in hematopoietic support, both individually and together. The emerging data reveal an increasing number of cell types involved, but the greater challenge will be in revealing their hierarchical and functional relationship and how these cell types integrate into an organ, sufficiently nimble and dynamic, to support the demands of hematopoiesis. It has to be recognized that these relationships are dynamic and interactive. Hematopoietic cells influence each other and can shape their environment but similarly, different developmental subsets of environmental cells have effects on hematopoietic cells and other ancillary cells, e.g. osteoprogenitor cells have emerged as regulators of the integrity of the bone marrow architecture in the mouse models available,19,54 generating secondary changes in other microenvironmental compartments such as endothelial cells or the tissue matrix.
As these investigations are likely to occur in animal model systems, it will be important to translate findings to the human bone marrow. Identification of cellular subsets in humans will then allow their molecular interrogation in normal versus diseased states.
In the years to come, these investigations may complement the well known schedule of differentiation and lineage progression in hematopoiesis with the microenvironmental cells and factors that govern these processes in the bone marrow. Deciphering this microenvironmental dimension of hematopoiesis will be essential to fully appreciate its role in hematopoietic disease, including leukemogenesis. The evolving concepts on the contribution of the environment to oncogenesis, partly instructed by other systems as outlined in this paper, warrant consideration in the design and interpretation of such investigations.
Footnotes
Authorship and Disclosures
The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
References
- 1.Till JE, McCulloch EA. A direct measurement of the radiation sensitivity of normal mouse bone marrow cells. Radiat Res. 1961;14:213–22. [PubMed] [Google Scholar]
- 2.Spangrude GJ, Heimfeld S, Weissman IL. Purification and characterization of mouse hematopoietic stem cells. Science. 1988;241(4861):58–62. doi: 10.1126/science.2898810. [DOI] [PubMed] [Google Scholar]
- 3.Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3(7):730–7. doi: 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- 4.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100(1):57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 5.Capdeville R, Buchdunger E, Zimmermann J, Matter A. Glivec (STI571, imatinib), a rationally developed, targeted anticancer drug. Nat Rev Drug Discov. 2002;1(7):493–502. doi: 10.1038/nrd839. [DOI] [PubMed] [Google Scholar]
- 6.Li L, Neaves WB. Normal stem cells and cancer stem cells: the niche matters. Cancer Res. 2006;66(9):4553–7. doi: 10.1158/0008-5472.CAN-05-3986. [DOI] [PubMed] [Google Scholar]
- 7.Nelson CM, Bissell MJ. Of extracellular matrix, scaffolds, and signaling: tissue architecture regulates development, home-ostasis, and cancer. Annu Rev Cell Dev Biol. 2006;22:287–309. doi: 10.1146/annurev.cellbio.22.010305.104315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tlsty TD, Coussens LM. Tumor stroma and regulation of cancer development. Annu Rev Pathol. 2006;1:119–50. doi: 10.1146/annurev.pathol.1.110304.100224. [DOI] [PubMed] [Google Scholar]
- 9.Flynn CM, Kaufman DS. Donor cell leukemia: insight into cancer stem cells and the stem cell niche. Blood. 2007;109(7):2688–92. doi: 10.1182/blood-2006-07-021980. [DOI] [PubMed] [Google Scholar]
- 10.Kerbauy DM, Lesnikov V, Torok-Storb B, Bryant E, Deeg HJ. Engraftment of distinct clonal MDS-derived hematopoietic precursors in NOD/SCID-beta2-microglobulin-deficient mice after intramedullary transplantation of hematopoietic and stromal cells. Blood. 2004;104(7):2202–3. doi: 10.1182/blood-2004-04-1518. [DOI] [PubMed] [Google Scholar]
- 11.Johansson B, Billstrom R, Broberg K, Fioretos T, Nilsson PG, Ahlgren T, et al. Cytogenetic polyclonality in hematologic malignancies. Genes Chromosomes Cancer. 1999;24(3):222–9. [PubMed] [Google Scholar]
- 12.Schofield R. The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells. 1978;4(1–2):7–25. [PubMed] [Google Scholar]
- 13.Raaijmakers MH, Scadden DT. Evolving concepts on the microenvironmental niche for hematopoietic stem cells. Curr Opin Hematol. 2008;15(4):301–6. doi: 10.1097/MOH.0b013e328303e14c. [DOI] [PubMed] [Google Scholar]
- 14.Calvi LM, Adams GB, Weibrecht KW, Weber JM, Olson DP, Knight MC, et al. Osteoblastic cells regulate the haematopoi-etic stem cell niche. Nature. 2003;425(6960):841–6. doi: 10.1038/nature02040. [DOI] [PubMed] [Google Scholar]
- 15.Zhang J, Niu C, Ye L, Huang H, He X, Tong WG, et al. Identification of the haematopoietic stem cell niche and control of the niche size. Nature. 2003;425(6960):836–41. doi: 10.1038/nature02041. [DOI] [PubMed] [Google Scholar]
- 16.Kiel MJ, Yilmaz OH, Iwashita T, Yilmaz OH, Terhorst C, Morrison SJ. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121(7):1109–21. doi: 10.1016/j.cell.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 17.Butler JM, Nolan DJ, Vertes EL, Varnum-Finney B, Kobayashi H, Hooper AT, et al. Endothelial cells are essential for the self-renewal and repopulation of Notch-dependent hematopoietic stem cells. Cell Stem Cell. 2010;6(3):251–64. doi: 10.1016/j.stem.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sugiyama T, Kohara H, Noda M, Nagasawa T. Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity. 2006;25(6):977–88. doi: 10.1016/j.immuni.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 19.Raaijmakers MH, Mukherjee S, Guo S, Zhang S, Kobayashi T, Schoonmaker JA, et al. Bone progenitor dysfunction induces myelodysplasia and secondary leukaemia. Nature. 2010;464(7290):852–7. doi: 10.1038/nature08851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Omatsu Y, Sugiyama T, Kohara H, Kondoh G, Fujii N, Kohno K, et al. The essential functions of adipo-osteogenic progenitors as the hematopoietic stem and progenitor cell niche. Immunity. 2010;33(3):387–99. doi: 10.1016/j.immuni.2010.08.017. [DOI] [PubMed] [Google Scholar]
- 21.Kollet O, Dar A, Shivtiel S, Kalinkovich A, Lapid K, Sztainberg Y, et al. Osteoclasts degrade endosteal components and promote mobilization of hematopoietic progenitor cells. Nat Med. 2006;12(6):657–64. doi: 10.1038/nm1417. [DOI] [PubMed] [Google Scholar]
- 22.Naveiras O, Nardi V, Wenzel PL, Hauschka PV, Fahey F, Daley GQ. Bone-marrow adipocytes as negative regulators of the haematopoietic microenvironment. Nature. 2009;460(7252):259–63. doi: 10.1038/nature08099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mendez-Ferrer S, Michurina TV, Ferraro F, Mazloom AR, Macarthur BD, Lira SA, et al. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010;466(7308):829–34. doi: 10.1038/nature09262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Adams GB, Martin RP, Alley IR, Chabner KT, Cohen KS, Calvi LM, et al. Therapeutic targeting of a stem cell niche. Nat Biotechnol. 2007;25(2):238–43. doi: 10.1038/nbt1281. [DOI] [PubMed] [Google Scholar]
- 25.Issigonis M, Tulina N, de Cuevas M, Brawley C, Sandler L, Matunis E. JAK-STAT signal inhibition regulates competition in the Drosophila testis stem cell niche. Science. 2009;326(5949):153–6. doi: 10.1126/science.1176817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jin Z, Kirilly D, Weng C, Kawase E, Song X, Smith S, et al. Differentiation-defective stem cells outcompete normal stem cells for niche occupancy in the Drosophila ovary. Cell Stem Cell. 2008;2(1):39–49. doi: 10.1016/j.stem.2007.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meads MB, Gatenby RA, Dalton WS. Environment-mediated drug resistance: a major contributor to minimal residual disease. Nat Rev Cancer. 2009;9(9):665–74. doi: 10.1038/nrc2714. [DOI] [PubMed] [Google Scholar]
- 28.Ishikawa F, Yoshida S, Saito Y, Hijikata A, Kitamura H, Tanaka S, et al. Chemotherapy-resistant human AML stem cells home to and engraft within the bone-marrow endosteal region. Nat Biotechnol. 2007;25(11):1315–21. doi: 10.1038/nbt1350. [DOI] [PubMed] [Google Scholar]
- 29.Saito Y, Uchida N, Tanaka S, Suzuki N, Tomizawa-Murasawa M, Sone A, et al. Induction of cell cycle entry eliminates human leukemia stem cells in a mouse model of AML. Nat Biotechnol. 2010;28(3):275–80. doi: 10.1038/nbt.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Colmone A, Amorim M, Pontier AL, Wang S, Jablonski E, Sipkins DA. Leukemic cells create bone marrow niches that disrupt the behavior of normal hematopoietic progeni-tor cells. Science. 2008;322(5909):1861–5. doi: 10.1126/science.1164390. [DOI] [PubMed] [Google Scholar]
- 31.Kai T, Spradling A. Differentiating germ cells can revert into functional stem cells in Drosophila melanogaster ovaries. Nature. 2004;428(6982):564–9. doi: 10.1038/nature02436. [DOI] [PubMed] [Google Scholar]
- 32.Sheng XR, Brawley CM, Matunis EL. Dedifferentiating spermatogonia outcom-pete somatic stem cells for niche occupancy in the Drosophila testis. Cell Stem Cell. 2009;5(2):191–203. doi: 10.1016/j.stem.2009.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barroca V, Lassalle B, Coureuil M, Louis JP, Le Page F, Testart J, et al. Mouse differentiating spermatogonia can generate germinal stem cells in vivo. Nat Cell Biol. 2009;11(2):190–6. doi: 10.1038/ncb1826. [DOI] [PubMed] [Google Scholar]
- 34.Wei J, Wunderlich M, Fox C, Alvarez S, Cigudosa JC, Wilhelm JS, et al. Microenvironment determines lineage fate in a human model of MLL-AF9 leukemia. Cancer Cell. 2008;13(6):483–95. doi: 10.1016/j.ccr.2008.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vermeulen L, De Sousa E, Melo F, van der Heijden M, Cameron K, de Jong JH, Borovski T, et al. Wnt activity defines colon cancer stem cells and is regulated by the microenvironment. Nat Cell Biol. 2010;12 (5):468–76. doi: 10.1038/ncb2048. [DOI] [PubMed] [Google Scholar]
- 36.Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol. 2002;3(5):349–63. doi: 10.1038/nrm809. [DOI] [PubMed] [Google Scholar]
- 37.Parsonage G, Filer AD, Haworth O, Nash GB, Rainger GE, Salmon M, et al. A stromal address code defined by fibroblasts. Trends Immunol. 2005;26(3):150–6. doi: 10.1016/j.it.2004.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer. 2006;6(5):392–401. doi: 10.1038/nrc1877. [DOI] [PubMed] [Google Scholar]
- 39.Orimo A, Gupta PB, Sgroi DC, Arenzana-Seisdedos F, Delaunay T, Naeem R, et al. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. 2005;121(3):335–48. doi: 10.1016/j.cell.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 40.Karnoub AE, Dash AB, Vo AP, Sullivan A, Brooks MW, Bell GW, et al. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature. 2007;449(7162):557–63. doi: 10.1038/nature06188. [DOI] [PubMed] [Google Scholar]
- 41.Yauch RL, Gould SE, Scales SJ, Tang T, Tian H, Ahn CP, et al. A paracrine requirement for hedgehog signalling in cancer. Nature. 2008;455(7211):406–10. doi: 10.1038/nature07275. [DOI] [PubMed] [Google Scholar]
- 42.Trimboli AJ, Cantemir-Stone CZ, Li F, Wallace JA, Merchant A, Creasap N, et al. Pten in stromal fibroblasts suppresses mammary epithelial tumours. Nature. 2009;461(7267):1084–91. doi: 10.1038/nature08486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ducy P, Schinke T, Karsenty G. The osteoblast: a sophisticated fibroblast under central surveillance. Science. 2000;289 (5484):1501–4. doi: 10.1126/science.289.5484.1501. [DOI] [PubMed] [Google Scholar]
- 44.Bruserud O, Ryningen A, Wergeland L, Glenjen NI, Gjertsen BT. Osteoblasts increase proliferation and release of pro-angiogenic interleukin 8 by native human acute myelogenous leukemia blasts. Haematologica. 2004;89(4):391–402. [PubMed] [Google Scholar]
- 45.Taichman RS, Reilly MJ, Verma RS, Emerson SG. Augmented production of interleukin-6 by normal human osteoblasts in response to CD34+ hematopoietic bone marrow cells in vitro. Blood. 1997;89(4):1165–72. [PubMed] [Google Scholar]
- 46.Sneddon JB, Werb Z. Location, location, location: the cancer stem cell niche. Cell Stem Cell. 2007;1(6):607–11. doi: 10.1016/j.stem.2007.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gonda TA, Tu S, Wang TC. Chronic inflammation, the tumor microenviron-ment and carcinogenesis. Cell Cycle. 2009;8(13):2005–13. doi: 10.4161/cc.8.13.8985. [DOI] [PubMed] [Google Scholar]
- 48.Campisi J. Senescent cells, tumor suppression, and organismal aging: good citizens, bad neighbors. Cell. 2005;120(4):513–22. doi: 10.1016/j.cell.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 49.Krtolica A, Parrinello S, Lockett S, Desprez PY, Campisi J. Senescent fibroblasts pro-mote epithelial cell growth and tumorigen-esis: a link between cancer and aging. Proc Natl Acad Sci USA. 2001;98(21):12072–7. doi: 10.1073/pnas.211053698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li L, Clevers H. Coexistence of quiescent and active adult stem cells in mammals. Science. 2010;327(5965):542–5. doi: 10.1126/science.1180794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wilson A, Laurenti E, Oser G, van der Wath RC, Blanco-Bose W, Jaworski M, et al. Hematopoietic stem cells reversibly switch from dormancy to self-renewal during homeostasis and repair. Cell. 2008;135(6):1118–29. doi: 10.1016/j.cell.2008.10.048. [DOI] [PubMed] [Google Scholar]
- 52.Bhowmick NA, Chytil A, Plieth D, Gorska AE, Dumont N, Shappell S, et al. TGF-beta signaling in fibroblasts modulates the onco-genic potential of adjacent epithelia. Science. 2004;303(5659):848–51. doi: 10.1126/science.1090922. [DOI] [PubMed] [Google Scholar]
- 53.Walkley CR, Olsen GH, Dworkin S, Fabb SA, Swann J, McArthur GA, et al. A microenvironment-induced myeloprolifera-tive syndrome caused by retinoic acid receptor gamma deficiency. Cell. 2007;129 (6):1097–110. doi: 10.1016/j.cell.2007.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Maes C, Goossens S, Bartunkova S, Drogat B, Coenegrachts L, Stockmans I, et al. Increased skeletal VEGF enhances beta-catenin activity and results in excessively ossified bones. EMBO J. 2010;29(2):424–41. doi: 10.1038/emboj.2009.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bristow RG, Hill RP. Hypoxia and metabolism. Hypoxia, DNA repair and genetic instability. Nat Rev Cancer. 2008;8(3):180–92. doi: 10.1038/nrc2344. [DOI] [PubMed] [Google Scholar]
- 56.Radisky DC, Bissell MJ. Matrix metallopro-teinase-induced genomic instability. Curr Opin Genet Dev. 2006;16(1):45–50. doi: 10.1016/j.gde.2005.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bindra RS, Glazer PM. Genetic instability and the tumor microenvironment: towards the concept of microenvironment-induced mutagenesis. Mutat Res. 2005;569(1–2):75–85. doi: 10.1016/j.mrfmmm.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 58.Wu M, Pastor-Pareja JC, Xu T. Interaction between Ras(V12) and scribbled clones induces tumour growth and invasion. Nature. 2010;463(7280):545–8. doi: 10.1038/nature08702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Deeg HJ. Marrow stroma in MDS: culprit or bystander? Leuk Res. 2002;26(7):687–8. doi: 10.1016/s0145-2126(02)00015-2. [DOI] [PubMed] [Google Scholar]
- 60.Mangi MH, Mufti GJ. Primary myelodys-plastic syndromes: diagnostic and prognostic significance of immunohistochemical assessment of bone marrow biopsies. Blood. 1992;79(1):198–205. [PubMed] [Google Scholar]
- 61.Flores-Figueroa E, Arana-Trejo RM, Gutiérrez-Espíndola G, Pérez-Cabrera A, Mayani H. Mesenchymal stem cells in myelodysplastic syndromes: phenotypic and cytogenetic characterization. Leuk Res. 2005;29(2):215–24. doi: 10.1016/j.leukres.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 62.Lopez-Villar O, Garcia JL, Sanchez-Guijo FM, Robledo C, Villaron EM, Hernandez-Campo P, et al. Both expanded and uncultured mesenchymal stem cells from MDS patients are genomically abnormal, showing a specific genetic profile for the 5q- syndrome. Leukemia. 2009;23(4):664–72. doi: 10.1038/leu.2008.361. [DOI] [PubMed] [Google Scholar]
- 63.Polyak K, Haviv I, Campbell IG. Co-evolution of tumor cells and their microenviron-ment. Trends Genet. 2009;25(1):30–8. doi: 10.1016/j.tig.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 64.Ximeri M, Galanopoulos A, Klaus M, Parcharidou A, Giannikou K, Psyllaki M, et al. Effect of lenalidomide therapy on hematopoiesis of patients with myelodys-plastic syndrome associated with chromo-some 5q deletion. Haematologica. 2010;95 (3):406–14. doi: 10.3324/haematol.2009.010876. [DOI] [PMC free article] [PubMed] [Google Scholar]