Abstract
Although Helicobacter pylori have been identified in the liver, the role of Helicobacter sp. in human liver diseases remains unclear. This study explored whether H. pylori were internalized and could persist in hepatocytes. The majority of an inoculum of H. pylori (1 × 107 colony forming units) adhered to hepatocytes. Using the gentamicin invasion assay we found that approximately 2% were internalized and persisted following passage for more than 2 months. Electron microscopy confirmed the presence of intracellular Helicobacter. The number of adherent or internalized H. pylori was significantly greater with hepatocytes than with gastric epithelial cells (P <0.05) and was also dependent on cag pathogenicity island (PAI), VacA, OipA, or BabA status. Transmission electron microscopy was used to confirm adherence and invasion of H. pylori into hepatocytes. Internalization of H. pylori was inhibited by antibodies to β1-integrin receptors, genistein, and cytochalasin D (P < 0.05) consistent with β1-integrin acting as a surface receptor with additional requirements for tyrosine kinase phosphorylation and actin polymerization. In summary, H. pylori both adhered to and invaded into hepatocytes in vitro, depending on the virulent factors, and persisted within hepatocytes during subcultures. β1-integrin is likely a receptor involved in internalization of H. pylori into hepatocytes.
Keywords: Helicobacter, Adherence, Internalization, Persistence, Hepatocytes
Introduction
There has been increasing interest in the possible role of Helicobacter sp. in hepatobiliary diseases in animals and humans [1–4]. Helicobacter DNA, or cross-reacting DNA, has been detected in hepatic tissues from both adults and children with and without liver disease [5–12]. Liver diseases in which Helicobacter sp. DNA has been detected include cirrhosis, hepatocellular carcinomas (HCC) [5–10], primary sclerosing cholangitis, and primary biliary cirrhosis [11]. Although the role of Helicobacter sp. in the pathogenesis of these disease remains unclear, the available data support the notion that Helicobacter co-infection may play a role in hepatic carcinogenesis associated with hepatitis viruses [8–10].
Helicobacter pylori is an established cause of gastroduodenal diseases [13, 14]. In the stomach the infection is persistent and produces decades-long chronic inflammation that may progress to atrophy, intestinal metaplasia, and in some cases to gastric carcinoma. The mechanisms of persistence of H. pylori remain unclear. While the majority of H. pylori remain in the intestinal lumen, H. pylori can also be found within gastric epithelial cells. Recently, H. pylori have also been described within metaplastic, dysplastic, and neoplastic gastric epithelial cells in vivo [15–20], and it has been suggested that the intracellular expression of H. pylori virulence genes might play a role in the development of H. pylori associated diseases [19].
HCC is one of the most common cancers worldwide and its incidence is increasing due in part to the increase in hepatitis C infections [21–23]. Immunohistochemistry and PCR have demonstrated the presence of H. pylori or related organisms within hepatocytes in human livers and in HCC [8]. Recent studies reported that Helicobacter sp. could be cultured from HCC tissues [24]. The investigators also reported viable rod-shaped bacteria by transmission electron microscopy (TEM) after culture of HCC tissue [24] and scanning electron microscope (SEM) revealed Helicobacter-like organisms attached on the surface of hepatocytes in HCC [24]. Here, we report in vitro studies regarding the interaction of H. pylori and human liver cells.
Materials and Methods
Bacterial Culture
Helicobacter pylori NCTC11637, a putative more virulent strain (positive for cag pathogenicity island [PAI], VacA, OipA, and BabA), and 401C, a less-virulent strain (negative for cag PAI, VacA, OipA, and BabA), were grown on blood agar plates containing brain heart infusion (BHI) agar (Difco, Sparks, MD) and 7% horse blood for 2 days in a CO2 incubator (12% CO2) at 37°C. Bacteria were then suspended in cell culture medium consisting of minimal essential medium (MEM) (Invitrogen, Carlsbad, CA) or RPMI 1640 (Invitrogen) with 10% fetal bovine serum (FBS) (Invitrogen). OD measurements were used to adjust bacterial concentrations before infection of cells.
Cell Culture
The human hepatocyte (hepatocellular carcinoma) cell line Huh7 (kindly provided by Dr. Boris Yoffe) was maintained in a 5% CO2 atmosphere in MEM supplemented with 10% FBS. The human gastric cancer epithelial cell line (AGS cells, American Type Culture Collection, Manassas, VA) was also maintained in a 5% CO2 atmosphere in RPMI 1640 medium supplemented with 10% FBS.
Quantitative Adherence and Gentamicin Invasion Assays
About 1 × 105 cells were seeded on 24-well plates and allowed to attach overnight. H. pylori were added to each well at a multiplicity of infection (MOI) of 100. After incubation for 1, 2, 4, 6, 24, and 48 h in the 5% CO2 atmosphere, the wells were gently washed eight times with culture medium to remove nonadherent H. pylori [25]. Attached cells were then harvested by trypsinization and sonicated for 5 s at 2 W (VerSonic 60, The Virtis Company Inc., Gardiner, NY). Preliminary experiments showed that 5 s of sonication resulted in disruption of all the epithelial cells but did not affect the viability of H. pylori (data not shown). Serial 10-fold dilutions of the cell lysates were plated on blood agar plates and incubated in a CO2 incubator (12% CO2) at 37°C. After 5 days, the number of colony forming units (CFU) was counted.
We used the gentamicin invasion assay to assess the presence of internalized H. pylori [25]. To eliminate extracellular H. pylori, infected epithelial cells were washed six times with culture medium supplemented with 100 μg/ml gentamicin and then incubated with the same medium as above for 2 h at 37°C in a 5% CO2 atmosphere. After this treatment, no bacteria were cultured from the supernatant. The cells were then washed four times with PBS to remove the gentamicin and harvested as described above. The number of intracellular H. pylori was determined as the proportion of bacteria surviving gentamicin treatment compared to the number of adherent bacteria. Each experiment was repeated at least in triplicate.
For the assays, to compare the number of adherent or intracellular bacteria and the proportion of intracellular bacteria against adherent bacteria between Huh7 and AGS cells, we used an MOI of 10 and 6 h incubations to avoid bacterial attachment or invasion reaching a plateau and to avoid harm to cells by the bacteria. The proportion of intracellular bacteria was calculated as the percentage of the total of the sum of the adherent and intracellular bacteria.
The minimum inhibitory concentration (100% MIC) of the H. pylori strains to gentamicin was assessed by agar dilution methods and was found to be 2 μg/ml. The concentration of gentamicin in our experiments was therefore 50-fold greater than the MIC and theoretically sufficient to eliminate all external H. pylori.
Transmission Electron Microscopy (TEM)
Epithelial cells (2 × 106) on plastic tissue culture dishes were infected by H. pylori at an MOI of 100 for 24 h. Cells were harvested by trypsinizations and fixed with 2.5% glutaraldehyde in 0.1 M sodium cacodylate buffer (Electron Microscopy Sciences, Hatfield, PA) at 4°C over night. After embedding, sections were observed by electron microscopy as described elsewhere [26].
Persistence of Intracellular H. pylori
To determine the fate of intracellular H. pylori, hepatocytes containing intracellular H. pylori were subcultured 24 h after inoculation of H. pylori at an MOI of 100. Noninternalized bacteria were killed by gentamicin treatment as described above before harvesting in each subculture. The presence of intracellular H. pylori was assessed by gentamicin invasion assay and TEM as described above.
Mechanism of Internalization of H. pylori
It has been suggested that H. pylori invasion into gastric epithelial cells is similar to the pathway used by Yersinia involving the β1-integrin receptor [27]. Helicobacter pylori expresses the invasin gene which is the adhesin used by Yersinia to bind to β1-integrin [28–30]. To test this hypothesis we used anti-β1-integrin antibody to examine the possible role of β1-integrin as a receptor for H. pylori internalization. Huh7 cells were seeded on 24-well plates as described above. Anti-β1-integrin antibody (0, 2.5, or 5 μg/ml) (Santa Cruz Bio-technology, Inc., Santa Cruz, CA) was applied to each well. After 40 min incubation, the bacterial suspension was added and incubated for 30 min [27]. The cells were then collected and seeded on blood agar as described above.
We evaluated the requirement of tyrosine phosphorylation for invasion of H. pylori into Huh7 cells. Genistein, a tyrosine kinase inhibitor, was used to assess the role of tyrosine kinase phosphorylation in invasion of H. pylori into hepatocytes [27]. Kwok et al. also suggested that tyrosine phosphorylation may play a role in the downstream events of β1-integrin-mediated signaling [31]. The gentamicin-invasion assay was performed as described above with pretreatment of the cells by culture medium containing 0, 100, or 200 μmol/l of genistein for 20 min before infection of bacterial suspension.
Cytochalasin D pretreatment blocks actin polymerization [32] and was used to determine whether actin polymerization was involved in the internalization of H. pylori into hepatocytes. Su et al. reported that H. pylori invaded into AGS cells using β1-integrin mediated signaling pathway, inducing actin polymerization [27]. Furthermore, other investigators have also reported that actin polymerization was associated with invasion of H. pylori into cells [25, 33]. We used cytochalasin D to assess whether invasion of H. pylori into Huh7 cells required actin polymerization. In the cytochalasin D invasion assay, pretreatment with culture medium containing 0, 1, 2.5, or 5 μg/ml of cytochalasin D (Sigma, St. Louis, MO) was performed before the addition of the bacterial suspension followed by 4 h incubation [25].
Statistical Analyses
The results are presented as mean ± SEM. Comparison of adherent and internalization between Huh7 cells and AGS cells or between H. pylori strains NCTC11637 and 401C was done using the student’s t test. The inhibition of internalization by anti-β1-integrin antibody, genistein, or cytochalasin D was evaluated by one-way analysis of variance (ANOVA). Analyses were done using Sigma Stat 3.0 (SPSS Inc., Chicago, Illinois). P < 0.05 was considered significant.
Results
Quantitative Adherence and Gantamicin Invasion Assay
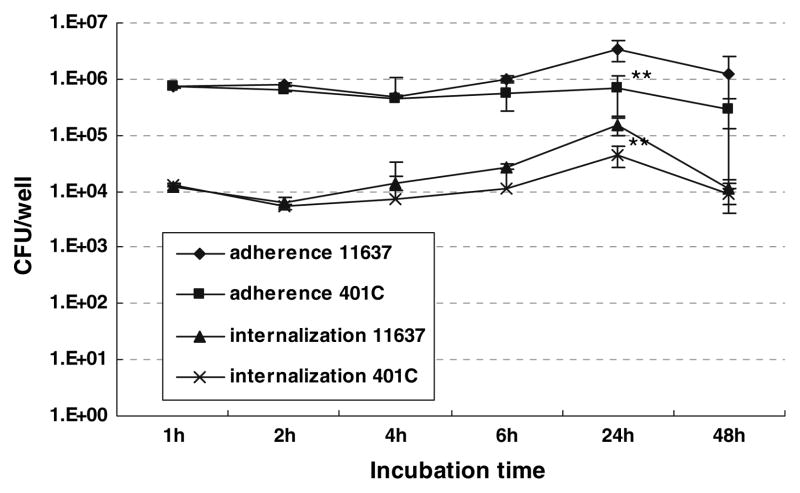
The number of H. pylori adherent and internalized into hepatocytes was assessed by the adherence assay and the gentamicin invasion assay, respectively (Fig. 1). Approximately 10% (7.2 ± 0.2 × 105 CFU) of H. pylori NCT C11637 was adherent to the hepatocytes after a 1 h incubation. Then, the number of adherent H. pylori was gradually increased to become maximal after 24 h (3.1 ± 1.3 × 106 CFU). The number of adherent H. pylori then decreased slightly (to 1.8 ± 1.2 × 106 CFU) after 48 h. The number of intracellular H. pylori was more than 100-fold less than adherent bacteria (Fig. 1). The number of adherent and intracellular H. pylori was independent of the presence of the putative virulence factors as the results were similar with strains NCTC11637 and 401C except after 24 h incubations (Fig. 1). After 24 h incubation, the number of adherent and internalized H. pylori strain NCTC11637 was significantly greater than those of strain 401C, suggesting a possible role of these virulent factors in attachments and invasion of H. pylori into hepatocytes.
Fig. 1.
Quantitative adherence and gentamicin invasion assay in Huh7 cells incubated with H. pylori NCTC11637 or 401C strain. 1 × 105 Huh7 cells were incubated with the putatively more virulent H. pylori NCTC11637 or the less-virulent H. pylori 401C strain at an MOI of 100. The number of adherent (diamond dots for NCTC11637, square dots for 401C) and internalized (triangle dots for NCTC11637, cross dots for 401C) H. pylori was assessed by CFU logarithmically after 1, 2, 4, 6, 24, and 48 h incubation. Data represent mean ± SEM of more than triplicate experiments. ** P < 0.001 compared with strain NCTC11637
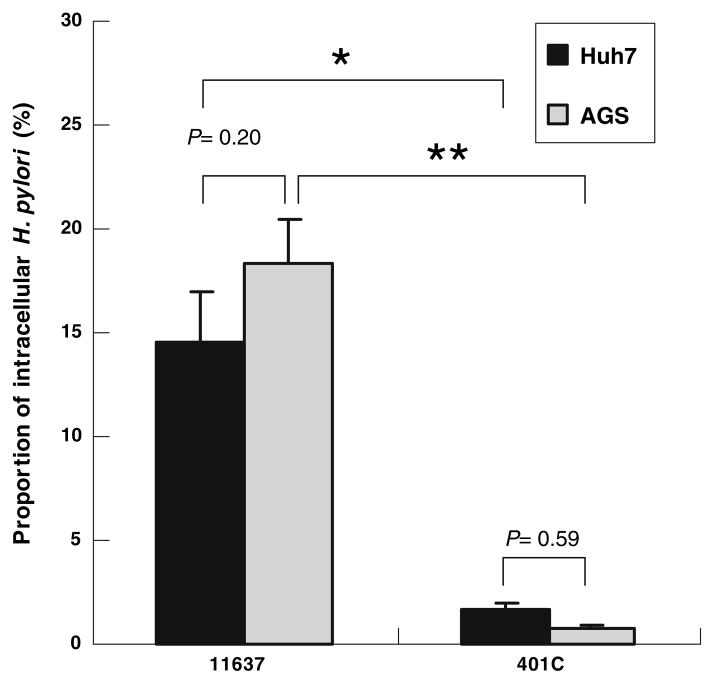
The proportion of intracellular H. pylori strain NCTC11637 versus the total of adherent and intracellular bacteria was significantly greater than those of strain 401C (P < 0.05; Fig. 2). The proportions of intracellular bacteria were 16 ± 2.7% and 2.3 ± 0.4% of adherent H. pylori strain NCTC11637 and 401C, respectively, and in Huh7 cells, 20 ± 2.3% and 0.8 ± 0.1%, respectively, in AGS cells. No significant difference was seen in the proportion of bacteria internalized into Huh7 cells and AGS cells, suggesting that efficient adherence of bacteria to the host cells contributes to greater invasion of bacteria in Huh7 cells compared with AGS cells.
Fig. 2.
Quantitative comparison of proportion of intracellular H. pylori with Huh7 cells and AGS cells using H. pylori NCTC11637 or 401C strain. Quantitative gentamicin invasion assay using 1 × 105 Huh7 cells (dark gray bars) or AGS cells (light gray bars) incubated with H. pylori NCTC11637 or 401C strain at MOI of 10 for 6 h. The proportion of intracellular H. pylori against adherent bacteria was shown by percentage. Data represent mean ± SEM of triplicate experiments. * P < 0.05, ** P ≤ 0.01
Helicobacter pylori Visualization
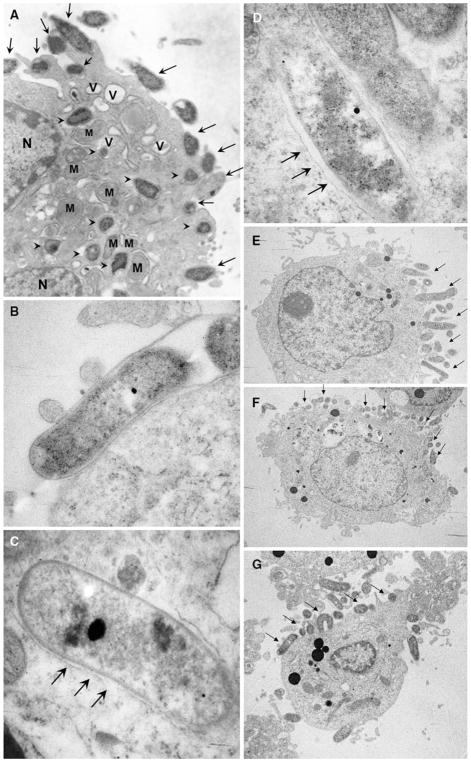
Transmission electron microscopy (TEM) showed both adherent and intracellular H. pylori (Fig. 3a for Huh7 cells). Figure 3b shows rod shaped H. pylori adhered very tightly to cell membrane in a highly magnified view. Careful observations revealed that intracellular H. pylori were surrounded by thin membranes which were suspected to be cell membranes similar to endocytotic vesicles (Fig. 3c, d) [34, 35]. Lysosomes were also seen within the vesicles. Intracellular H. pylori were noted to be rod shaped and not coccoid forms (i.e., putative resting or injured forms of H. pylori) in Huh7 cells (Fig. 3c, d). Under lower magnification, TEM revealed that cultured hepatocytes showed polarity with one side showing many microvilli, which is presumably the side facing sinusoid in vivo, and a side with few microvilli, which was presumably the basal side (Fig. 3e–g). Most of the bacteria seemed to be present on the microvillus side compared with the presumed basal side. No differences were seen between the H. pylori NCTC11637 strain and the 401C strain.
Fig. 3.
Transmission electron microscopy (TEM) of H. pylori NCTC11637 adhering and internalized into Huh7 cells. (a) Huh7 cells incubated with H. pylori for 24 h at MOI of 100. Adherent (arrows) and internalized (arrow heads) H. pylori were observed (×10,000). (b) Rod shaped H. pylori adhered to the surface of a Huh7 cell tightly (×40,000). (c, d) The viable bacilli forming intracellular H. pylori were observed. Helicobacter pylori were in endocytotic vesicles. The vesicle membrane was observed very closely surrounding the bacterial body (arrow) (×50,000). (e–g) Huh7 cells showed polarity with one side with many microvilli and the other with few microvilli. Most H. pylori (arrow) adhered to and invade from the side with many microvilli (e ×4,000; f ×3,000; g ×5,000). N nucleus; M mitochondria; V vacuole
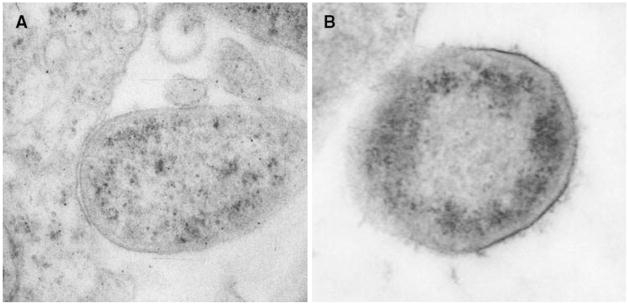
Further, filamentous structures [36] were sparse on bacteria that adhered to or were near the cell membrane of Huh7 cells (Fig. 4a). In contrast, a fine filamentous structure was seen surrounding H. pylori when they adhered or were near AGS cells (Fig. 4b).
Fig. 4.
Difference in the presence of a filamentous structure covering H. pylori adhering to liver or gastric cells. (a) H. pylori with little filamentous structure adhering to Huh7 cells (×10,000). (b) H. pylori close to an AGS cell and covered with a fine filamentous structure (×15,000)
Long Term Internalization Assay
We performed repeated subculture to determine whether H. pylori persisted within epithelial cells. Helicobacter pylori (both strains NCTC11637 and 401C) persisted within hepatocytes for more than 56 days (i.e., 13 passages) (Table 1). In contrast, persistence within AGS gastric epithelial cells could only be confirmed for three passages (10 days). TEM showed degraded H. pylori in endocytotic vesicles (lysosomal bodies) after one or five passages of Huh7 cells (data not shown). Nondegraded intracellular H. pylori was also observed after one passage (data not shown).
Table 1.
Long term internalization assay (units in CFU/dish)
| Day 3 | Day 7 | Day 10 | Day 17 | Day 22 | Day 56 | Day 60 | ||
|---|---|---|---|---|---|---|---|---|
| Huh7 | 11637 | > 1,000 | 100–1,000 | 100–1,000 | 100–1,000 | 0–100 | 0–100 | - |
| 401C | > 1,000 | 100–1,000 | 100–1,000 | 100–1,000 | 0–100 | 0–100 | 0–100 | |
| AGS | 11637 | > 1,000 | 100–1,000 | 0–100 | - | - | - | - |
| 401C | > 1,000 | 100–1,000 | 0–100 | - | - | - | - |
Studies of the Mechanism of Internalization
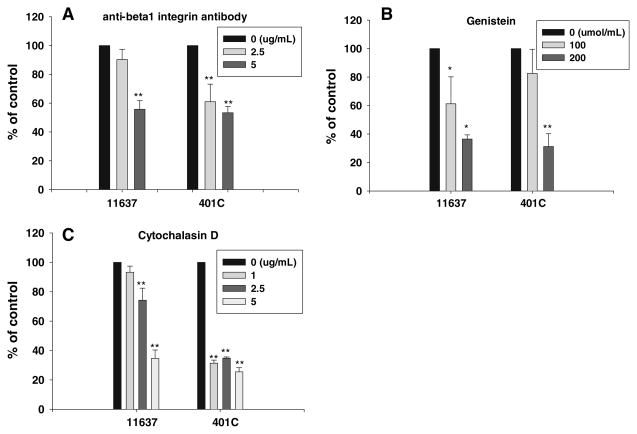
β1-integrin is a cell membrane protein thought to possibly be involved in H. pylori adherence and internalization within gastric epithelial cells [27]. We assessed the role of β1-integrin internalization of H. pylori into hepatocytes using anti-β1-integrin antibody to block the β1-integrin receptor on the surface of the cell membrane and thus potentially block bacterial adherence. Anti-β1-integrin antibody inhibited H. pylori invasion into Huh 7 cells in a dose-dependent manner (Fig. 5a) with both strains NCTC11637 and 401C. Inhibition of internalization was greater with strain 401C than with strain NCTC11637 when using 2.5 μg/ml of anti-β1-integrin antibody, and the results were similar with 5 μg/ml (Fig. 5a).
Fig. 5.
Effect of anti-β1-integrin antibody, genistein, or cytochalasin D on internalizations of H. pylori into Huh 7 cells. Internalization was inhibited by anti-β1-integrin antibody (a), genistein (b), or cytochalasin D (c). Huh7 cells were co-cultured with H. pylori in the medium including above regents at the specified concentrations. Results are presented as the proportion of internalized bacteria versus control. The proportion of internalized bacteria was calculated as the rate of the number of internalized bacteria divided by the number of adherent bacteria. Data are presented as mean ± SEM of triplicate experiments.
*P < 0.05; ** P < 0.01
Genistein, a tyrosine kinase inhibitor, also inhibited internalization of H. pylori in a dose-dependent manner (Fig. 5b) [27]. Cytochalasin D inhibits internalization by blocking actin polymerization [27, 32] and also resulted in a dose-dependent reduction in internalization (from 93%, 74%, and 35% of control; P < 0.01; Fig. 5c). Cytochalasin D inhibited invasion by both H. pylori strains in Huh7 cells. However, at low concentrations of cytochalasin D the invasion of NCTC11637 was inhibited less than with strain 401C. This result suggests that there are possibly mechanisms of invasion by strain NCTC11637 which are not dependent on actin polymerization. The virulent factors that are present in these bacteria (e.g., the cag PAI) may play a role in the mechanisms of invasion.
Discussion
Although there are a number of reports suggesting an association between Helicobacter sp. and hepatobiliary diseases, including hepatocellular carcinoma in humans, there are limited in vitro studies regarding the hepatotoxicity of Helicobacter sp. [5–12, 37, 38]. We found differences between hepatocytes and gastric epithelial cells in terms of both adherence and internalization of H. pylori. Of interest, H. pylori adhered and were internalized into hepatocytes more efficiently than into gastric epithelial cells. This efficient invasion was primarily related to efficient adherence. These findings were consistent with the notion that H. pylori could be internalized into hepatocytes efficiently even if very small amounts of bacteria actually reached the liver.
We found that the pattern of adherence also differed between hepatocytes and gastric epithelial cells with more broad and random attachment of H. pylori on hepatocytes, which may have assisted in more efficient internalization. Several investigators have previously noted that intracellular H. pylori are located within vacuoles in gastric epithelial cells [31, 39–43]. Amieva et al. even reported finding H. pylori moving within large vacuoles in gastric epithelial cells [39]. Although we were able to confirm the presence of H. pylori within vacuoles, we did not observe vacuoles of sufficient size to allow H. pylori to move within hepatocytes. The fact that we were able to culture H. pylori after internalization into hepatocytes is also consistent with several prior reports suggesting that H. pylori could survive within mammalians cells [25, 31, 39, 40, 42].
Furthermore, the vacuole membrane seen was close to the bacterial body, which is similar to that seen with endocytosis vesicles [33, 34]. Amieva et al. reported that VacA was important for H. pylori to survive in the gastric epithelial cells [39, 40]. However, in our study vacuoles were produced by both VacA negative and VacA positive strains, leading us to surmise that the vacuoles were not produced by VacA but were likely endocytotic vesicles. We also showed that H. pylori were able to survive long term passage within hepatocytes (i.e., for more than 56 days) independent of the presence of VacA. Recently, Terebiznik et al. showed that the vacuolating cytotoxin was involved with the generation of the large H. pylori compartment but not the capacity to invade AGS cells [43]. In hepatocytes, our data are consistent with VacA possibly having a role in the invasion of H. pylori but does not show persistence within hepatocytes.
The mechanism of persistence of H. pylori during multiple passages of cells remains unclear. It appears clear that the organisms can survive inside hepatocytes but we did not find evidence of them replicating within the cells. It is therefore unclear whether the intracellular H. pylori replicated outside the cells followed by re-internalization (e.g., during cell division of the hepatocytes), or whether they replicated inside the cells and were then released outside, or both. Semino-Mora et al. observed that H. pylori could be released from inside host cells with mucus into the lumen of metaplastic glands and sometimes adhered to goblet cells [19]. They hypothesized that the coccoid bacteria released from inside could restart growth and division and thus re-enter other metaplastic cells.
In preliminary experiments we found that growing H. pylori in culture media containing gentamicin was associated with a decrease in the number of intracellular H. pylori and no viable H. pylori were recovered after three passages (data not shown). While gentamicin is an extracellular antibiotic, it is unknown whether it might obtain access to intracellular H. pylori during cell division. Amieva et al. also showed the disappearance of H. pylori 24 h after 12 h gentamicin treatment [39]. These data suggest that the organisms either undergo an extracellular phase or contact gentamicin during division of the hepatocytes. Another possible reason is that intracellular bacteria could be killed by gentamicin which gained access to the cell interior by diffusion through the cell membrane, possibly during cell division.
The ultrastructual adhesion patterns of H. pylori have been classified as (a) filamentous binding, (b) adhering to microvilli, (c) abutting on the cell membrane, (d) associated with adhesion pedestals, (e) occupying a depression in the cell membrane, and (f) invading the cell [17]. Evans et al. noted that intimate contact was observed between adherent H. pylori and the human laryngeal epithelial cell line HEp-2 cell, whereas no intimate contacts were observed between formalin-killed H. pylori and HEp-2 cells [25]. Liu et al. reported the presence of a fine filamentous structure surrounding H. pylori when it adhered to gastric epithelial cells [36] and suggested that the filamentous structure was used to make tight junctions between H. pylori and cells. They found two types of adhesion to epithelial cells were recognized connecting the bacteria to the plasma membrane of the epithelial cells: (1) by fine filaments thinly arranged, leaving a wide space between the bacterial membrane and epithelial cell membrane, and (2) with a contact zone, where the space between the two membranes was very narrow. In our study, TEM revealed that H. pylori adherence to hepatocytes and to gastric epithelial cells differed in terms of the presence of the amount and presence of a filamentous structure consistent with the different pattern of adherence previously observed. Adherent bacteria did not leave a wide space between the bacterial membrane and the epithelial cell membrane with fine filaments thinly arranged. Our results are consistent with the presence of adhesion loci with gastric epithelial cells reported by Liu et al. [36]. The fine filamentous structure on the surface of H. pylori was not seen when H. pylori adhered to hepatocytes.
The steps in H. pylori adherence and invasion of hepatocytes are not yet completely understood. However, the β1-integrin receptor and signaling pathways for actin polymerizations appear to be required for internalization of H. pylori into hepatocytes. β1-integrin plays a crucial role in focal adhesion [44] and the involvement of β1-integrin has also been implicated in the entry of H. pylori into AGS cells [27]. Our results are supported by a previous report from Zhang et al. showing that β1-integrin was up-regulated in H. pylori-treated hepatocytes [38]. Upon binding to ligands, integrins are known to cluster on the plasma membrane and interact with the cytoskeleton, thereby promoting the assembly of adhesive structures such as focal adhesions and hemidesmosomes [45]. Binding of H. pylori to αβ1-integrins can also induce phosphotyrosine signaling, and it has been suggested that H. pylori binding to αβ1-integrin elicits the tyrosine phosphorylation of proteins participating in focal adhesion zones and a more efficient multivalent interaction with theses receptors [27]. Kwok et al. reported that intracellular H. pylori were associated with tyrosine phosphorylation located at characteristic focal adhesion plaques [31] and suggested that tyrosine phosphorylation may play a role in the downstream events of β1-integrin-mediated signaling. Yersinia invade host cells following attachment of invasin, which is a Yersinia adhesin, to the β1-integrin receptor on the cell membrane as described above [29]. This model is suggested as the most likely mechanism for H. pylori invasion as H. pylori also expresses the invasin gene [30]. The fact that anti-β1 integrin antibody reduced but did not fully block H. pylori invasion suggests the presence of additional and as yet uninvestigated pathways. Of interest, H. pylori virulent factors appear to possibly play a role in the bacterial internalizations as shown by the difference in the concentrations of anti-β1-integrin antibody or cytochalasin D required to inhibit invasion of the virulent strain, NCTC 11637, compared with the less-virulent strain, 401C. BabA and OipA are adhesins and may possibly play a role in the internalization. Our results did not confirm a role for VacA in relation to survival of H. pylori within host cells [39, 40, 43]. In this study, we used two wild-type H. pylori which differed in relation to the presence of the putative virulence factors including the cag PAI, VacA, OipA, and BabA. We used wild-type stains as controls because isogenic mutants lacking each of these factors were not available. The role of each factor in the internalizations remains unclear and awaits clarification using isogenic mutants.
In conclusion, we showed that Helicobacter can adhere to and invade hepatocytes in vitro, and further, they can persist during multiple subcultures. Internalization of Helicobacter within hepatocytes may be a possible strategy of Helicobacter to avoid host immuno-reaction and stay in the liver, resulting in changes in hepatocytes morphologically, physiologically, biologically, or pathologically.
Acknowledgments
We are grateful to Dr. Gretchen Darlington, Dr. Borris Yoffe, Dr. Norman Sussman, and Dr. Milton Finegold for insightful discussions as assistance. Also, we thank Jian Wu and James Barrish for excellent technical assistance. This material is based upon work supported in part by the Office of Research and Development Medical Research Service Department of Veterans Affairs and by Public Health Service grant DK56338 which funds the Texas Gulf Coast Digestive Diseases Center. Dr. Yoshio Yamaoka is supported in part by NIH grant DK 62813. Dr. Kyoko Ito was supported in part by an International GI Training Grant from the American College of Gastroenterology.
Contributor Information
Kyoko Ito, Department of Medicine, Michael E. DeBakey VA Medical Center, Baylor College of Medicine, 2002 Holcombe Blvd. 3A-320, Houston, TX 77030, USA, Division of Gastroenterology and Hepatology, Department of Internal Medicine, The Jikei School of Medicine, Tokyo, Japan.
Yoshio Yamaoka, Department of Medicine, Michael E. DeBakey VA Medical Center, Baylor College of Medicine, 2002 Holcombe Blvd. 3A-320, Houston, TX 77030, USA.
Hiroyoshi Ota, Department of Biomedical Laboratory Sciences, School of Health Sciences, Shinshu University, School of Medicine, Nagano, Japan.
Hala El-Zimaity, Department of Medicine, Michael E. DeBakey VA Medical Center, Baylor College of Medicine, 2002 Holcombe Blvd. 3A-320, Houston, TX 77030, USA.
David Y. Graham, Email: dgraham@bcm.edu, Department of Medicine, Michael E. DeBakey VA Medical Center, Baylor College of Medicine, 2002 Holcombe Blvd. 3A-320, Houston, TX 77030, USA
References
- 1.Solnick JV, Schauer DB. Emergence of the Helicobacter genus in the pathogenesis of gastrointestinal disease. Clin Microbiol Rev. 2001;14:59–97. doi: 10.1128/CMR.14.1.59-97.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rogers AB, Boutin SR, Whary MT, Sundina N, Ge Z, Cormier K, Fox JG. Progression of chronic hepatitis and preneoplasia in Helicobacter hepaticus-infected A/JCr mice. Toxicol Pathol. 2004;32(6):668–677. doi: 10.1080/01926230490524247. [DOI] [PubMed] [Google Scholar]
- 3.Fox JG, Drolet R, Higgins S, Messier S, Yan L, Coleman BE, Paster BJ, Dewhirst FE. Helicobacter canis isolated from a dog liver with multifocal necrotizing hepatitis. J Clin Microbiol. 1997;34:2479–2482. doi: 10.1128/jcm.34.10.2479-2482.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nilsson HO, Pietroiusti A, Gabrielli M, Zocco MA, Gasbarrini G, Gasbarrini A. Helicobacter pylori and extragastric diseases; other Helicobacters. Helicobacter. 2005;10(suppl 1):54–65. doi: 10.1111/j.1523-5378.2005.00334.x. [DOI] [PubMed] [Google Scholar]
- 5.Stalke P, Al-Soud WA, Bielawski KP, Bakowska A, Trocha H, Stepinski J, Wadström T. Detection of Helicobacter species in liver and stomach tissues of patients with chronic liver diseases using polymerase chain reaction-denaturing gradient gel electrophoresis and immunohistochemistry. Scan J Gastroenterol. 2005;40:1032–1041. doi: 10.1080/00365520510023251. [DOI] [PubMed] [Google Scholar]
- 6.Avenaud P, Marais A, Monteiro L, Le Bail B, Bioulac Sage P, Balabaud C, Mégraud F. Detection of Helicobacter species in the liver of patients with and without primary liver carcinoma. Cancer. 2000;89:1431–1439. [PubMed] [Google Scholar]
- 7.Verhoef C, Pot RG, de Man RA, Zondervan PE, Kuipers EJ, IJzermans JN, Kusters JG. Detection of identical Helicobacter DNA in the stomach and in the non-cirrhotic liver of patients with hepatocellular carcinoma. Eur J Gastroenterol Hepatol. 2003;15(11):1171–1174. doi: 10.1097/00042737-200311000-00004. [DOI] [PubMed] [Google Scholar]
- 8.Ito K, Nakamura M, Toda G, Negishi M, Torii A, Ohno T. Potential role of Helicobacter pylori in hepatocarcinogenesis. Int J Mol Med. 2004;13:221–227. [PubMed] [Google Scholar]
- 9.Dore MP, Realdi G, Mura D, Graham DY, Sepulveda AR. Helicobacter infection in patients with HCV-related chronic hepatitis, cirrhosis, and hepatocellular carcinoma. Dig Dis Sci. 2002;47(7):1638–1643. doi: 10.1023/a:1015848009444. [DOI] [PubMed] [Google Scholar]
- 10.Rocha M, Avenaud P, Menard A, Le Bail B, Balabaud C, Bioulac-Sage P, de Magalhães Queiroz DM, Mégraud F. Association of Helicobacter species with hepatitis C cirrhosis with or without hepatocellular carcinoma. Gut. 2005;54:396–401. doi: 10.1136/gut.2004.042168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nilsson H-O, Taneera J, Castedal M, Glatz E, Olsson R, Wadström T. Identification of Helicobacter pylori and other Helicobacter species by PVR, hybridization, and partial DNA sequencing in human liver samples from patients with primary sclerosing cholangitis or primary biliary cirrhosis. J Clin Microbiol. 2000;38(3):1072–1076. doi: 10.1128/jcm.38.3.1072-1076.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tolia V, Nilsson H-O, Boyer K, Wuerth A, Al-Soud WA, Rabah R, Wadström T. Detection of Helicobacter ganmani-like 16S rDNA in pediatric liver tissue. Helicobacter. 2004;9(5):460–468. doi: 10.1111/j.1083-4389.2004.00266.x. [DOI] [PubMed] [Google Scholar]
- 13.Suerbaum S, Michetti P. Helicobacter pylori infection. N Engl J Med. 2002;347:175–186. doi: 10.1056/NEJMra020542. [DOI] [PubMed] [Google Scholar]
- 14.Peek RM, Jr, Blaser MJ. Helicobacter pylori and gastrointestinal tract adenocarcinoma. Nat Rev Cancer. 2002;2:28–37. doi: 10.1038/nrc703. [DOI] [PubMed] [Google Scholar]
- 15.Björkholm B, Zhunkovitsky V, Löfman C, Hultén K, Enroth H, Block M, Rigo R, Falk P, Engstrand L. Helicobacter pylori entry into human gastric epithelial cells: a potential determinant of virulence, persistence, and treatment failures. Helicobacter. 2000;5(3):148–154. doi: 10.1046/j.1523-5378.2000.00023.x. [DOI] [PubMed] [Google Scholar]
- 16.Noach LA, Rolf TM, Tyget GN. Electron microscopy study of association between Helicobacter pylori and gastric and duodenal mucosa. J Clin Pathol. 1994;47:699–704. doi: 10.1136/jcp.47.8.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Papadogiannakis N, Willen R, Carlen B, Sjostedt S, Wadström T, Gad A. Models of adherence of Helicobacter pylori to gastric surface epithelium in gastroduodenal diseases: a possible sequence of events leading to internalization. APMIS. 2000;108(6):439–447. doi: 10.1034/j.1600-0463.2000.d01-80.x. [DOI] [PubMed] [Google Scholar]
- 18.Ko GH, Kang SM, Kim YK, Lee JH, Park CK, Youn HS, Baik SC, Cho MJ, Lee WK, Rhee KH. Invasiveness of Helicobacter pylori into human gastric mucosa. Helicobacter. 1999;4(2):77–81. doi: 10.1046/j.1523-5378.1999.98690.x. [DOI] [PubMed] [Google Scholar]
- 19.Semino-Mora C, Doi SQ, Marty A, Simko V, Carlstedt I, Dubois A. Intracellular and interstitial expression of Helicobacter pylori virulence genes in gastric precancerous intestinal metaplasia and adenocarcinoma. J Infect Dis. 2003;187(8):1165–1177. doi: 10.1086/368133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Necchi V, Candusso ME, Tava F, Luinetti O, Ventura U, Fiocca R, Ricci V, Solcia E. Intracellular, intercellular, and stromal invasion of gastric mucosa, preneoplastic lesions, and cancer by Helicobacter pylori. Gastroenterology. 2007;132(3):1009–1023. doi: 10.1053/j.gastro.2007.01.049. [DOI] [PubMed] [Google Scholar]
- 21.Taylor-Robinson SD, Foster GR, Arora S, Hargreaves S, Thomas HC. Increase in primary liver cancer in the UK, 1979–94. Lancet. 1997;350(9085):1142–1143. doi: 10.1016/S0140-6736(05)63789-0. [DOI] [PubMed] [Google Scholar]
- 22.Deuffic S, Poynard T, Buffat L, Valleron AJ. Trends in primary liver cancer. Lancet. 1998;351(9097):214–215. doi: 10.1016/S0140-6736(05)78179-4. [DOI] [PubMed] [Google Scholar]
- 23.El-Serag HB, Mason AC. Rising incidence of hepatocellular carcinoma in the United States. N Engl J Med. 1999;340:745–750. doi: 10.1056/NEJM199903113401001. [DOI] [PubMed] [Google Scholar]
- 24.Xuan SY, Li N, Qiang X, Zhoou RR, Shi YX, Jiang WJ. Helicobacter infection in hepatocellular carcinoma tissue. World J Gastroenterol. 2006;12(15):2335–2340. doi: 10.3748/wjg.v12.i15.2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Evans DG, Evans DJ, Graham DY. Adherence and internalization of Helicobacter pylori by Hep-2 cells. Gastroenterology. 1992;102:1557–1567. doi: 10.1016/0016-5085(92)91714-f. [DOI] [PubMed] [Google Scholar]
- 26.Carison JA, Rogers BB, Sifers RN, Hawkins HK, Finegold MJ, Woo SL. Multiple tissues express alpha1-antitrypsin in transgenic mice and man. J Clin Invest. 1988;82(1):26–36. doi: 10.1172/JCI113580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Su B, Johansson S, Fällman M, Patrroyo M, Granström M, Normark S. Signal transduction-mediated adherence and entry of Helicobacter pylori into cultured cells. Gastroenterology. 1999;117(3):595–604. doi: 10.1016/s0016-5085(99)70452-x. [DOI] [PubMed] [Google Scholar]
- 28.Boyle EC, Finlay BB. Bacterial pathogenesis: exploiting cellular adherence. Curr Opin Cell Biol. 2003;15:633–639. doi: 10.1016/s0955-0674(03)00099-1. [DOI] [PubMed] [Google Scholar]
- 29.Pizarro-Cerda J, Cossart P. Bacterial adhesion and entry into host cells. Cell. 2006;124:715–727. doi: 10.1016/j.cell.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 30.Tomb JF, White O, Kerlavage AR, Clayton RA, Sutton GG, Fleischmann RD, Ketchum KA, Klenk HP, Gill S, Dougherty BA, Nelson K, Quackenbush J, Zhou L, Kirkness EF, Peterson S, Loftus B, Richardson D, Dodson R, Khalak HG, Glodek A, McKenney K, Fitzegerald LM, Lee N, Adams MD, Hickey EK, Berg DE, Gocayne JD, Utterback TR, Peterson JD, Kelley JM, Cotton MD, Weidman JM, Fujii C, Bowman C, Watthey L, Wallin E, Hayes WS, Borodovsky M, Karp PD, Smith HO, Fraser CM, Venter JC. The complete genome sequence of gastric pathogen Helicobacter pylori. Nature. 1997;388:539–547. doi: 10.1038/41483. [DOI] [PubMed] [Google Scholar]
- 31.Kwok T, Backert S, Schwarz H, Berger J, Meyer TF. Specific entry of Helicobacter pylori into cultured gastric epithelial cells via a zipper-like mechanism. Infect Immun. 2002;70(4):2108–2120. doi: 10.1128/IAI.70.4.2108-2120.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cooper JA. Effects of cytochalasin and phalloidin on actin. J Cell Biol. 1987;105:1473–1478. doi: 10.1083/jcb.105.4.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Birkness KA, Gold BD, White EH, Bartlett JH, Quinn FD. In vitro models to study attachment and invasion of Helicobacter pylori. Ann NY Acad Sci. 1996;797:293–295. doi: 10.1111/j.1749-6632.1996.tb52983.x. [DOI] [PubMed] [Google Scholar]
- 34.Yamauchi K, Snel J. Transmission electron microscopic demonstration of phagocytosis and intracellular processing of segmented filamentous bacteria by intestinal epithelial cells of the chick ileum. Infect Immun. 2000;68(11):6496–6504. doi: 10.1128/iai.68.11.6496-6504.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ringø E, Løndemel JB, Myklebust R, Kaino T, Mayhew TM, Olsen RE. Epithelium-associated bacteria in the gastrointestinal tract of Arctic charr (Salvelinus alpinus L.). An electron microscopical study. J Appl Microbiol. 2001;90(2):294–300. doi: 10.1046/j.1365-2672.2001.01246.x. [DOI] [PubMed] [Google Scholar]
- 36.Liu Y, Hidaka E, Kaneko Y, Akamatsu T, Ota H. Ultra-structure of Helicobacter pylori in human gastric mucosa and H. pylori-infected human gastric mucosa using transmission electron microscopy and the high-pressure freezing-freeze substitution technique. J Gastroenterol. 2006;41(6):569–574. doi: 10.1007/s00535-006-1813-2. [DOI] [PubMed] [Google Scholar]
- 37.Taylor NS, Fox JG, Yan L. In-vitro hepatotoxic factor in Helicobacter hepaticus, H. pylori and other Helicobacter species. J Med Microbiol. 1995;42:48–52. doi: 10.1099/00222615-42-1-48. [DOI] [PubMed] [Google Scholar]
- 38.Zhang Y, Fan XG, Chen R, Xiao ZO, Feng XP, Tian XF, Chen ZH. Comparative proteome analysis of untreated and Helicobacter pylori-treated HepG2. World J Gastroenterol. 2005;11(22):3485–3489. doi: 10.3748/wjg.v11.i22.3485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Amieva MR, Salama NR, Tompkins LS, Falkow S. Helicobacter pylori enter and survive within multivesicular vacuoles of epithelial cells. Cell Microbiol. 2002;4(10):677–690. doi: 10.1046/j.1462-5822.2002.00222.x. [DOI] [PubMed] [Google Scholar]
- 40.Petersen AM, Sorensen K, Blom J, Krogfelt KA. Reduced intracellular survival of Helicobacter pylori vacA mutants in comparison with their wild-types indicated the role of VacA in pathogenesis. FEMS Immunol Med Microbiol. 2001;30(2):103–108. doi: 10.1111/j.1574-695X.2001.tb01556.x. [DOI] [PubMed] [Google Scholar]
- 41.Wyle FA, Tarnawsi A, Schulman D, Dabros W. Evidence for gastric cell invasion by C. pylori and ultrastructural study. J Clin Gastroenterol. 1990;12(suppl 1):S92–598. doi: 10.1097/00004836-199001001-00016. [DOI] [PubMed] [Google Scholar]
- 42.Wilkinson SM, Uhl JR, Kline BC, Cockerill FR., 3rd Assessment of invasion frequencies of cultured HEp-2 cells by clinical isolates of Helicobacter pylori using an acridine orange assay. J Clin Pathol. 1998;51:127–133. doi: 10.1136/jcp.51.2.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Terebiznik MR, Vazquez CL, Torbicki K, Banks D, Wang T, Hong W, Blanke SR, Colombo MI, Jones NL. Helicobacter pylori VacA toxin promotes bacterial intracellular survival in gastric epithelial cells. Infect Immun. 2006;74(12):6599–6614. doi: 10.1128/IAI.01085-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Murgia C, Blaikie P, Kim N, Dans M, Petrie HT, Giancotti FG. Cell cycle and adhesion defected in mice carrying a targeted deletion of the integrin beta4 cytoplasmic domain. EMBO J. 1998;17(14):3940–3951. doi: 10.1093/emboj/17.14.3940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Burridge K, Chrzanowska-Wodnicka M. Focal adhesions, contractility and signaling. Annu Rev Cell Dev Biol. 1996;12:463–519. doi: 10.1146/annurev.cellbio.12.1.463. [DOI] [PubMed] [Google Scholar]