Abstract
Helicobacter pylori is a serious chronic transmissible pathogen that causes gastric structural and functional damage and is a major cause of morbidity and mortality worldwide. H. pylori is one of the digestive tract pathogens that has traveled with mankind since before humans moved out of Africa. Some have suggested that the long association of H. pylori with humans means there must be a benefit and suggest dire consequences will follow its eradication. Fortunately, there are now sufficient experiences with the outcome of treatment and with populations where the infection disappeared long ago to support the conclusion that H. pylori is a serious chronic transmissible pathogen that no one needs, deserves, or desires. The dire consequence hypotheses have proven to be erroneous attempts to blame some of the problems facing the modern world on a pathogen that was and is responsible for much suffering, morbidity, and mortality. It is time to join together to eradicate it and to let H. pylori join smallpox and polio on the hit list of undesirables.
Keywords: prevalence, nongastric manifestations, obesity, asthma, esophageal carcinoma, epidemiology
Helicobacter pylori is a serious chronic transmissible pathogen that causes gastric structural and functional damage and is a major cause of morbidity and mortality worldwide. At least 20% of those infected will eventually experience a clinical outcome [1]. Table 1 shows the diseases in which H. pylori is a major cause. None are exclusive to the infection. The lifetime risk for an H. pylori-infected individual to develop peptic ulcer disease has been estimated at one in six, and 10–25% of those with peptic ulcers are expected to develop complications such as gastrointestinal hemorrhage or perforation. H. pylori infection is a necessary but not sufficient cause of gastric cancer. Gastric cancer is the second most common cancer worldwide. The lifetime risk of developing gastric cancer is related to the prevalence of H. pylori-associated atrophic gastritis and in high-risk countries is as high as 19.6% in Changle, China, and as low as 1.5% in the UK [2]. These differences reflect the variation in H. pylori prevalence, in strain virulence, and in host and particularly environmental factors [3].
Table 1.
Diseases in which Helicobacter pylori infection is a major cause
| Atrophic gastritis |
| Increased susceptibility to gastrointestinal infections |
| B12 deficiency |
| Gastric carcinoma |
| Primary gastric B-cell lymphoma |
| Peptic ulcer disease |
| Bleeding, obstruction, perforation |
| Iron deficiency anemia |
| Thrombocytopenic purpura |
Primitive man was home to many intestinal parasites [4–8]. Improvements in the standards of living over the last several millennia and particularly the improvements in sanitation, availability of clean water, and better understanding of the practices of household hygiene have resulted in the disappearance of these previously common intestinal pathogens. H. pylori is one of the last major worldwide gut pathogens that has accompanied man on his many migrations [9,10]. The 19th century witnessed the appearance of a number of “new diseases” including paralytic polio, acute infectious hepatitis, and duodenal ulcer disease. In retrospect, the first two were related to major changes in sanitation that resulted in a change in the age of acquisition of polio virus and hepatitis A virus from infancy to young adulthood and the appearance of paralytic polio and acute infectious hepatitis as recognizable diseases. It is likely that this also marked the beginning of the loss of H. pylori as well as a change in the pattern of gastritis and the appearance of antral predominant gastritis. This change was enhanced by changes in standards of living and diet that resulted in the appearance of duodenal ulcer disease as another significant new disease. Duodenal ulcer was initially a disease of the upper classes as they could take the advantages of improved standards of living and improved diets. The improvements in household hygiene, sanitation, diet, and general standards of living led to a break in the chain of transmission of H. pylori such that the infection became increasingly less common among the middle and upper classes. Loss of the infection led to loss of duodenal ulcer disease that switched from being an upper-class problem to one of the lower classes where H. pylori was still common and for whom there was a trickle down of improved socioeconomic conditions. Further improvements in sanitation and household hygiene led to a continuing decline in H. pylori prevalence to where now the infection is present in only a small proportion of the upper and middle social classes in most Western societies.
New Problems Caused by Absence of H. pylori
The fact that H. pylori has been part of man’s burden for millennia has been considered as evidence that it “must” play some positive role and might even be a commensal [11–13]. It has been suggested that the loss of H. pylori from the population may be in part responsible for the epidemic of obesity, childhood asthma, gastroesophageal reflux, and esophageal adenocarcinoma. Generally, commensals are difficult or impossible to eliminate, whereas H. pylori is in the process of disappearing from every population where sanitation and standards of living have improved.
H. pylori infection may play a role in the development of symptomatic gastroesophageal reflux disease (GERD). H. pylori infection of the gastric corpus can impair the stomach’s ability to make acid and this provides a physiologic basis for loss of the infection playing a role in development of symptomatic GERD. However, H. pylori can also play a role in producing GERD. For example, H. pylori-associated duodenal ulcer (which is associated with the presence of CagA-positive strains) has also long been known to be strongly associated with GERD [14–31]. Thus, both the presence and absence of H. pylori may play roles in GERD. Gastroesophageal reflux is a function of the effectiveness of the barrier mechanisms at the esophagogastric junction preventing reflux of gastric contents into the esophagus. The effectiveness of this barrier is influenced by anatomic features (presence of hiatal hernia, body mass index) as well as by drugs and foods that impair lower esophageal function. GERD is directly related to esophageal acid load [30,31]. Therefore, people living in regions of the world with a high prevalence of atrophic gastritis experience a high incidence of gastric cancer and a low incidence of GERD. Loss of H. pylori prevents the development of atrophic gastritis and allows those with preexisting asymptomatic or minimally symptomatic gastroesophageal reflux to develop more significant disease. GERD has been a common disease in the United States for at least 100 years. Although there is little evidence to support the notion that it has increased in incidence recently, there is evidence that the recognition of gastroesophageal reflux has increased since the 1970s [29]. The recent increase in average body mass and the absence of H. pylori in the majority of stomachs in Western populations ensure that the incidence of symptomatic gastroesophageal reflux will not decline and will likely increase somewhat. It has become clear that in Western countries neither the presence of H. pylori infection nor H. pylori eradication causes GERD. At least three large studies have shown that any concern regarding the risk of H. pylori eradication provoking gastroesophageal reflux in the general Western population was unfounded [32–34]. H. pylori eradication also does not impede or change the amount or type of antisecretory drug therapy required for patients with symptomatic gastroesophageal reflux. In fact, on average, symptoms in patients with GERD are more likely to improve than worsen following H. pylori eradication [29,31,35].
In contrast to Western countries where atrophic gastritis is uncommon, cure of the infection in patients with preexisting asymptomatic gastroesophageal reflux and moderate to severe corpus gastritis will likely remove the inflammation-associated inhibition of parietal cell function and produce an increase in gastric acidity [35]. This increase in acid concentration will increase the toxicity of any material refluxing into the esophagus through an already impaired antireflux barrier. Whether the patient develops symptomatic GERD depends on the interplay between host factors and the resulting esophageal acid load. One can easily imagine that some patients with asymptomatic reflux will convert to symptomatic reflux by this mechanism [31,35].
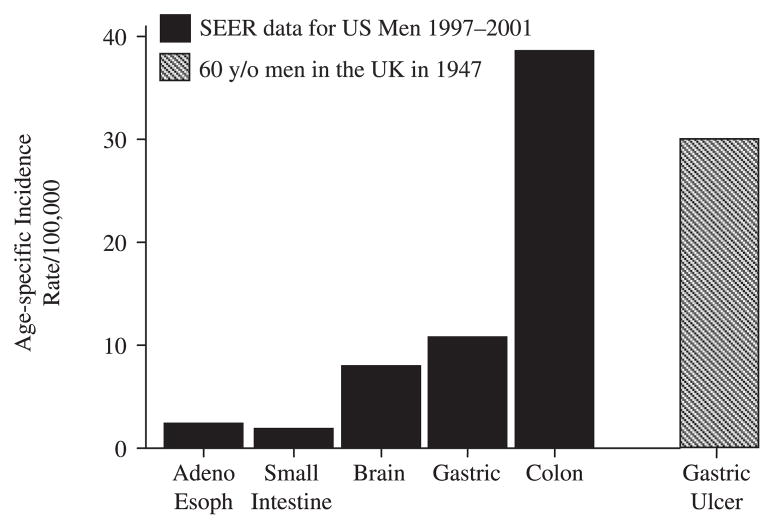
Adenocarcinoma of the distal esophagus is a complication of GERD and has been increasing in incidence. Although the incidence has increased rapidly, it has actually changed from being very rare disease (e.g. around 0.3/100,000 per year in 1970 in the highest risk population of White men) to becoming a rare disease (~2.5/100,000 per year) with approximately the same incidence as brain or small bowel cancers [31,35]. This might be compared to the fact that the mortality rate from gastric ulcer in the UK in 1947 was more than 10 times higher than adenocarcinoma of the esophagus currently (i.e. approximately 30/100,000 per year in 60-year-olds) (Fig. 1). The change in the pattern of gastritis and the loss of H. pylori from Western populations was associated with a reduction of the incidence of atrophic pan-gastritis. This loss led to gastric cancer changing from the most common cancer in the USA in 1930 to becoming an uncommon disease (from approximately 40/100,000 in the general population to approximately 7/100,000 deaths per year).
Figure 1.
Age-related incidence (per 100,000 per year) of different cancers among men in the United States is compared with the mortality related to gastric ulcer among men in the UK in 1947 [46]. The cancer data show that adenocarcinoma of the esophagus, despite its marked increase, is a very rare disease with an incidence approximately equal to small bowel cancer. Even after the marked decline in gastric cancer in the US, it remains a much more important problem than adenocarcinoma of the esophagus. Data from the SEER database for the period of 1997–2001 [47]. Clearly the trade-off of loss of gastric cancer and peptic ulcer for higher acid secretion favors eradication. Considering the low prevalence of H. pylori in the high-risk group of White men, all of the effects of H. pylori eradication have likely already occurred. However, effects from diet and obesity will continue to be felt.
Expectations from Current and Past Populations
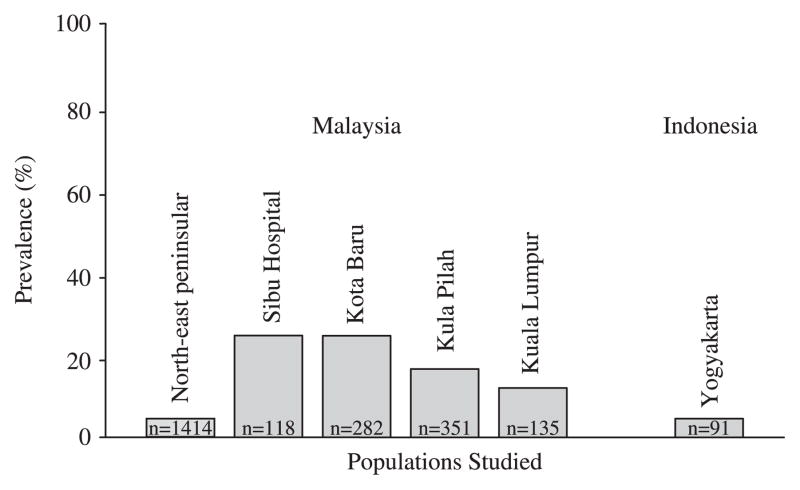
One can reasonably expect to find isolated populations where H. pylori is absent. Such a population could easily arise via a bottle neck where the founding population by chance was H. pylori negative. One such population may be the Malays. In the early 20th century it was recognized that what are now known to be H. pylori-related diseases were very rare among Malays but were common among the Indian and Chinese immigrants living in the same region. These data suggested that the Malay population was possibly H. pylori negative prior to the immigration of Chinese and Indians at the turn of the 20th century and that infections in Malays are likely the result of contamination from the Indian and Chinese populations. Of course that hypothesis (i.e. H. pylori infection in Malays with Chinese or Indian strains) can easily be tested by genetic comparison of the strains in those populations. Recent epidemiologic studies have confirmed the hypothesis that H. pylori infection is uncommon in Malays and is even uncommon among the rare individuals presenting with peptic ulcer disease [36–38] (Fig. 2). A study of the prevalence of H. pylori among the general population of Malays showed that the prevalence ranged from 11.9 to 29.2% [37]. Another study in northeastern peninsular Malaysia reported a prevalence of only about 4% [39]. Of interest, the prevalence of H. pylori infection was only about 4.5% in Javanese [40]. The presence of low prevalence among non-Western populations allows one to test the dire consequences hypotheses concerning the loss of H. pylori. Clearly, neither the Malays nor the Javanese are known for their propensity to be obese, to have adenocarcinoma of the esophagus as a major cause of death or for childhood asthma. Yet peptic ulcer and gastric cancer are rare, suggesting that the presence of H. pylori infection did convey some advantages to the population. It is also important to remember that in the West, the presence of H. pylori has not prevented Black and Hispanic children from becoming obese or the adults from either becoming obese or from experiencing GERD [41,42]. GERD is a reflection of the ability to make acid and in Malaysia GERD is not rare. The fact that GERD is more common in Indians than Chinese or Malays suggests that environmental and genetic factors are more important than H. pylori in the pathogenesis of GERD [43,44]. Adenocarcinoma of the esophagus is still very rare in Singapore (0.5 per 100,000 men), which consists of a mixed Chinese, Indian, and Malay population. However, the incidence of adenocarcinoma has been increasing along with an increase in the incidence of esophagitis, the amount of fat in the diet and probably, most importantly, obesity [45]. The incidence of adenocarcinoma of the esophagus has not been broken out for the different subpopulations; however, the precursor, Barrett’s esophagus, is approximately twice as common among Indians as in Malays [44].
Figure 2.
Prevalence of Helicobacter pylori infection in low-prevalence populations (healthy and blood donor Malays [37,39] and Javanese [40]) in whom H. pylori infection is hypothesized to have been reintroduced following the immigration of Indians and Chinese into the region.
In summary, there are no data to support any conclusion other than H. pylori being a serious chronic transmissible pathogen that no one needs, deserves, or desires. Absence of H. pylori is to be desired and the dire consequences hypotheses have proven to be simply erroneous attempts to blame some of the problems facing the modern world on a pathogen that was and is responsible for much suffering, morbidity, and mortality. It is time to join together to eradicate it and to let H. pylori join small pox and polio on the hit list of undesirables.
Footnotes
Conflicts of interest: This material is based upon work supported in part by the Office of Research and Development Medical Research Service Department of Veterans Affairs and by Public Health Service grant DK56338, which funds the Texas Gulf Coast Digestive Diseases Center. Dr Graham has received small amounts of grant support and/or free drugs or urea breath tests from Meretek, Jannsen/Eisai, and TAP, and BioHit for investigator initiated and completely investigator controlled research. Dr Graham is a consultant for Novartis in relation to vaccine development for treatment or prevention of H. pylori infection. Dr Graham is also a paid consultant for Otsuka Pharmaceuticals and a member of the Board of Directors of Meretek, Diagnostics, the manufacturer of the 13C-urea breath test. Dr Graham also receives royalties on the Baylor College of Medicine patent covering the serologic test, HM-CAP. Dr Malaty has received research support from AstraZeneca and Jannsen/Eisai. Dr Yamaoka is supported in part by NIH grant DK 62813.
References
- 1.Axon A, Forman D. Helicobacter gastroduodenitis: a serious infectious disease [editorial] BMJ. 1997;314:1430–1. doi: 10.1136/bmj.314.7092.1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scientific Publications no. 155. Lyon: IARC; 2004. Cancer incidence in five continents. [PubMed] [Google Scholar]
- 3.Breuer T, Malaty HM, Graham DY. The epidemiology of H. pylori-associated gastroduodenal diseases. In: Ernst P, Michetti P, Smith PD, editors. The Immunobiology of H. pylori from Pathogenesis to Prevention. Philadelphia: Lippincott-Raven; 1997. pp. 1–14. [Google Scholar]
- 4.Evans AC, Markus MB, Mason RJ, Steel R. Late Stone Age coprolite reveals evidence of prehistoric parasitism. S Afr Med J. 1996;86:274–5. [PubMed] [Google Scholar]
- 5.Reinhard KJ. Cultural ecology of prehistoric parasitism on the Colorado Plateau as evidenced by coprology. Am J Phys Anthropol. 1988;77:355–66. doi: 10.1002/ajpa.1330770308. [DOI] [PubMed] [Google Scholar]
- 6.Faulkner CT, Patton S, Johnson SS. Prehistoric parasitism in Tennessee: evidence from the analysis of desiccated fecal material collected from Big Bone Cave, Van Buren County, Tennessee. J Parasitol. 1989;75:461–3. [PubMed] [Google Scholar]
- 7.Reinhard KJ. Archaeoparasitology in North America. Am J Phys Anthropol. 1990;82:145–63. doi: 10.1002/ajpa.1330820204. [DOI] [PubMed] [Google Scholar]
- 8.Moore JG, Grundmann AW, Hall HJ, Fry GF. Human fluke infection in Glen Canyon at AD 1250. Am J Phys Anthropol. 1974;41:115–7. doi: 10.1002/ajpa.1330410115. [DOI] [PubMed] [Google Scholar]
- 9.Falush D, Wirth T, Linz B, et al. Traces of human migrations in Helicobacter pylori populations. Science. 2003;299:1582–5. doi: 10.1126/science.1080857. [DOI] [PubMed] [Google Scholar]
- 10.Linz B, Balloux F, Moodley Y, et al. An African origin for the intimate association between humans and Helicobacter pylori. Nature. 2007;445:915–8. doi: 10.1038/nature05562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chow WH, Blaser MJ, Blot WJ, et al. An inverse relation between cagA+ strains of Helicobacter pylori infection and risk of esophageal and gastric cardia adenocarcinoma. Cancer Res. 1998;58:588–90. [PubMed] [Google Scholar]
- 12.Ani AE, Malu AO. Helicobacter pylori: pathogen and symbiont. Niger Postgrad Med J. 2003;10:121–4. [PubMed] [Google Scholar]
- 13.Blaser MJ. An endangered species in the stomach. Sci Am. 2005;292:38–45. doi: 10.1038/scientificamerican0205-38. [DOI] [PubMed] [Google Scholar]
- 14.Boyd EJ. The prevalence of esophagitis in patients with duodenal ulcer or ulcer-like dyspepsia. Am J Gastroenterol. 1996;91:1539–43. [PubMed] [Google Scholar]
- 15.Moraes-Filho JP, Zaterka S, Pinotti HW, Bettarello A. Esophagitis and duodenal ulcer. Digestion. 1974;11:338–46. doi: 10.1159/000197601. [DOI] [PubMed] [Google Scholar]
- 16.Moraes-Filho JP. Lack of specificity of the acid perfusion test in duodenal ulcer patients. Am J Dig Dis. 1974;19:785–90. doi: 10.1007/BF01071936. [DOI] [PubMed] [Google Scholar]
- 17.Flook D, Stoddard CJ. Gastro-oesophageal reflux and oesophagitis before and after vagotomy for duodenal ulcer. Br J Surg. 1985;72:804–7. doi: 10.1002/bjs.1800721010. [DOI] [PubMed] [Google Scholar]
- 18.Earlam RJ, Amerigo J, Kakavoulis T, Pollock DJ. Histological appearances of oesophagus, antrum and duodenum and their correlation with symptoms in patients with a duodenal ulcer. Gut. 1985;26:95–100. doi: 10.1136/gut.26.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goldman MS, Jr, Rasch JR, Wiltsie DS, Finkel M. The incidence of esophagitis in peptic ulcer disease. Am J Dig Dis. 1967;12:994–9. doi: 10.1007/BF02233258. [DOI] [PubMed] [Google Scholar]
- 20.Behar J, Biancani P, Sheahan DG. Evaluation of esophageal tests in the diagnosis of reflux esophagitis. Gastroenterology. 1976;71:9–15. [PubMed] [Google Scholar]
- 21.Winkelstein A. Peptic esophagitis with duodenal ulcer. Am J Surg. 1957;93:234–7. doi: 10.1016/0002-9610(57)90772-9. [DOI] [PubMed] [Google Scholar]
- 22.Casula G, Jordan PH., Jr Is an antireflux procedure necessary in conjunction with parietal cell vagotomy in the absence of preoperative reflux? Am J Surg. 1987;153:215–20. doi: 10.1016/0002-9610(87)90818-x. [DOI] [PubMed] [Google Scholar]
- 23.Csendes A, Oster M, Moller JT, Flynn J, Funch-Jensen P, Overgaard H, Amdrup E. Gastroesophageal reflux in duodenal ulcer patients before and after vagotomy. Ann Surg. 1978;188:804–8. doi: 10.1097/00000658-197812000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cruze K, Byron FX, Hill JT. The association of peptic ulcers and asymptomatic hiatal hernia. Surgery. 1959;46:664–8. [PubMed] [Google Scholar]
- 25.Winkelstein A, Wolf BS, Som ML, Marshak RH. Peptic esophagitis with duodenal or gastric ulcer. JAMA. 1954;154:885–9. doi: 10.1001/jama.1954.02940450005002. [DOI] [PubMed] [Google Scholar]
- 26.Benedict EB, Sweet RH. Benign stricture of the esophagus. Gastroenterology. 1948;11:618–28. [PubMed] [Google Scholar]
- 27.Wallin L. Acid gastro-oesophageal reflux pattern in duodenal ulcer patients related to dyspeptic symptoms. Scand J Gastroenterol. 1980;15:151–5. doi: 10.3109/00365528009181447. [DOI] [PubMed] [Google Scholar]
- 28.Wallin L. The influence of cimetidine on the acid gastro-oesophageal reflux in duodenal ulcer patients. Scand J Gastroenterol. 1980;15:157–63. doi: 10.3109/00365528009181448. [DOI] [PubMed] [Google Scholar]
- 29.Dent J. Review article: from1906 to 2006 – a century of major evolution of understanding of gastro-oesophageal reflux disease. Aliment Pharmacol Ther. 1906;24:1269–81. doi: 10.1111/j.1365-2036.2006.03122.x. [DOI] [PubMed] [Google Scholar]
- 30.Graham DY, Yamaoka Y. H. pylori and cagA. Relationships with gastric cancer, duodenal ulcer, and reflux esophagitis and its complications. Helicobacter. 1998;3:145–51. doi: 10.1046/j.1523-5378.1998.08031.x. [DOI] [PubMed] [Google Scholar]
- 31.Liu Y, Akiyama J, Graham DY. Current understandings of Helicobacter pylori, peptic ulcer and gastroesophageal reflux disease. Minerva Gastroenterol Dietol. 2006;52:235–48. [PubMed] [Google Scholar]
- 32.Hobbs FD, Delaney BC, Rowsby M, Kenkre JE. Effect of Helicobacter pylori eradication therapy on dyspeptic symptoms in primary care. Fam Pract. 1996;13:225–8. doi: 10.1093/fampra/13.3.225. [DOI] [PubMed] [Google Scholar]
- 33.Lane JA, Murray LJ, Noble S, Egger M, Harvey IM, Donovan JL, Nair P, Harvey RF. Impact of Helicobacter pylori eradication on dyspepsia, health resource use, and quality of life in the Bristol Helicobacter project: randomised controlled trial. BMJ. 2006;332:199–204. doi: 10.1136/bmj.38702.662546.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moayyedi P, Feltbower R, Brown J, Mason S, Mason J, Nathan J, Richards ID, Dowell AC, Axon AT. Effect of population screening and treatment for Helicobacter pylori on dyspepsia and quality of life in the community: a randomised controlled trial. Leeds HELP Study Group. Lancet. 2000;355:1665–9. doi: 10.1016/s0140-6736(00)02236-4. [DOI] [PubMed] [Google Scholar]
- 35.Graham DY. The changing epidemiology of GERDGeography and Helicobacter pylori. Am J Gastroenterol. 2003;98:1462–70. doi: 10.1111/j.1572-0241.2003.07533.x. [DOI] [PubMed] [Google Scholar]
- 36.Goh KL. Prevalence of and risk factors for Helicobacter pylori infection in a multi-racial dyspeptic Malaysian population undergoing endoscopy. J Gastroenterol Hepatol. 1997;12:S29–S35. doi: 10.1111/j.1440-1746.1997.tb00455.x. [DOI] [PubMed] [Google Scholar]
- 37.Goh KL, Parasakthi N. The racial cohort phenomenonseroepidemiology of Helicobacter pylori infection in a multiracial South-East Asian country. Eur J Gastroenterol Hepatol. 2001;13:177–83. doi: 10.1097/00042737-200102000-00014. [DOI] [PubMed] [Google Scholar]
- 38.Raj SM, Yap K, Haq JA, Singh S, Hamid A. Further evidence for an exceptionally low prevalence of Helicobacter pylori infection among peptic ulcer patients in north-eastern peninsular Malaysia. Trans R Soc Trop Med Hyg. 2001;95:24–7. doi: 10.1016/s0035-9203(01)90319-0. [DOI] [PubMed] [Google Scholar]
- 39.Uyub AM, Raj SM, Visvanathan R, Nazim M, Aiyar S, Anuar AK, Mansur M. Helicobacter pylori infection in north-eastern peninsular Malaysia. Evidence for an unusually low prevalence. Scand J Gastroenterol. 1994;29:209–13. doi: 10.3109/00365529409090465. [DOI] [PubMed] [Google Scholar]
- 40.Tokudome S, Soeripto N, Triningsih FX, et al. Rare Helicobacter pylori infection as a factor for the very low stomach cancer incidence in Yogyakarta, Indonesia. Cancer Lett. 2005;219:57–61. doi: 10.1016/j.canlet.2004.09.043. [DOI] [PubMed] [Google Scholar]
- 41.El-Serag HB, Petersen NJ, Carter J, Graham DY, Richardson P, Genta RM, Rabeneck L. Gastroesophageal reflux among different racial groups in the United States. Gastroenterology. 2004;126:1692–9. doi: 10.1053/j.gastro.2004.03.077. [DOI] [PubMed] [Google Scholar]
- 42.El-Serag HB, Graham DY, Satia JA, Rabeneck L. Obesity is an independent risk factor for GERD symptoms and erosive esophagitis. Am J Gastroenterol. 2005;100:1243–50. doi: 10.1111/j.1572-0241.2005.41703.x. [DOI] [PubMed] [Google Scholar]
- 43.Rosaida MS, Goh KL. Gastro-oesophageal reflux disease, reflux oesophagitis and non-erosive reflux disease in a multiracial Asian population: a prospective, endoscopy based study. Eur J Gastroenterol Hepatol. 2004;16:495–501. doi: 10.1097/00042737-200405000-00010. [DOI] [PubMed] [Google Scholar]
- 44.Rajendra S, Kutty K, Karim N. Ethnic differences in the prevalence of endoscopic esophagitis and Barrett’s esophagus: the long and short of it all. Dig Dis Sci. 2004;49:237–42. doi: 10.1023/b:ddas.0000017444.30792.94. [DOI] [PubMed] [Google Scholar]
- 45.Fernandes ML, Seow A, Chan YH, Ho KY. Opposing trends in incidence of esophageal squamous cell carcinoma and adenocarcinoma in a multi-ethnic Asian country. Am J Gastroenterol. 2006;101:1430–6. doi: 10.1111/j.1572-0241.2006.00570.x. [DOI] [PubMed] [Google Scholar]
- 46.Jamieson RA, Smith WE, Scott LDW. Peptic ulcer in Glasgow. A hospital survey. BMJ. 1949;1:298–300. doi: 10.1136/bmj.1.4598.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Surveillance, Epidemiology and End Results (SEER) Program. doi: 10.1016/j.ijrobp.2008.02.065. ( www.peer.cancer.gov) [DOI] [PubMed]