Abstract
Objective
To investigate the phenotypic characters of carcinoma cells and the response of gastric epithelial cells to Helicobacter pylori (H. pylori) infection using the gastric carcinoma cell lines.
Material and methods
Real-time reverse transcription-polymerase chain reaction (RT-PCR) was used to assess the effect of H. pylori infection on mRNA levels of transcription factors (SOX2 and CDX2), mucin core proteins (MUC2, MUC5AC, and MUC6), and trefoil factor family peptides (TFF) (TFF1, TFF2, and TFF3) in gastric carcinoma cells (AGS, MKN45, and KATO III cells). H. pylori ATCC 43504 and its isogenic cag pathogenicity island (PAI) deleted mutant were used.
Results
These cell lines expressed mixed gastric and intestinal phenotypes. The intestinal phenotype predominated in AGS cells and gastric phenotypes in MKN45 and KATO III cells. In all three cell lines, H. pylori infection inhibited SOX2 mRNA expression, but induced the three TFFs mRNAs. In AGS cells, H. pylori induced cag PAI-dependent mRNA expression of CDX2, MUC2, MUC5AC, and MUC6. mRNA expressions of CDX2, MUC5AC, and MUC6 were inhibited in KATO III cells, whereas MUC2 mRNA expression was unchanged. In MKN45 cells, H. pylori induced the three MUCs mRNAs but inhibited CDX2 mRNA expression.
Conclusions
This study provides a useful platform for selecting appropriate cell lines to model H. pylori-related changes in the gastric epithelium that mirror the changes seen in vivo. The outcome of H. pylori infection may reflect changes in the mucus gel layer caused by altered expression of mucins and TFF peptides.
Keywords: CDX2, Helicobacter pylori, MUC, PDX1, real-time PCR, TFF
Introduction
Helicobacter pylori (H. pylori) infection is identified as the major etiologic factor in gastritis, gastroduodenal ulcer, gastric atrophy, intestinal metaplasia, and gastric cancer [1,2]. A pathogenicity island (PAI) has been identified within the H. pylori genome which contains a cluster of genes, the cag (cytotoxin-associated gene) PAI. The cag PAI, a 40 kb stretch of DNA, encodes a type IV secretory apparatus which injects the CagA protein and possibly other unknown proteins into the eukaryotic cells and induces intracellular responses in the H. pylori infected cells [3–5].
H. pylori infection is associated with alteration in the secretion of mucins and the trefoil factor family (TFF) peptides from the gastric epithelial cells [6] and these changes may contribute to H. pylori-associated gastric mucosal damage. The mucins secreted by gastric mucous cells form a mucous gel layer covering the gastric mucosa. This gel layer is considered to be the first line of gastric mucosal defense against luminal noxious agents [7–9] and damage to the mucous gel is thought to precede gastric mucosal injury. The gastric surface mucous cells and gland mucous cells express secretory mucin, MUC5AC and MUC6, respectively [10]. In contrast, the secretory mucin MUC2 is aberrantly expressed in gastric carcinoma, its closely related lesion (intestinal metaplasia) [10], and in the intestinal goblet cells [11]. In addition to secreting mucins, the mucous cells secrete TFF peptides, TFF1, TFF2, and TFF3, which are mucin-associated and along with mucins, provide a structural mucosal barrier function and also participate in the repair and healing of the damaged mucosa [12–14]. TFF1 (formerly designated as pS2) is produced by the gastric surface mucous cells [15] and TFF2 (human spasmolytic peptide: hSP) is produced by the gastric gland mucous cells [16].TFF3 (intestinal trefoil factor: ITF) is mainly produced by the intestinal goblet cells [17] and has also been demonstrated in surface mucous cells in the gastric pyloric mucosa [18].
Cell differentiation is controlled by the transcription factors encoded by the homeobox genes [19]. SOX2 is one of the candidate factors for controlling gastric differentiation. SOX2 mRNA expression in the human gastrointestinal tract is confined to the gastric mucosa [20] and strong immunoreactivity of SOX2 has been reported in the nuclei of the gastric surface mucous cells [20,21]. In addition, gastric carcinoma with a gastric phenotype expresses SOX2 mRNA and SOX2 protein [21,22]. In contrast, the caudal homeobox 2 (CDX2) gene, which regulates the development of the intestine, is expressed in the intestinal epithelial cells, intestinal metaplastic cells, and gastric carcinoma cells [23,24].
Gastric carcinoma is histologically classified into two types, differentiated and undifferentiated type or intestinal and diffuse type, based on the prevalent gland formation [25,26]. Recently, human gastric carcinomas have been phenotypically classified into gastric, gastric and intestinal mixed, or intestinal type, depending on the expression of phenotypic markers of gastrointestinal epithelial cells. This phenotypic classification is useful for investigating carcinogenesis and the clinicopathological characteristics of gastric carcinoma [27–29].
A variety of gastric carcinoma cell lines have been used as in vitro systems to model gastric epithelial response to H. pylori. However, there is a lack of information on the quantitative analysis of the expression of mucins and TFF peptides in human gastric cancer cell lines. The present study was undertaken to characterize the phenotype expression in three gastric carcinoma cell lines: AGS, MKN45, and KATO III cells, which are widely used to model the gastric epithelial responses to H. pylori infection. We also examined the response of gastric epithelial cells to H. pylori infection by assessing the mRNA levels of the transcription factors (SOX2 and CDX2), mucin core proteins (MUC5AC, MUC6, and MUC2), and TFF peptides (TFF1, TFF2, and TFF3) in the three gastric carcinoma cell lines using wild-type H. pylori ATCC 43504 infection and its isogenic cag PAI totally deleted mutant.
Material and methods
Cell culture
The cell lines used in this experiment were derived from human gastric cancers and included AGS (American Type Culture Collection (ATCC), Manassas, Va., USA), MKN45 (Riken Cell Bank, Tsukuba, Japan) and KATO III (ATCC). These cells were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM; Gibco-BRL, Grand Island, N.Y., USA) containing 10% heat-inactivated fetal bovine serum, 100 units/ml penicillin, and 100 µg/ml streptomycin in a humidified incubator at 37°C under 5% CO2. The cells were starved for 18 h with DMEM without any supplements before co-culture with H. pylori.
Bacterial strains and inoculation
The H. pylori strain ATCC 43504 (ATCC) and its isogenic cag PAI totally deleted mutant were cultured for 3 days on horse blood agar plates in a microaerophilic milieu (15% CO2) at 37°C. The strains were grown in 2 ml brucella broth (Becton Dickinson, Cockeysville, Md., USA) supplemented with 10% horse serum at 37°C under microaerophilic conditions with shaking at 120 rpm. After 40 h, the medium was removed, and the H. pylori cells were re-suspended in DMEM without any supplements and added to the AGS cells at a multiplicity of infection (MOI) described below.
To investigate the effect of the concentration of H. pylori on mRNA expression, the bacteria cells that had been re-suspended in DMEM were added to the 1 × 106 AGS cells at different MOIs (0, 50, 100, and 300), and mRNA expression was analyzed by real-time polymerase chain reaction (PCR) 12 h after H. pylori infection. To examine the cell viability under each condition, the number of viable cells was determined by the trypan-blue exclusion test.
The isogenic cag PAI totally deleted mutant was constructed according to the method previously reported [30]. Briefly, the regions upstream (hp0518-hp0519; 545,254-547,164 bp: hp number and location from H. pylori strain 26695: Genbank accession number: AE000511) and downstream (hp0549-hp0550; 584,570–586,563 bp) of the cag PAI were amplified to delete the entire cag PAI from the H. pylori chromosome. These fragments were separated by a chloramphenicol resistance cassette and cloned into the T7Blue vector (Novagen, Madison, Wisc., USA). All of the plasmids (1 mg) were used for inactivation of the chromosomal genes by natural transformation. Inactivation of the genes was confirmed by PCR amplification followed by Southern blot as well as by Western blot for CagA (Austral Biologicals, San Ramon, Calif., USA).
Real-time PCR analysis
The quantification of each of the transcription factors (SOX2 and CDX2), mucin core proteins (MUC2, MUC5AC, and MUC6), and TFF peptides (TFF1, TFF2, and TFF3) mRNA levels in the AGS cells, MKN45 cells and KATO III cell was performed in the unstimulated state and 12 h after H. pylori infection. Total RNA was isolated using the QIAamp RNA Blood Mini Kit (Qiagen, Valencia, Calif., USA). The first-strand cDNA was synthesized from approximately 1 µg RNA by using a random primer and the Moloney murine leukemia virus reverse transcriptase. Additionally, 5 µl of the reverse transcription (RT) reaction mixture was used for quantitative PCR.
The primers and probe mixture that were used to amplify each of the targeted genes and the housekeeping gene (glyceraldehyde-3-phosphate dehydrogenase; GAPDH) were purchased from Applied Biosystems (Foster City, Calif., USA).
The reaction solution for real-time PCR was prepared by mixing 5 µl of synthesized cDNA solution with 25 µl TaqMan Universal PCR Master Mix (Applied Biosystems) and 2.5 µl of the reaction mixture. Real-time PCR was carried out using the ABI PRISM 7700 Sequence Detection System (Applied Biosystems) at 50°C for 2 min, 95°C for 10 min, followed by 50 cycles at 95°C for 15 s, and at 60°C for 1 min. Each assay was done in triplicate. The expression of GAPDH was used to normalize that of the target genes. Before using the comparative threshold cycle (Ct) method for the relative quantification, a validation experiment was performed according to the manufacturer’s instructions to verify that the efficiencies of the target and GAPDH genes were approximately equal. The change in the Ct (ΔCt) of the target genes was calculated as ΔCt = (Ct of target genes)–(Ct ofGAPDH).We calculated the ratio of the target genes to GAPDH as 2−ΔCt, expressed as 2−ΔCt × 105; this ratio was used to evaluate the expression level within each target gene in each gastric carcinoma cell in the unstimulated state. The abundance of the target genes relative to that of GAPDH was calculated as ΔΔCt = (ΔCt of target genes)–(ΔCt of GAPDH). The ratio was calculated as 2−ΔΔCt to evaluate the alteration of the target genes 12 h after co-culture with the gastric cancer cells and H. pylori.
Statistics
The data were presented as mean values±standard deviation. We used the one-way analysis of variance, followed by post hoc contrasts using the Bonferroni limitation for the statistical analysis. P-values of less than 0.05 were considered significant.
Results
Characterization of human gastric cancer cell lines by RT-PCR
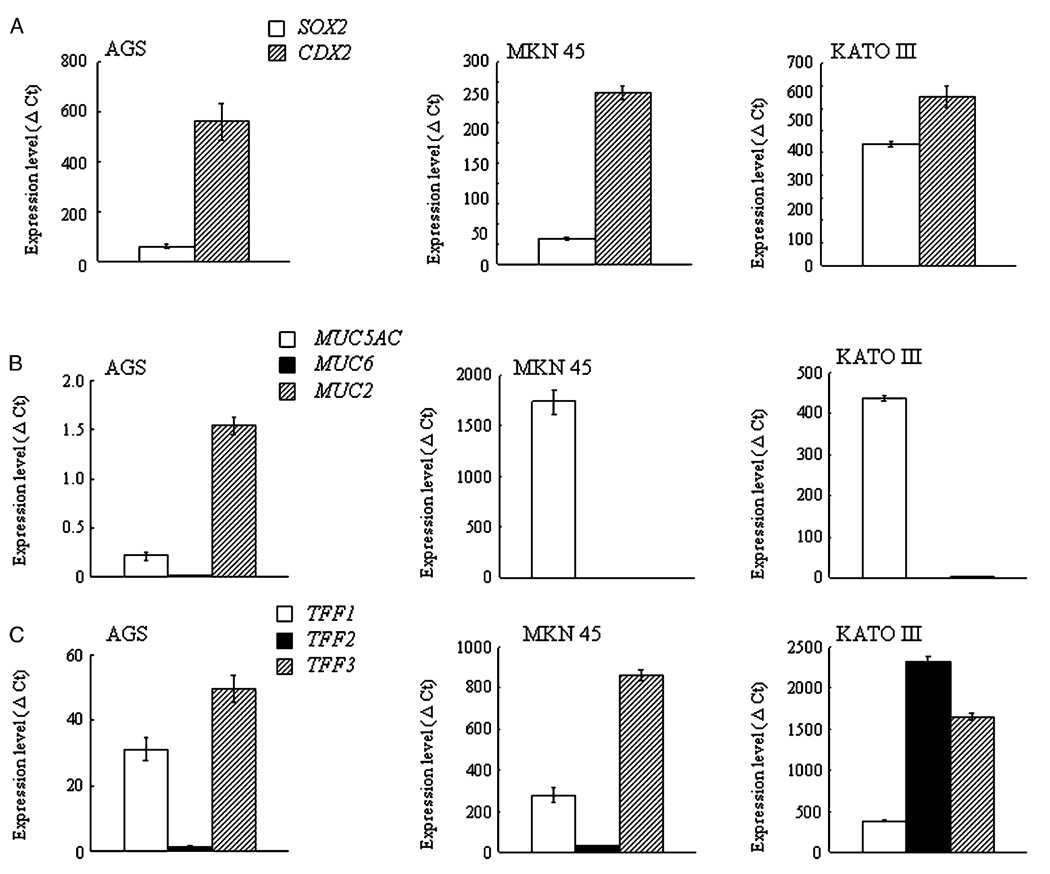
The mRNA levels of SOX2, CDX2, MUCs, and TFFs in the AGS, MKN45, and KATO III cells at the stage of early confluency are shown in Figure 1.
Figure 1.
Unstimulated mRNA levels of transcription factors (SOX2 and CDX2) (A), mucin core proteins (MUC5AC, MUC6, and MUC2) (B), and trefoil factor family peptides (TFF1, TFF2, TFF3) (C) in the AGS, MKN45 and KATO III cells. The change in the comparative threshold cycle (ΔCt) for the target genes was calculated as ΔCt = (Ct of target genes)–(Ct of GAPDH). The ratio of the target genes to GAPDH was calculated as 2−ΔCt and expressed as 2−ΔCt × 105; this ratio was used to evaluate the expression level within each target gene (SOX2, CDX2, MUCs, and TFFs) in the AGS cells, MKN45 cells, and KATO III cells in the unstimulated state. Data are expressed as mean values±SD.
CDX2 mRNA levels were higher than those of the SOX2 mRNA in all three cell lines (Figure 1A). MUC2 mRNA levels were the highest in the AGS cells followed by MUC5AC mRNA and MUC6 mRNA levels (Figure 1B). In both the MKN45 and KATO III cells, the MUC5AC mRNA levels (expression level; 1741±120 in MKN45 cells, 439±7.0 in KATO III cells) were higher than those of the MUC6 (0.01±0.001 in MKN45 cells, 0.26±0.02 in KATO III cells) or MUC2 mRNA (3.07±0.05 in MKN45 cells, 1.16±0.24 in KATO III cells).
TFF3 mRNA levels were highest among three TFF mRNAs in the AGS cells and the MKN45 cells, followed by TFF1 mRNA and the TFF2 mRNA levels. In the KATO III cells, the TFF2 mRNA levels were the highest, followed by the TFF3 mRNA and the TFF1 mRNA levels (Figure 1C). Both the MKN45 and KATO III cells showed much higher amounts of MUC mRNAs and TFF mRNAs than the AGS cells (Figure 1B, C).
Effect of H. pylori infection on mRNA expression of transcription factors, mucin core proteins, and TFF peptides
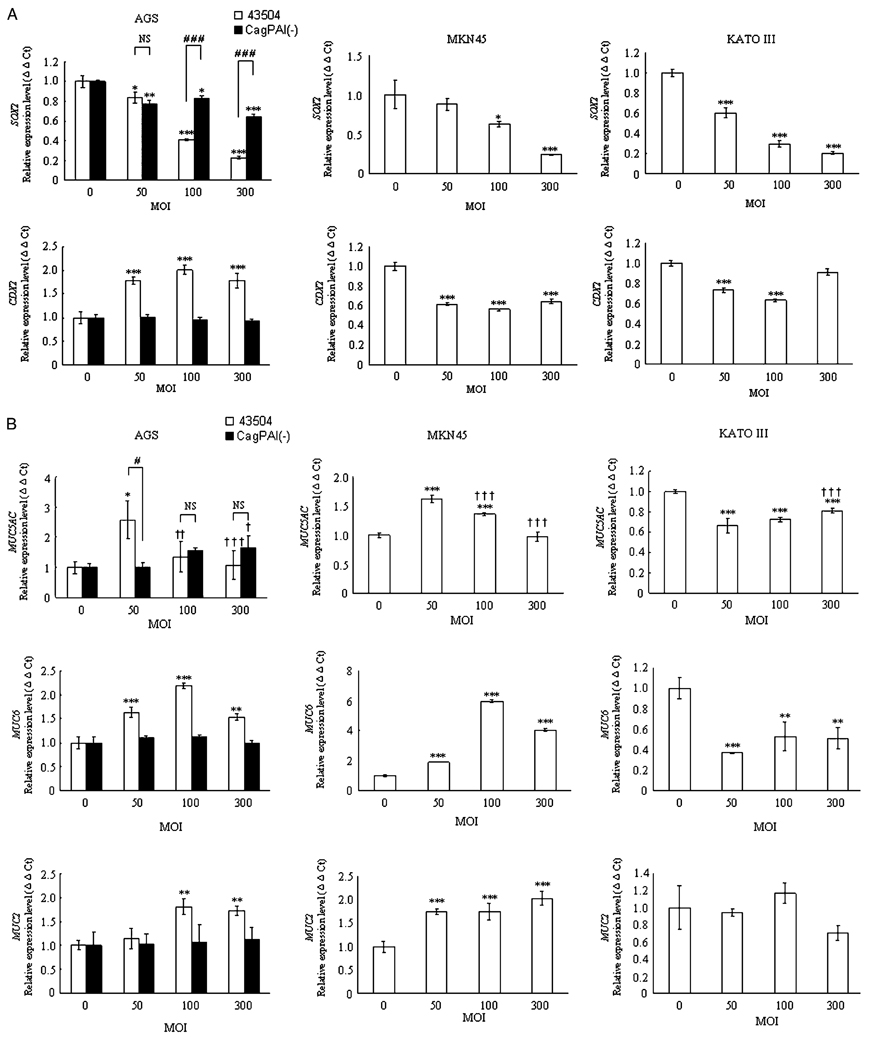
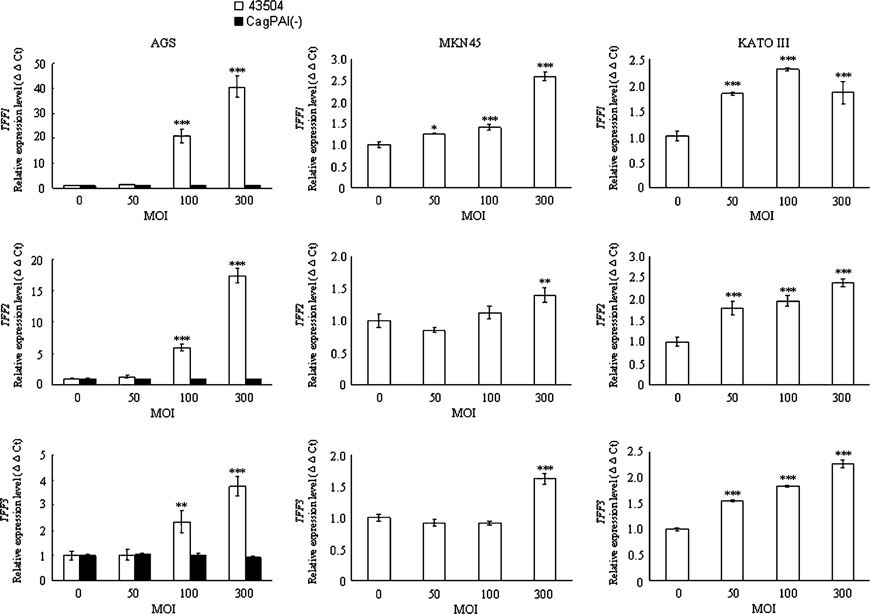
Wild-type H. pylori significantly inhibited SOX2 mRNA expression in a concentration-dependent manner in all three cell lines (Figure 2A). In contrast, wild-type H. pylori significantly induced mRNA expression of the three TFFs in a concentration-dependent manner in all three cell lines (Figure 2C). Importantly, the expression patterns of CDX2, MUC5AC, MUC6, and MUC2 mRNA by wild- type H. pylori infection were gastric cell line specific. Wild-type H. pylori significantly induced the expression of these four mRNAs in AGS cells (Figure 2A–C), whereas H. pylori significantly inhibited the expression of these three mRNAs in KATOIII cells (except for MUC2 mRNA, which was unchanged) (Figure 2A–C). In MKN45 cells, wild-type H. pylori significantly induced the expression of the three MUC mRNAs, but significantly inhibited that of CDX2 mRNA (Figure 2A–C).
Figure 2.
Effect of treatment with the wild-type H. pylori strain and the cag-PAI deleted mutant strain on the expression levels of the mRNAs of the transcription factors (SOX2 and CDX2) (A), MUCs (MUC5AC, MUC6, and MUC2) (B), and TFFs (TFF1, TFF2, and TFF3) (C) in gastric cancer cells at different H. pylori multiplicities of infection (MOIs). The abundance of the target genes relative to that of GAPDH was calculated as ΔΔCt = (ΔCt of target genes)–(ΔCt of GAPDH). The ratio was calculated as 2−ΔΔCt to evaluate the alteration of the target genes 12 h after co-culture with the gastric cancer cells and H. pylori. MOI. *p <0.05; **p <0.01; ***p <0.001 in comparison with that of cells cultured at a MOI of 0. †p <0.05; ††p <0.01; †††p <0.001 in comparison with that of cells cultured at a MOI of 50. #p < 0.05; ###p <0.001 in comparison with that of cells cultured with wild-type H. pylori
Under each condition, there was no significant alteration in the number of viable carcinoma cells determined by the trypan-blue exclusion test (data not shown).
Effect of cag-PAI on mRNA expression of transcription factors, mucin core proteins, and TFF peptides in AGS cells
We next examined the effects of the cag PAI on the expression of the transcription factors of interest, mucin core proteins, and TFF peptides in AGS cells. AGS cells were chosen because expression of MUC5AC mRNA and MUC2 mRNA paralleled that of the corresponding transcription factors (SOX2 and CDX2) suggesting that AGS cells should be ideal for investigating the effects of the cag PAI on expression of these mRNAs. With the exception of the SOX2 mRNA and MUC5AC mRNA levels, infection with the cag-PAI mutant did not alter the mRNA levels of the transcription factors, mucin core proteins, or TFF peptides (Figure 2A–C). The cag PAI mutant also inhibited the expression of SOX2 mRNA; however, the SOX2 mRNA levels at a MOI of 100 and 300 were significantly higher in cells infected with the cag PAI mutant than in those infected with wild-type H. pylori suggesting that SOX2 mRNA expression was partially cag PAI dependent (p <0.001) (Figure 2A). The MUC5AC mRNA levels were significantly higher in cells infected with wild-type H. pylori than in those with the cag PAI mutant at a MOI of 50 (p <0.05) (Figure 2B), whereas there was no difference in the levels between the strains at a MOI of 100 and 300, which suggests that MUC5AC mRNA expression was also partially cag PAI dependent.
Discussion
In this report, we showed that gastric cancer cell lines expressed mixed gastric and intestinal phenotypes. The intestinal phenotype predominated in AGS cells, whereas gastric phenotype predominated in MKN45 and KATO III cells. H. pylori infection altered the expression of the mRNA of both gastric and intestinal transcription factors as well as MUCs and TFFs.
In AGS cells, expression of the intestinal transcription factor CDX2 was predominant compared with that of the gastric transcription factor SOX2. Accordingly, goblet cell-specific MUC2 mRNA and TFF3 mRNA levels were also predominant in AGS cells. These data clearly show that coordinated expression of transcription factor mRNAs, MUCs mRNAs, and TFFs mRNAs was observed in AGS cells under uninfected conditions, and are in agreement with previous studies that SOX2 and CDX2 are putative regulators for MUC5AC [21] and MUC2 [31], respectively. In addition, these co-expression patterns reflect the regulatory function of the transcription factors and correspond to those observed in in vivo expression [22].
In the MKN45 and KATO III cells, gastric surface mucous cell-specific MUC5AC mRNA levels were much higher than those of goblet cell-specific MUC2 mRNA, whereas goblet cell-specific TFF3 mRNA levels were higher than those of gastric surface mucous cell-specific TFF1. This discrepancy can be explained by the fact that human gastric carcinoma tissues express a gastric pyloric mucosal phenotype rather than a gastric fundic mucosal phenotype [32] and that TFF3 mRNA and TFF3 protein are normally expressed in surface mucous cells in human normal gastric pyloric mucosa [18]. Thus, predominant expression of the MUC5AC and TFF3 mRNA in the MKN45 and KATO III cells shows that both cell lines express a predominantly gastric phenotype.
The mixed gastric and intestinal phenotype expression in these gastric carcinoma cell lines expands the results previously reported regarding mucins and mucin gene expression in gastric cancer cell lines [33,34]. For example, Carvalho et al. [33] used RT-PCR to show that MKN45 cells expressed MUC5AC mRNA but not MUC2 or MUC6 mRNA and that KATO III cells expressed both MUC5AC and MUC2 mRNAs but not MUC6 mRNA. Cornberg et al. [34] used RT-PCR and reported that both AGS and KATO III cells expressed both MUC5AC and MUC2 mRNAs but not MUC6 mRNAs. The lack of MUC2 and MUC6 mRNAs expression described in these reports could be explained by the relatively small amounts of MUC2 and MUC6 mRNAs expressed in these cells as confirmed in this study. A similar mixed gastric and intestinal phenotype expression has also been reported in human gastric adenocarcinoma tissues [22].
Interestingly, wild-type H. pylori strains inhibited SOX2 mRNA expression in a dose-dependent manner in all three cell lines examined. In contrast, H. pylori at a MOI of 50 induced MUC5AC mRNA expression, whereas greater amounts of H. pylori inhibited the expression in AGS cells and MKN45 cells. Previously, Li et al. reported that the SOX2 protein was observed in the nuclei of the gastric surface mucous cells of normal human gastric mucosa and that MUC5AC mRNAwas induced in COS-7 cells transfected with a SOX2 construct [21]. Taken together, the putative transcriptional regulator function of the SOX2 protein should influence the expression of the MUC5AC mRNA. Furthermore, there is a possibility that a regulatory factor other than SOX2 is also involved in the regulation of MUC5AC mRNA expression in AGS and MKN45 cells.
In contrast to AGS cells and MKN45 cells, MUC5AC mRNA levels were consistently inhibited by H. pylori infection irrespective of the MOI in KATO III cells. Similarly, MUC6 mRNA levels were also induced by H. pylori infection of AGS cells and MKN45 cells, whereas the levels were inhibited in KATO III cells. The reason for the opposite function of H. pylori on the expression of MUC5AC mRNA and MUC6 mRNA remains unknown. However, the data suggest that H. pylori infection activates different signaling pathways related to the expression of the MUC5AC mRNA and MUC6 mRNA in the different cell lines. It is interesting that these differences appear independent of the original phenotype, since both MKN45 cells and KATO III cells were of gastric predominance. However, MKN45 cells and KATO III cells were derived from different types of carcinoma: MKN45 cells from a poorly differentiated adenocarcinoma of the medullary type [35] and KATO III cells from a signet-ring cell carcinoma [35,36]. This difference in histological type of the parent tumors might also be reflected in the differences in MUC5AC mRNA and MUC6 mRNA expression patterns.
The down-regulation of MUC5AC in H. pylori-infected human gastric mucosa has been reported by using immunohistochemistry and in situ hybridization [37]. In contrast, the up-regulation of MUC6 mRNA has been reported in the human gastric surface mucous cells of H. pylori-infected patients [38]. Since MUC5AC is a mucin core protein of the gastric surface mucous cells and a major mucin core protein of the mucins forming the gastric surface mucous gel layer covering the gastric mucosa [9], a decrease in MUC5AC synthesis after H. pylori infection may impair the protective gastric mucous gel layer. However, as shown in this study, different cell types showed different reactions by H. pylori infection. Further studies are necessary to investigate the MUC5AC induction patterns in vivo in the gastric mucosa.
Different reactions by H. pylori infection in different cells were also observed in CDX2 mRNA levels. Wild-type H. pylori infection induced CDX2 mRNA expression in the AGS cells, whereas it inhibited the expression in the MKN45 cells and KATO III cells. Our data for AGS cells were in agreement with a recent report that H. pylori induced CDX2 mRNA expression in AGS cells [39]. In an in vivo study, immunohistochemistry was used to examine the expression of CDX2 and it was reported to be enhanced in H. pylori-infected human gastric mucosa [40]. Importantly, we found that wild-type H. pylori induced both CDX2 mRNA and MUC2 mRNA expressions in AGS cells. This coordinated upregulation of the expression of CDX2 mRNA and MUC2 mRNA in AGS cells could be explained by the putative transcriptional regulator function of CDX2 on the MUC2 mRNA expression [31]. In addition, the up-regulation of the CDX2 and MUC2 mRNA in the AGS cells incubated with H. pylori confirmed in this study suggests a close relationship between H. pylori infection and intestinal metaplasia, which is one of the lesions preceding the development of gastric carcinoma [41].
However, the expression of MUC2 mRNA was independent of the expression of CDX2 mRNA in both the MKN45 and KATO III cells. The mechanism involved in MUC2 mRNA expression may be different between intestinal phenotype predominant AGS cells and gastric phenotype predominant MKN45 and KATO III cells.
In this study, wild-type H. pylori induced TFF1, TFF2, and TFF3 mRNA expression in all three cell lines. These findings are consistent with those of previous reports on the increased immunohistochemical and mRNA expression of TFF1 in H. pylori-infected human gastric mucosa [6,42] and with the report of the increased TFF1 mRNA levels in AGS cells following infection with H. pylori [43]. A recent study has reported that the up-regulation of TFF1 and TFF2 mRNA levels was carried out by incubation of MKN45 cells with peroxisome proliferator- activated receptor gamma (PPARγ) ligands [44]. PPARγ is a member of the nuclear hormone receptor superfamily that plays an important role in cell differentiation and regulation of metabolism [45]. It is notable that PPARγ expression was up-regulated by Kato III cells incubated with H. pylori [46]. Moreover, the hypothesis that strains containing the cag PAI augment PPARγ activation was reported [46]. We therefore hypothesize that the cag PAI-positive H. pylori could up-regulate PPARγ expression in gastric carcinoma cells and should contribute to the up-regulation of TFF1 and TFF2 mRNA levels in gastric carcinoma cells. This upregulation of TFF1 expression by H. pylori infection may facilitate the adhesion of H. pylori to the gastric mucosa and its colonization in the gastric surface mucous gel layer. On the one hand, TFF1 has been reported to act as a receptor for H. pylori, in vivo, and to explain the tropism of H. pylori for the gastric surface mucous cells and its co-localization with the gastric mucin MUC5AC [47]. On the other hand, from the perspective of a mucosal defense mechanism, this up-regulation of TFF mRNAs could be interpreted as a defensive reaction of the host cells against H. pylori-related gastric mucosal injuries. The mucosal protective functions of TFF peptides include stabilization of the gastric mucous gel layer, promotion of the gastric epithelial restitution, and suppression of apoptosis [12–14]. Interestingly, gender differences in TFF1 mRNA expression in the gastric mucosa with H. pylori infection have been reported in the human stomach [42]. Differences in the expression of TFF peptide proteins as cellular responses to H. pylori infection in the stomach may be related to differences in disease outcomes of H. pylori infection.
In conclusion, we have shown that the intestinal phenotype predominated in AGS cells, whereas the gastric phenotype predominated in MKN45 and KATO III cells and that H. pylori cells alter the expression of the transcription factors mRNA, MUCs mRNA, and TFFs mRNA levels in H. pylori-infected cells in a pattern common to cell lines or in a pattern specific to cell lines. These alterations were largely cag PAI dependent and may contribute to the outcomes of H. pylori infections. The present study provides a useful platform for selecting appropriate cell lines to model H. pylori-related changes in the gastric epithelium that mirror the changes seen in vivo.
Acknowledgements
We express our gratitude to Professor Tsutomu Katsuyama (Department of Laboratory Medicine, Shinshu University School of Medicine) and Professor David Y. Graham (Department of Medicine/Gastroenterology, Michael E. DeBakey Veterans Affairs Medical Center and Baylor College of Medicine) for their advice and encouragement. This report is based on work supported in part by a Grant-in-Aid for Scientific Research C-17590488 (to H.O.) from the Ministry of Education, Culture, Sports, Sciences and Technology of Japan and grants from the National Institutes of Health DK62813 (to Y.Y.).
References
- 1.Marshall BJ, Warren JR. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet. 1984;1:1311–1315. doi: 10.1016/s0140-6736(84)91816-6. [DOI] [PubMed] [Google Scholar]
- 2.Parsonnet J, Friedman GD, Vandersteen DP, Chang Y, Vogelman JH, Orentreich N, et al. Helicobacter pylori infection and the risk of gastric carcinoma. N Engl J Med. 1991;325:1127–1131. doi: 10.1056/NEJM199110173251603. [DOI] [PubMed] [Google Scholar]
- 3.Asahi M, Azuma T, Ito S, Ito Y, Suto H, Nagai Y, et al. Helicobacter pylori CagA protein can be tyrosine phosphorylated in gastric epithelial cells. J Exp Med. 2000;191:593–602. doi: 10.1084/jem.191.4.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Backert S, Ziska E, Brinkmann V, Zimny-Arndt U, Fauconnier A, Jungblut PR, et al. Translocation of the Helicobacter pylori CagA protein in gastric epithelial cells by a type IV secretion apparatus. Cell Microbiol. 2000;2:155–164. doi: 10.1046/j.1462-5822.2000.00043.x. [DOI] [PubMed] [Google Scholar]
- 5.Odenbreit S, Puls J, Sedlmaier B, Gerland E, Fischer W, Haas R. Translocation of Helicobacter pylori CagA into gastric epithelial cells by type IV secretion. Science. 2000;287:1497–1500. doi: 10.1126/science.287.5457.1497. [DOI] [PubMed] [Google Scholar]
- 6.Van De Bovenkamp JH, Korteland-Van Male AM, Buller HA, Einerhand AW, Dekker J. Infection with Helicobacter pylori affects all major secretory cell populations in the human antrum. Dig Dis Sci. 2005;50:1078–1086. doi: 10.1007/s10620-005-2708-4. [DOI] [PubMed] [Google Scholar]
- 7.Ota H, Katsuyama T. Alternating laminated array of two types of mucin in the human gastric surface mucous layer. Histochem J. 1992;24:86–92. doi: 10.1007/BF01082444. [DOI] [PubMed] [Google Scholar]
- 8.Allen A, Flemstrom G, Garner A, Kivilaakso E. Gastro-duodenal mucosal protection. Physiol Rev. 1993;73:823–857. doi: 10.1152/physrev.1993.73.4.823. [DOI] [PubMed] [Google Scholar]
- 9.Ho SB, Takamura K, Anway R, Shekels LL, Toribara NW, Ota H. The adherent gastric mucous layer is composed of alternating layers of MUC5AC and MUC6 mucin proteins. Dig Dis Sci. 2004;49:1598–1606. doi: 10.1023/b:ddas.0000043371.12671.98. [DOI] [PubMed] [Google Scholar]
- 10.Ho SB, Shekels LL, Toribara NW, Kim YS, Lyftogt C, Cherwitz DL, et al. Mucin gene expression in normal, preneoplastic, and neoplastic human gastric epithelium. Cancer Res. 1995;55:2681–2690. [PubMed] [Google Scholar]
- 11.Weiss AA, Babyatsky MW, Ogata S, Chen A, Itzkowitz SH. Expression of MUC2 and MUC3 mRNA in human normal, malignant, and inflammatory intestinal tissues. J Histochem Cytochem. 1996;44:1161–1166. doi: 10.1177/44.10.8813081. [DOI] [PubMed] [Google Scholar]
- 12.Sands BE, Podolsky DK. The trefoil peptide family. Annu Rev Physiol. 1996;58:253–273. doi: 10.1146/annurev.ph.58.030196.001345. [DOI] [PubMed] [Google Scholar]
- 13.Wong WM, Poulsom R, Wright NA. Trefoil peptides. Gut. 1999;44:890–895. doi: 10.1136/gut.44.6.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoffmann W. Trefoil factor family (TFF) peptides: regulators of mucosal regeneration and repair, and more. Peptides. 2004;25:727–730. doi: 10.1016/j.peptides.2004.03.019. [DOI] [PubMed] [Google Scholar]
- 15.Tomasetto C, Rio MC, Gautier C, Wolf C, Hareuveni M, Chambon P, et al. hSP, the domain-duplicated homolog of pS2 protein, is co-expressed with pS2 in stomach but not in breast carcinoma. Embo J. 1990;9:407–414. doi: 10.1002/j.1460-2075.1990.tb08125.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hanby AM, Poulsom R, Singh S, Elia G, Jeffery RE, Wright NA. Spasmolytic polypeptide is a major antral peptide: distribution of the trefoil peptides human spasmolytic polypeptide and pS2 in the stomach. Gastroenterology. 1993;105:1110–1116. doi: 10.1016/0016-5085(93)90956-d. [DOI] [PubMed] [Google Scholar]
- 17.Podolsky DK, Lynch-Devaney K, Stow JL, Oates P, Murgue B, DeBeaumont M, et al. Identification of human intestinal trefoil factor. Goblet cell-specific expression of a peptide targeted for apical secretion. J Biol Chem. 1993;268:6694–6702. [PubMed] [Google Scholar]
- 18.Kouznetsova I, Peitz U, Vieth M, Meyer F, Vestergaard EM, Malfertheiner P, et al. A gradient of TFF3 (trefoil factor family 3) peptide synthesis within the normal human gastric mucosa. Cell Tissue Res. 2004;316:155–165. doi: 10.1007/s00441-004-0854-1. [DOI] [PubMed] [Google Scholar]
- 19.Abate-Shen C. Deregulated homeobox gene expression in cancer: cause or consequence? Nat Rev Cancer. 2002;2:777–785. doi: 10.1038/nrc907. [DOI] [PubMed] [Google Scholar]
- 20.Tsukamoto T, Inada K, Tanaka H, Mizoshita T, Mihara M, Ushijima T, et al. Down-regulation of a gastric transcription factor, Sox2, and ectopic expression of intestinal homeobox genes, Cdx1 and Cdx2: inverse correlation during progression from gastric/intestinal-mixed to complete intestinal metaplasia. J Cancer Res Clin Oncol. 2004;130:135–145. doi: 10.1007/s00432-003-0519-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li XL, Eishi Y, Bai YQ, Sakai H, Akiyama Y, Tani M, et al. Expression of the SRY-related HMG box protein SOX2 in human gastric carcinoma. Int J Oncol. 2004;24:257–263. [PubMed] [Google Scholar]
- 22.Tsukamoto T, Mizoshita T, Mihara M, Tanaka H, Takenaka Y, Yamamura Y, et al. Sox2 expression in human stomach adenocarcinomas with gastric and gastric-and-intestinal-mixed phenotypes. Histopathology. 2005;46:649–658. doi: 10.1111/j.1365-2559.2005.02170.x. [DOI] [PubMed] [Google Scholar]
- 23.Bai YQ, Yamamoto H, Akiyama Y, Tanaka H, Takizawa T, Koike M, et al. Ectopic expression of homeodomain protein CDX2 in intestinal metaplasia and carcinomas of the stomach. Cancer Lett. 2002;176:47–55. doi: 10.1016/s0304-3835(01)00753-4. [DOI] [PubMed] [Google Scholar]
- 24.Eda A, Osawa H, Yanaka I, Satoh K, Mutoh H, Kihira K, et al. Expression of homeobox gene CDX2 precedes that of CDX1 during the progression of intestinal metaplasia. J Gastroenterol. 2002;37:94–100. doi: 10.1007/s005350200002. [DOI] [PubMed] [Google Scholar]
- 25.Lauren P. The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. An attempt at a histo-clinical classification. Acta Pathol Microbiol Scand. 1965;64:31–49. doi: 10.1111/apm.1965.64.1.31. [DOI] [PubMed] [Google Scholar]
- 26.Nakamura K, Sugano H, Takagi K. Carcinoma of the stomach in incipient phase: its histogenesis and histological appearances. Gan. 1968;59:251–258. [PubMed] [Google Scholar]
- 27.Sepulveda AR, Wu L, Ota H, Gutierrez O, Kim JG, Genta RM, et al. Molecular identification of main cellular lineages as a tool for the classification of gastric cancer. Hum Pathol. 2000;31:566–574. doi: 10.1053/hp.2000.6684. [DOI] [PubMed] [Google Scholar]
- 28.Shibata N, Watari J, Fujiya M, Tanno S, Saitoh Y, Kohgo Y. Cell kinetics and genetic instabilities in differentiated type early gastric cancers with different mucin phenotype. Hum Pathol. 2003;34:32–40. doi: 10.1053/hupa.2003.2. [DOI] [PubMed] [Google Scholar]
- 29.Tatematsu M, Tsukamoto T, Inada K. Stem cells and gastric cancer: role of gastric and intestinal mixed intestinal metaplasia. Cancer Sci. 2003;94:135–141. doi: 10.1111/j.1349-7006.2003.tb01409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kudo T, Lu H, Wu JY, Graham DY, Casola A, Yamaoka Y. Regulation of RANTES promoter activation in gastric epithelial cells infected with Helicobacter pylori. Infect Immun. 2005;73:7602–7612. doi: 10.1128/IAI.73.11.7602-7612.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yamamoto H, Bai YQ, Yuasa Y. Homeodomain protein CDX2 regulates goblet-specific MUC2 gene expression. Biochem Biophys Res Commun. 2003;300:813–818. doi: 10.1016/s0006-291x(02)02935-2. [DOI] [PubMed] [Google Scholar]
- 32.Fujimori Y, Akamatsu T, Ota H, Katsuyama T. Proliferative markers in gastric carcinoma and organoid differentiation. Hum Pathol. 1995;26:725–734. doi: 10.1016/0046-8177(95)90219-8. [DOI] [PubMed] [Google Scholar]
- 33.Carvalho F, David L, Aubert JP, Lopez-Ferrer A, De Bolos C, Reis CA, et al. Mucins and mucin-associated carbohydrate antigens expression in gastric carcinoma cell lines. Virchows Arch. 1999;435:479–485. doi: 10.1007/s004280050431. [DOI] [PubMed] [Google Scholar]
- 34.Cornberg M, Enss ML, Makkink MK, Beil W, Bock CT, Sobek-Klocke I, et al. Variation of human mucin gene expression in gastric cancer cell lines and gastric mucous cell primary cultures. Eur J Cell Biol. 1999;78:832–841. doi: 10.1016/s0171-9335(99)80034-x. [DOI] [PubMed] [Google Scholar]
- 35.Motoyama T, Hojo H, Watanabe H. Comparison of seven cell lines derived from human gastric carcinomas. Acta Pathol Jpn. 1986;36:65–83. doi: 10.1111/j.1440-1827.1986.tb01461.x. [DOI] [PubMed] [Google Scholar]
- 36.Sekiguchi M, Sakakibara K, Fujii G. Establishment of cultured cell lines derived from a human gastric carcinoma. Jpn J Exp Med. 1978;48:61–68. [PubMed] [Google Scholar]
- 37.Byrd JC, Yunker CK, Xu QS, Sternberg LR, Bresalier RS. Inhibition of gastric mucin synthesis by Helicobacter pylori. Gastroenterology. 2000;118:1072–1079. doi: 10.1016/s0016-5085(00)70360-x. [DOI] [PubMed] [Google Scholar]
- 38.Byrd JC, Yan P, Sternberg L, Yunker CK, Scheiman JM, Bresalier RS. Aberrant expression of gland-type gastric mucin in the surface epithelium of Helicobacter pylori-infected patients. Gastroenterology. 1997;113:455–464. doi: 10.1053/gast.1997.v113.pm9247464. [DOI] [PubMed] [Google Scholar]
- 39.Manzo BA, Crabtree JE, Campbell FM, Tweedle D, Potten CS, Bajaj-Elliott M, et al. Helicobacter pylori regulates the expression of inhibitors of DNA binding (Id) proteins by gastric epithelial cells. Microbes Infect. 2006;8:1064–1074. doi: 10.1016/j.micinf.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 40.Satoh K, Mutoh H, Eda A, Yanaka I, Osawa H, Honda S, et al. Aberrant expression of CDX2 in the gastric mucosa with and without intestinal metaplasia: effect of eradication of Helicobacter pylori. Helicobacter. 2002;7:192–198. doi: 10.1046/j.1523-5378.2002.00080.x. [DOI] [PubMed] [Google Scholar]
- 41.Correa P. A human model of gastric carcinogenesis. Cancer Res. 1988;48:3554–3560. [PubMed] [Google Scholar]
- 42.Kato S, Matsukura N, Togashi A, Masuda G, Matsuda N, Yamada N, et al. Sex differences in mucosal response to Helicobacter pylori infection in the stomach and variations in interleukin-8, COX-2 and trefoil factor family 1 gene expression. Aliment Pharmacol Ther. 2004;20 Suppl 1:17–24. doi: 10.1111/j.1365-2036.2004.01985.x. [DOI] [PubMed] [Google Scholar]
- 43.Guillemin K, Salama NR, Tompkins LS, Falkow S. Cag pathogenicity island-specific responses of gastric epithelial cells to Helicobacter pylori infection. Proc Natl Acad Sci USA. 2002;99:15136–15141. doi: 10.1073/pnas.182558799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shimada T, Koitabashi A, Kuniyoshi T, Hashimoto T, Yoshiura K, Yoneda M, et al. Up-regulation of TFF expression by PPARgamma ligands in gastric epithelial cells. Aliment Pharmacol Ther. 2003;18 Suppl 1:119–125. doi: 10.1046/j.1365-2036.18.s1.14.x. [DOI] [PubMed] [Google Scholar]
- 45.Michalik L, Desvergne B, Wahli W. Peroxisome-proliferator-activated receptors and cancers: complex stories. Nat Rev Cancer. 2004;4:61–70. doi: 10.1038/nrc1254. [DOI] [PubMed] [Google Scholar]
- 46.Konturek PC, Kania J, Kukharsky V, Raithel M, Ocker M, Rembiasz K, et al. Implication of peroxisome proliferator-activated receptor gamma and proinflammatory cytokines in gastric carcinogenesis: link to Helicobacter pylori-infection. J Pharmacol Sci. 2004;96:134–143. doi: 10.1254/jphs.fpj04016x. [DOI] [PubMed] [Google Scholar]
- 47.Clyne M, Dillon P, Daly S, O’Kennedy R, May FE, Westley BR, et al. Helicobacter pylori interacts with the human singledomain trefoil protein TFF1. Proc Natl Acad Sci USA. 2004;101:7409–7414. doi: 10.1073/pnas.0308489101. [DOI] [PMC free article] [PubMed] [Google Scholar]