Abstract
Pro-inflammatory cytokines and anti-inflammatory cytokines are produced in gastric mucosa from inflammatory cells activated by Helicobacter pylori (H. pylori) infection. Of the inflammatory cytokines, interleukin (IL)-1β and tumor necrosis factor (TNF)-α have a potent inhibitive effect on gastric acid production. Polymorphisms in these genes are associated with individual differences in cytokine messenger RNA levels, which result in different gastric mucosal inflammation, different acid inhibition and different gastroduodenal disease risks in response to H. pylori infection. The sustained higher intragastric pH during an eradication therapy is known to be one of the therapeutic determinants of the H. pylori eradication as well as antibiotics resistance and poor compliance. The IL-1B-511 polymorphism is related to eradication rate, and, in combined analysis of previous reports, the eradication rate in patients with the IL-1B-511 C/C genotype (77.4%, 209/270), low IL-1β producer genotype, is lower than that of the IL-1B-511 C/T and T/T genotypes (87.2%, 631/724) (Odds ratio for eradication failure: 1.98, 95% confidence interval: 1.38–2.84, P = 0.0002). Moreover, the odds ratio of combined CYP2C19 rapid metabolizer-IL-1B-511 C/C type for eradication failure is 11.15 (5.23–23.78) times that of the CYP2C19 poor metabolizer-IL-1B-511 non-C/C type. However, there is no positive data indicating the role of other inflammatory cytokine polymorphisms (e.g. IL-1RN, TNF-A or IL-10) in eradication therapy. Nevertheless, the studies show that inflammatory cytokine polymorphisms, especially the IL-1B-511 T/T genotype, are the determinants of eradication by affecting gastric acid secretion and mucosal inflammation. Therefore, the tailored eradication therapy, considering inflammatory cytokine polymorphisms, may be effective for the higher eradication rates.
Keywords: eradication therapy, Helicobacter pylori, IL-1β, inflammatory cytokine, polymorphism, TNF-α
I. Introduction
Helicobacter pylori (H. pylori) is such an ancient bacteria that its genetic variations can be used to trace migrations during the past 100 000 years. In fact, it is now believed that humans probably acquired H. pylori quite early in their history, long before the migration of modern humans out of Africa.1,2 H. pylori colonize the gastric mucosa in human populations with a prevalence that ranges from approximately 25% in developed countries to as high as 80–95% in developing countries.3–5 Therefore, for the prevention of the development of H.-pylori-related diseases, an eradication therapy of H. pylori has been carried out not only for the patients with H.-pylori-related upper gastrointestinal diseases (e.g. peptic ulcer diseases, gastric adenoma, and gastric-mucosa-associated lymphoid tissue lymphoma), but also for the patients with some extra-gastrointestinal disorders (e.g. such as idiopathic thrombocytopenic purpura, chronic idiopathic urticaria and iron-deficiency anemia).6–12 Currently, the eradication of H. pylori is known to be an important strategy for the prevention of gastric cancer development.6,8,13 As with other bacterial infections, however, successful treatment of H. pylori infection depends on the use of antibiotics to which the organism is susceptible. Additionally, the cure rate is also affected by several major factors, such as genotypes of host genetic factors (e.g. CYP2C19 and multidrug-resistant transporter-1 [MDR1]), lifestyle (smoking habits), treatment compliance, duration of eradication therapy, H. pylori virulent factors and gastric emptying.14–17 Moreover, the importance of potent acid inhibition during eradication therapy has recently been demonstrated.18–20
H. pylori virulence factors (e.g. cagA and vacA) play important roles in gastric mucosal inflammation and injury in relation to activated inflammatory cell infiltration.21,22 Activated neutrophils and mononuclear cells infiltrating into gastric mucosa with H. pylori infection produce several pro-inflammatory cytokines (e.g. interleukin [IL]-1β, IL-6, IL-8 and tumor necrosis factor [TNF]-α) and anti-inflammatory cytokines (e.g. IL-4 and IL-10). The cagA-positive, vacA s1 and m1 strains produce significantly higher IL-1β in gastric mucosa compared with that of cagA-negative, vacA s2 and m2 strains.23,24 Interestingly, IL-1β and TNF-α are potent inhibitors of gastric acid secretion.25 Therefore, the increased gastric mucosal production of IL-1β and/or TNF-α in response to H. pylori infection results in a potent suppression of gastric acid secretion, as well as an enhanced gastric mucosal inflammation.26–29
With the abovementioned evidences, it raises the question of whether the cure rates of H. pylori eradication are associated with the pro-inflammatory cytokine polymorphisms.30–33 Therefore, we decided to review the association of H. pylori infection and inflammatory cytokines, the roles of cytokine gene polymorphisms for gastric mucosal changes, and then summarize the efficacy of sufficient acid inhibition during the eradication in relation to inflammatory cytokine gene polymorphisms.
II. Inflammatory cytokine and H. pylori infection
The key physiological event in H. pylori infection is the initiation of a gastric mucosal inflammatory response. H. pylori, its products and many non-microbial agents stimulate transcription and synthesis of inflammatory cytokines, especially in IL-1β and TNF-α. Inflammatory cytokines are soluble peptide molecules that mediate the interaction between immunocompetent and hematopoietic cells, and between the immune and neuroendocrine systems.34 After H. pylori infects the gastric mucosa, neutrophils and mononuclear cells activated by H. pylori infection infiltrate the gastric mucosa, and produce several inflammatory cytokines.35 These cytokines exert their biological effects through binding to specific receptors on target cells.
Of the inflammatory cytokines, IL-1β and TNF-α, which are produced locally in the gastric mucosa, are potent inhibitors of gastric acid secretion.25,36,37 IL-1β is 100 times more potent an inhibitor than a proton pump inhibitor (PPI), and 6000 times more potent than histamine-2 receptor antagonists on a molar basis.25,36 The intracisternal injection of IL-1β induces longstanding inhibition of gastric acid secretion, an effect that is dependent on the integrity of the prostaglandin (PG) pathways, in particular PGE2.38 As a biological mechanism, IL-1β stimulates gastrin release from gastric G cells and histamine release in resting fundic enterochromaffin-like (ECL) cells, whereas pre-incubation of these cells with the cytokine markedly inhibits gastrin-stimulated histamine secretion.39,40 This modulation of the physiological function of G cells and ECL cells by IL-1β can explain the hypergastrinemia and the decreased gastric mucosal histamine levels as observed in H.-pylori-induced gastritis. IL-1β also has profound effects on parietal cells. For example, IL-1β modulates parietal cells transcription of the H,K-ATPase a-subunit gene via an ERK1/2 kinase signal pathway by H. pylori infection. Moreover, in animal studies, decreased acid secretion is reported to be accompanied by an elevation of IL-1β mRNA levels in the H.-pylori-infected gastric mucosa, and the effect is reversed after injection of an IL-1 endogenous receptor antagonist.26 TNF-α is reported to have many pathways of gastric acid inhibition.41 This inhibition occurs at the post-receptor level and involves pertussis toxin and tyrosine kinase dependent and independent pathways. Therefore, the increased production of IL-1β and/or TNF-α in gastric mucosa by H. pylori infection results in enhanced gastric acid suppression.26–29
III. Inflammatory cytokine polymorphism of IL-1β and TNF-α
Most inflammatory cytokine genes have genetic variations, which influence cytokine production into gastric mucosa.
The IL-1B gene has three diallelic polymorphisms at positions −511, −31, and +3954 base pairs (bp) from the transcriptional start site.42 The gene for IL-1 receptor antagonist (IL-RN) has a variable number of identical tandem repeats of 86 bp in length in intron 2.43 The mucosal IL-1β levels significantly differ among different genotypes of IL-1B-511, -31 and IL-RN. Moreover, the carriers of the IL-1B-511 T, -31 C and IL-RN *2 allele have significantly higher IL-1β levels than carriers of the other genotype.35 According to this difference, H.-pylori-infected carriers with the IL-1B-511 T allele have lower levels of gastric acid secretion compared with those with the IL-1B-511 C allele.28 Furuta et al.29 reported that the fasting intragastric pH is 6.5 in H.-pylori-positive patients with the IL-1B-511 T/T genotype and 3.8 in those with the C/T genotype, which is significantly higher than those with the C/C genotype (i.e. pH 2.4). Moreover, in histology, the scores for inflammatory cell infiltration are significantly higher in the carriers of the IL-1B-511 T/T genotype than those of the T/C or C/C genotype.29 A difference also occurred in the score of neutrophil infiltration between IL-1RN*1/*1 and IL-1RN*1/*2 genotypes. Although there is some controversy,44 recent epidemiological studies have revealed that the carriers of the IL-1B-511 T, IL-1B-31 C allele or IL-1RN *2/*2 (2 repeats of 86 bp) genotypes, high producer alleles/genotypes, have an increased risk of gastric atrophy, peptic ulcer and gastric cancer compared with those with the IL-1B-511C, IL-1B-31 T or non-IL-1RN*2 alleles.28,29,35,37,45 The combination of IL-B-511 and IL-1RN genotypes additively increases gastroduodenal disease risks as well as histological gastric mucosal inflammation risks.29 Increased IL-1 levels will theoretically result in enhanced suppression of gastric acid secretion allowing more rapid development of gastric atrophy and an increased risk of developing gastric cancer.
TNF-A encodes TNF-α, and is polymorphic.46,47 The TNF-A-238 G/A and -308 G/A polymorphisms are relevant to different transcriptional intra-individual activities, and these polymorphisms are associated with the development of duodenal ulcers, gastric ulcers and gastric cancers, as observed in various inflammatory and autoimmune diseases in Western populations.46,47 However, these polymorphisms are not found in Asian populations because most of their patients have lower producer alleles, TNF-A-238 G/G and -308 G/G genotypes.48–50 Recently, three polymorphisms of the TNF-A–1031 T/C, -863 C/A, and –857 C/T, which are related to high transcriptional promoter activity, have been identified in Japanese patients.51,52 Concanavalin A-stimulated TNF-α production from peripheral mononuclear cells in subjects with the TNF-A–857/-863/-1031 TAC haplotype is 1.8-fold higher than in subjects with dominant genotypes.51 In fact, TNF-A-857/-863/-1031 TAC genotypes are correlated with rugal hyperplastic gastritis, peptic ulcer and gastric cancer developments.48–50
Thus, on a theoretical basis, the polymorphisms of these inflammatory cytokines, not only IL-1B, IL-1RN and TNF-A, but also IL-853 and IL-1054 might be related to the intraindividual differences in gastric mucosal inflammation and gastric acid inhibition in response to H. pylori infection.
IV. Gastric acid and eradication therapy
Recently, H. pylori eradication rates of the traditional clarithromycin (CAM)-containing triple therapy have steadily been eroded over time largely due to the increasing prevalence of the CAM-resistant strain (resistance rate: more than 20–25%), because of the increasing usage chance of CAM (e.g. chronic obstructive pulmonary diseases and chronic otitis media).55 Therefore, the current recommended therapies that increase the success rates of initial treatments are sequential therapies (sequential administration of a dual therapy PPI plus amoxicillin [AMPC] for 5 days followed by the PPI plus CAM and tinidazole or metronidazole for 5 days), bismuth-containing quadruple therapy and therapy with a PPI plus AMPC, CAM and tinidazole or metronidazole (MNZ) for 7–14 days.56
An insufficient gastric acid inhibition during the eradication treatment also causes eradication failure. CAM and AMPC, which are key antibiotics of the H. pylori eradication regimen, are acid-sensitive, and therefore, the gastric acid secretion must be potently inhibited during the treatment for preventing their degradation under a low pH condition.20 The potent acid inhibition by acid inhibitory drugs makes antibiotics more stable and bioavailable in the stomach and increases the concentration of antibiotics in gastric mucosa.57–59 Raising pH from 3.5 to 5.5 increases the in vitro effectiveness of AMPC more than 10-fold.57 Moreover, acid inhibition allows H. pylori to reach the growth phase and become more sensitive to antibiotics, such as AMPC.59
In dual therapy with omeprazole (OPZ) 20 mg bid and AMPC 1 g bid for 7 days, sustained intragastric pH (> 5.5) levels during eradication therapy are reported to be required for therapeutic success.60 The success rates of triple eradication therapy are also closely related to the degree of acid suppression during treatment irrespective of adding a second antibiotic (Fig. 1a,b).20 In cases with a percentage time of intragastric pH < 4.0 and an average 24-hour intragastric pH less than 10% and more than 6.0, respectively, the majority of patients can overcome H. pylori infection irrespective of their bacterial susceptibility to CAM in triple therapy with lansoprazole (LPZ), CAM and AMPC (Fig. 2).20
Figure 1.
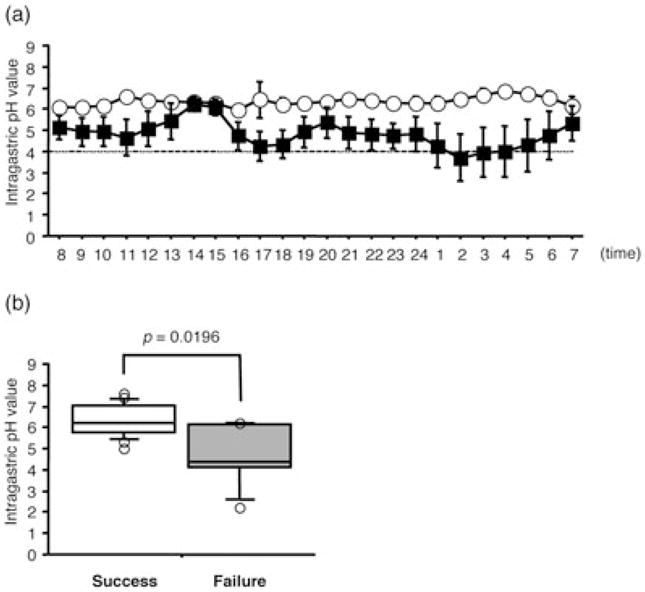
The (a) 24-hour pH-profile and the (b) median 24-hour pH in the patients with success and failure in Helicobacter pylori (H. pylori) eradication.20 In patients with successful cure of H. pylori infection, the median 24-hour pH is significantly higher than that in patients with therapeutic failure (a,b).
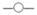 Success;
Success;
 Failure.
Failure.
Figure 2.
The association with 24-hour pH and percent times of pH < 4.0 during the 24-hour dosing period.20 The majority of patients can eradicate Helicobacter pylori (H. pylori) infection with a triple therapy, when the percent time of pH < 4.0 during the 24-hour post-dose period is less than 10% and the 24-hour pH is higher than 6.0 (shaded area). On the other hand, when the percent time of pH < 4.0 is longer than 40% or when the 24-hour pH is lower than 4.4 during treatment (dotted area), the eradication with a triple therapy appears to be quite difficult. ○ Success/clarithromycin (CAM)-sensitive or unknown; ▽ Success/CAM-resistant; ✕ Failure/CAM-sensitive; ▼ Failure/CAM-resistant or unknown.
This raises the question of which regimen of eradication reveals a sufficient acid inhibition throughout a 24-hour period. Recently, Villoria et al.61 reported a meta-analysis asking whether high-dose PPI (2-times-doses twice daily dosing) increase cure rates of triple eradication therapy consisting of a PPI and CAM plus AMPC or tinidazole/MNZ for 7 days. The intention-to-treat cure rate is 82% with the high-dose PPI regimen compared to 74% with standard (relative risk: 1.09; 95% confidence interval (CI): 1.01–1.17). Therefore, the high-dose PPI regime seems more effective than the standard-dose regime, suggesting that increased PPI doses have additive effects.61 However, the cure rate with the high-dose PPI therapy is poor, less than 85%. The half-life of most PPI, OPZ, LPZ and rabeprazole (RPZ), is 2–3 hours, and therefore, PPI are rapidly eliminated from the systemic circulation after 2–3 hours. Then, the H+,K ± ATPase newly generated or activated in gastric parietal cells after rapid elimination of PPI can secrete gastric acid. To overcome this situation, the usefulness of high frequent PPI dosing regimen is being considered, and, in fact, the four-time-daily dosing therapy of PPI plus AMPC is reported to attain high re-eradication rates (96.8–100%).62 High frequent PPI dosing sustains a higher PPI level throughout a 24-hour period and can continue to inactivate H+,K ± ATPase for 24 hours with high frequent PPI, resulting in sufficient acid inhibition.63
Moreover, the effects of PPI depend on metabolic enzyme, cytochrome P450 (CYP) enzymes, and CYP2C19 with genetic differences in the activity of this enzyme.64 In rapid metabolizers (RM) of CYP2C19, PPI are rapidly eliminated from the systemic circulation, and therefore, acid inhibition attained by a PPI in the RM genotype is often insufficient in comparison with poor metabolizers (PM).18,63,65,66 The CYP2C19-related differences in acid inhibition attained influence eradication rates by a PPI-based regimen. The eradication rate by a triple therapy for 1 week in RM is significantly lower in comparison with those attained in PM (72.7% in RM and 97.8% in PM).67 This analysis also supports the theory that potent acid inhibition is required for treatment to be successful.
Therefore, physicians are recommended not to increase the dosing of PPI at one time, but to build an optimal regimen by considering CYP2C19 polymorphisms, a PPI dosing schedule, and other factors influencing gastric acid secretion (e.g. cytokine polymorphism) in each patient as well as antibiotics-resistance. In RM, the intragastric pH value with RPZ 10 mg qid, not 20 mg bid or 40 mg oid, could keep intragastric pH > 4.0 throughout a 24-hour period.63
V. Inflammatory cytokine polymorphism and eradication therapy
In 2003, although Take et al.30 reported the association with the IL-1B-511 polymorphism and eradication therapy for H. pylori infection, there was no significant difference in the cure rates between different IL-1B-511 genotype groups (Table 1). However, the cure rate of 1-week triple OPZ-AMPZ-CAM therapy by different IL-1B-511 genotypes is influenced in CYP2C19 genotype status, especially in the IL-1B-511 C/C genotype. These findings suggest that the IL-1B-511 polymorphism affects eradication therapy through gastric acid inhibition.30
Table 1.
The association with the success rate of Helicobacter pylori eradication therapy and cytokine polymorphisms
| Authors | Year | Number | Regimen | Gene | Genotype | OR | 95%CI | |
|---|---|---|---|---|---|---|---|---|
| Take30 | 2003 | 231 | OAC, LAC, RAC | IL-1B-511 | CT, TT | 138/172 (80%) | 1.00 | |
| CC | 47/59 (80%) | 1.04 | 0.50–2.17 | |||||
| Furuta31 | 2004 | 336 | OAC, LAC | IL-1B-511 | TT | 71/75 (95%) | 1.00 | |
| CT | 147/164 (90%) | 2.05 | 0.67–6.33 | |||||
| CC | 75/97 (77%) | 5.21 | 1.71–15.86 | |||||
| Sugimoto32 | 2006 | 360 | OAC, LAC, RAC | IL-1B-511 | TT | 67/76 (88%) | 1.00 | |
| CT | 164/187 (88%) | 1.04 | 0.46–2.38 | |||||
| CC | 70/97 (72%) | 2.31 | 1.00–5.35 | |||||
| IL-1RN | *2 carrier | 28/37 (76%) | 1.00 | |||||
| Non-*2 | 273/323 (85%) | 0.52 | 0.23–1.17 | |||||
| TNF-A-857 | T carrier | 94/115 (82%) | 1.00 | |||||
| CC | 207/245 (84%) | 0.72 | 0.40–1.31 | |||||
| TNF-A-863 | A carrier | 99/117 (85%) | 1.00 | |||||
| CC | 202/243 (83%) | 0.99 | 0.54–1.82 | |||||
| TNF-A-1031 | C carrier | 99/118 (84%) | 1.00 | |||||
| TT | 202/242 (83%) | 0.91 | 0.50–1.67 | |||||
| IL-10-1082 | G carrier | 65/76 (86%) | 1.00 | |||||
| AA | 236/284 (83%) | 1.08 | 0.53–2.22 | |||||
| IL-10-819 | C carrier | 156/181 (85%) | 1.00 | |||||
| TT | 145/179 (81%) | 1.26 | 0.71–2.24 | |||||
| IL-10-592 | C carrier | 145/167 (87%) | 1.00 | |||||
| AA | 156/193 (81%) | 1.36 | 0.76–2.44 | |||||
| Ishida68 | 2006 | 67 | LAC | IL-1B-31 | CC | 14/14 (100%) | 1.00 | |
| CT | 30/36 (83%) | NA | – | |||||
| TT | 13/17 (77%) | NA | – | |||||
| TNF-A-1031 | CC | 2/2 (100%) | 1.00 | |||||
| T carrier | 55/65 (80%) | NA | – | |||||
| Zambon33 | 2007 | 102 | OAC | IL-10-1082/-892/-592 | ATA/ATA | 8/9 (89%) | 1.00 | |
| Non-ATA/ATA | 49/93 (51%) | 7.18 | 0.86–59.76 | |||||
Although Zambon et al.33 performed IL-1B-31, IL-1RN, IFN-γ+874, TNF-A-238, -308, -376, -857, -1031, IL-12A+6686 and IL-12B+15485 genotyping, there were no detail data.
CI, confidence interval; IL, interleukin; LAC, lansoprazole, amoxicillin and clarithromycin; NA, no analysis; OAC, omeprazole, amoxicillin and clarithromycin; OR, odds ratio; RAC, rabeprazole, amoxicillin and clarithromycin; TNF, tumor necrosis factor.
Furuta et al.31 and Sugimoto et al.32 reported that the IL-1B-511 polymorphism is associated with different acid inhibition levels in response to H. pylori infection as well as H. pylori eradication rates. The eradication rates in patients with IL-1B-511 C/C, C/T and T/T genotypes are 72%, 87% and 88%, respectively (P = 0.0017).32 In patients infected with CAM-resistant strains, the IL-1B-511 T/T genotype significantly increases the eradication rate to 78%, which appears greater than for those with the IL-1B-511 C/C and C/T genotypes (35% and 50%, respectively).31 On the other hand, Ishida et al.68 and Zambon et al.33 reported no significant association with the IL-1B-31 genotypes or with the cure rate for eradication therapy. In a former report by Ishida et al.,68 although the eradication rate is highest for that with the IL-1B-31C/C genotype tightly linked with the IL-1B-511 T/T genotype, the P-value is marginal among the whole subjects. The sample sizes of these two studies are small, and therefore it will likely be difficult to show statistically significant differences among different genotypes.
When the IL-1B-31 T/C polymorphism is displaced in IL-1B-511 C/T, because the polymorphism of IL-1B T-31C is tightly linked with 511 C/T, the IL-1B-511 polymorphisms significantly influence the eradication rates using data of previous reports (Fig. 3).30–33,68 The H. pylori eradication rate in the IL-1B-511 C/C genotype is 77.4% (209/270, 95%CI: 71.9–92.3%), which is significantly lower than IL-1B-511 C/T and T/T genotypes (87.2%, 631/724, 95%CI: 84.5–89.5%, P = 0.0002) (Fig. 3). The risk of failure for eradication therapy in the IL-1B-511 C/C genotype is 1.98 (95%CI: 1.38–2.84, P = 0.0002) times that of the IL-1B-511 C/T and T/T genotypes, and therefore the IL-1B-511 C/C genotype is considered to be a risk factor for eradication failure.
Figure 3.
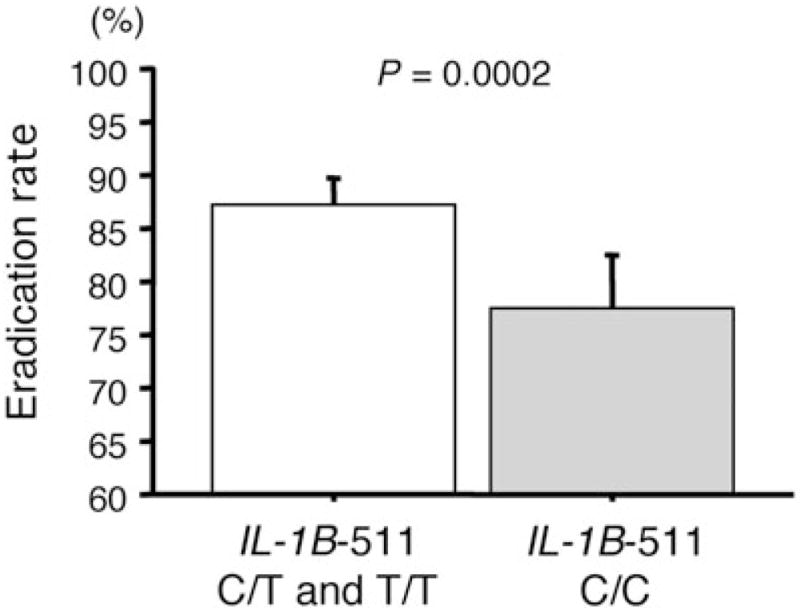
The association with IL-1B-511 polymorphisms and the eradication rates for Helicobacter pylori infection. The eradication rate in IL-1B-511 C/T and T/T genotypes is significantly higher than that in the C/C genotype.
There are no significant relationships between the eradication rates and polymorphisms of TNF-A-857/-863/-1031 and IL-10-1082/-819/-592 (Table 1).32,68 Zambon et al.33 reported that a significant association was found between the IL-10-819 or IL-10-592 polymorphism and eradication success: the IL-10-819 T/T or IL-10-592 A/A genotypes are more frequently found among patients with eradication (13.3%) than among subjects without eradication (1.8%). Moreover, a significant association is found among the cure rate for CAM-sensitive strains and the IL-12B+15485 polymorphism (e.g. C/C genotype: absent for eradicated patients and 13.3% for not eradicated subjects) and TNF-A-238 genotypes.33 However, the sample size of this report is too small and statistical analysis seems to have many mistakes. Anyway, TNF-α also inhibits basal gastric acid and histamine secretions, and gastrin- and carbachol-stimulated acid secretions of gastric parietal cells in a dose-dependent manner.41 IL-10 is a multifunctional anti-inflammatory cytokine that inhibits the production of pro-inflammatory cytokine by inhibition of Th1 lymphocytes and stimulation of B lymphocytes and Th2 lymphocytes.69,70 Although TNF-α potently upregulates the active inflammation of gastric mucosa and IL-10 decreases TNF-α and IL-1 productions in gastric mucosa, the acid inhibition-related effects of TNF-α and IL-10 may not be as potent as that of IL-1β.
H. pylori virulence factors (e.g. cagA and vacA) play important roles in gastric mucosal injury in relation to inflammatory cytokine production.21,22 The cure rates in patients infected with cagA-positive and vacA s1 genotype strains, which enhanced gastric mucosal inflammation, are significantly higher than those with cagA-negative and vacA s2 genotype strains.17 The relationship between the success/failure of H. pylori eradication therapy and virulence factors has been explained by the enhanced gastric mucosal inflammation, resulting in a higher level of inflammatory cytokines. Good correlation between cagA positivity and severe gastric inflammation has been confirmed.71,72 The cagA-positive strains produce significantly higher IL-1β in gastric mucosa compared with that of cagA-negative strains.23,24 Therefore, the cure rate in cagA status and vacA s1 type is considered to be influenced via different cytokine levels in the gastric mucosa, the same as cytokine gene polymorphisms.
VI. Association with CYP2C19 genotype and cytokine polymorphism
The genotypes of CYP2C19 and IL-1B-511 are independent factors for H. pylori eradication therapy. Therefore, patients with CYP2C19 RM and IL-1B-511 C/C genotype are considered to have lower bioavailability of a PPI, as well as a higher potential gastric acid secretion, resulting in the insufficient acid inhibition by a PPI at the standard dose during the eradication therapy. Moreover, in combined analysis of both genotypes of CYP2C19 and IL-1B-511, additive effects for eradication are also being considered.
IL-1B-511 genotype-dependent differences in the cure rates of H. pylori infection are observed only in patients with RM of CYP2C19, but not in those with IM or PM genotype status (Table 2).31,32 In combined analysis of previous reports, the eradication rate is 52% (51/98, 95%CI: 42–62%) for the IL-1B-511 C/C genotype in CYP2C19 RM, which is lower than the IL-1B-511 C/T and T/T genotype (80%, 185/231, 95%CI: 74–85%) (Table 3). Similarly, CYP2C19 genotype-dependent differences in the cure rates of H. pylori infection are observed in patients with the IL-1B-511 C/C genotype.30–32 Take et al. also reported that in the IL-1B-511 C/T and T/T group there was no statistically significant difference in the cure rate among the three CYP2C19 genotype subgroups. However, in the IL-1B-511 C/C group the cure rate among CYP2C19 PM patients (93.3%) was significantly higher than among the CYP2C19 RM (60.0%) and IM patients (63.6%).30 CYP2C19 RM in the IL-1B-511 C/C genotype has an eradication rate of 52%, which is lower than that in the CYP2C19 IM (83%, 110/132, 95%CI: 76–89%) and PM genotype (97%, 31/32, 95%CI: 84–100%) (Table 3). The OR of the CYP2C19 IM-IL-1B-511 C/C type, CYP2C19 RM-IL-1B-511 T/C and T/T type and the CYP2C19 RM-IL-1B-511 C/C type are 2.43, 3.00 and 11.15, respectively, which significantly increases compared with that of CYP2C19 PM-IL-1B-511 T/C and T/T types (Table 3).
Table 2.
The association with IL-IB-511 polymorphism and CYP2C19 genotypes for Helicobacter pylori eradication therapy
| Authors | Number | IL-1B-511 | CYP2C19 RM | IM | PM |
|---|---|---|---|---|---|
| Take30 | 231 | C/T, T/T | 44/61 (72%) | 72/92 (78%) | 22/28 (79%) |
| C/C | 12/20 (60%) | 21/33 (64%) | 14/15 (93%)* | ||
| Furuta31 | 336 | T/T | 20/22 (91%)** | 36/37 (97%) | 31/32 (97%) |
| C/T | 44/56 (79%)** | 77/82 (94%) | 26/26 (100%) | ||
| C/C | 17/35 (49%) | 49/53 (92%)* | 9/9 (100%)* | ||
| Sugimoto32 | 360 | T/T | 27/33 (82%)** | 26/27 (96%) | 14/16 (88%) |
| C/T | 50/59 (85%)** | 86/99 (87%) | 28/29 (97%) | ||
| C/C | 22/43 (51%) | 40/46 (87%)* | 8/8 (100%)* |
P < 0.05 compared with RM genotype of CYP2C19 and
P < 0.05 compared with IL-1B-511 C/C genotype.
Table 3.
The odds risk for eradication failure of Helicobacter pylori infection
| CYP2C19 | IL-1B-511 | Number | Cure rate | OR | 95%CI | P-value |
|---|---|---|---|---|---|---|
| PM | T/T and T/C | 131 | 92.4% | 1.00 | ||
| C/C | 32 | 96.9% | 0.39 | 0.05–3.17 | 0.38 | |
| IM | T/T and T/C | 337 | 88.1% | 1.63 | 0.79–3.36 | 0.18 |
| C/C | 132 | 83.3% | 2.42 | 1.10–5.34 | 0.03 | |
| RM | T/T and T/C | 231 | 80.1% | 3.00 | 1.46–6.19 | < 0.01 |
| C/C | 98 | 52.0% | 11.15 | 5.23–23.78 | < 0.01 |
CI, confidence interval; IM, intermediate metabolizer; OR, odds ratio; PM, poor metabolizer; RM, rapid metabolizer.
Therefore, patients with such combined genotypes (i.e. RM of CYP2C19 plus IL-1B-511 C/C) will require another advanced treatment for a sufficient acid inhibition throughout a 24-hour period, such as treatment with a more frequent PPI dosing (e.g. RPZ 10 mg 4-times-daily)62,63,73 and concomitant therapy of PPI and H2RA.74
VII. Conclusion
We have reviewed H. pylori eradication therapy mainly in relation to the inflammatory cytokine and its polymorphism. There are many genetic factors associated with therapeutic outcomes of H. pylori eradication therapy with a PPI and antimicrobial agents. We would like to recommend the tailored regimens based on the genetic inflammatory cytokine polymorphism the same as relevant pharmacogenomics and microbiological factors (e.g. antibiotic resistance and H. pylori virulent factors).18 However, because the two major factors, antibiotics resistance and CYP2C19 RM, potently influence the success/failure of eradication compared with the genetic inflammatory cytokine polymorphisms, we are not sure that all factors should be determined before the treatment. Moreover, there is genetic variation of cytokine genotypes among different populations, and it is unclear whether this evidence, that the cure rate of eradication is influenced by inflammatory cytokine polymorphisms, is adapted to both the Asian population and Western populations. Therefore, advanced data to evaluate above the hypothesis will be required.
Acknowledgments
Grant support: Grant Number R01 DK62813 from National Institutes of Health (NIH).
We thank Jared Pinkston for his advice and grammatical editing of this paper. This study is supported by Grant Number R01 DK62813 (National Institute of Health).
Abbreviations
- AMPC
amoxicillin
- CAM
clarithromycin
- H. pylori
Helicobacter pylori
- IM
intermediate metabolizer
- LPZ
lansoprazole
- OPZ
omeprazole
- PM
poor metabolizer
- RM
rapid metabolizer
- RPZ
rabeprazole
Footnotes
None of the authors had conflicts of interest related to this study.
References
- 1.Falush D, Wirth T, Linz B, et al. Traces of human migrations in Helicobacter pylori populations. Science. 2003;299:1582–5. doi: 10.1126/science.1080857. [DOI] [PubMed] [Google Scholar]
- 2.Moodley Y, Linz B, Yamaoka Y, et al. The peopling of the Pacific from a bacterial perspective. Science. 2009;323:527–30. doi: 10.1126/science.1166083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yamaoka Y, Orito E, Mizokami M, et al. Helicobacter pylori in North and South America before Columbus. FEBS Lett. 2002;517:180–4. doi: 10.1016/s0014-5793(02)02617-0. [DOI] [PubMed] [Google Scholar]
- 4.Van Doorn LJ, Figueiredo C, Megraud F, et al. Geographic distribution of vacA allelic types of Helicobacter pylori. Gastroenterology. 1999;116:823–30. doi: 10.1016/s0016-5085(99)70065-x. [DOI] [PubMed] [Google Scholar]
- 5.Sugimoto M, Yamaoka Y. The association of vacA genotype and Helicobacter pylori-related disease in Latin American and African populations. Clin Microbiol Infect. 2009;15:835–42. doi: 10.1111/j.1469-0691.2009.02769.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Uemura N, Okamoto S, Yamamoto S, et al. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med. 2001;345:784–9. doi: 10.1056/NEJMoa001999. [DOI] [PubMed] [Google Scholar]
- 7.Wong BC, Lam SK, Wong WM, et al. Helicobacter pylori eradication to prevent gastric cancer in a high-risk region of China: A randomized controlled trial. JAMA. 2004;291:187–94. doi: 10.1001/jama.291.2.187. [DOI] [PubMed] [Google Scholar]
- 8.Take S, Mizuno M, Ishiki K, et al. The effect of eradicating Helicobacter pylori on the development of gastric cancer in patients with peptic ulcer disease. Am J Gastroenterol. 2005;100:1037–42. doi: 10.1111/j.1572-0241.2005.41384.x. [DOI] [PubMed] [Google Scholar]
- 9.Hopkins RJ, Girardi LS, Turney EA. Relationship between Helicobacter pylori eradication and reduced duodenal and gastric ulcer recurrence: A review. Gastroenterology. 1996;110:1244–52. doi: 10.1053/gast.1996.v110.pm8613015. [DOI] [PubMed] [Google Scholar]
- 10.Uemura N, Mukai T, Okamoto S, et al. Effect of Helicobacter pylori eradication on subsequent development of cancer after endoscopic resection of early gastric cancer. Cancer Epidemiol Biomarkers Prev. 1997;6:639–42. [PubMed] [Google Scholar]
- 11.Wotherspoon AC, Doglioni C, de Boni M, Spencer J, Isaacson PG. Antibiotic treatment for low-grade gastric MALT lymphoma. Lancet. 1994;343:1503. [PubMed] [Google Scholar]
- 12.Sugimoto M, Kajimura M, Shirai N, et al. Outcome of radiotherapy for gastric mucosa-associated lymphoid tissue lymphoma refractory to Helicobacter pylori eradication therapy. Intern Med. 2006;45:405–9. doi: 10.2169/internalmedicine.45.1473. [DOI] [PubMed] [Google Scholar]
- 13.Fukase K, Kato M, Kikuchi S, et al. Effect of eradication of Helicobacter pylori on incidence of metachronous gastric carcinoma after endoscopic resection of early gastric cancer: An open-label, randomised controlled trial. Lancet. 2008;372:392–7. doi: 10.1016/S0140-6736(08)61159-9. [DOI] [PubMed] [Google Scholar]
- 14.Furuta T, Ohashi K, Kosuge K, et al. CYP2C19 genotype status and effect of omeprazole on intragastric pH in humans. Clin Pharmacol Ther. 1999;65:552–61. doi: 10.1016/S0009-9236(99)70075-5. [DOI] [PubMed] [Google Scholar]
- 15.Graham DY, Lu H, Yamaoka Y. Therapy for Helicobacter pylori infection can be improved: Sequential therapy and beyond. Drugs. 2008;68:725–36. doi: 10.2165/00003495-200868060-00001. [DOI] [PubMed] [Google Scholar]
- 16.Kamada T, Haruma K, Komoto K, et al. Effect of smoking and histological gastritis severity on the rate of H. pylori eradication with omeprazole, amoxicillin, and clarithromycin. Helicobacter. 1999;4:204–10. doi: 10.1046/j.1523-5378.1999.99299.x. [DOI] [PubMed] [Google Scholar]
- 17.Sugimoto M, Yamaoka Y. Virulence factor genotypes of Helicobacter pylori affect cure rates of eradication therapy. Arch Immunol Ther Exp (Warsz) 2009;57:45–56. doi: 10.1007/s00005-009-0007-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Furuta T, Shirai N, Kodaira M, et al. Pharmacogenomics-based tailored versus standard therapeutic regimen for eradication of. H pylori Clin Pharmacol Ther. 2007;81:521–8. doi: 10.1038/sj.clpt.6100043. [DOI] [PubMed] [Google Scholar]
- 19.Sugimoto M, Furuta T, Shirai N, et al. Treatment strategy to eradicate Helicobacter pylori infection: impact of pharmacogenomics-based acid inhibition regimen and alternative antibiotics. Expert Opin Pharmacother. 2007;8:2701–17. doi: 10.1517/14656566.8.16.2701. [DOI] [PubMed] [Google Scholar]
- 20.Sugimoto M, Furuta T, Shirai N, et al. Evidence that the degree and duration of acid suppression are related to Helicobacter pylori eradication by triple therapy. Helicobacter. 2007;12:317–23. doi: 10.1111/j.1523-5378.2007.00508.x. [DOI] [PubMed] [Google Scholar]
- 21.Broutet N, Tchamgoue S, Pereira E, Lamouliatte H, Salamon R, Megraud F. Risk factors for failure of Helicobacter pylori therapy—results of an individual data analysis of 2751 patients. Aliment Pharmacol Ther. 2003;17:99–109. doi: 10.1046/j.1365-2036.2003.01396.x. [DOI] [PubMed] [Google Scholar]
- 22.van der Hulst RW, Weel JF, Verheul SB, et al. Treatment of Helicobacter pylori infection with low or high dose omeprazole combined with amoxycillin and the effect of early retreatment. Aliment Pharmacol Ther. 1996;10:165–71. doi: 10.1046/j.1365-2036.1996.715895000.x. [DOI] [PubMed] [Google Scholar]
- 23.Yamaoka Y, Kita M, Kodama T, Sawai N, Imanishi J. Helicobacter pylori cagA gene and expression of cytokine messenger RNA in gastric mucosa. Gastroenterology. 1996;110:1744–52. doi: 10.1053/gast.1996.v110.pm8964399. [DOI] [PubMed] [Google Scholar]
- 24.Yamaoka Y, Kodama T, Gutierrez O, Kim JG, Kashima K, Graham DY. Relationship between Helicobacter pylori iceA, cagA, and vacA status and clinical outcome: Studies in four different countries. J Clin Microbiol. 1999;37:2274–9. doi: 10.1128/jcm.37.7.2274-2279.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wolfe MM, Nompleggi DJ. Cytokine inhibition of gastric acid secretion—a little goes a long way. Gastroenterology. 1992;102:2177–8. doi: 10.1016/0016-5085(92)90360-b. [DOI] [PubMed] [Google Scholar]
- 26.Takashima M, Furuta T, Hanai H, Sugimura H, Kaneko E. Effects of Helicobacter pylori infection on gastric acid secretion and serum gastrin levels in Mongolian gerbils. Gut. 2001;48:765–73. doi: 10.1136/gut.48.6.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang M, Furuta T, Takashima M, et al. Relation between interleukin-1beta messenger RNA in gastric fundic mucosa and gastric juice pH in patients infected with Helicobacter pylori. J Gastroenterol. 1999;34 (Suppl 11):10–17. [PubMed] [Google Scholar]
- 28.Furuta T, Shirai N, Takashima M, Xiao F, Sugimura H. Effect of genotypic differences in interleukin-1 beta on gastric acid secretion in Japanese patients infected with Helicobacter pylori. Am J Med. 2002;112:141–3. doi: 10.1016/s0002-9343(01)01036-1. [DOI] [PubMed] [Google Scholar]
- 29.Furuta T, El-Omar EM, Xiao F, et al. Interleukin 1beta polymorphisms increase risk of hypochlorhydria and atrophic gastritis and reduce risk of duodenal ulcer recurrence in Japan. Gastroenterology. 2002;123:92–105. doi: 10.1053/gast.2002.34156. [DOI] [PubMed] [Google Scholar]
- 30.Take S, Mizuno M, Ishiki K, et al. Interleukin-1beta genetic polymorphism influences the effect of cytochrome P 2C19 genotype on the cure rate of 1-week triple therapy for Helicobacter pylori infection. Am J Gastroenterol. 2003;98:2403–8. doi: 10.1111/j.1572-0241.2003.07707.x. [DOI] [PubMed] [Google Scholar]
- 31.Furuta T, Shirai N, Xiao F, et al. Polymorphism of interleukin-1beta affects the eradication rates of Helicobacter pylori by triple therapy. Clin Gastroenterol Hepatol. 2004;2:22–30. doi: 10.1016/s1542-3565(03)00288-x. [DOI] [PubMed] [Google Scholar]
- 32.Sugimoto M, Furuta T, Shirai N, Ikuma M, Hishida A, Ishizaki T. Influences of proinflammatory and anti-inflammatory cytokine polymorphisms on eradication rates of clarithromycin-sensitive strains of Helicobacter pylori by triple therapy. Clin Pharmacol Ther. 2006;80:41–50. doi: 10.1016/j.clpt.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 33.Zambon CF, Fasolo M, Basso D, et al. Clarithromycin resistance, tumor necrosis factor alpha gene polymorphism and mucosal inflammation affect H. pylori eradication success. J Gastrointest Surg. 2007;11:1506–14. doi: 10.1007/s11605-007-0246-4. discussion 1514. [DOI] [PubMed] [Google Scholar]
- 34.Fridman WH, Tartour E. Cytokines and cell regulation. Mol Aspects Med. 1997;18:3–90. doi: 10.1016/s0098-2997(96)00012-x. [DOI] [PubMed] [Google Scholar]
- 35.Hwang IR, Kodama T, Kikuchi S, et al. Effect of interleukin 1 polymorphisms on gastric mucosal interleukin 1beta production in Helicobacter pylori infection. Gastroenterology. 2002;123:1793–803. doi: 10.1053/gast.2002.37043. [DOI] [PubMed] [Google Scholar]
- 36.Kondo S, Shinomura Y, Kanayama S, et al. Interleukin-1 beta inhibits gastric histamine secretion and synthesis in the rat. Am J Physiol. 1994;267:G966–71. doi: 10.1152/ajpgi.1994.267.6.G966. [DOI] [PubMed] [Google Scholar]
- 37.El-Omar EM, Carrington M, Chow WH, et al. Interleukin-1 polymorphisms associated with increased risk of gastric cancer. Nature. 2000;404:398–402. doi: 10.1038/35006081. [DOI] [PubMed] [Google Scholar]
- 38.Saperas ES, Yang H, Rivier C, Tache Y. Central action of recombinant interleukin-1 to inhibit acid secretion in rats. Gastroenterology. 1990;99:1599–606. doi: 10.1016/0016-5085(90)90463-b. [DOI] [PubMed] [Google Scholar]
- 39.Weigert N, Schaffer K, Schusdziarra V, Classen M, Schepp W. Gastrin secretion from primary cultures of rabbit antral G cells: Stimulation by inflammatory cytokines. Gastroenterology. 1996;110:147–54. doi: 10.1053/gast.1996.v110.pm8536851. [DOI] [PubMed] [Google Scholar]
- 40.Prinz C, Neumayer N, Mahr S, Classen M, Schepp W. Functional impairment of rat enterochromaffin-like cells by interleukin 1 beta. Gastroenterology. 1997;112:364–75. doi: 10.1053/gast.1997.v112.pm9024290. [DOI] [PubMed] [Google Scholar]
- 41.Beales IL, Calam J. Interleukin 1 beta and tumour necrosis factor alpha inhibit acid secretion in cultured rabbit parietal cells by multiple pathways. Gut. 1998;42:227–34. doi: 10.1136/gut.42.2.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stokkers PC, van Aken BE, Basoski N, Reitsma PH, Tytgat GN, van Deventer SJ. Five genetic markers in the interleukin 1 family in relation to inflammatory bowel disease. Gut. 1998;43:33–9. doi: 10.1136/gut.43.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tarlow JK, Blakemore AI, Lennard A, et al. Polymorphism in human IL-1 receptor antagonist gene intron 2 is caused by variable numbers of an 86-bp tandem repeat. Hum Genet. 1993;91:403–4. doi: 10.1007/BF00217368. [DOI] [PubMed] [Google Scholar]
- 44.Kato S, Onda M, Yamada S, Matsuda N, Tokunaga A, Matsukura N. Association of the interleukin-1 beta genetic polymorphism and gastric cancer risk in Japanese. J Gastroenterol. 2001;36:696–9. doi: 10.1007/s005350170033. [DOI] [PubMed] [Google Scholar]
- 45.Machado JC, Pharoah P, Sousa S, et al. Interleukin 1B and interleukin 1RN polymorphisms are associated with increased risk of gastric carcinoma. Gastroenterology. 2001;121:823–9. doi: 10.1053/gast.2001.28000. [DOI] [PubMed] [Google Scholar]
- 46.Kroeger KM, Carville KS, Abraham LJ. The -308 tumor necrosis factor-alpha promoter polymorphism effects transcription. Mol Immunol. 1997;34:391–9. doi: 10.1016/s0161-5890(97)00052-7. [DOI] [PubMed] [Google Scholar]
- 47.Negoro K, Kinouchi Y, Hiwatashi N, et al. Crohn’s disease is associated with novel polymorphisms in the 5′-flanking region of the tumor necrosis factor gene. Gastroenterology. 1999;117:1062–8. doi: 10.1016/s0016-5085(99)70390-2. [DOI] [PubMed] [Google Scholar]
- 48.Sugimoto M, Furuta T, Shirai N, et al. Different effects of polymorphisms of tumor necrosis factor-alpha and interleukin-1 beta on development of peptic ulcer and gastric cancer. J Gastroenterol Hepatol. 2007;22:51–9. doi: 10.1111/j.1440-1746.2006.04442.x. [DOI] [PubMed] [Google Scholar]
- 49.Ohyama I, Ohmiya N, Niwa Y, et al. The association between tumour necrosis factor-alpha gene polymorphism and the susceptibility to rugal hyperplastic gastritis and gastric carcinoma. Eur J Gastroenterol Hepatol. 2004;16:693–700. doi: 10.1097/01.meg.0000108315.52416.bf. [DOI] [PubMed] [Google Scholar]
- 50.Zambon CF, Basso D, Navaglia F, et al. Pro- and anti-inflammatory cytokines gene polymorphisms and Helicobacter pylori infection: interactions influence outcome. Cytokine. 2005;29:141–52. doi: 10.1016/j.cyto.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 51.Higuchi T, Seki N, Kamizono S, et al. Polymorphism of the 5′-flanking region of the human tumor necrosis factor (TNF)-alpha gene in Japanese. Tissue Antigens. 1998;51:605–12. doi: 10.1111/j.1399-0039.1998.tb03002.x. [DOI] [PubMed] [Google Scholar]
- 52.Soga Y, Nishimura F, Ohyama H, Maeda H, Takashiba S, Murayama Y. Tumor necrosis factor-alpha gene (TNF-alpha) -1031/-863, -857 single-nucleotide polymorphisms (SNPs) are associated with severe adult periodontitis in Japanese. J Clin Periodontol. 2003;30:524–31. doi: 10.1034/j.1600-051x.2003.00287.x. [DOI] [PubMed] [Google Scholar]
- 53.Taguchi A, Ohmiya N, Shirai K, et al. Interleukin-8 promoter polymorphism increases the risk of atrophic gastritis and gastric cancer in Japan. Cancer Epidemiol Biomarkers Prev. 2005;14:2487–93. doi: 10.1158/1055-9965.EPI-05-0326. [DOI] [PubMed] [Google Scholar]
- 54.El-Omar EM, Rabkin CS, Gammon MD, et al. Increased risk of noncardia gastric cancer associated with proinflammatory cytokine gene polymorphisms. Gastroenterology. 2003;124:1193–201. doi: 10.1016/s0016-5085(03)00157-4. [DOI] [PubMed] [Google Scholar]
- 55.Graham DY, Lu H, Yamaoka Y. A report card to grade Helicobacter pylori therapy. Helicobacter. 2007;12:275–8. doi: 10.1111/j.1523-5378.2007.00518.x. [DOI] [PubMed] [Google Scholar]
- 56.Graham DY, Shiotani A. New concepts of resistance in the treatment of Helicobacter pylori infections. Nat Clin Pract Gastroenterol Hepatol. 2008;5:321–31. doi: 10.1038/ncpgasthep1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Grayson ML, Eliopoulos GM, Ferraro MJ, Moellering RC., Jr Effect of varying pH on the susceptibility of Campylobacter pylori to antimicrobial agents. Eur J Clin Microbiol Infect Dis. 1989;8:888–9. doi: 10.1007/BF01963775. [DOI] [PubMed] [Google Scholar]
- 58.Goddard AF, Jessa MJ, Barrett DA, et al. Effect of omeprazole on the distribution of metronidazole, amoxicillin, and clarithromycin in human gastric juice. Gastroenterology. 1996;111:358–67. doi: 10.1053/gast.1996.v111.pm8690200. [DOI] [PubMed] [Google Scholar]
- 59.Scott D, Weeks D, Melchers K, Sachs G. The life and death of Helicobacter pylori. Gut. 1998;43 (Suppl 1):S56–60. doi: 10.1136/gut.43.2008.s56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Labenz J, Stolte M, Blum AL, et al. Intragastric acidity as a predictor of the success of Helicobacter pylori eradication: A study in peptic ulcer patients with omeprazole and amoxicillin. Gut. 1995;37:39–43. doi: 10.1136/gut.37.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Villoria A, Garcia P, Calvet X, Gisbert JP, Vergara M. Meta-analysis: High-dose proton pump inhibitors vs. standard dose in triple therapy for Helicobacter pylori eradication. Aliment Pharmacol Ther. 2008;28:868–77. doi: 10.1111/j.1365-2036.2008.03807.x. [DOI] [PubMed] [Google Scholar]
- 62.Furuta T, Shirai N, Xiao F, et al. High-dose rabeprazole/amoxicillin therapy as the second-line regimen after failure to eradicate H. pylori by triple therapy with the usual doses of a proton pump inhibitor, clarithromycin and amoxicillin. Hepatogastroenterology. 2003;50:2274–8. [PubMed] [Google Scholar]
- 63.Sugimoto M, Furuta T, Shirai N, et al. Different dosage regimens of rabeprazole for nocturnal gastric acid inhibition in relation to cytochrome P450 2C19 genotype status. Clin Pharmacol Ther. 2004;76:290–301. doi: 10.1016/j.clpt.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 64.Ishizaki T, Horai Y. Review article: Cytochrome P450 and the metabolism of proton pump inhibitors—emphasis on rabeprazole. Aliment Pharmacol Ther. 1999;13 (Suppl 3):27–36. doi: 10.1046/j.1365-2036.1999.00022.x. [DOI] [PubMed] [Google Scholar]
- 65.Shirai N, Furuta T, Moriyama Y, et al. Effects of CYP2C19 genotypic differences in the metabolism of omeprazole and rabeprazole on intragastric pH. Aliment Pharmacol Ther. 2001;15:1929–37. doi: 10.1046/j.1365-2036.2001.01108.x. [DOI] [PubMed] [Google Scholar]
- 66.Shirai N, Furuta T, Xiao F, et al. Comparison of lansoprazole and famotidine for gastric acid inhibition during the daytime and night-time in different CYP2C19 genotype groups. Aliment Pharmacol Ther. 2002;16:837–46. doi: 10.1046/j.1365-2036.2002.01229.x. [DOI] [PubMed] [Google Scholar]
- 67.Furuta T, Shirai N, Takashima M, et al. Effect of genotypic differences in CYP2C19 on cure rates for Helicobacter pylori infection by triple therapy with a proton pump inhibitor, amoxicillin, and clarithromycin. Clin Pharmacol Ther. 2001;69:158–68. doi: 10.1067/mcp.2001.113959. [DOI] [PubMed] [Google Scholar]
- 68.Ishida Y, Goto Y, Kondo T, et al. Eradication rate of Helicobacter pylori according to genotypes of CYP2C19, IL-1B, and TNF-A. Int J Med Sci. 2006;3:135–40. doi: 10.7150/ijms.3.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.de Waal Malefyt R, Abrams J, Bennett B, Figdor CG, de Vries JE. Interleukin 10(IL-10) inhibits cytokine synthesis by human monocytes: An autoregulatory role of IL-10 produced by monocytes. J Exp Med. 1991;174:1209–20. doi: 10.1084/jem.174.5.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nelson DR, Lauwers GY, Lau JY, Davis GL. Interleukin 10 treatment reduces fibrosis in patients with chronic hepatitis C: A pilot trial of interferon nonresponders. Gastroenterology. 2000;118:655–60. doi: 10.1016/s0016-5085(00)70134-x. [DOI] [PubMed] [Google Scholar]
- 71.van der Hulst RW, van der Ende A, Dekker FW, et al. Effect of Helicobacter pylori eradication on gastritis in relation to cagA: A prospective 1-year follow-up study. Gastroenterology. 1997;113:25–30. doi: 10.1016/s0016-5085(97)70076-3. [DOI] [PubMed] [Google Scholar]
- 72.De Francesco V, Zullo A, Margiotta M, et al. Sequential treatment for Helicobacter pylori does not share the risk factors of triple therapy failure. Aliment Pharmacol Ther. 2004;19:407–14. doi: 10.1046/j.1365-2036.2004.01818.x. [DOI] [PubMed] [Google Scholar]
- 73.Bayerdorffer E, Miehlke S, Mannes GA, et al. Double-blind trial of omeprazole and amoxicillin to cure Helicobacter pylori infection in patients with duodenal ulcers. Gastroenterology. 1995;108:1412–17. doi: 10.1016/0016-5085(95)90689-4. [DOI] [PubMed] [Google Scholar]
- 74.Okudaira K, Furuta T, Shirai N, Sugimoto M, Miura S. Concomitant dosing of famotidine with a triple therapy increases the cure rates of Helicobacter pylori infections in patients with the homozygous extensive metabolizer genotype of CYP2C19. Aliment Pharmacol Ther. 2005;21:491–7. doi: 10.1111/j.1365-2036.2005.02353.x. [DOI] [PubMed] [Google Scholar]


