Abstract
Objectives
To explore the percentage enhancement wash-out ratio (PEW) and relative PEW (RPEW) of low-dose multi-phasic computed tomography (CT) in distinguishing benign from malignant parotid gland tumours.
Methods
This study was approved by the ethics committee, and informed patient consent was obtained. 51 patients with parotid tumours proven by histopathology received CT, including 18 with pleomorphic adenomas, 14 with Warthin’s tumours and 19 with malignant tumours. Size and attenuation of parotid tumours were measured. Compared with 5-min attenuation, the 30-s and 90-s PEW (PEW30, PEW90) and RPEW (RPEW30, RPEW90) were calculated.
Results
There was a significant difference in PEW30, RPEW30, PEW90 and RPEW90 in the parotid neoplasms groups (P < 0.01), and statistical significance existed simultaneously in pleomorphic adenomas vs malignant tumours and Warthin’s tumours vs malignant tumours according to SNK-q test. The optimal diagnosis results of malignancy with 100% specificity (32/32) was obtained by using a combination of the following criteria: −70% > PEW30 < 36%, −30% > PEW30 < 19%, PEW90 > 12%, and the sensitivity (74%) for diagnosis of malignancy was yield.
Conclusions
Wash-out ratio may assist in differentiating the benign from malignant parotid gland tumours. Combining the percentage of enhanced wash-out ratios of CT protocols can yield diagnostic results for malignancy.
Keywords: Low-dose CT·parotid gland neoplasm, Dynamic contrast-enhanced CT, Percentage enhancement wash-out ratio
Introduction
Salivary gland neoplasms account for approximately 3% of all head and neck tumours. About 70% of all salivary gland neoplasms occur in the parotid gland [1, 2]. Several studies [3–6] have investigated the value of computed tomography (CT) in distinguishing parotid gland tumours. The results of some documents have demonstrated that CT could identify patients with pleomorphic adenomas, Warthin’s tumours and malignant tumours, and evaluate the extent of parotid gland tumours. Moreover, detailed information on signal intensity and wash-out ratio in parotid gland tumours by contrast-enhanced MRI has been reported for pleomorphic adenomas, Warthin’s tumours, and malignant tumours [7–10]. To our knowledge, very few data on the percentage enhanced wash-out ratio in low-dose multiple-phasic CT among pleomorphic adenomas, Warthin’s tumours and malignant tumours have already been analysed. Consequently, this study focused on strategies to differentiate benign from malignant neoplasms by measuring the percentage enhanced washout ratio of patients with parotid gland tumours.
Materials and methods
Study cohort
This study was approved by our institutional review board, and informed patient consent was obtained before study participation according to institutional and native guidelines. Between August 2002 and December 2009, the 51 patients (29 men and 22 women aged 25–68 years; mean age, 44 ± 8.5 years) fulfilled the criteria for inclusion. 18 patients (13 men and 5 women aged 26–58 years; mean age, 41 ± 7.4 years) had pleomorphic adenomas, and 14 (6 men and 8 women aged 33–78 years; mean age, 55 ± 9.1 years) had Warthin’s tumours. 19 patients (11 men and 8 women aged 25–68 years; mean age, 46 ± 8.6 years) had malignancies, including adenoid cystic carcinoma (n = 6), mucoepidermoid carcinoma (n = 4), carcinoma ex pleomorphic adenoma (n = 2), ductal carcinoma (n = 2), acinic cell carcinoma (n = 1), sebaceous carcinoma (n = 1), lymphoma (n = 2) and squamous carcinoma (n = 1). Diagnoses for 51 patients with parotid gland neoplasms were established when histological proof was obtained at surgery.
Imaging protocol
All patients underwent multi-detector CT (LightSpeed ultra; GE Medical Systems, USA). All patients gave written informed consent to undergo CT. To reduce the radiation doses and preserve the diagnostic quality of the imaging, the protocol for the helical CT examinations performed in 51 patients with acquisition parameters: 5-mm slice, a 7.5 mm/s table speed, 120 kVp, 60 mA, a 0.8-s rotation time, a pitch of 0.625, and 5-mm reconstruction intervals before and after the intravenous bolus injection of contrast material (Ultravist 300, Schering, Germany; 300 mg of iodine per millilitre). Imaging began at the skull base and continued to the thyroid cartilage. To optimise the reproducibility of the starting measurements, each image was obtained while the patient held their breath and stopped deglutition.
First, a non-enhanced image was obtained through the parotid gland. An 18- or 20-gauge intravenous catheter was placed in an antecubital vein and manually tested by rapidly infusing 10 ml of saline. Subsequently, 80–120 ml of non-ionic contrast material (1.5 ml per kg) was administered at 2 ml/s by using a power injector (Medrad, Warrendale, PA, USA). In all patients, the second, third, and fourth images were obtained 30 s, 90 s, and 5 min after the start of the contrast material injection respectively. The imaging protocol was preprogrammed so that these images were obtained by using the same parameters that were used to obtain the non-enhanced images. The images were obtained with standard soft-tissue settings (window width, 300 HU; window level, 40 HU).
Image and data analyses
The CT were interpreted and values of diagnostic parameters were measured by using Advantage Windows 4.2 (GE Medical systems, USA). These tasks were performed by two radiologists who were experienced in performing CT of the parotid glands. Two radiologists had no knowledge of the clinical or histological findings, and they worked independently without consultation with one another.
To determine the size of the masses, a distance cursor was used to measure the diameter in the transverse plane. The section of the largest square area of the parotid gland mass was used for analysis. The measurements obtained by the two radiologists were averaged.
The attenuation values of all parotid gland masses detected at CT were measured by using circular region-of-interest (ROI) cursors placed over the area of disease (the biggest mass was selected if multiple lesions were present). The ROI circle was made as large as possible with sufficient margin to avoid partial volume effects. Cystic, necrotic and haemorrhagic components, as well as calcifications of the parotid gland masses, were excluded if they were present. Necrosis was defined as a region (within the mass) with an attenuation value similar to that of water (−20HU ~ 20HU) on the non-enhanced CT. Calcification was defined as a region with an attenuation value greater than 120 HU on non-enhanced CT. Attenuation values were recorded and averaged for final data analysis.
We calculated the following diagnostic parameters for all masses: the absolute percentage enhanced wash-out ratio (PEW) was calculated as 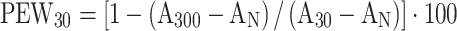 or
or 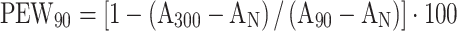 , and the relative percentage enhanced wash-out ratio (RPEW30 or RPEW90) was calculated as follows:
, and the relative percentage enhanced wash-out ratio (RPEW30 or RPEW90) was calculated as follows: 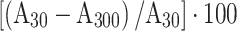 or
or 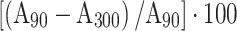 , where AN is the attenuation on non-enhanced CT, A
30 or A
90 is 30 or 90-s enhanced attenuation, and A
300 is 5-min enhanced attenuation.
, where AN is the attenuation on non-enhanced CT, A
30 or A
90 is 30 or 90-s enhanced attenuation, and A
300 is 5-min enhanced attenuation.
Statistical analyses
Statistical analyses were performed with software (SPSS 13.0; Chicago, IL, USA). Primary statistical analysis of the pooled data (mean±standard deviations) was performed with the analysis of variance (ANOVA) to determine mean differences in objective measurements of parotid gland tumour groups by helical CT, followed by Student-Newman-Keuls –q (SNK-q) test for multiple comparisons. P < 0.01 was considered to indicate statistical significance. Receiver operating characteristic (ROC) analysis was employed to investigate the differentiation capability of the diagnostic parameters value for distinguishing malignant from benign parotid tumours when significant differences existed simultaneously in the malignant tumours versus pleomorphic adenoma groups and malignant tumour versus Warthin’s tumour groups. The optimal threshold value, determined by the maximal Youden index defined as sensitivity plus specificity minus 1, was selected and the area under the ROC (AUC) was calculated. Then, corresponding sensitivity and specificity were calculated for individual parameters. In addition, we examined whether the diagnostic accuracy could be improved by combining these individual diagnostic parameters.
Results
Mass size
The biggest range of pleomorphic adenoma diameters was from 0.8 cm to 8.1 cm, compared with those of Warthin’s tumours (2.2–3.3 cm) and malignant tumours (1.9–4.5 cm). There were no significant differences in mean diameters among the pleomorphic adenoma, Warthin’s tumour and malignant tumour groups (P = 0.41).
Non-enhanced CT, dual contrast-enhanced CT and 5-minute delay contrast-enhanced CT
The means and the ranges of attenuation of different groups of parotid gland tumours are listed in Table 1. There were no significant differences among the mean attenuations of pleomorphic adenomas, Warthin’s tumours and malignant tumours on non-enhanced CT (P = 0.047) and the fourth CT (P = 0.21). Although significant differences were found in the attenuation of the pleomorphic adenomas, Warthin’s tumours and malignant tumours groups (P < 0.01) on the second and the third CT, there were simultaneously no differences between pleomorphic adenomas vs malignant tumours and Warthin’s tumours vs malignant tumours by SNK-q test.
Table 1.
CT parameters for each group and statistical results of ANOVA
| CT Parameters | Pleomorphic adenomas (n = 18) | Warthin’s tumours (n = 14) | Malignant tumours (n = 19) | P value* |
|---|---|---|---|---|
| Non-enhanced attenuation (HU) | 37 ± 6.3(28–46) | 42 ± 5.6(33–49) | 38 ± 5(32–48) | 0.047 |
| 30-s enhanced attenuation (HU) | 57 ± 11 (32–75) | 93 ± 22(38–120) | 69 ± 10(52–88) | <0.01 |
| 90-s enhanced attenuation (HU) | 69 ± 15(31–95) | 80 ± 16(37–105) | 88 ± 18(56–126) | <0.01 |
| 5-min enhanced attenuation (HU) | 79 ± 18(30–102) | 69 ± 13(35–87) | 76 ± 13(49–101) | 0.21 |
Data are mean ± standard deviations, with ranges in parentheses
The P value levels are for comparison among pleomorphic adenomas, Warthin’s tumours and malignant tumours groups by using the analysis of variance (P < 0.01)
Sixty-two masses in 51 patients were detected by the identical imaging protocol, including 18 pleomorphic adenomas (n = 18), 18 Warthin’s tumours (n = 14) and 26 malignant tumours (n = 19). Calcifications were found in two pleomorphic adenomas, but not in any of the Warthin’s tumours or malignant tumours. 8 of the 14 Warthin’s tumours, 6 of the 18 pleomorphic adenomas and 4 of the 19 malignant tumours had regions of necrosis.
Diagnostic ratios
Diagnostic ratios of parotid gland neoplasms are listed in Table 2. There were significant differences in PEW30 and RPEW30 among these parotid gland tumour groups (P < 0.01). By using a threshold value of −70% PEW30 and −30% RPEW30 between pleomorphic adenomas and malignant tumours, the sensitivity could reach up to 89% and 89% respectively, but the specificity was low for malignant tumours. At the threshold value of 36% PEW30 and 19% RPEW30 between Warthin’s tumours and malignant tumours, the sensitivity-specificity ratios were 100%:93% and 100%:86% respectively.
Table 2.
Diagnostic ratios for each group and statistical results of ANOVA
| CT Parameters | Pleomorphic adenomas (n = 18) | Warthin’s tumours (n = 14) | Malignant tumours (n = 19) | P value* |
|---|---|---|---|---|
| PEW30 (%) | −188 ± 341(−1400–67) | 49 ± 11(29–75) | −30 ± 46(−121–33) | <0.01 |
| RPEW30 (%) | −38 ± 32(−93–18) | 24 ± 8(8–39) | −11 ± 18(−51–18) | <0.01 |
| PEW90 (%) | −39 ± 98(−411–50) | 32 ± 13(12–67) | 26 ± 10(3–47) | <0.01 |
| RPEW90 (%) | −14 ± 21(−80–9) | 13 ± 5(5–20) | 14 ± 1(1–24) | <0.01 |
Data are mean ± standard deviations, with ranges in parentheses
The P value levels are for comparison among pleomorphic adenomas, Warthin’s tumours, and malignant tumours groups by using the analysis of variance (P < 0.01)
There were significant differences in PEW90 and RPEW90 among the three parotid gland tumour groups. Applying a threshold value of 12% PEW90 and 4% RPEW90 between pleomorphic adenomas and malignant tumours, the ratios of sensitivity to specificity were 95/89% and 95/94%. At the cut-off value of 31% PEW90 between Warthin’s tumours and malignant tumours, the ratio of sensitivity-specificity was 63/71%. The optimal threshold values of RPEW90 were not obtained between the Warthin’s tumours and malignant tumours (Table 3).
Table 3.
ROC analysis of percentage enhanced wash-out ratios of pleomorphic adenomas (n = 18), Warthin’s tumours (n = 14), and malignant tumours (n = 19) of the parotid gland
| Parameters | AUCa | Optimal cut-off value | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|
| Differentiation between pleomorphic adenomas and malignant tumoursb | ||||
| PEW30 (%) | 0.75(0.58–0.91) | −70 | 89(17/19) | 56(10/18) |
| RPEW30 (%) | 0.76(0.60–0.92) | −30 | 89(17/19) | 61(11/18) |
| PEW90 (%) | 0.92(0.82–1.00) | 12 | 95(18/19) | 89(16/18) |
| RPEW90 (%) | 0.98(0.94−1.00) | 4 | 95(18/19) | 94(17/18) |
| Differentiation between Warthin’s tumours and malignant tumoursb | ||||
| PEW30 (%) | 0.99(0.98–1.00) | 36 | 100(19/19) | 93(13/14) |
| RPEW30 (%) | 0.98(0.95–1.00) | 19 | 100(19/19) | 86(12/14) |
| PEW90 (%) | 0.64(0.45–0.84) | 31 | 63(12/19) | 71(10/14) |
| RPEW90 (%) | 0.47(0.27–0.68) | – | – | – |
aData in parentheses are 95% confidence intervals
bSensitivity and specificity in the identification of malignant tumours. Data in parentheses are numbers used to calculate percentages
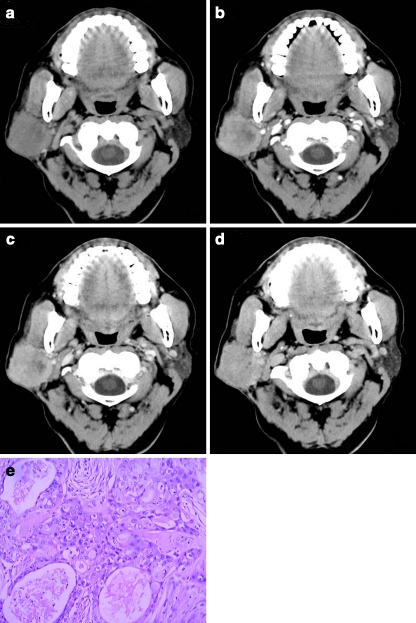
To obtain 100% specificity and maximal sensitivity in malignant gland tumours by using the combination of PEW30, RPEW30 and PEW90 (Table 4), we obtained the threshold value criteria as follows: −70% > PEW30 < 36%, −30% > PEW30 < 19%, PEW90 > 12%, and 74% sensitivity was yielded (Fig. 1a–e).
Table 4.
Best diagnostic values for percentage enhanced wash-out ratios for differentiating benign from malignant tumours of the parotid gland
| Parameter | Threshold criterion | Parotid gland tumours | |
|---|---|---|---|
| Sensitivity (%) | Specificity (%) | ||
| PEW30 (%) | −70> and <36 | 89(17/19) | 78(25/32) |
| RPEW30 (%) | −30> and <19 | 89(17/19) | 69(22/32) |
| PEW90 (%) | >12 | 84(16/19) | 72(23/32) |
| Combined threshold values | –- | 74(14/19) | 100(32/32) |
Sensitivity and specificity in the identification of malignant tumours. Data in parentheses are numbers used to calculate percentages
Fig. 1.
a–e Transverse CT obtained in a 65-year-old man with mucoepidermoid carcinoma. a Non-enhanced CT obtained at level of the right parotid gland shows ill-defined hypo-attenuation mass relative to the jugomaxillary muscle. 30-s and 90-s contrast enhancement, 5-min delay (b, c, d) obtained at the same level as in a. The tumour has heterogeneous enhancement on the 30-s (b), 90-s (c) and the 5-min delay phase (d) images. Tumour attenuation had 39.2 HU in a, 68.4 HU in b, 76.7 HU in c, and 67.4 HU in d. Thus, the absolute percentage enhanced wash-out ratio of malignant tumours is 3.4% of PEW30 and 24.8% of PEW90, and the relative percentage enhanced wash-out ratio of the malignancy is 1.5% of RPEW30 and 12.1% of RPEW90. e Surgical histopathology revealed mucoepidermoid carcinoma; H&E × 100
Discussion
Parotid gland masses are common: about 80% of parotid tumours are benign, and most of them, about 60%, are pleomorphic adenoma, followed by Warthin’s tumour (about 10%). Malignant tumours comprise about 15 to 30% of all kinds of parotid tumours. The most common parotid gland malignancy is mucoepidermoid carcinoma [11]. The cystic adenoid carcinoma is the second most frequent malignancy; other parotid tumours include acinar cell carcinomas, adenocarcinoma not otherwise specified (NOS), lymphoma, and the carcinoma ex pleomorphic adenoma [12].
The main symptom in patients with parotid neoplasms is a lump in the parotid gland area. Other symptoms such as pain, facial palsy and skin ulcers may emerge in malignancies. According to the extent of the tumour, partial or total parotidectomy is the mainstream treatment measure for benign and malignant parotid tumours. Radiotherapy may aid in malignant cases as adjuvant therapy; chemotherapy is rarely applied. Parotid gland neoplasm recurrence rates of local, regional and distant are 40%, 15% and 11%, respectively. Patients with recurrence have a poor prognosis [13].
The use of CT and MR imaging has resulted in the detection of parotid gland masses. More recent literature [3–10, 14, 15] has focused on different approaches to characterising parotid masses, including assessing lesion size, describing morphological criteria, measuring attenuation or signal intensity, calculating the contrast material change of single or dual enhancement. Kress et al. [14] have demonstrated that CT sialography is dispensable in the diagnosis of suspected malignant tumours. Casselman and Mancuso [15] reported that CT and MR imaging were the first choices for providing the same diagnostic information in all cases of parotid gland neoplasms. Choi et al. [4] demonstrated that the ratio of CT numbers in enhancement patterns and the attenuation change was significantly different between Warthin’s tumours and pleomorphic adenomas, and between Warthin’s tumours and malignant tumours. Yabuuchi et al. [7] showed that parotid neoplasms could be differentiated by the 120 s of MR time of peak enhancement and a 30% wash-out ratio (WR), and benign and malignant tumours were recognised by a combined classification of time signal intensity curve and 30% WR. However, to our knowledge, there are no reports of comparisons for benign and malignant parotid gland tumours by the attenuation and percentage enhancement wash-out ratios.
The results of our study indicated that the mean attenuation values of pleomorphic adenomas were similar to those of malignant tumours, but lower than those of Warthin’s tumours on non-enhanced CT. However, there was considerable overlap in attenuation among pleomorphic adenomas, Warthin’s tumours and malignant tumours, and, hence, there was no difference between benign and malignant parotid gland tumours (P = 0.047). In our limited data, multiple masses were found in patients with Warthin’s tumours and malignant tumours. Necrotic portions were viewed in three types of parotid tumours. Calcifications were detected in some pleomorphic adenomas, we presumed that the finding was related to ossification of cartilage structure in pleomorphic adenomas.
From our limited data, the mean attenuation of Warthin’s tumour attained the peak of enhancement during 30-s imaging, and most malignant tumours attain peak attenuation in 90-s imaging, but significant differences did not exist simultaneously between the malignant tumour vs the pleomorphic adenoma groups and the malignant tumour vs the Warthin’s tumour groups for a sustainable overlap. On the 5-min delay imaging, there was no difference among the parotid gland tumour groups. The discrepancy of mean attenuations between ours and a previous study [4] was attributed to CT parameters, such the total volume of contrast material and the injection rate of contrast material. Our findings suggested that reliable cut-off values were not helpful in differentiating benign from malignant tumours of the parotid gland on non-enhanced, 30-s, 90-s and 5-min imaging series.
The PEW and RPEW are the percentage enhanced wash-out ratio of enhanced attenuation according to the timing of the early and delayed imaging. Our results showed that there were significant differences in PEW and RPEW of parotid gland tumour groups, and significant differences simultaneously in the malignant tumour versus pleomorphic adenoma groups and the malignant tumour versus Warthin’s tumour groups.
From 30-s to 5-min helical CT of parotid gland tumours, applying the threshold value of −70% of PEW30 and −30% of RPEW30 between pleomorphic adenomas and malignant tumours, 89% and 89% of sensitivity, 56% and 61% of specificity were procured for the diagnosis of malignant tumours, relatively, 36% of PEW30 and 19% of RPEW30 between Warthin’s tumours and malignant tumours, 93% and 86% of specificity company with 100% sensitivity was diagnosed for malignancies. Likewise, from 90-s to 5-min helical CT of parotid gland tumours, using an optimal cut-off value of 12% of PEW90 and 4% of RPEW90 between pleomorphic adenomas and malignant tumours, 95% and 95% of sensitivity, 89% and 94% of specificity were obtained for diagnosis of malignant tumours, and 31% of PEW90 between Warthin’s tumours and malignant tumours, 63% of sensitivity and 71% of specificity were diagnosed for malignancies; conversely, with RPEW90 there was much more of an overlap between Warthin’s tumours and malignant tumours.
Although, the enhanced wash-out ratio of tumours was altered for CT parameters [16], for instance, volume, injection rate and time of contrast material, the foundation of different enhanced wash-out ratios was determined by many inherent traits of parotid gland neoplasms, such as angiogenesis [17, 18]. With pleomorphic adenoma it is widely accepted that both epithelial and mesenchymal (myxoid, hyaline, chondroid, osseous) elements often arise from the same cell clone, which may be a myoepithelial or ductal reserve cell [19, 20]. Warthin’s tumours, also known as papillary cystadenoma lymphomatosum, consist of an epithelial, monomorphic, oncocytic component and a lymphoid stroma [21, 22]. Parotid gland malignancies include diverse tumour types, with the mucoepidermoid carcinoma being the most common tumour. The incidence of adenocarcinoma NOS, the acinar cell carcinoma, the cystic adenoid carcinoma, the carcinoma ex pleomorphic adenoma, and the undifferentiated carcinoma is obviously disparate in different research [23–27]. The enhancement wash-out ratio may mirror different histological components of tumour cells and different architecture of tumoral angiogenesis in parotid gland tumours. Hereby, the percentage-enhanced change could provide the pathological information and differentiate benign from malignant tumours in the parotid gland.
Although we set the optimal threshold criteria to obtain diagnostic values of individual percentage enhanced wash-out ratios, they had much more distinction with regard to sensitivity and specificity. We believe that identification of patients with malignant parotid tumours is more important than missing benign parotid tumours. For this reason, we reanalysed cut-off values of the individual percentage enhanced washout ratios to yield 100% specificity, and examined whether a combination of some of these parameters improved the sensitivity. We tried to employ one or two of three percentages enhanced changes to get 100% specificity, but failed. The final research demonstrated that a parotid gland tumour was defined as a malignant mass when the following parameters were satisfied: −70% > PEW30 < 36%, −30% > PEW30 < 19%, PEW90 > 12%, and 74% sensitivity.
Our study had some limitations. First, parotid gland malignancies include various types of tumours, such as mucoepidermoid carcinoma, adenoid cystic carcinoma, carcinoma ex pleomorphic adenoma, squamous cell carcinoma, acinic cell carcinoma, duct carcinoma and lymphoma. Second, some selected masses were biased in Warthin’s tumours and malignant tumours, only the largest masses of the parotid gland were evaluated. Third, because of the small sample size and overlap in data among the three study subgroups, validation of the proposed cut-off values of CT diagnostic parameters needs to be performed in a larger scale trial. Lastly, if multiple-phasic CT were performed along with a diagnostic study, the patients would be exposed to an additional radiation dose.
In summary, the percentage wash-out ratios in contrast material-enhanced CT may reflect various characters of parotid gland neoplasms, and assist in differentiating the benign from malignant tumours. Combining the percentage enhanced wash-out ratios of the CT protocol can yield diagnostic results in malignant parotid gland tumours.
Acknowledgments
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.Nagler RM, Laufer D. Tumors of the major and minor salivary glands: review of 25 years of experience. Anticancer Res. 1997;17:701–707. [PubMed] [Google Scholar]
- 2.Pinkston JA, Cole P. Incidence rates of salivary gland tumors: results from a population-based study. Otolaryngol Head Neck Surg. 1999;120:834–840. doi: 10.1016/S0194-5998(99)70323-2. [DOI] [PubMed] [Google Scholar]
- 3.McGahan JP, Walter JP, Bernstein L. Evaluation of the parotid gland. Comparison of sialography, non-contrast computed tomography, and CT sialography. Radiology. 1984;52:453–458. doi: 10.1148/radiology.152.2.6739814. [DOI] [PubMed] [Google Scholar]
- 4.Choi DS, Na DG, Byun HS, et al. Salivary gland tumors: evaluation with two-phase helical CT. Radiology. 2000;214:231–236. doi: 10.1148/radiology.214.1.r00ja05231. [DOI] [PubMed] [Google Scholar]
- 5.Lev MH, Khanduja K, Morris PP, et al. Parotid pleomorphic adenomas: delayed CT enhancement. AJNR Am J Neuroradiol. 1988;19:1835–1839. [PMC free article] [PubMed] [Google Scholar]
- 6.Yerli H, Aydin E, Coskun M, et al. Dynamic multislice computed tomography findings for parotid gland tumors. J Comput Assist Tomogr. 2007;31:309–316. doi: 10.1097/01.rct.0000236418.82395.b3. [DOI] [PubMed] [Google Scholar]
- 7.Yabuuchi H, Fukuya T, Tajima T, et al. Salivary gland tumors: diagnostic value of gadolinium-enhanced dynamic MR imaging with histopathologic correlation. Radiology. 2003;226:345–354. doi: 10.1148/radiol.2262011486. [DOI] [PubMed] [Google Scholar]
- 8.Ikeda M, Motoori K, Hanazawa T, et al. Warthin tumor of the parotid gland: diagnostic value of MR imaging with histopathologic correlation. AJNR Am J Neuroradiol. 2004;25:1256–1262. [PMC free article] [PubMed] [Google Scholar]
- 9.Alibek S, Zenk J, Bozzato A, et al. The value of dynamic MRI studies in parotid tumors. Acad Radiol. 2007;14:701–710. doi: 10.1016/j.acra.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 10.Okahara M, Kiyosue H, Hori Y, et al. Parotid tumors: MR imaging with pathological correlation. Eur Radiol. 2003;13:125–133. doi: 10.1007/s00330-003-1999-0. [DOI] [PubMed] [Google Scholar]
- 11.Spiro RH. Salivary neoplasms: overview of a 35-year experience with 2,807 patients. Head Neck Surg. 1986;8:177–184. doi: 10.1002/hed.2890080309. [DOI] [PubMed] [Google Scholar]
- 12.Yu GY, Ma DQ. Carcinoma of the salivary gland: a clinicopathologic study of 405 cases. Semin Surg Oncol. 1987;3:240–244. doi: 10.1002/ssu.2980030405. [DOI] [PubMed] [Google Scholar]
- 13.Fitzpatrick PJ, Black KM. Salivary gland tumors. J Otolaryngol. 1985;14:296–300. [PubMed] [Google Scholar]
- 14.Kress E, Schulz HG, Neumann T. Diagnosis of diseases of the large salivary glands of the head by ultrasound, sialography and CT-sialography. A comparison of methods. HNO. 1993;41:345–351. [PubMed] [Google Scholar]
- 15.Casselman JW, Mancuso AA. Major salivary gland masses: comparison of MR imaging and CT. Radiology. 1987;165:183–189. doi: 10.1148/radiology.165.1.3628768. [DOI] [PubMed] [Google Scholar]
- 16.Jeong YJ, Lee KS, Jeong SY, et al. Solitary pulmory nodule: characterization with combined wash-in and washout features at dynamic multi-detector row CT. Radiology. 2005;237:675–83. doi: 10.1148/radiol.2372041549. [DOI] [PubMed] [Google Scholar]
- 17.Passe TJ, Bluemke DA, Siegelman SS. Tumor angiogenesis: tutorial on implications for imaging. Radiology. 1997;203:593–600. doi: 10.1148/radiology.203.3.9169673. [DOI] [PubMed] [Google Scholar]
- 18.Wang JH, Min PQ, Wang PJ, et al. Dynamic CT evaluation of tumor vascularity in renal cell carcinoma. AJR Am J Roentgenol. 2006;186:1423–1430. doi: 10.2214/AJR.04.1408. [DOI] [PubMed] [Google Scholar]
- 19.Kondo T. A case of lipomatous pleomorphic adenoma in the parotid gland. Diagn Pathol. 2009;4:16. doi: 10.1186/1746-1596-4-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ide F, Kusama K. Myxolipomatous pleomorphic adenoma: an unusual oral presentation. J Oral Pathol Med. 2004;33:53–55. doi: 10.1111/j.1600-0714.2004.00110.x. [DOI] [PubMed] [Google Scholar]
- 21.Hatch RL, Shah S. Warthin tumor: a common, benign tumor presenting as a highly suspicious mass. J Am Board Fam Pract. 2005;18:320–322. doi: 10.3122/jabfm.18.4.320. [DOI] [PubMed] [Google Scholar]
- 22.Russo C, Brunelli M, Baroli P. Cytogenetic patterns in Warthin tumor. Acta Otorhinolaryngol. 1993;13:551–558. [PubMed] [Google Scholar]
- 23.Wahlberg P, Anderson H, Biörklund A, et al. Carcinoma of the parotid and submandibular glands–a study of survival in 2465 patients. Oral Oncol. 2002;38:706–713. doi: 10.1016/S1368-8375(02)00007-6. [DOI] [PubMed] [Google Scholar]
- 24.Goode RK, Auclair PL, Ellis GL. Mucoepidermoid carcinoma of the major salivary glands: clinical and histopathologic analysis of 234 cases with evaluation of grading criteria. Cancer. 1998;82:1217–1224. doi: 10.1002/(SICI)1097-0142(19980401)82:7<1217::AID-CNCR2>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 25.Ostman J, Anneroth G, Gustafsson H. Malignant salivary gland tumours in Sweden 1960–1989–an epidemiological study. Oral Oncol. 1997;33:169–176. doi: 10.1016/S0964-1955(96)00077-2. [DOI] [PubMed] [Google Scholar]
- 26.Subhashraj K. Salivary gland tumors: a single institution experience in India. Br J Oral Maxillofac Surg. 2008;46:635–638. doi: 10.1016/j.bjoms.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 27.Ito FA, Ito K, Vargas PA. Salivary gland tumors in a Brazilian population: a retrospective study of 496 cases. Int J Oral Maxillofac Surg. 2005;34:533–536. doi: 10.1016/j.ijom.2005.02.005. [DOI] [PubMed] [Google Scholar]