Abstract
Representational difference analysis (RDA) cloning has identified transcriptional intermediary factor 1 beta (TIF1β) as a gene inducibly expressed early during myeloid differentiation of the promyelocytic cell lines HL-60 and U937. To assess the role of TIF1β, U937 cell lines were made that expressed antisense-hammerhead ribozymes targeted specifically against TIF1β mRNA. These cells failed to differentiate into macrophages, as determined by several criteria: a nonadherent morphology, a failure to arrest cell cycle, lowered levels of macrophage-specific cell surface markers, resistance to Legionella pneumophila infection, a loss of the ability to phagocytose and chemotax, and decreased expression of chemokine mRNAs. One way TIF1β acts in macrophage differentiation is to augment C/EBPβ transcriptional activity. Furthermore, we show by EMSA supershifts and coimmunoprecipitation that C/EBPβ and TIF1β physically interact. Although TIF1β is necessary for macrophage differentiation of U937 cells, it is not sufficient, based on the inability of ectopically expressed TIF1β to induce or augment phorbol ester-induced macrophage differentiation. We conclude that TIF1β plays an important role in the terminal differentiation program of macrophages, which involves the coactivation of C/EBPβ and induction of C/EBPβ-responsive myeloid genes.
Keywords: TIF1β, C/EBPβ, U937 cells, macrophage, antisense
Macrophages play a critical role in development, physiology, innate and adaptive immunity, and in the pathogenesis of many infectious, immunologic, and degenerative disease processes. They possess many specialized cellular functions such as phagocytosis, chemotaxis, antibody-dependent cell cytotoxicity, antigen presentation, and specific expression of a repertoire of cytokines, chemokines, and cell surface markers (Gordon 1995).
Several myelocytic cell lines have been established which, when differentiated with a variety of agents, make the study of these diverse functions tractable. HL-60 is a human myeloid cell line derived from a patient with acute promyelocytic leukemia (Collins et al. 1977). In response to different treatments, HL-60 cells can be induced to differentiate toward mature granulocyte-like cells or monocyte/macrophage-like cells (Collins et al. 1978). HL-60 cells show bilineage differentiation similar to normal granulocyte-macrophage progenitor cells (CFU-GM; Koeffler 1983; Koeffler et al. 1985). The U937 line, established from a patient with histiocytic lymphoma (Sundstrom and Nilsson 1976), has properties consistent with an immature monocyte (Ralph et al. 1976; Sundstrom and Nilsson 1976) and can be induced by phorbol esters to undergo differentiation to a macrophage (Ralph et al. 1982). Thus, both the HL-60 and U937 cell lines provide useful model systems for the study of macrophage differentiation.
Several transcription factors are known to be involved in macrophage differentiation (Clarke and Gordon 1998). The ets-family member PU.1 is critical for myeloid differentiation. Both the granulocyte and monocyte lineages are absent in PU.1-deficient mice (McKercher et al. 1996), and the activation of several macrophage-specific genes requires PU.1 (Pahl et al. 1993; Feinman et al. 1994; Moulton et al. 1994; Perez et al. 1994; Zhang et al. 1994; Rosmarin et al. 1995). The C/EBP family has also been shown to play a direct role in macrophage differentiation. Cotransfection of C/EBPα, β, and δ expression plasmids can activate transcription from macrophage-specific promoters (Ness et al. 1993; Hohaus et al. 1995; Mink et al. 1996; Zhang et al. 1996). C/EBPβ, in particular, has been shown to be induced upon macrophage differentiation and to be a potent activator of cytokine gene transcription. The proteins Blimp-1 and ICSBP were recently shown to be potent regulators of myeloid differentiation. Ectopic expression of Blimp-1 is sufficient to trigger myeloid differentiation (Chang et al. 2000). Mice lacking ICSBP lack macrophages and develop a Chronic myeloid leukemia (CML)-like leukemia (Tamura et al. 2000). Hoxa9, when overexpressed, blocks myeloid differentiation (Calvo et al. 2000).
Our objective was to identify early genes expressed during myeloid differentiation, in the hope that such genes might encode regulators of the differentiation process. Toward this end, we used the subtractive cloning technique known as representational difference analysis (RDA) (Hubank and Schatz 1994) to isolate genes expressed during differentiation of the promyelocytic cell line HL-60. One of the cDNAs isolated was that encoding transcriptional intermediary factor 1 beta (TIF1β). To study the functional role of TIF1β, antisense-hammerhead ribozymes were designed and used to make stable myelomonocytic U937 cell lines in which endogenous TIF1β mRNA is ablated. U937 stable clones expressing the antisense-ribozymes were unable to differentiate into macrophages. They have deficiencies in cell cycle arrest, macrophage-specific cell surface marker expression, Legionella pneumophila parasitism, phagocytosis, chemotaxis, and chemokine expression. Since C/EBPβ has been implicated in the regulation of genes involved in these processes, we investigated the effect of TIF1β on C/EBPβ transcriptional activity. TIF1β was found to interact with C/EBPβ in coimmunoprecipitations and in EMSAs, and functions as a coactivator of transiently transfected and endogenous C/EBPβ-responsive genes. We propose that TIF1β is a critical regulator of macrophage differentiation and functions, at least in part, by augmenting the expression of C/EBPβ-dependent genes.
Results
A representational difference analysis of early genes in macrophage differentiation isolates the cDNA for TIF1β
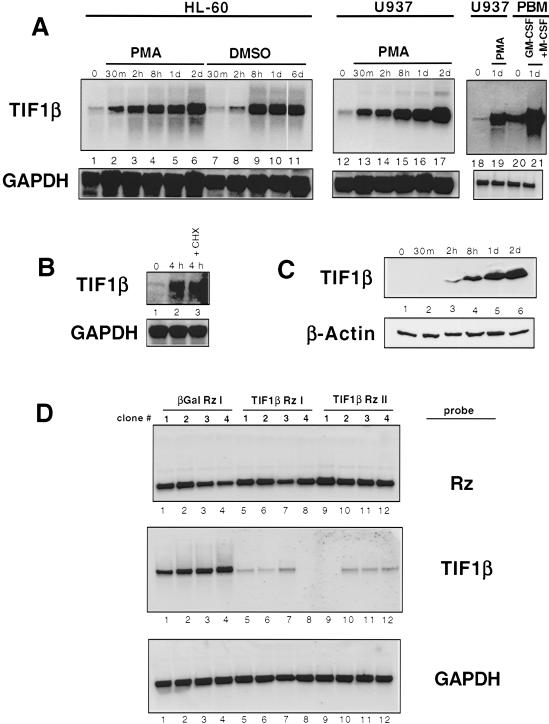
The promyelocytic line HL-60 was used for the RDA. HL-60 cells become fully differentiated in response to the phorbol ester phorbol 12-myristate 13-acetate (PMA) after 2 d (Rovera et al. 1979). To ensure that early genes were isolated, the HL-60 cells used were treated with PMA for only 2 h. RDA subtraction was performed using uninduced HL-60 cDNA as the driver and 2 h PMA-treated HL-60 cDNA as the tester. One of the resulting amplified fragments, when subjected to a BLAST sequence homology search (Altschul et al. 1990), was 100% homologous to the cDNA for TIF1β. Northern blots of HL-60 RNA (Fig. 1A) showed that TIF1β mRNA was induced rapidly (by 30 min) and sustained throughout 2 d of PMA-dependent macrophage differentiation (Fig. 1A, lanes 1–6). It was also strongly induced when HL-60 cells underwent granulocytic differentiation in response to treatment with DMSO (Fig. 1A, lanes 7–11). An identical expression pattern of TIF1β mRNA was seen with PMA-treated U937 cells (Fig. 1A, lanes 12–17).
Figure 1.
TIF1β expression during macrophage differentiation. (A) Northern blot of TIF1β mRNA in differentiating HL-60, U937, and human peripheral blood monocytes (PBMs). HL-60 cells were induced with PMA (lanes 2–6) or DMSO (lanes 7–11). U937 cells were induced with PMA (lanes 12–17). Human PBMs were induced with GM-CSF and M-CSF (lanes 20–21). (B) TIF1β mRNA is not affected by cycloheximide. Northern analysis was performed on untreated U937 cells untreated (lane 1), treated for 4 h with PMA (lane 2) and treated with PMA and cycloheximide for 4 h (lane 3). (C) Immunoblots of extracts from differentiating U937 cells using antibodies specific for TIF1β (top) and β-actin (bottom). (D) Expression of TIF1β antisense-hammerhead ribozymes ablates TIF1β mRNA levels. U937 cells were stably transfected with plasmids expressing β-gal or TIF1β antisense-ribozymes and induced with PMA for 24 h. Four hygromicin-resistant cell clones were isolated and analyzed for expression of the mRNAs encoding antisense-ribozymes (top), endogenous TIF1β (middle), and GAPDH (bottom).
When human peripheral mononuclear cells (PMNs) were analyzed, TIF1β mRNA levels were significantly induced upon macrophage differentiation in response to GM-CSF and M-CSF (Fig. 1A, cf. lanes 20 and 21 with U937 levels in lanes 18 and 19). PMA-induced TIF1β mRNA in U937 cells was unaffected by the addition of the protein inhibitor cycloheximide, thus defining TIF1β as an immediate-early gene (Fig. 1B). Additionally, TIF1β protein levels correlated with TIF1β mRNA expression (Fig. 1C). Thus, TIF1β is identified as an early response gene for the differentiation of HL-60 cells to either macrophages or granulocytes, for the differentiation of U937 cells to macrophages, and for the differentiation of PMNs to macrophages.
Antisense-hammerhead ribozymes suppress endogenous TIF1β expression
We sought to determine whether TIF1β induction was important for macrophage differentiation. Antisense-hammerhead ribozymes were designed to suppress endogenous TIF1β mRNA. The RNA folding program mfold (Patzel et al. 1998; Mathews et al. 1999; Zuker et al. 1999) was used to predict regions of single-strandedness within the TIF1β RNA molecule, and two antisense constructs were made corresponding to these regions. To further ensure inhibition of the endogenous TIF1β transcript, catalytic hammerhead ribozyme sequences were incorporated into the antisense constructs (Homann et al. 1993; Tabler et al. 1994; Hormes et al. 1997). As a negative control, a comparable antisense-ribozyme was directed against the β-galactosidase mRNA. Expression plasmids for these three antisense-ribozymes were stably transfected into U937 cells, and four clones expressing each antisense-ribozyme were selected for further study. To monitor expression of the antisense-ribozymes, Northern analyses were performed using as a probe the hammerhead ribozyme sequence (Fig. 1D, top panel, lanes 1–12). To test for the ability of the antisense-ribozymes to degrade endogenous TIF1β mRNA, the Northern blots were hybridized with a cDNA probe for TIF1β mRNA (Fig. 1D, middle panel). Whereas the antisense-ribozymes directed against β-gal mRNA had no effect on TIF1β mRNA levels (Fig. 1D, middle panel, lanes 1–4), each of the four clones with the ribozymes directed against TIF1β mRNA significantly suppressed expression (Fig. 1D, middle panel, lanes 5–8 and 9–12). Conversely, because the TIF1β antisense-ribozymes had no effect on GAPDH mRNA levels (Fig. 1D, bottom panel), they appear to be specific for the TIF1β mRNAs. These data show that the TIF1β antisense-ribozymes specifically ablate expression of endogenous TIF1β mRNA.
TIF1β is required for many aspects of myeloid differentiation
Cell cycle arrest during macrophage differentiation of U937 cells
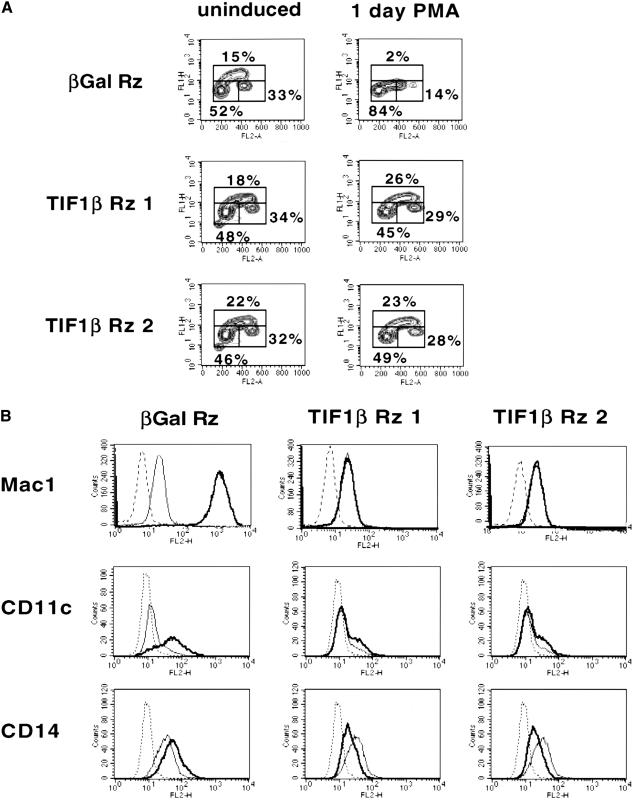
Ribozyme-induced degradation of TIF1β mRNA in these clones allowed the functional role of TIF1β in U937 differentiation to be determined. One distinguishing feature of differentiating macrophages is that they undergo cell cycle arrest. To analyze the state of the cell cycle in the antisense-ribozyme-expressing clones, DNA content was determined by propidium iodine staining, and DNA replication was measured by BrdU incorporation (Fig. 2A). After one day of PMA treatment, the β-gal antisense-ribozyme stable U937 cell lines have undergone cell cycle arrest, as seen by the decrease in number of cells in S phase. In contrast, in the TIF1β antisense-ribozyme cell lines, no difference was seen in the status of cell cycle progression between untreated and PMA-treated cells. TIF1β antisense-ribozyme stable U937 cell lines continue to progress through S phase and fail to undergo G1 arrest. These results demonstrate that TIF1β is required for G1 arrest during U937 differentiation into macrophages.
Figure 2.
(A) Cell cycle analysis of ribozyme-expressing U937 cell lines. BrdU/PI FACS analysis of representative β-gal and TIF1β antisense-ribozyme stable cell lines. Untreated (left column) and 24 h PMA-treated (right column) cells of each clone were analyzed. Propidium iodide is on the horizontal axis, and BrdU incorporation is on the vertical axis. (B) Expression of macrophage cell surface proteins in stable U937 cell lines. Individual stable transfected clones expressing β-gal or TIF1β antisense-ribozymes were analyzed by FACS for expression of Mac1 (CD11b–CD18 heterodimer), CD11c, and CD14. Unstained cells (dashed line), untreated (normal line), and 24 h PMA-treated cells (bold line).
Macrophage-specific cell surface protein expression
Another consequence of macrophage differentiation is the expression of specific cell surface proteins. Among these are the β2-integrin receptor for ICAM-1 and the CR3 complement receptor, CD11b (Mac1), the β2-integrin receptor for fibrinogen, CD11c, and the LPS receptor, CD14. CD11b and CD11c both mediate macrophage attachment to endothelial cells and subsequent extravasation to areas of inflammation or infection. In addition, CD11b and CD11c both serve as complement receptors, activating an inflammatory response when bound by complement, and facilitating phagocytosis of complement-bound organisms. CD14, the LPS receptor, allows macrophages to recognize, bind to, and phagocytose bacteria. As seen in Figure 2B, CD11b, CD11c, and CD14 levels increased in response to PMA-treatment of U937 clones expressing the β-gal antisense-ribozyme. However, expression did not increase in the TIF1β antisense-ribozyme stable U937 cell lines. Indeed, the levels of CD14 were suppressed to levels below the untreated state. These data show that TIF1β is required for inducible expression of cell surface myeloid differentiation markers.
L. pneumophila parasitism
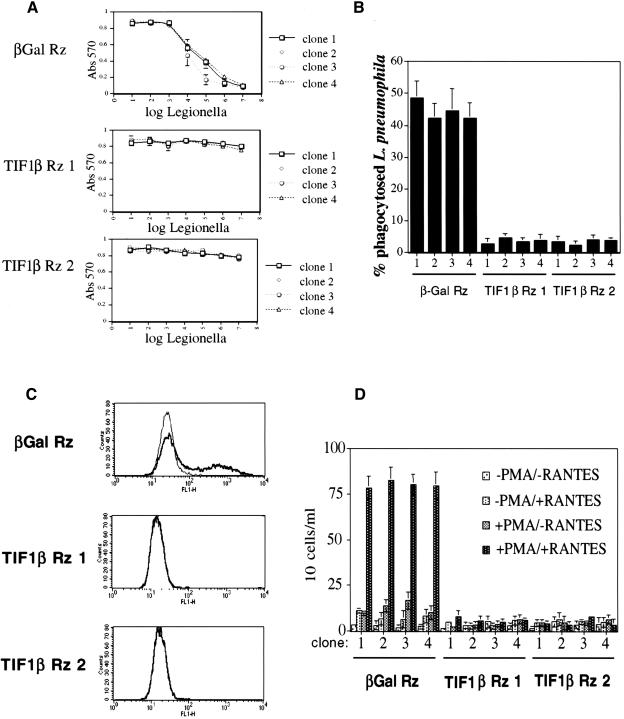
The cell surface expression of CD11b is required for the infection of macrophages by L. pneumophila, a facultative intracellular pathogen which parasitizes human alveolar macrophages (Horwitz and Silverstein 1980). Antibodies specific for CD11b have been shown to block L. pneumophila infection of the HL60 cell line (Marra et al. 1990). Since the suppression of TIF1β blocked CD11b expression, we predicted it would block L. pneumophila infection and killing, which requires functional CD11b. To test this prediction, we performed a L. pneumophila infection/killing assay. Live L. pneumophila were coincubated with PMA-treated U937 antisense-ribozyme stable cell lines for 4 d, and cell survival was assayed for the ability to reduce MTT, an indication of cell viability. As seen in Figure 3A, stable U937 cell lines expressing the antisense-ribozyme against β-gal were effectively killed by L. pneumophila, whereas the stable U937 cell lines expressing the TIF1β antisense-ribozymes were completely resistant to killing.
Figure 3.
(A) Susceptibility of stable U937 cell lines to infection and killing by L. pneumophila. PMA-differentiated U937 stable cell clones were coincubated with the indicated number of L. pneumophila bacteria for 4 d, and then assayed for cell survival by the ability to reduce MTT. Each point represents three independent results. (B) Measurement of L. pneumophila uptake by stable U937 cell lines. The ability of U937 stable cell lines to phagocytose L. pneumophila was determined by assaying for the number of viable intracellular L. pneumophila bacteria after a 2 h coincubation of PMA-treated U937 stable cell lines with L. pneumophila. (C) Phagocytosis of FITC-labeled S. aureus bacteria. Cell clones expressing β-gal and TIF1β antisense-ribozymes were analyzed for the ability to phagocytose FITC-labeled S. aureus bacteria. Results using untreated (normal line) and 24 h PMA-treated (bold line) are shown for each clone. (D) Chemotaxis. Shown are the number of cells/mL that have migrated through the transwell membrane towards the chemoattractant chemokine, RANTES, after 6 h.
We also investigated the ability of PMA-treated U937 cells to phagocytose L. pneumophila. U937 cells were assayed for their ability to phagocytose L. pneumophila (Fig. 3B) by determining the number of viable intracellular bacteria after a 2 h coincubation of L. pneumophila and stable U937 cell lines. The stable U937 cell lines expressing the antisense-ribozyme against β-gal effectively phagocytosed bacteria, whereas the stable U937 cell lines expressing the TIF1β antisense-ribozymes did not.
Phagocytosis of opsonized S. aureus
Whereas phagocytosis of L. pneumophila occurs through the CD11b complement receptor, phagocytosis may also occur via Fc receptors (Aderem and Underhill 1999). To investigate the effect of suppressing TIF1β expression on Fc receptor-dependent phagocytosis, the stable cell lines were used in phagocytosis assays using opsonized FITC-labeled S. aureus, followed by FACS analysis. Whereas the β-gal antisense-ribozyme stable U937 cell lines were able to phagocytose upon differentiation with PMA, both TIF1β antisense-ribozyme stable cells lines were completely blocked in the ability to phagocytose (Fig. 3C).
Chemotaxis in response to RANTES chemokine
Differentiated macrophages are able to undergo chemotaxis towards an area of inflammation or infection. To test for chemotaxis ability, the β-gal and TIF1β antisense-ribozyme stable cell lines were differentiated with PMA, placed on top of transwell membranes and tested for the ability to migrate to the lower chambers containing the chemoattractant chemokine, RANTES. As seen in Figure 3D, although the β-gal antisense-ribozyme stable cell lines retained the ability to undergo chemotaxis, both of the TIF1β antisense-ribozyme stable cell lines were completely blocked.
Induction of chemokine mRNAs
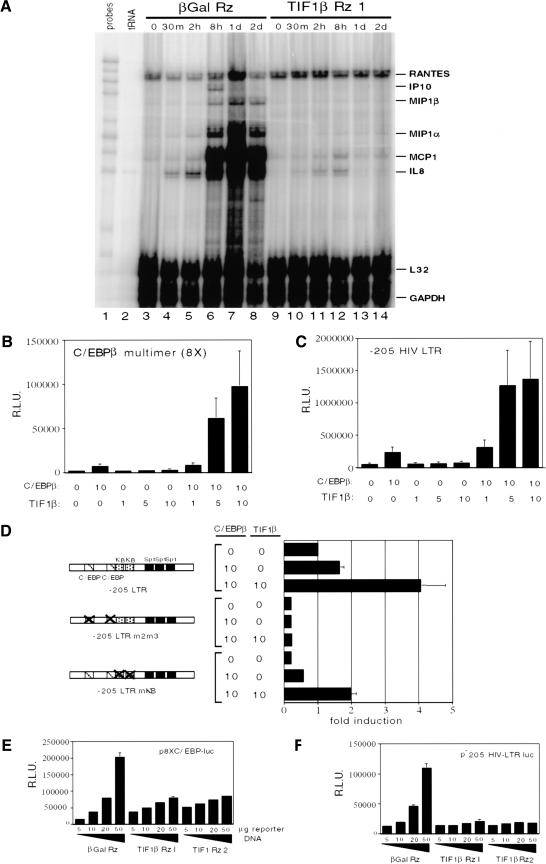
Activated macrophages exhibit a distinct pattern of cytokine and chemokine expression. These products serve to recruit other members of the immune system to combat infection and inflammation. Chemokine mRNA expression in the β-gal and TIF1β antisense-ribozyme stable cell lines was assayed using a ribonuclease protection assay (RPA) (Fig. 4A). Whereas IP-10, MIP1β, MIP1α, MCP1, and IL8 mRNA levels are induced upon PMA treatment of the β-gal antisense-ribozyme stable U937 cell line, there was a complete failure in the TIF1β antisense-ribozyme stable U937 cell lines to induce these cytokines.
Figure 4.
(A) Chemokine mRNA levels. Ribonuclease protection assay (RPA) was performed using probes specific for the mRNAs encoding chemokines RANTES, IP10, MIP1β, MIP1α, MCP1, IL8, and for the control L32 and GAPDH transcripts. Total RNA samples were used from cells treated with PMA for the indicated times. (B) Cotransfection of C/EBPβ and TIF1β expression plasmids with the 8XC/EBPβ-luciferase reporter plasmid into U937 cells followed by a 24 h PMA differentiation. (C) Cotransfection of C/EBPβ and TIF1β expression plasmids with the −205 HIV–LTR-luciferase reporter plasmid in U937 cells followed by a 24 h PMA differentiation. (D) Cotransfection of C/EBPβ and TIF1β expression plasmids with −205 HIV–LTR-luciferase reporter plasmids with mutations in the two C/EBPβ binding sites (−205 HIV LTR m2m3) or in the two NF-κB binding sites (−205 HIV LTR mκB) in U937 cells followed by a 24 h PMA differentiation. The absolute luciferase values were 250,000 light units with C/EBPβ alone, and 1.5 × 106 light units with both TIF1β and C/EBPβ cotransfected. (E) Transfections of U937 stable cell lines expressing β-gal and TIF1β antisense-ribozymes with increasing amounts of the 8XC/EBPβ-luciferase reporter plasmid followed by a 24 h PMA differentiation. (F) Transfections of U937 stable cell lines expressing β-gal and TIF1β antisense-ribozymes with increasing amounts of the −205 HIV–LTR-luciferase reporter plasmid followed by a 24 h PMA differentiation.
TIF1β functions as a coactivator of C/EBPβ in U937 cells
C/EBPβ is an important transcriptional activator that is induced during PMA treatment of U937 cells and activation of primary macrophages (Akira and Kishimoto 1992; Natsuka et al. 1992). Many of the macrophage functions assayed in this work depend on genes whose transcription is known to be regulated by C/EBPβ. For example, expression of MIP1α (Matsumoto et al. 1998), MCP1 (Yamamoto et al. 1999), and IL-8 (Kunsch et al. 1994) are in part regulated by C/EBPβ. C/EBPβ also regulates genes encoding cell surface molecules CD14 (Fig. 3; Matsumoto et al. 1998) and CD16 (FcγRIII receptor) (Feinman et al. 1994). A previous report showed that TIF1β and C/EBPβ coimmunoprecipitate in the P388D1 macrophage cell line (Chang et al. 1998). In transient transfections of BHK cells, TIF1β functions as a coactivator with C/EBPβ of the alpha-1 acid glycoprotein (AGP) gene (Chang et al. 1998). Thus, we suspected that one mechanism by which TIF1β may function during U937 differentiation is as a coactivator of C/EBPβ.
To investigate this hypothesis, we performed cotransfections with C/EBPβ and TIF1β expression plasmids and C/EBPβ-dependent luciferase reporters in U937 cells, followed by a 24-h PMA treatment. As seen in Figure 4B (bars 3–5), expression of C/EBPβ alone resulted in a modest activation of the p8XC/EBPβ-luc promoter (containing eight C/EBPβ-responsive elements), and expression of TIF1β alone had no effect. However, coexpression of TIF1β with C/EBPβ expression synergistically increased promoter activity (Fig. 4B, bars 6–8). Identical results were obtained using a natural C/EBPβ-responsive promoter/reporter which contains −205 to +1 bp of the HIV LTR (Henderson et al. 1995). This region contains two C/EBPβ binding sites, two NF-κB sites, and three SP-1 sites. As seen in Figure 4C (bar 2), ectopic expression of C/EBPβ in PMA-treated U937 cells alone activated the p−205 HIV LTR-luc promoter. Although TIF1β alone had no effect (Fig. 4C, bars 3–5) on the p−205 HIV LTR-luc promoter, in combination with C/EBPβ, it synergistically activated p−205 HIV LTR-luc promoter activity (Fig. 4C, bars 6–8). This increase was further shown to depend on C/EBPβ binding sites, since no augmentation was observed in a promoter harboring mutant C/EBPβ sites (Fig. 4D, bars 3–6). The promoter construct with mutated C/EBPβ sites also shows that other transcription factors binding to the two intact NF-κB-response elements and the three intact Sp1-response elements are unaffected by either C/EBPβ or TIF1β cotransfections, and suggests that the coactivator function of TIF1β is specific to C/EBPβ. Further evidence of the specificity of TIF1β for C/EBPβ is seen in the persistent augmentation of the reporter construct with both NF-κB binding sites mutated (see Fig. 6C, lanes 7–9). Thus, TIF1β specifically augments C/EBPβ-dependent transcription in PMA-differentiated U937 cells in transient cotransfection experiments.
Figure 6.
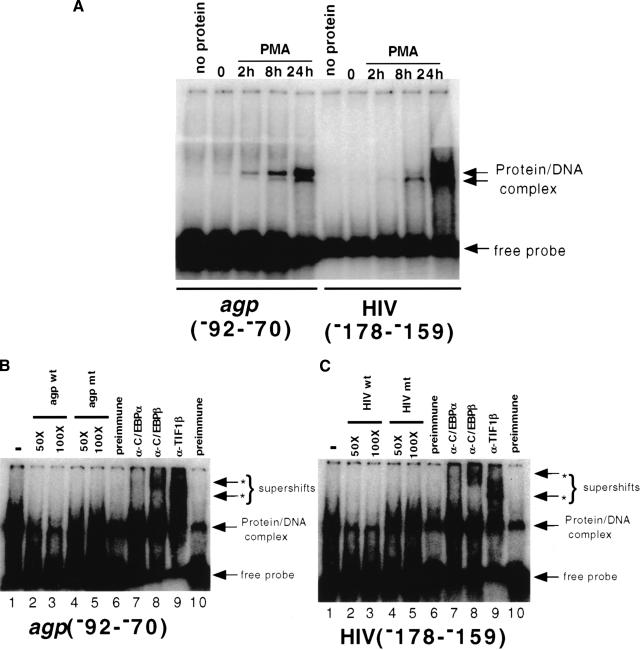
Detection of C/EBPβ- and TIF1β-containing complexes in EMSA analyses. (A) Nuclear extracts were prepared from untreated and PMA-treated U937 cells and used in EMSA analyses with oligonucleotides containing C/EBPβ binding sites from the α1-acid glycoprotein (AGP) promoter and the HIV LTR. (B) The shifted complex is sequence-specific and contains C/EBPβ, TIF1β, but not C/EBPα. The protein/DNA complex is competed with excess unlabeled wild-type (lanes 2,3), but not mutated (lanes 3,4), AGP oligonucleotide. EMSAs were intentionally overexposed to reveal the supershifts. Supershifted complexes are seen using antibodies against C/EBPβ (lane 8) and TIF1β (lane 9), but not with preimmune (lanes 6,10) or an antibody against C/EBPα (lane 7). (C) The experiment was identical to that shown in (B), but used the HIV LTR oligonucleotide.
To determine whether endogenous C/EBPβ transcriptional activity requires TIF1β, we transfected the β-gal Rz, TIF1β Rz1, and TIF1β Rz2 stable cell lines with p8XC/EBPβ-luc and p−205 HIV LTR-luc, followed by treatment with PMA. Transfection efficiency of the different cell clones was normalized using a cotransfected renilla luciferase plasmid. With both reporters, p8XC/EBPβ-luc (Fig. 4E) and p−205 HIV LTR-luc (Fig. 4F), maximal activity was seen using 50 μg of reporter when transfected into the β-gal Rz cell line (Fig. 4E,F, bar 4). In contrast, little activation of the two reporters was seen in the TIF1β Rz1 and TIF1β Rz2 U937 cell clones lacking TIF1β. Essentially no activation (or repression) was seen when undifferentiated U937 cells were used. Therefore, we conclude that TIF1β augments the transcriptional activity of endogenous C/EBPβ on reporter constructs in differentiating U937 cells.
TIF1β and C/EBPβ associate in vivo and in vitro
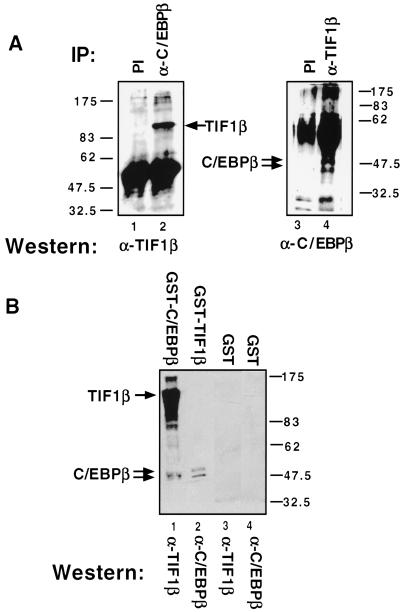
TIF1β and C/EBPβ cooperate to induce transcription. To determine whether they physically associate (directly or indirectly), we used total cellular extracts from U937 cells treated with PMA for 24 h in coimmunoprecipitation studies using antibodies against TIF1β and C/EBPβ. As seen in Figure 5A, whole-cell extracts were immunoprecipitated with either preimmune (lane 1) or an antibody directed against C/EBPβ (lane 2). When separated by PAGE and immunoblotted with an antibody directed against TIF1β, a band of the size of TIF1β (109 kD) was found in anti-C/EBPβ immunoprecipitated samples. In a reciprocal experiment, anti-TIF1β immunoprecipitated samples, but not preimmune immunoprecipitated, were shown by Western analysis to have coimmunoprecipitated C/EBPβ, which appears as a doublet of ∼48 kD (Fig. 5A, lanes 3 and 4).
Figure 5.
TIF1β and C/EBPβ associate. (A) Whole-cell extracts from 24 h PMA-treated U937 cells were immunoprecipitated with preimmune (PI) (lanes 1,3), anti-C/EBPβ (lane 2) or anti-TIF1β (lane 4). Western analysis was then performed using anti-TIF1β (lane 2) or anti-C/EBPβ (lane 4) antibodies. (B) Whole-cell extracts from 24 h PMA-treated U937 cells were incubated with prewashed GST-C/EBPβ (lane 1), GST-TIF1β (lane 2), or GST alone (lanes 3,4). After extensive washes, the protein complexes were subjected to SDS-PAGE and immunoblotted with anti-TIF1β antibody (lanes 1,3) or anti-C/EBPβ antibody (lanes 2,4).
Another test for the association of TIF1β and C/EBPβ was performed by incubating PMA-stimulated U937 cell extracts with immobilized GST-C/EBPβ or GST-TIF1β. After extensive washes, retained proteins were analyzed by immunoblotting. Endogenous TIF1β associated with GST-C/EBPβ (Fig. 5B, lane 1). Similarly, as seen in lane 2, endogenous C/EBPβ associated with GST-TIF1β. Thus, endogenous C/EBPβ and TIF1β physically associate (directly or indirectly) both in vivo and in vitro.
TIF1β and C/EBPβ associate on C/EBP binding sites
Since TIF1β augments C/EBPβ-dependent transcription, we reasoned that a complex of the two proteins might bind to C/EBPβ binding sites. To test this hypothesis, we performed EMSAs using two different C/EBPβ binding site oligonucleotides: one from the HIV LTR and another from the AGP promoter, together with nuclear extracts from U937 cells untreated or treated with PMA for various times (Fig. 6A). A protein–DNA complex appeared at 2 h and reached a maximum at 24 h. Cold competitions (Fig. 5B,C) established the specificity of this complex. Furthermore, antisera to TIF1β as well as that to C/EBPβ supershifted the complex (Fig. 6, lanes 8 and 9). Since TIF1β alone cannot bind DNA, this shows that a C/EBPβ/TIF1β complex is bound to the DNA. In contrast, control mouse antisera, or antisera specific to C/EBPα (not present in U937 cells) (Henderson et al. 1995) had no effect on binding (Fig. 6, lanes 6 and 7). Identical results were obtained using nuclear extracts from the promyelocytic cell line HL-60 (data not shown).
TIF1β is not sufficient for myeloid differentiation of the myelocytic cell line U937
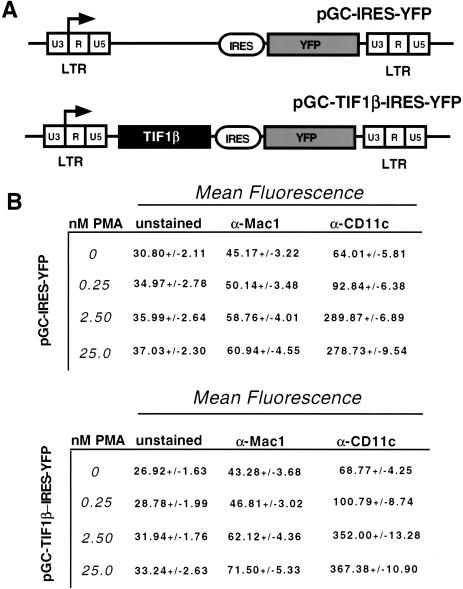
To determine whether ectopic expression of TIF1β alone is sufficient to cause differentiation of U937 cells, TIF1β was overexpressed in U937 cells using bicistronic retroviruses. Cells were transduced with a retrovirus encoding the cDNAs for TIF1β and yellow fluorescent protein (YFP) or a control expression only YFP (Fig. 7A). They were untreated, or treated with suboptimal amounts of PMA, and allowed to differentiate for 24 h. TIF1β alone did not cause myeloid differentiation, based on cell morphology, and FACS staining of surface Mac-1 and CD11c (Fig. 7B). TIF1β was also unable to augment differentiation of transduced cells treated with suboptimal doses of PMA. We conclude that TIF1β is necessary, but not sufficient, for the differentiation of the myelocytic cell line U937.
Figure 7.
Ectopic expression of TIF1β in U937 cells. (A) Retroviral constructs used to transduce U937 cells. An IRES (internal ribosomal entry site) ensures that TIF1β and YFP are coexpressed from a bicistronic mRNA message. (B) Effect of ectopic expression of TIF1β on myeloid cell surface markers Mac1 and CD11c. U937 cells were transduced with pGC-IRES-YFP (top table) or pGC-TIF1β-IRES-YFP (bottom table) induced for 24 h with optimal and suboptimal amounts of PMA. YFP-positive cells (expressing TIF1β) were assayed for levels of Mac1 and CD11c by FACS using PE-conjugated antibodies.
Discussion
Identification of TIF1β as necessary for myeloid differentiation
Our data provide the first indication that TIF1β mRNA is induced upon monocyte activation and that it plays a role in macrophage differentiation. Steady-state levels of TIF1β mRNA are significantly increased when HL-60 cells differentiate in response to PMA or DMSO treatment. Similarly, TIF1β is expressed when the slightly more mature line, U937, differentiates into macrophages in response to PMA treatment. TIF1β mRNA is also found in peripheral blood monocytes, and levels increase following treatment with GM-CSF and M-CSF. These data clearly demonstrate that TIF1β mRNA is induced during activation and differentiation of promyelocytic cells lines and primary monocytes.
Expression of antisense-hammerhead ribozymes was used to ablate TIF1β mRNA in U937 cells. Cell lines lacking TIF1β mRNA were unable to differentiate in response to PMA treatment, thus establishing a requirement for TIF1β in U937 differentiation. However, TIF1β is apparently not sufficient for U937 differentiation, based on the inability of ectopic TIF1β expression to induce or augment PMA-dependent differentiation. Due to technical limitations, we have not been able to express TIF1β and C/EBPβ together in U937 cells, so we do not know if this would be sufficient to drive their differentiation. However, based on the results from the U937 system and the expression and induction of TIF1β mRNA observed in PBMs, it is reasonable to suggest that TIF1β is required for macrophage differentiation in vivo. Formal proof of this awaits analysis of mice lacking TIF1β in their monocytes.
Myeloid differentiation is accompanied by cell cycle arrest, and other screens for myeloid-specific genes have identified such cell cycle genes as the cdk inhibitors p21 (Liu et al. 1996) and p27 (Freedman 1999). However, of the 50 cDNAs cloned, only one that encoded a protein associated with cell cycle arrest, CLN3, was cloned. We also isolated two cDNAs that encode proteins associated with cell cycle progression: CYCLIN1 and CDC2/CDK1. This apparent discrepancy is probably due to differences in the times when the cloning was performed. Unlike other studies where cDNAs were isolated toward the end of differentiation, our study isolated cDNAs expressed very early. It is known that U937 cells (and possibly HL-60 cells) undergo an early burst in proliferation before the onset of cell cycle arrest (Rots et al. 1999).
Functional aspects of TIF1β during myeloid development
We have shown that TIF1β functions as a coactivator for C/EBPβ. Expression of C/EBPβ-responsive promoters was augmented by TIF1β. Either mutation of the C/EBPβ binding sites or ablation of TIF1β by antisense-ribozymes eliminated this augmentation. Further, we showed by two methods, coimmunoprecipitation and GST-pull downs, that endogenous TIF1β and C/EBPβ physically associate (directly or indirectly). EMSA experiments showed that an inducible complex that binds to C/EBPβ response elements can be supershifted with antibodies to TIF1β and C/EBPβ. We conclude that TIF1β associates with (directly or indirectly) and functions as a transcriptional coactivator of C/EBPβ.
However, this does not rule out other possible mechanisms by which TIF1β may function in myeloid cells. TIF1β binds to and acts as a corepressor for Kruppel-associated box (KRAB) domain-containing proteins (Friedman et al. 1996; Moosmann et al. 1996; Agata et al. 1999). The KRAB domain is a conserved motif at the N-termini of proteins that contain multiple Kruppel-class (C2H2) zinc fingers in their carboxyl termini (Bellefroid et al. 1991). Approximately 700 human genes encode C2H2 zinc finger proteins (Klug and Schwabe 1995), and one-third of these contain KRAB domains (Bellefroid et al. 1991). Because consensus binding sequences are not currently known for any of the KRAB domain proteins, their putative targets are unknown. However, the differential expression of several KRAB domain-containing zinc finger proteins during myeloid differentiation suggests they could be important in this developmental process. If so, another function for TIF1β might be to modulate their function (Bellefroid et al. 1991).
Additionally, TIF1β may also be involved in remodeling chromatin structure to alter gene expression during macrophage differentiation. It was originally isolated based on its ability to associate with the mouse homologue of the Drosophila heterochromatinic protein 1 alpha (HP1α) (Le Douarin et al. 1996). HP1α is a nonhistone chromosomal protein that exerts dose-dependent effects on heterochromatin-mediated gene silencing (Eissenberg et al. 1995; Elgin 1996) and shares a conserved N-terminal domain with Drosophila Pc, a repressor of homeotic gene expression (Koonin et al. 1995). TIF1β colocalizes with HP1α in heterochromatin, and also with HP1γ, present in euchromatin (Ryan et al. 1999). TIF1β contains a bromo domain, and in yeast, bromo domain–containing proteins associate with chromatin remodeling complexes (Aasland et al. 1995; Jeanmougin et al. 1997 and references therein), consistent with a role for TIF1β in determining chromatin structure. Also, TIF1β activity can be blocked by trichostatin A, an inhibitor of histone deacetylases (Nielsen et al. 1999).
Although all three known activities of TIF1β may be involved in macrophage differentiation, we have provided evidence of its ability to function as a coactivator of C/EBPβ. C/EBPβ is known to be a key transcriptional activator that is induced during macrophage activation and differentiation (Natsuka et al. 1992; Akira and Kishimoto 1996; Akira 1997). Indeed, mice lacking C/EBPβ have defective macrophage function (Tanaka et al. 1995; Poli 1998), showing the critical importance of C/EBPβ in macrophage biology. Our data show that TIF1β augments C/EBPβ-dependent transcription in U937 cells from both natural and artificial promoters. Furthermore, we have shown that induction of endogenous chemokine mRNAs known to depend on C/EBPβ, such as MIP1α (Matsumoto et al. 1998), MCP1 (Yamamoto et al. 1999), and IL8 (Kunsch et al. 1994) is ablated in U937 lines lacking TIF1β. An exception is the chemokine RANTES, which has been shown to be regulated by C/EBPβ in dengue-2-virus-infected human liver cells (Lin et al. 2000) and by respiratory syncytial virus (RSV)-infected airway epithelial cells (Casola et al. 2001), but whose expression in our hands is unaffected by the loss of TIF1β. This discrepancy may be due to tissue-specific differences in RANTES expression levels. We have shown that expression of CD14, also known to depend on C/EBPβ (Matsumoto et al. 1998), is inhibited in cells lacking TIF1β. Finally, antisera specific for C/EBPβ and TIF1β produce supershifts in EMSA experiments using oligonucleotides containing C/EBPβ binding sites and nuclear extracts from differentiated U937 cells. Thus, our data provide strong evidence that one function of TIF1β during U937 differentiation is to be a coactivator for C/EBPβ, augmenting C/EBPβ-dependent transcription.
Materials and methods
Representational difference analysis
RDA was performed as described previously (Hubank and Schatz 1994). HL-60 cells, untreated or treated with PMA for 2 h, were used to prepare total RNA by the Guanidinium/CsCl method (Ausubel et al. 1994). Poly A+ mRNA was selected using Oligotex beads (QIAGEN), and cDNA was synthesized using Superscript RT (GIBCO BRL). Two rounds of RDA were performed.
Plasmids
−205 ΔLTR-luciferase, mκB LTR-luciferase, and mC2,3 LTR-luciferase have been described previously (Henderson et al. 1995). pE4A10E4 (called p8XCRE luciferase in the study) was described previously (Artandi et al. 1994). pMSCV-NF-IL6-IRES-GFP was a gift from A.J. Henderson (Veternary Science, Pennsylvania State University, University Park).
Antisense-hammerhead ribozymes and construction of stable U937 cell lines
Modeling of TIF1β mRNA secondary structure was performed using mfold v.3.0 at the Washington University server address (http://mfold1.wustl.edu/∼mfold/rna//form11.cgi). For TIF1β Rz1, the antisense region corresponding from 907 to 1002 (relative to the transcriptional start site) was amplified, incorporating a three-nucleotide helix I hammerhead ribozyme (Tabler et al. 1994), using the primers 5′-GCTCTAGAGCGGGTGAAG TACACC-3′ and 5′-CCCCCCCAAGCTTATTCCTGATGAG GCCTCGAGGCCGAATGCTTGTGTACGTTG-3′. For TIF1β Rz2, the antisense region corresponding from 1442 to 1537 was amplified using the primers 5′-GCTCTAGACTTTTGCTTTC TAAGA-3′ and 5′-CCCCCCAAGCTTAGGACTGATGAGGC CTAGAGGCCGAACTGAAACTTCATCTC-3′. For β-gal Rz, the antisense region corresponding from 1220 to 1315 was amplified using the primers 5′-GCTCTAGAATGAAGCCAATAT TGA-3′ and 5′-CCCCCCAAGCTTAGCTCTGATGAGGCCT CGAGGCCGAATTCGCGTTACGCGTT-3′.
PCR reactions were carried out in an MJ thermocycler using as the template a plasmid containing full-length TIF1β cDNA (a kind gift from W. Schaffner, Institute fur Molejularbiologie II der Universitat Zurich, Zurich, Switzerland) or β-gal (pcDNAHygro(+)lacZ) (Invitrogen). Amplified products were digested with XbaI and HindIII and ligated into the pcDNAHygro(+) (Invitrogen) driven by the CMV promoter. Polymerase fidelity was verified by DNA sequencing.
Stable, Hygromicinr U937 cell lines containing the pcDNA–antisense-ribozyme constructs were produced by electroporation and cloned by limiting dilution. HL-60 and U937 cells (obtained from ATCC) were cultured in RPMI-1640 media supplemented with 10% fetal calf serum and 50 μg/mL gentamicin. Cells were induced using 25 nM PMA or 1.25% DMSO.
Isolation of human PMNs
Blood from normal healthy human volunteers was collected and pooled and used to isolate PMNs as described (Denholm and Wolber 1991). Cell viability (>95%) was assessed by trypan blue exclusion. The purity of the monocytes (80%) was determined by differential counts of Wright's stained cytocentrifuge preparations. PMNs were treated for 24 h with (20 ng/mL) GM-CSF and (50 ng/mL) M-CSF (both from R&D Systems).
Northern analysis
Northern analysis was performed as described previously (Ausubel et al. 1994). Total RNA (20 μg) was blotted onto Hybond-X Nylon membranes (Amersham), and hybridizations were performed using random primed probes (>108 cpm/μg) according to the manufacturer's instructions. Northern analysis for antisense-ribozyme expression employed a riboprobe corresponding to the hammerhead ribozyme.
Flow cytometry
Cells were washed with ice-cold PBS containing 0.5% BSA, preincubated for 60 min with mouse whole Ig to block Fc receptors, washed, and incubated with fluorochrome-conjugated monoclonal antibody for 60 min. Cells were stained with 7-AAD to facilitate selection of the viable cell population and analyzed on a Becton-Dickinson FACScan using CellQuest software. The monoclonal antibodies used were R-phycoerythrin (PE)-conjugated anti-CD11c, R-phycoerythrin (PE)-conjugated anti-CD14 (both from Pharmingen), and R-phycoerythrin (PE)-conjugated anti-Mac1 (Boehringer Mannheim). BrdU/PI staining was done using an in situ cell proliferation labeling kit (Roche/Boehringer Mannheim).
Infection of U937 cells with L. pneumophila
The L. pneumophila strain used in this study was the wild-type Philadelphia-1 strain. Growth conditions were as described previously (Horwitz and Silverstein 1980). U937 cells were used as host cells as described (Marra et al. 1990). Dye [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide, MTT] reduction assays to quantitate cytotoxicity of bacteria were performed as described (Marra et al. 1990) Uptake of L. pneumophila was done as described (Moore and Humbert 1986).
Phagocytosis
Heat-killed Staphylococcus aureus (ATCC) were labeled with 0.01% fluorescein isothiocyanate (FITC) isomer I (Sigma), then sonicated, and opsonized in an equal volume of human serum with mixing for 30 min at 37°C. Next, 108 FITC-labeled S. aureus were added to 106 U937 cells in 10 mL RPMI-1640 supplemented with 10% human serum and allowed to incubate at 37°C for 3 h. Cells were washed three times in PBS with 0.5% BSA. Fluorescence by extracellular bacteria was quenched by adding trypan blue (final concentration of 30 μg/mL) for 5 min, and then analyzed on a Becton-Dickinson FACScan.
Chemotaxis assay
Cell migration assays were performed as previously described (Bleul et al. 1996). Briefly, 15 × 104 U937 cells in 150 μL of RPMI-1640 medium containing 0.25% human serum transmigrated through 5 μm pore-size bare filter Transwell inserts (Costar) for 6 h. Migrated cells were counted using a hemacytometer. Chemotaxis was performed in the presence of optimized ligand concentrations of RANTES (75 ng/mL; R&D Systems) present in the lower chamber.
Ribonuclease protection assay
The ribonuclease protection assay was performed using a kit and templates from Pharmingen and carried out following the instructions of the manufacturer using 20 μg of total RNA. Protected probe fragments were analyzed by 5% denaturing PAGE.
Transient transfections and reporter assays
U937 transient transfections and luciferase assays were performed as described (Henderson et al. 1995). In all experiments, luciferase activity was normalized to the amount of protein used in the assay. In transfections of ribozyme-expressing stable cell lines, luciferase activity was normalized to renilla luciferase activity (1μg pTK-Renilla/transfection).
Preparation of whole-cell extracts, immunoprecipitations, and Western blotting
Whole-cell extracts from U937 cells, coimmunoprecipitations, and Westerns were preformed as described (Chang et al. 1998). GST pull-down experiments using GST-TIF1β, GST-C/EBPβ, and GST with whole-cell extracts were done as described (Chang et al. 1998).
Retroviral transduction of U937 cells
The full-length TIF1β cDNA was subcloned into the pGC-IRES-YFP retroviral plasmid (Costa et al. 2000). Infection retroviral supernatant was generated as described previously (Chang et al. 2000). Concentrated VSV-G pseudotyped virus containing pGC-IRES-YFP or pGC-TIF1β-IRES-YFP was produced and used to infect U937 cells using protocols from G. Nolan et al. (http://www.stanford.edu/group/nolan/NL-phnxr.html).
EMSA
Preparation of nuclear extracts and EMSAs were performed as described (Rooney et al. 1994). Antibodies against C/EBPα were from Santa Cruz Biotechnology. Antisera against TIF1β and C/EBPβ was a kind gift from Sheng-Chung Lee (Academia Sinica, College of Medicine, National Taiwan University, Taipei, Taiwan). Five microliter binding reactions were preincubated for 30 min at room temperature with 1 μL (2μg) of antibody.
Oligonucleotides used: HIV (-178 to -159) C/EBPwt top, 5′-GATCGCCTAGCATTTCATCACACGT-3′; HIV (-178 to -159) C/EBPwt consensus bottom, 3′-CGGATCGTAAAGTAGTGT GCACTAG-5′; HIV (-178 to -159) C/EBPmt consensus top, 5′-GATCGCCTAGCtgcaggggACACGT-3′; HIV (-178 to -159) C/EBPmt consensus bottom, 3′-CGGATCGacgtccccTGTG CACTAG-5′; AGP wt top (-92 to -70), 5′-GATCGCTGGTGA GATTGTGCCACAGCT-3′; AGP wt bottom (-92 to -70), 3′-CGACCACTCTAACACGGTGTCGACTAG-5′; AGP mt top (-92 to -70), 5′-GATCGCTGGTGAcagctgGCCACAGCT-3′; AGP mt bottom (-92 to -70), 3′-CGACCACTgtcgacCGGTGTC GACTAG-5′.
Acknowledgments
We thank Drs. Walter Schaffner, Sheng-Chung Lee, Andrew Henderson, Garry P. Nolan, Howard Shuman, and Henry Ginsberg. J.W.R. was funded in part by the Cancer Research Institute and an NIH Arteriosclerosis training grant (T32-HL07343). This work was supported by RO1 GM29361 and RO1 A143567 to K.L.C.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Footnotes
E-MAIL Klc1@columbia.edu; FAX (212) 305-1468.
Article and publication are at http://www.genesdev.org/cgi/doi/10.1101/gad.937201.
References
- Aasland R, Gibson TJ, Stewart AF. The PHD finger: Implication for chromatin-mediated transcriptional regulation. Trends Biochem Sci. 1995;20:56–59. doi: 10.1016/s0968-0004(00)88957-4. [DOI] [PubMed] [Google Scholar]
- Aderem A, Underhill DM. Mechanisms of phagocytosis in macrophages. Annu Rev Immunol. 1999;17:593–623. doi: 10.1146/annurev.immunol.17.1.593. [DOI] [PubMed] [Google Scholar]
- Agata Y, Matsuda E, Shimizu A. Two novel Kruppel-associated box-containing zinc-finger proteins, KRAZ1 and KRAZ2, repress transcription through functional interaction with the corepressor KAP-1 (TIF1β/KRIP-1) J Biol Chem. 1999;274:16412–16422. doi: 10.1074/jbc.274.23.16412. [DOI] [PubMed] [Google Scholar]
- Akira S. IL-6-regulated transcription factors. Int J Biochem Cell Biol. 1997;29:1401–1418. doi: 10.1016/s1357-2725(97)00063-0. [DOI] [PubMed] [Google Scholar]
- Akira S, Kishimoto T. IL-6 and NF-IL6 in acute-phase response and viral infection. Immunol Rev. 1992;127:25–50. doi: 10.1111/j.1600-065x.1992.tb01407.x. [DOI] [PubMed] [Google Scholar]
- Akira S, Kishimoto T. Role of interleukin-6 in macrophage function. Curr Opin Hematol. 1996;3:87–93. doi: 10.1097/00062752-199603010-00013. [DOI] [PubMed] [Google Scholar]
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Artandi S, Cooper C, Shrivastava A, Calame K. The basic helix-loop-helix-zipper domain of TFE3 mediates enhancer-promoter interaction. Mol Cell Biol. 1994;14:7704–7716. doi: 10.1128/mcb.14.12.7704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K. Current protocols in molecular biology. New York, NY: John Wiley and Sons; 1994. [Google Scholar]
- Bellefroid EJ, Poncelet DA, Lecocq PJ, Revelant O, Martial JA. The evolutionarily conserved Kruppel-associated box domain defines a subfamily of eukaryotic multifingered proteins. Proc Nat Acad Sci. 1991;88:3608–3612. doi: 10.1073/pnas.88.9.3608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleul CC, Fuhlbrigge RC, Casasnovas JM, Aiuti A, Springer TA. A highly efficacious lymphocyte chemoattractant, stromal cell-derived factor 1 (SDF-1) J Exp Med. 1996;184:1101–1109. doi: 10.1084/jem.184.3.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvo KR, Sykes DB, Pasillas M, Kamps MP. Hoxa9 immortalizes a granulocyte-macrophage colony-stimulating factor-dependent promyelocyte capable of biphenotypic differentiation to neutrophils or macrophages, independent of enforced Meis expression. Mol Cell Biol. 2000;20:3274–3285. doi: 10.1128/mcb.20.9.3274-3285.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casola A, Garofalo RP, Haeberle H, Elliott TF, Lin R, Jamaluddin M, Brasier AR. Multiple cis-regulatory elements control RANTES promoter activity in alveolar epithelial cells infected with respiratory syncytial virus. J Virol. 2001;75:6428–6439. doi: 10.1128/JVI.75.14.6428-6439.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C-J, Chen Y-L, Lee S-C. Coactivator TIF1β interacts with transcription factor C/EBPβ and glucocorticoid receptor to induce α1-acid glycoprotein gene expression. Mol Cell Biol. 1998;18:5880–5887. doi: 10.1128/mcb.18.10.5880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang DH, Angelin-Duclos C, Calame K. BLIMP-1: Trigger for differentiation of myeloid lineage. Nat Immunol. 2000;1:69–176. doi: 10.1038/77861. [DOI] [PubMed] [Google Scholar]
- Clarke S, Gordon S. Myeloid-specific gene expression. J Leuk Biol. 1998;63:153–168. doi: 10.1002/jlb.63.2.153. [DOI] [PubMed] [Google Scholar]
- Collins SJ, Gallo RC, Gallagher RE. Continuous growth and differentiation of human myeloid leukaemic cells in suspension culture. Nature. 1977;270:347–349. doi: 10.1038/270347a0. [DOI] [PubMed] [Google Scholar]
- Collins SJ, Ruscetti FW, Gallagher RE, Gallo RC. Terminal differentiation of human promyelocytic leukemia cells induced by dimethyl sulfoxide and other polar compounds. Proc Natl Acad Sci. 1978;75:2458–2462. doi: 10.1073/pnas.75.5.2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa GL, Benson JM, Seroogy CM, Achacoso P, Fathman CG, Nolan GP. Targeting rare populations of murine antigen-specific T lymphocytes by retroviral transduction for potential application in gene therapy for autoimmune disease. J Immunol. 2000;164:3581–3590. doi: 10.4049/jimmunol.164.7.3581. [DOI] [PubMed] [Google Scholar]
- Denholm EM, Wolber FM. A simple method for the purification of human peripheral blood monocytes: A substitute for Sepracell-MN. J Immunol Meth. 1991;144:247–251. doi: 10.1016/0022-1759(91)90092-t. [DOI] [PubMed] [Google Scholar]
- Eissenberg JC, Elgin SCR, Paro R. Epigenetic regulation in Drosophila: A conspiracy of silence. In: Elgin SCR, editor. Chromatin structure and gene expression. Oxford, UK: Oxford University Press; 1995. pp. 147–171. [Google Scholar]
- Elgin SRC. Heterochromatin and gene regulation in Drosophila. Curr Opin Genet Dev. 1996;6:193–202. doi: 10.1016/s0959-437x(96)80050-5. [DOI] [PubMed] [Google Scholar]
- Feinman R, Qin W, Pearse RN, Nikolajczyk BS, Sen R, Sheffery M, Ravetch JV. PU.1 and an HLH family member contributes to the myeloid-specific transcription of the FcγRIIIA promoter. EMBO J. 1994;13:3852–3860. doi: 10.1002/j.1460-2075.1994.tb06696.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman LP. Transcriptional targets of the vitamin D3 receptor-mediating cell cycle arrest and differentiation. J Nutrition. 1999;129:581–586. doi: 10.1093/jn/129.2.581S. [DOI] [PubMed] [Google Scholar]
- Friedman JR, Fredericks WJ, Jensen DE, Speicher DW, Huang W-P, Neilson E, Rauscher FJ., III KAP-1, a novel corepressor for the highly conserved KRAB repression domain. Genes & Dev. 1996;10:2067–2078. doi: 10.1101/gad.10.16.2067. [DOI] [PubMed] [Google Scholar]
- Gordon S. The macrophage. BioEssays. 1995;17:977–986. doi: 10.1002/bies.950171111. [DOI] [PubMed] [Google Scholar]
- Henderson AJ, Zou X, Calame KL. C/EBP proteins activate transcription from the human immunodeficiency virus type 1 long terminal repeat in macrophages/monocytes. J Virol. 1995;69:5337–5344. doi: 10.1128/jvi.69.9.5337-5344.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohaus S, Petrovick MS, Voso MT, Sun Z, Zhang DE, Tenen DG. PU.1 (Spi-1) and C/EBPα regulate the expression of the granulocyte-macrophage colony-stimulating factor receptor α gene. Mol Cell Biol. 1995;15:5830–5845. doi: 10.1128/mcb.15.10.5830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homann M, Tzortzakaki S, Rittner K, Sczakiel G, Tabler M. Incorporation of the catalytic domain of a hammerhead ribozyme into antisense RNA enhances its inhibitory effect on the replication of human immunodeficiency virus type 1. Nucleic Acids Res. 1993;21:2809–2814. doi: 10.1093/nar/21.12.2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hormes R, Homann M, Oeize I, Marschall P, Tabler M, Eckstein F, Sczakiel G. The subcellular localization and length of hammerhead ribozymes determine efficacy in human cells. Nucleic Acids Res. 1997;25:769–775. doi: 10.1093/nar/25.4.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz MA, Silverstein SC. Legionnaires' disease bacterium (Legionella pneumophila) multiplies intracellularly in human monocytes. J Clin Invest. 1980;60:441–450. doi: 10.1172/JCI109874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubank M, Schatz DG. Identifying differences in mRNA expression by representational difference analysis of cDNA. Nucleic Acids Res. 1994;22:5640–5648. doi: 10.1093/nar/22.25.5640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeanmougin F, Wurtz J-M, Le Douarin B, Chambon P, Losson R. The bromodomain revisited. Trends Biochem Sci. 1997;22:151–153. doi: 10.1016/s0968-0004(97)01042-6. [DOI] [PubMed] [Google Scholar]
- Klug A, Schwabe JW. Protein motifs 5. Zinc fingers. FASEB J. 1995;9:597–604. [PubMed] [Google Scholar]
- Koeffler HP. Induction of differentiation of human myelogenous leukemia cells: Therapeutic implications. Blood. 1983;62:709–715. [PubMed] [Google Scholar]
- Koeffler HP, Yelton L, Prokocimer M, Hirji K. Study of differentiation of fresh myelogenous leukemic cells by compounds that induce a human promyelocytic leukemic line (HL-60) to differentiation. Leuk Res. 1985;7:117–126. doi: 10.1016/0145-2126(85)90022-0. [DOI] [PubMed] [Google Scholar]
- Koonin EV, Zhou S, Lucchesi JC. The chromo superfamily: New members, duplication of the chromo domain and possible role in delivering transcription regulators to chromatin. Nucleic Acids Res. 1995;23:4229–4233. doi: 10.1093/nar/23.21.4229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunsch C, Lang RK, Rosen CA, Shannon MF. Synergistic transcriptional activation of the IL8 gene by NF-κB p65 (RelA) and NF-IL-6. J Immunol. 1994;153:153–164. [PubMed] [Google Scholar]
- Le Douarin B, Nielsen AL, Garnier J-M, Ichinose H, Jeanmougin F, Losson R, Chambon P. A possible involvement of TIF1α and TIF1β in the epigenetic control of transcription by nuclear receptors. EMBO J. 1996;15:6701–6715. [PMC free article] [PubMed] [Google Scholar]
- Lin Y-L, Liu C-C, Chuang J-I, Lei H-Y, Yeh T-M, Lin Y-S, Huang Y-H, Liu H-S. Involvement of oxidative stress, NF-IL-6, and RANTES expression in dengue-2-virus-infected human liver cells. Virology. 2000;276:114–126. doi: 10.1006/viro.2000.0524. [DOI] [PubMed] [Google Scholar]
- Liu M, Ivarone A, Freedman LP. Transcriptional activation of the human p21(WAF1/CIP1) gene by retinoic acid receptor. Correlation with retinoid induction of U937 cell differentiation. J Biol Chem. 1996;271:31723–31728. doi: 10.1074/jbc.271.49.31723. [DOI] [PubMed] [Google Scholar]
- Marra A, Horwitz MA, Shuman HA. The HL-60 model for the interaction of human macrophages with the legionnaires' disease bacterium. J Immunol. 1990;144:2738–2744. [PubMed] [Google Scholar]
- Mathews DH, Sabina J, Zuker M, Turner DH. Expanded sequence dependence of thermodynamic parameters provides robust prediction of RNA secondary structure. J Mol Biol. 1999;288:911–940. doi: 10.1006/jmbi.1999.2700. [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Sakao Y, Akira S. Inducible expression of nuclear factor IL-6 increases endogenous gene expression of macrophage inflammatory protein-1 alpha, osteopontin and CD14 in a monocytic leukemia cell line. Int Immunol. 1998;10:1825–1835. doi: 10.1093/intimm/10.12.1825. [DOI] [PubMed] [Google Scholar]
- McKercher SR, Torbett BE, Anderson KL, Henkel GW, Vestal D, Baribault H, Klemsz M, Feeney AJ, Wu GE, Paige CJ, et al. Disruption of the PU.1 gene leads to multiple hematopoietic defects. EMBO J. 1996;15:5647–5658. [PMC free article] [PubMed] [Google Scholar]
- Mink S, Kerber U, Klempnauer K-H. Interaction of C/EBPβ and v-Myb is required for synergistic activation of the mim-1 gene. Mol Cell Biol. 1996;16:1316–1325. doi: 10.1128/mcb.16.4.1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore L, Humbert J. Intracellular killing of bacteria and fungi. Methods Enzymol. 1986;132:520–528. doi: 10.1016/s0076-6879(86)32037-8. [DOI] [PubMed] [Google Scholar]
- Moosmann PG, Le Douarin B, Bourquin J-P, Schaffner W. Transcriptional repression by RING finger protein TIF1β that interacts with the KRAB repressor domain of KOX1. Nucleic Acids Res. 1996;24:4859–4867. doi: 10.1093/nar/24.24.4859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulton KS, Semple K, Wu H, Glass CK. Cell-specific expression of the macrophage scavenger receptor gene is dependent on PU.1 and a composite AP-1/ets motif. Mol Cell Biol. 1994;14:4408–4418. doi: 10.1128/mcb.14.7.4408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natsuka S, Akira S, Nishio Y, Hashimoto S, Sugita T, Isshiki H, Kishimoto T. Macrophage differentiation-specific expression of NF-IL6, a transcription factor for interleukin-6. Blood. 1992;79:460–466. [PubMed] [Google Scholar]
- Nielsen AL, Ortiz JA, You J, Oulad-Abdelghani M, Khechumian R, Gansmuller A, Chambon P, Losson R. Interaction with members of the heterochromatin protein 1 (HP1) family and histone deacetylation are differentially involved in transcriptional silencing by members of the TIF1 family. EMBO J. 1999;18:6385–6395. doi: 10.1093/emboj/18.22.6385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ness SA, Kowenz-Leutz E, Casini T, Graf T, Leutz A. Myb and NF-M: Combinatorial activators of myeloid genes in heterologous cell types. Genes & Dev. 1993;7:749–759. doi: 10.1101/gad.7.5.749. [DOI] [PubMed] [Google Scholar]
- Pahl HL, Scheibe RJ, Zhang D, Chen H, Galson DL, Maki RA, Tenen DG. The protooncogene PU.1 regulates expression of the myeloid-specific CD11b promoter. J Biol Chem. 1993;268:5014–5020. [PubMed] [Google Scholar]
- Patzel V, Sczakiel G. Theoretical design of antisense RNA structures substantially improves annealing kinetics and efficacy in human cells. Nat Biotechnol. 1998;16:64–68. doi: 10.1038/nbt0198-64. [DOI] [PubMed] [Google Scholar]
- Perez C, Coeffier E, Moreau-Gachelin F, Wietzerbin J, Benech PD. Involvement of the transcription factor PU.1/Spi-1 in myeloid cell-restricted expression of an interferon-inducible gene encoding the human high affinity Fc γ receptor. Mol Cell Biol. 1994;14:5023–5031. doi: 10.1128/mcb.14.8.5023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poli V. The role of C/EBP isoforms in the control of inflammatory and native immunity functions. J Biol Chem. 1998;273:29279–29282. doi: 10.1074/jbc.273.45.29279. [DOI] [PubMed] [Google Scholar]
- Ralph P, Moore MA, Nilsson K. Lysozyme synthesis by established human and murine histiocytic lymphoma cell lines. J Exp Med. 1976;143:1528–1533. doi: 10.1084/jem.143.6.1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralph P, Williams N, Moore MA, Litcofsky PB. Antibody-dependent and nonspecific tumor killing in human monocytic leukemia cells by non-lymphocyte factors and phorbol ester. Cell Immunol. 1982;71:215–232. doi: 10.1016/0008-8749(82)90257-x. [DOI] [PubMed] [Google Scholar]
- Rooney JW, Hodge MR, McCaffrey PG, Rao A, Glimcher LH. A common factor regulates both Th1- and Th2-specific cytokine gene expression. EMBO J. 1994;3:625–633. doi: 10.1002/j.1460-2075.1994.tb06300.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosmarin AG, Caprio D, Levy R, Simkevich C. CD18(β2 leukocyte integrin) promoter requires PU.1 transcription factor. Proc Natl Acad Sci. 1995;92:801–805. doi: 10.1073/pnas.92.3.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rots NY, Iavarone A, Bromleigh V, Freedman LP. Induced differentiation of U937 cells by 1,25-dihydroxyvitamin D3 involves cell cycle arrest in G1 that is preceded by a transient proliferative burst and an increase in cyclin expression. Blood. 1999;93:2721–2729. [PubMed] [Google Scholar]
- Rovera G, Santoli D, Damsky C. Human promyelocytic leukemia cells in culture differentiate into macrophage-like cells when treated with a phorbol diester. Proc Natl Acad Sci. 1979;76:2779–2783. doi: 10.1073/pnas.76.6.2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan RF, Schultz DC, Ayyanathan K, Singh PB, Friedman JR, Fredericks WJ, Rauscher FJ., III KAP-1 corepressor protein interacts and colocalizes with heterochromatic and euchromatic HP1 proteins: A potential role for Kruppel-associated box-zinc finger proteins in heterochromatin-mediated gene silencing. Mol Cell Biol. 1999;19:4366–4378. doi: 10.1128/mcb.19.6.4366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundstrom C, Nilsson K. Establishment and characterization of a human histiocytic lymphoma cell line (U-937) Int J Cancer. 1976;17:565–577. doi: 10.1002/ijc.2910170504. [DOI] [PubMed] [Google Scholar]
- Tabler M, Homann M, Tzortzakaki S, Sczakiel G. A three-nucleotide helix I is sufficient for full activity of a hammerhead ribozyme: Advantages of an asymmetric design. Nucleic Acids Res. 1994;22:3958–3965. doi: 10.1093/nar/22.19.3958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura T, Nagamura-Inoue T, Shmeltzer Z, Kuwata T, Ozato K. ICSBP directs bipotential myeloid progenitor cells to differentiate into mature macrophages. Immunity. 2000;13:155–165. doi: 10.1016/s1074-7613(00)00016-9. [DOI] [PubMed] [Google Scholar]
- Tanaka T, Akira S, Yoshida K, Umemoto M, Yoneda Y, Shirafuji N, Fujiwara H, Suematsu S, Yoshida N, Kishimoto T. Targeted disruption of the NF-IL6 gene discloses its essential role in bacteria killing and tumor cytotoxicity by macrophages. Cell. 1995;80:353–361. doi: 10.1016/0092-8674(95)90418-2. [DOI] [PubMed] [Google Scholar]
- Yamamoto K, Takeshima H, Hamada K, Nakao M, Kino T, Nishi T, Kochi M, Kuratsu J, Yoshimura T, Ushio Y. Cloning and functional characterization of the 5′-flanking region of the human monocyte chemoattractant protein-1 receptor (CCR2) gene. Essential role of 5′-untranslated region in tissue-specific expression. J Biol Chem. 1999;274:4646–4654. doi: 10.1074/jbc.274.8.4646. [DOI] [PubMed] [Google Scholar]
- Zhang D, Hetherington CJ, Tan S, Dziennis SE, Gonzalez DA, Chen H, Tenen DG. Sp1 is a critical factor for the monocytic specific expression of human CD14. J Biol Chem. 1994;269:11425–11434. [PubMed] [Google Scholar]
- Zhang EE, Hetherington CJ, Meyers S, Rhoades K, Larson CJ, Chen HM, Hiebert SW, Tenen DG. CCAAT enhancer-binding protein (C/EBP) and AML1 (CBFα2) synergistically activate the macrophage colony-stimulation factor receptor promoter. Mol Cell Biol. 1996;16:1231–1240. doi: 10.1128/mcb.16.3.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuker M, Mathews DH, Turner DH. Algorithms and thermodynamics for RNA secondary structure prediction: A practical guide. In: Barciszewski J, Clark BFC, editors. RNA biochemistry and biotechnology. New York, NY: Kluwer Academic Publishers; 1999. pp. 11–43. [Google Scholar]