Abstract
The TIM23 complex mediates translocation of proteins across, and their lateral insertion into, the mitochondrial inner membrane. Translocation of proteins requires both the membrane-embedded core of the complex and its ATP-dependent import motor. Insertion of some proteins, however, occurs in the absence of ATP, questioning the need for the import motor during lateral insertion. We show here that the import motor associates with laterally inserted proteins even when its ATPase activity is not required. Furthermore, our results suggest a role for the import motor in lateral insertion. Thus, the import motor is involved in ATP-dependent translocation and ATP-independent lateral insertion.
Keywords: mitochondria, TIM23, protein insertion
Introduction
A large fraction of proteins synthesized on cytosolic ribosomes are sorted by protein translocases to the site of their activity in one of the cell organelles. Whereas some protein translocases exclusively transport proteins across or insert them into membranes, other translocases—such as the SEC61 complex in the endoplasmic reticulum and the TIM23 complex in the mitochondria—can sort proteins both across and into membranes. The molecular mechanisms underlying switching between different modes of protein transport have remained largely unclear.
The TIM23 complex, the main protein translocase of the mitochondrial inner membrane, uses the energy of the membrane potential and ATP to transport almost all matrix proteins and many inner-membrane proteins (Neupert & Herrmann, 2007; Endo & Yamano, 2009; Schmidt et al, 2010). Its subunits are usually classified as constituents of either the membrane-embedded core of the complex or the import motor. The membrane-embedded core comprises the intermembrane space (IMS)-exposed receptors and the translocation channel. It is comprised of inner-membrane proteins Tim17, Tim23 and Tim50. The import motor is responsible for ATP-dependent translocation across the inner membrane. It consists of mitochondrial Hsp70 (mtHsp70)—the ATP-consuming subunit of the complex—and its cochaperones Tim44, Tim14(Pam18), Tim16(Pam16) and Mge1, which regulate the ATP-hydrolysis-driven cycle of mtHsp70. Tim21 and Pam17 are the only subunits that are dispensable for the viability of yeast cells. They both seem to interact with the membrane-embedded part of the complex (Popov-Čeleketić et al, 2008).
Most of the precursors transported by the TIM23 complex contain amino-terminal presequences (Neupert & Herrmann, 2007; Endo & Yamano, 2009; Schmidt et al, 2010). These are necessary and sufficient to transport proteins into the matrix through the TOM complex in the outer membrane and the TIM23 complex in the inner membrane. Some of the precursors transported by the TIM23 complex also contain a stop-transfer signal (Glick et al, 1992). These poorly defined hydrophobic segments arrest translocation at the level of the TIM23 complex and the complex opens laterally, leading to insertion of the transmembrane segment into the inner membrane. Translocation into the matrix of all precursors analysed so far depends on both the membrane-embedded core of the TIM23 complex and its import motor (Neupert & Herrmann, 2007; Endo & Yamano, 2009; Schmidt et al, 2010). Conversely, the requirements for the ATP-dependent activity of the import motor during transport of laterally sorted precursors differ between precursors. Proteins that have a long stretch between the presequence and the stop-transfer signal and/or have folded domains behind the stop-transfer signal need the ATPase activity of mtHsp70 for import into the mitochondria (Voos et al, 1993; Gartner et al, 1995; Mokranjac et al, 2003; Chacinska et al, 2010). Insertion of other precursors can occur even in its absence (Voos et al, 1993; Rojo et al, 1998).
There are two proposed models for protein transport by the TIM23 complex. According to the single-entity model, all essential subunits of the translocase function as one complex that is actively remodelled on recognition of targeting signals in the translocating chain (Popov-Čeleketić et al, 2008; Tamura et al, 2009). Thus, several conformational changes enable the TIM23 complex to sort proteins into two mitochondrial subcompartments. Tim21 and Pam17 facilitate these conformational changes, but are not required for them to take place. Conversely, the modular model suggests that the TIM23 complex exists in two forms (Chacinska et al, 2005, 2010). One contains Tim21 but lacks the import motor, and is responsible for lateral insertion. The other lacks Tim21 but contains the import motor, and is responsible for transport into the matrix. Both models reflect the highly dynamic nature of the TIM23 complex and agree that transport into the matrix and motor-dependent lateral insertion require both sectors of the translocase.
We reasoned that the two models could be distinguished by studying the import pathway of ATP-independent, laterally sorted precursors. The single-entity model would predict that the components of the import motor are present in the translocase during lateral insertion, even if its ATPase activity is not required. By contrast, the modular model would suggest that the components of the import motor are absent from the translocase during the same process. Analysis of the molecular environment of productive translocation intermediates of ATP-independent, laterally sorted precursors showed that components of both the import motor and the membrane-embedded core of the complex are found in the vicinity of such laterally sorted proteins. Furthermore, the data presented here suggest a specific role of Tim14 during lateral insertion. Our results provide evidence for the role of the import motor beyond the ATP-dependent translocation, and thus support the single-entity model.
Results and Discussion
Arrest of laterally sorted ATP-independent proteins
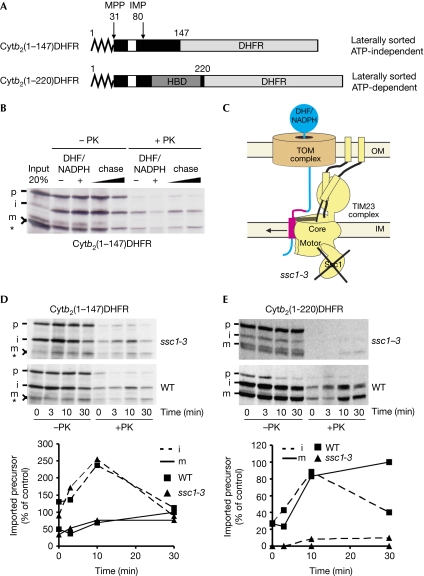
Chimeras that consist of up to 167 residues of yeast cytochrome b2 and mouse dihydrofolate reductase (DHFR) are laterally sorted by the TIM23 complex in an ATP-independent manner (Fig 1A; supplementary Fig S1A online; Glick et al, 1993; Voos et al, 1993). A construct that contains 220 residues of b2 is also laterally sorted but requires the ATP-dependent action of the import motor, due to the presence of a folded haem-binding domain (Fig 1A) (Glick et al, 1993; Voos et al, 1993). During import, these constructs are first processed by the matrix-processing peptidase—which removes the presequence in the matrix—and subsequently by the inner-membrane protease in the IMS, which cleaves the stop-transfer signal. The latter cleavage requires folding of the haem-binding domain, resulting in incomplete maturation of shorter constructs (Glick et al, 1993). A particularly useful characteristic of these chimeras is the possibility of generating intermediates that span two membranes by stably folding the DHFR domain in the presence of DHF, or its analogues, and nicotinamide adenine dinucleotide phosphate (reduced form; NADPH). On removal of DHF and NADPH, in vitro arrested ATP-dependent fusion proteins can be chased to their final location within the mitochondria, irrespective of whether they are sorted laterally into the inner membrane or translocated into the matrix (Chacinska et al, 2010). We asked whether the same is true for ATP-independent constructs. Indeed, both cytb2(1–147)DHFR and cytb2(1–167)DHFR could be arrested in this manner and subsequently chased to the protease-protected location in the mitochondria (Fig 1B; supplementary Fig S1B online). We reasoned that stabilization of a folded DHFR domain in this two-step import reaction might lead to an altered import pathway, by which their transport becomes dependent on the ATPase activity of the import motor. To test this, we used ssc1-3 mitochondria, which carry a temperature-sensitive mutant form of mtHsp70 that, on inactivation, cannot support ATP-dependent import (Fig 1C; Voos et al, 1993). Arrest and subsequent chase of cytb2(1–147)DHFR took place in ssc1-3 mitochondria as efficiently as in wild type (Fig 1D). The same was observed for chimeras that contain 107, 127 and 167 residues of b2 (supplementary Fig S1C–E online). By contrast, arrest and chase of ATP-dependent precursor cytb2(1–220)DHFR were impaired (Fig 1E). We conclude that these ATP-independent, laterally sorted precursors can be arrested and subsequently chased into the mitochondria, and that folding of the DHFR domain does not change their import pathway into an ATP-dependent one.
Figure 1.
Translocation arrest of substrates laterally sorted by the TIM23 complex. (A) Schematic representation of fusion proteins consisting of different segments of yeast cytochrome b2 (Cytb2; black box) and mouse DHFR (grey box). Zigzag line denotes the presequence and white boxes denote the transmembrane segment of b2. (B) 35S-labelled cytb2(1–147)DHFR was imported into wild-type mitochondria in the presence or absence of DHF and NADPH. DHF/NADPH-treated samples were reisolated, washed and incubated further for 5 and 20 min (chase). Samples were treated with PK where indicated and analysed by SDS–PAGE and autoradiography. Asterisk indicates a translation product arising from an internal methionine. (C) Schematic representation of the translocation arrest and lateral release in the ssc1-3 mutant mitochondria, which carry a temperature-sensitive mutant of mitochondrial Hsp70. (D,E) Wild-type and ssc1-3 mitochondria were preincubated for 10 min at 37 °C and used for import of indicated precursor proteins in the presence of DHF and NADPH. Mitochondria were reisolated, incubated further without NADPH/DHF for the indicated time periods and subsequently treated as described in (B). Autoradiographs are shown in the upper panels and quantifications of PK-protected material in the lower panels. PK-protected mature form at 30 min in WT was set to 100%. DHFR, dihydrofolate reductase; HBD, haem-binding domain; i, intermediate form of imported protein; IM, inner membrane; IMP, inner membrane protease; m, mature form of imported protein; MPP, mitochondrial processing peptidase; NADPH, nicotinamide adenine dinucleotide phosphate (reduced form); OM, outer membrane; p, precursor form of imported protein; PK, proteinase K; SDS–PAGE, SDS–polyacrylamide gel electrophoresis; WT, wild-type.
Molecular environment of arrested intermediates
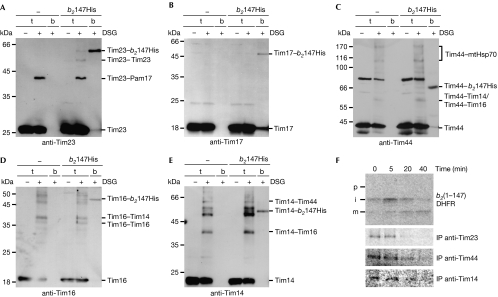
We analysed the molecular environment of in vivo-arrested, laterally sorted precursors by chemical crosslinking in intact mitochondria. A version of the above-described fusion protein cytb2(1–147)DHFRHis that has a C-terminal His-tag was expressed in yeast cells in the presence of aminopterin, a folate analogue, so that it became arrested as a two-membrane spanning intermediate in vivo (Wienhues et al, 1991; Popov-Čeleketić et al, 2008). Crosslinking was performed with isolated, intact mitochondria followed by isolation of arrested proteins and their crosslinking adducts on NiNTA-Agarose beads. Tim23 and Tim17—components of the membrane-embedded core of the translocase—were crosslinked to the arrested protein (Fig 2A,B). Crosslinks were detected if proteins were arrested in the translocase, but not if they were expressed under non-arrest conditions (supplementary Fig S2 online). Interestingly, we also detected crosslinks of cytb2(1–147)DHFRHis with Tim44 (Fig 2C), Tim16 (Fig 2D) and Tim14 (Fig 2E). This suggests that components of the import motor are also found in the vicinity of laterally sorted precursors. The sizes of the crosslinking adducts of cytb2(1–147)DHFRHis were indistinguishable from those of cytb2(1–167)Δ19DHFRHis, the matrix-targeted precursor, suggesting that it is either the precursor or the intermediate form of cytb2(1–147)DHFRHis that is crosslinked (supplementary Fig S3 online).
Figure 2.
Molecular environment of arrested, laterally sorted precursor. (A–E) Mitochondria isolated from cells containing arrested cytb2(1–147)DHFRHis (b2147His) or no precursor (control) were incubated with the crosslinker DSG, solubilized with SDS and incubated with NiNTA-Agarose beads. Bound material was eluted with Laemmli buffer containing imidazole and analysed by SDS–PAGE and immunodecoration with antibodies to (A) Tim23, (B) Tim17, (C) Tim44, (D) Tim16 and (E) Tim14. Known crosslinking adducts are indicated. (F) 35S-labelled cytb2(1–147)DHFR was imported for 15 min into mitochondria in the presence of NADPH/DHF. Mitochondria were reisolated, resuspended in import buffer and incubated further. At the indicated time points, aliquots were removed, and either treated with proteinase K and directly analysed by SDS–PAGE and autoradiography (top panel) or first subjected to crosslinking with DSG followed by solubilization with SDS and IP with antibodies to Tim23, Tim44 or Tim14, as indicated, before analysis by SDS–PAGE and autoradiography (lower panels). b, bound fraction (100%); DSG, disuccinimidyl glutarate; i, intermediate form of imported protein; IP, immunoprecipitation; m, mature form of imported protein; mtHsp70, mitochondrial Hsp70; NADPH, nicotinamide adenine dinucleotide phosphate (reduced form); p, precursor form of imported protein; SDS–PAGE, SDS–polyacrylamide gel electrophoresis; t, total (5%).
To analyse whether the observed crosslinks were derived from the productive, on-pathway intermediates we combined the in vitro arrest and chase assay using isolated mitochondria—as described above—with crosslinking followed by immunoprecipitation with affinity-purified antibodies to various subunits of the TIM23 complex. Crosslinks between Tim23, Tim44 and Tim14 and the in vitro arrested cytb2(1–147)DHFR were found (Fig 2F). This supports the results obtained with the same precursor arrested in vivo. Importantly, the crosslinks to the various TIM23 components disappeared as the protein was chased into the mitochondria. Thus, the observed crosslinks are the crosslinks of productive on-pathway intermediates. This further supports the notion that the components of the import motor are a genuine part of the translocase during lateral insertion of transmembrane segments.
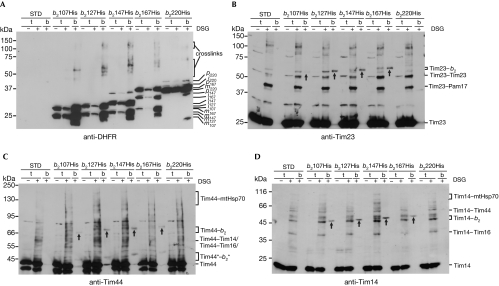
To determine which stage of lateral insertion the components of the import motor are involved in, we performed crosslinking of a series of laterally sorted precursors of different lengths. Precursors comprising 107, 127, 147, 167 and 220 residues of b2 fused to DHFR were expressed at similar levels (Fig 3A). However, their crosslinking patterns differed. Furthermore, the intensities of their crosslinking adducts increased with precursor length, yet disappeared in the longest one. This suggests that the different precursors occupy different positions in the TIM23 complex. Cytb2(1–220)DHFR progressed to a later transport step and probably left the TIM23 complex. Precursors comprising 107, 127, 147 and 167 residues of b2 were crosslinked to Tim23 (Fig 3B). Notably, the same precursors were also crosslinked to Tim44 (Fig 3C) and Tim14 (Fig 3D). Thus, the components of the import motor remain in the vicinity of the translocating chain during the entire process of lateral insertion.
Figure 3.
Molecular environment of arrested, laterally sorted precursors of different lengths. (A–D) Chimeras consisting of indicated segments of b2 and full-length mouse DHFR with a C-terminal His tag were expressed in yeast cells as translocation intermediates in mitochondria. Cells not expressing any chimera acted as a control (STD). Isolated mitochondria were incubated with the crosslinker DSG, solubilized with SDS and incubated with NiNTA-Agarose beads. Bound material was eluted with Laemmli buffer containing imidazole and analysed by SDS–polyacrylamide gel electrophoresis and immunodecoration with antibodies to (A) DHFR, (B) Tim23, (C) Tim44 and (D) Tim14. Arrows indicate the crosslinking adducts of TIM23 components with the arrested precursors. b, bound fraction (100%); DHFR, dihydrofolate reductase; DSG, disuccinimidyl glutarate; i, intermediate form of imported protein; m, mature form of imported protein; mtHsp70, mitochondrial Hsp70; p, precursor form of imported protein; t, total fraction (5%).
Role of Tim14 in lateral insertion
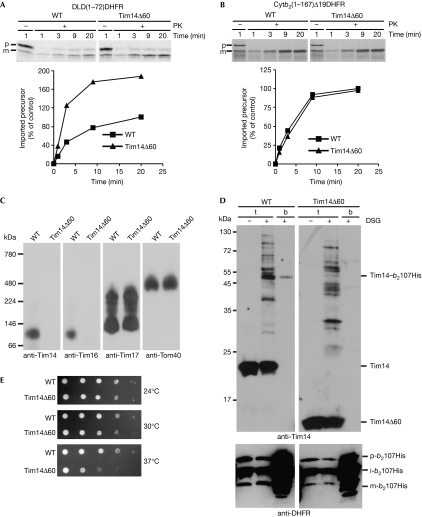
The import motor is exposed largely to the matrix. Yet, Tim14 is integrated into the inner membrane and exposes an approximately 60-residue-long segment to the IMS (Mokranjac et al, 2003). This segment of Tim14 has been shown to interact with Tim17 and the IMS domain of Tim23 (Chacinska et al, 2005; D’Silva et al, 2008; Tamura et al, 2009). We therefore reasoned that it might have a role in lateral insertion. To test this, a laterally sorted, ATP-independent precursor, DLD(1–72)DHFR (Rojo et al, 1998), was imported into wild-type mitochondria and mitochondria lacking the IMS domain of Tim14 (Tim14Δ60). Surprisingly, removal of the IMS domain of Tim14 resulted in a considerably higher import efficiency of this precursor (Fig 4A). As missorting of this precursor into the matrix might have occurred, we checked its submitochondrial location. Mitochondria were subjected to hypotonic swelling to rupture the outer membrane and treated with protease (supplementary Fig S4A online). The mature form of DLD(1–72)DHFR was degraded in both wild-type and Tim14Δ60 mitoplasts, showing that DLD(1–72)DHFR was correctly sorted into the inner membrane. We asked whether the same effect of Tim14Δ60 mitochondria would be observed with other laterally sorted precursors. Indeed, import of both cytb2(1–127)DHFR and cytb2(1–147)DHFR was more efficient in Tim14Δ60 than in wild type (supplementary Fig S4B,C online). By contrast, the import of matrix-targeted precursor cytb2(1–167)Δ19DHFR and a TIM23 complex-independent precursor, ATP/ADP carrier, were indistinguishable between the two types of mitochondria (Fig 4B; supplementary Fig S4D online). Thus, the IMS domain of Tim14 seems to modulate the rate of lateral release of transmembrane segments into the lipid bilayer.
Figure 4.
Role of Tim14 in lateral insertion. (A,B) Mitochondria were preincubated in import buffer for 10 min at 37 °C before the addition of indicated 35S-labelled precursors. Import reactions were performed at 25 °C. At the indicated time points, samples were removed and treated with PK where indicated. Samples were analysed by SDS–PAGE and autoradiography (upper panels). Quantifications of the PK-protected mature forms are shown in the lower panels. The signal obtained at the longest time point in wild-type mitochondria was set to 100%. (C) Mitochondria were preincubated for 10 min at 37 °C, solubilized with digitonin and analysed by BN-PAGE and immunodecoration with the depicted antibodies. (D) Wild-type and Tim14Δ60 mitochondria containing in vivo arrested cytb2(1–107)DHFRHis were subjected to crosslinking with DSG, solubilized with SDS and incubated with NiNTA-Agarose. Total (t, 5%) and bound (b, 100%) material were analysed by SDS–PAGE followed by immunodecoration with antibodies to Tim14 (upper panel) and DHFR (lower panel). (E) Serial dilutions of wild-type and Tim14Δ60 cells were plated on lactate medium and incubated for 60 h at the indicated temperatures. DHFR, dihydrofolate reductase; DSG, disuccinimidyl glutarate; i, intermediate form of imported protein; m, mature form of imported protein; p, precursor form of imported protein; PK, proteinase K; SDS–PAGE, SDS–polyacrylamide gel electrophoresis; WT, wild-type.
How can this be explained? The IMS domain of Tim14 might influence the rate of lateral insertion by affecting the conformation of the TIM23 complex. We therefore compared the crosslinking patterns of Tim23 in wild-type and Tim14Δ60 mitochondria. This assay is sensitive to changes in the conformation of the translocase (Popov-Čeleketić et al, 2008). The crosslinking patterns of Tim23 in the two types of mitochondria were indistinguishable (supplementary Fig S4E online). Furthermore, coimmunoprecipitation experiments from digitonin-solubilized mitochondria revealed no effect of removal of the IMS domain of Tim14 (Mokranjac et al, 2007; data not shown). Even the Triton X100-stable dimer of Tim14 and Tim16 (Kozany et al, 2004) was present in Tim14Δ60 mitochondria (supplementary Fig S4F online). However, the oligomeric complex of Tim14 and Tim16 on the blue native–PAGE, that is present in wild type, was absent in Tim14Δ60 mitochondria (Fig 4C). The other complexes, including the Tim17–Tim23 complex or the TOM complex, were not affected by removal of the IMS domain of Tim14. In contrast to full-length Tim14, Tim14Δ60 was not found in the crosslinking distance to the in vivo arrested cytb2(1–107)DHFRHis (Fig 4D, upper panel), although the expression levels and the processing pattern of the arrested protein did not differ between the two types of mitochondria (Fig 4D, lower panel). In addition, the Tim14Δ60 cells grew more slowly than wild type on lactate medium at higher temperatures (Fig 4E). Taken together, these results suggest an active role of Tim14 in modulating the rate of lateral insertion of transmembrane segments by the TIM23 complex.
The stop-transfer signal is specifically recognized by the TIM23 complex, although the molecular mechanisms for this remain unknown. Data presented here raise the possibility that Tim14 might be part of this recognition element.
Conclusion
The TIM23 complex mediates translocation of precursor proteins across, and their lateral insertion into, the mitochondrial inner membrane. Previous data have demonstrated that both the membrane-embedded part of the complex and its import motor are involved in translocation of precursors across the inner membrane. However, lateral insertion of some precursors can occur even in the absence of the ATPase activity of the import motor. It was thus not clear whether the import motor has a role in the process of lateral insertion. We show here that components of the import motor are in the vicinity of laterally sorted proteins and affect the efficiency of insertion even when the ATPase activity of the import motor is not required. Thus, the function of the import motor of the TIM23 complex extends beyond its ATP-dependent action during translocation of proteins across the inner membrane into the matrix.
Methods
Plasmids, yeast strains and growth conditions. Chimeras consisting of different segments of yeast cytochrome b2 and mouse DHFR were made using standard techniques. For in vitro transcription and translation, all constructs were cloned under the Sp6 promoter in pGEM4 (Promega). For expression in yeast they were cloned in pYES2 (Invitrogen) and transformed into wild-type yeast strain YPH499. Expression and in vivo arrest of chimeric constructs was performed as described previously (Popov-Čeleketić et al, 2008). The generation and growth conditions of Tim14Δ60 yeast strain have been described previously (Mokranjac et al, 2007).
Chemical crosslinking and isolation of crosslinking adducts. Chemical crosslinking and isolation of crosslinking adducts were performed as described previously (Mokranjac et al, 2003). Briefly, isolated mitochondria were incubated in the presence of disuccinimidyl glutarate for 30 min on ice. Disuccinimidyl glutarate is an amino-group-specific crosslinker with a spacer arm of 7.7 Å. After quenching the excess crosslinker, mitochondria were reisolated and solubilized in SDS-containing buffer to dissociate all non-covalent interactions. Samples were diluted with Triton X-100-containing buffer and incubated with either NiNTA-Agarose beads (for isolation of His-tagged precursors and their crosslinking adducts) or affinity-purified antibodies to TIM23 components bound to ProteinA-Sepharose (for isolation of crosslinking adducts of translocation intermediates arrested in vitro). Bound proteins were eluted with Laemmli buffer and samples were analysed by SDS–polyacrylamide gel electrophoresis and transferred to nitrocellulose membrane, followed by immunodecoration or autoradiography.
Miscellaneous. Previously described methods were used for protein import into isolated mitochondria (Mokranjac et al, 2003), in vitro arrest of precursor proteins with NADPH/DHF and subsequent chase into mitochondria (Chacinska et al, 2010) and coimmunoprecipitation from Triton-X100-solubilized mitochondria (Kozany et al, 2004). NativePAGE 4–16% Bis–Tris Gel system (Invitrogen) was used for BN-PAGE analysis. Mitochondria were solubilized with digitonin and further processed according to the manufacturer's instructions. For all figures, one of at least three independent experiments are shown.
Supplementary information is available at EMBO reports online (http://www.emboreports.org).
Supplementary Material
Acknowledgments
We acknowledge the expert technical assistance of M. Malesic, P. Robisch and H. Germeroth, K. Hell for stimulating discussions and the financial support of the Deutsche Forschungsgemeinschaft (SFB 594, B18 and B3) and German–Israeli Foundation.
Footnotes
The authors declare that they have no conflict of interest.
References
- Chacinska A et al. (2005) Mitochondrial presequence translocase: switching between TOM tethering and motor recruitment involves Tim21 and Tim17. Cell 120: 817–829 [DOI] [PubMed] [Google Scholar]
- Chacinska A et al. (2010) Distinct forms of mitochondrial TOM–TIM supercomplexes define signal-dependent states of preprotein sorting. Mol Cell Biol 30: 307–318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Silva PR, Schilke B, Hayashi M, Craig EA (2008) Interaction of the j-protein heterodimer pam18/pam16 of the mitochondrial import motor with the translocon of the inner membrane. Mol Biol Cell 19: 424–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo T, Yamano K (2009) Multiple pathways for mitochondrial protein traffic. Biol Chem 390: 723–730 [DOI] [PubMed] [Google Scholar]
- Gartner F, Voos W, Querol A, Miller BR, Craig EA, Cumsky MG, Pfanner N (1995) Mitochondrial import of subunit Va of cytochrome c oxidase characterized with yeast mutants. J Biol Chem 270: 3788–3795 [DOI] [PubMed] [Google Scholar]
- Glick BS, Beasley EM, Schatz G (1992) Protein sorting in mitochondria. Trends Biochem Sci 17: 453–459 [DOI] [PubMed] [Google Scholar]
- Glick BS, Wachter C, Reid GA, Schatz G (1993) Import of cytochrome b2 into intermembrane space: the tightly folded haem-binding domain makes import dependent upon matrix ATP. Prot Sci 2: 1901–1917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozany C, Mokranjac D, Sichting M, Neupert W, Hell K (2004) The J domain-related cochaperone Tim16 is constituent of the mitochondrial TIM23 preprotein translocase. Nat Struct Mol Biol 11: 234–241 [DOI] [PubMed] [Google Scholar]
- Mokranjac D, Sichting M, Neupert W, Hell K (2003) Tim14, a novel key component of the import motor of the TIM23 protein translocase of mitochondria. EMBO J 22: 4945–4956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mokranjac D, Berg A, Adam A, Neupert W, Hell K (2007) Association of the Tim14.Tim16 subcomplex with the TIM23 translocase is crucial for function of the mitochondrial protein import motor. J Biol Chem 282: 18037–18045 [DOI] [PubMed] [Google Scholar]
- Neupert W, Herrmann JM (2007) Translocation of proteins into mitochondria. Annu Rev Biochem 76: 723–749 [DOI] [PubMed] [Google Scholar]
- Popov-Čeleketić D, Mapa K, Neupert W, Mokranjac D (2008) Active remodelling of the TIM23 complex during translocation of preproteins into mitochondria. EMBO J 27: 1469–1480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojo EE, Guiard B, Neupert W, Stuart RA (1998) Sorting of D-lactate dehydrogenase to the inner membrane of mitochondria. Analysis of topogenic signal and energetic requirements. J Biol Chem 273: 8040–8047 [DOI] [PubMed] [Google Scholar]
- Schmidt O, Pfanner N, Meisinger C (2010) Mitochondrial protein import: from proteomics to functional mechanisms. Nat Rev Mol Cell Biol 11: 655–667 [DOI] [PubMed] [Google Scholar]
- Tamura Y, Harada Y, Shiota T, Yamano K, Watanabe K, Yokota M, Yamamoto H, Sesaki H, Endo T (2009) Tim23-Tim50 pair coordinates functions of translocators and motor proteins in mitochondrial protein import. J Cell Biol 184: 129–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voos W, Gambill BD, Guiard B, Pfanner N, Craig EA (1993) Presequence and mature part of preproteins strongly influence the dependence of mitochondrial protein import on heat shock protein 70 in the matrix. J Cell Biol 123: 119–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wienhues U, Becker K, Schleyer M, Guiard B, Tropschug M, Horwich AL, Pfanner N, Neupert W (1991) Protein folding causes an arrest of preprotein translocation into mitochondria in vivo. J Cell Biol 115: 1601–1609 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.