Abstract
The bacterium Bacillus subtilis produces the DNA integrity scanning protein (DisA), a checkpoint protein that delays sporulation in response to DNA damage. DisA scans the chromosome and pauses at sites of DNA lesions. Structural analysis showed that DisA synthesizes the small molecule cyclic diadenosine monophosphate (c-di-AMP). Here, we demonstrate that the intracellular concentration of c-di-AMP rises markedly at the onset of sporulation in a DisA-dependent manner. Furthermore, exposing sporulating cells to DNA-damaging agents leads to a global decrease in the level of this molecule. This drop was associated with stalled DisA complexes that halt c-di-AMP production and with increased levels of the c-di-AMP-degrading enzyme YybT. Reduced c-di-AMP levels cause a delay in sporulation that can be reversed by external supplementation of the molecule. Thus, c-di-AMP acts as a secondary messenger, coupling DNA integrity with progression of sporulation.
Keywords: B. subtilis , sporulation, DisA, c-di-AMP, DNA damage checkpoint
Introduction
To ensure accurate transmission of genomic information, cells have evolved complex surveillance mechanisms to monitor genomic integrity (Weinert & Hartwell, 1988; Elledge, 1996). In eukaryotes, replication and DNA damage checkpoints include components that detect DNA lesions and transduce signalling cascades that prevent progression through the cell cycle and activate DNA repair (Reinhardt & Yaffe, 2009). Although DNA damage checkpoints are known to exist in bacteria (Cairns, 2002), little is known about the cellular components that participate in these pathways. In Bacillus subtilis, replication block and DNA damage induce checkpoint responses that control the initiation of the developmental process of sporulation (Ireton & Grossman, 1992; Burkholder et al, 2001; Bejerano-Sagie et al, 2006).
During sporulation, B. subtilis undergoes complex morphological changes, culminating in the formation of a dormant spore. Sporulation is initiated by the construction of a polar septum that divides the cell into a small forespore compartment and a larger mother cell. Subsequently, the forespore is engulfed by the mother cell to form the fully mature spore (Piggot & Hilbert, 2004). The durability of the spore and its ability to germinate and propagate depend on its genomic integrity. Indeed, several mechanisms are used to proofread the genome at the onset of sporulation (Burkholder et al, 2001; Bejerano-Sagie et al, 2006). On replication block, a checkpoint protein called Sda binds to and inhibits a histidine kinase involved in activating Spo0A, the master regulator for entry into sporulation (Burkholder et al, 2001; Rowland et al, 2004). In a similar manner, we have shown that the DNA integrity scanning protein DisA triggers a sporulation DNA damage checkpoint response (Bejerano-Sagie et al, 2006).
DisA is a nonspecific DNA-binding protein that forms a focus which moves dynamically along chromosomes, scanning for lesions. When damage is encountered, DisA pauses at the lesion site and induces a cellular response that delays activation of Spo0A and culminates in a temporary block in sporulation before asymmetric division (Bejerano-Sagie et al, 2006). Elucidation of the crystal structure of DisA revealed that the molecules form an octamer comprising three domains: an amino-terminal globular domain with diadenylate-cyclase (DAC) activity, a central helical domain and a carboxy-terminal DNA-binding domain. The DAC domain converts a pair of ATP molecules into cyclic diadenosine monophosphate (c-di-AMP; Witte et al, 2008; Fig 1A). Four c-di-AMP moieties were found to be located between each of the four pairs within the octamer. Remarkably, c-di-AMP synthesis is inhibited when DisA binds to branched DNA, a structure that mimics DNA repair intermediates (Witte et al, 2008). By monitoring and modulating the c-di-AMP concentrations in vivo, we demonstrate here that the c-di-AMP intracellular level is utilized by DisA to report DNA integrity.
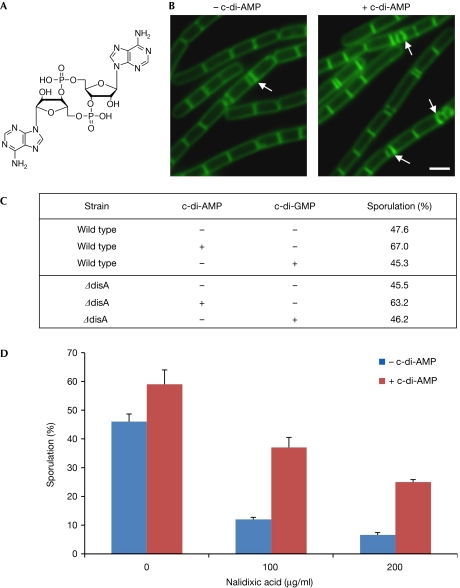
Figure 1.
Externally added c-di-AMP promotes progression of sporulation. (A) The chemical structure of c-di-AMP. (B) Fluorescence microscopy images of PY79 (wild-type) cells at 2 h of sporulation in the absence (–) and presence (+) of c-di-AMP (1.4 μM) added at 0 h. Cells were treated with the membrane stain FM1-43. Arrows indicate, positions of polar septa. Scale bar, 1 μm. (C) The percentage of sporulating PY79 (wild-type) and YA5 (ΔdisA) cells at 2 h of sporulation in the absence and presence of c-di-AMP or c-di-GMP (1.4 μM) added at 0 h. (D) The percentage of sporulating PY79 (wild-type) cells at 2.5 h of sporulation in the absence and presence of c-di-AMP (1.4 μM) and various concentrations of nalidixic acid as indicated. c-di-AMP and nalidixic acid were added at 0 h. (C,D) Cells were visualized using fluorescence microscopy, with the appearance of polar septa serving as the marker of sporulation. One representative experiment out of three independent experiments is shown. Standard deviation was calculated from the means of several fields of cells. At least 600 cells were counted for each treatment. c-di-AMP, cyclic diadenosine monophosphate; c-di-GMP, bis-(3’–5’)-cyclic dimeric guanosine monophosphate.
Results And Discussion
c-di-AMP promotes progression of sporulation
The assumption that DisA synthesizes c-di-AMP when scanning for DNA lesions, and stalls production when it ceases on a damage site, raised the idea that c-di-AMP positively signals sporulation to initiate. To examine this possibility, we supplemented sporulating B. subtilis cells with external c-di-AMP, and assayed the progression of sporulation. As c-di-AMP is negatively charged and does not cross the plasma membrane, we devised a method for c-di-AMP uptake (see Methods section). Wild-type cells were induced to sporulate in the presence and absence of c-di-AMP, and sporulation was monitored by fluorescence microscopy. Sporulation was stimulated in the presence of c-di-AMP, with asymmetric septa exhibited by 67.0% of the cells exposed to c-di-AMP compared with 47.6% of untreated cells (Fig 1B,C). Subsequently, we examined the effect of c-di-AMP on the expression of the spoIIE and racA genes, which are expressed before asymmetric division under Spo0A regulation (York et al, 1992; Ben-Yehuda et al, 2003). Monitoring fluorescence from strains harbouring spoIIE-gfp and racA-gfp fusions showed an approximately twofold increase in the number of cells showing a detectable signal in the presence of c-di-AMP than in its absence (supplementary Fig S1 online). When c-di-AMP was added at later stages of sporulation, no effect on the process was found (data not shown). Importantly, the addition of a similar molecule, bis-(3’–5’)-cyclic dimeric guanosine monophosphate (c-di-GMP), had no specific phenotype, implying that c-di-AMP has a specific impact on sporulation (Fig 1C). Finally, when ΔdisA cells were induced to sporulate in the presence of c-di-AMP, sporulation was promoted, similarly to wild-type cells (Fig 1C). Thus, an intracellular increase in c-di-AMP level leads to a rapid entry into sporulation, in a manner independent of DisA.
c-di-AMP bypasses the DNA damage checkpoint response
Next, we examined whether the addition of c-di-AMP could bypass the DisA-mediated DNA damage checkpoint. Wild-type cells were treated with different concentrations of the DNA-damaging agent nalidixic acid (NAL), and were induced to sporulate in the presence or absence of c-di-AMP. Consistent with previous results, entry into sporulation was delayed in NAL-treated cells, as indicated by the reduced asymmetric septa (Fig 1D; Bejerano-Sagie et al, 2006). Remarkably, the addition of c-di-AMP to NAL-treated cells increased polar septa formation (Fig 1D). Similar results were observed when the cells were exposed to the DNA-damaging agent Mitomycin C (MMC; data not shown). We conclude that the addition of c-di-AMP to sporulating cells overrides the inhibition of sporulation induced by the DNA damage checkpoint response.
Endogenous c-di-AMP levels correspond to DNA damage
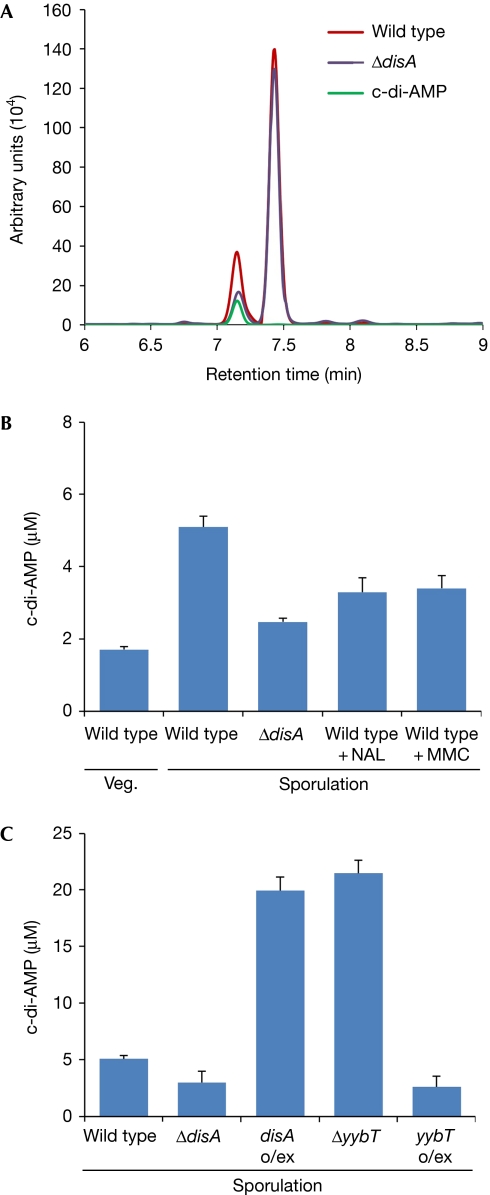
As sporulation can be induced by artificially increasing c-di-AMP levels, its cellular concentration might control the progression of the process. To explore this possibility, we developed a procedure to monitor the endogenous intracellular levels of c-di-AMP. Sporulating cells were lysed, and cell extract was enriched for nucleotides and analysed by high-performance liquid chromatography (HPLC; see Methods section). A peak with a retention time identical to that of purified c-di-AMP was readily detected (Fig 2A), indicating that sufficient amounts of c-di-AMP are produced by sporulating cells to be monitored by HPLC. A sequential analysis of the peak by high-resolution mass spectrometry was consistent with the molecular mass of c-di-AMP (658.4), confirming that the size of the peak corresponds to the cellular level of the investigated molecule (supplementary Fig S2 online). Importantly, when the HPLC profiles of extracts from sporulating wild-type and ΔdisA cells were compared, the c-di-AMP peak was found to be considerably larger for the wild type, indicating that DisA noticeably contributes to the cellular c-di-AMP pool (Fig 2A).
Figure 2.
Endogenous c-di-AMP levels correspond to DNA damage. (A) HPLC analysis of extracts from PY79 (wild type) and YA5 (ΔdisA) cells and commercially available c-di-AMP (see Methods section). (B,C) Intracellular c-di-AMP concentrations, as determined by HPLC (see Methods section). Each column represents the mean c-di-AMP concentration calculated from three independent biological samples, with bars representing standard deviation. (B) PY79 (wild-type) cells were collected during vegetative (Veg) growth and at 2 h of sporulation. Sporulation was carried out in the presence of 350 μg/ml NAL or 50 ng/ml MMC, as indicated. YA5 (ΔdisA) cells were collected at 2 h of sporulation. (C) PY79 (wild type), YA5 (ΔdisA), YA188 (ΔyybT), MB21 (Phyper−spank-disA; disA o/ex) and YA214 (Phyper−spank-yybT; yybT o/ex) were collected at 2 h of sporulation. c-di-AMP, cyclic diadenosine monophosphate; HPLC, high-performance liquid chromatography; MMC, Mitomycin C; NAL, nalidixic acid.
Quantification analysis was carried out to compare the cellular c-di-AMP levels during growth and sporulation (Fig 2B). c-di-AMP was detectable during vegetative growth (1.7 μM), implying that proteins other than DisA produce c-di-AMP throughout this phase. An increase in c-di-AMP levels was observed in cells undergoing sporulation, reaching a concentration (5.1 μM) threefold higher than that measured in vegetative cells. This elevation was dependent on DisA; as only a slight increase in c-di-AMP levels was observed in sporulating ΔdisA cells (Fig 2B). Consistently, overexpressing DisA during sporulation resulted in a fourfold increase in the concentration of endogenous c-di-AMP (Fig 2C).
As DisA molecules that are stalled at sites of DNA damage halt c-di-AMP production, we predicted that a drop in the concentration of this molecule would occur in the presence of DNA lesions. Indeed, when sporulating wild-type cells were treated with either NAL or MMC, the concentration of endogenous c-di-AMP dropped to a level close to that monitored in sporulating ΔdisA cells (Fig 2B).
These results are consistent with the view that the in vivo level of c-di-AMP during sporulation correlates with DNA integrity. When DisA complexes pause at sites of DNA lesions, c-di-AMP synthesis ceases, leading to a rapid decrease in the cellular concentration of the molecule.
DAC activity is required for DisA foci assembly
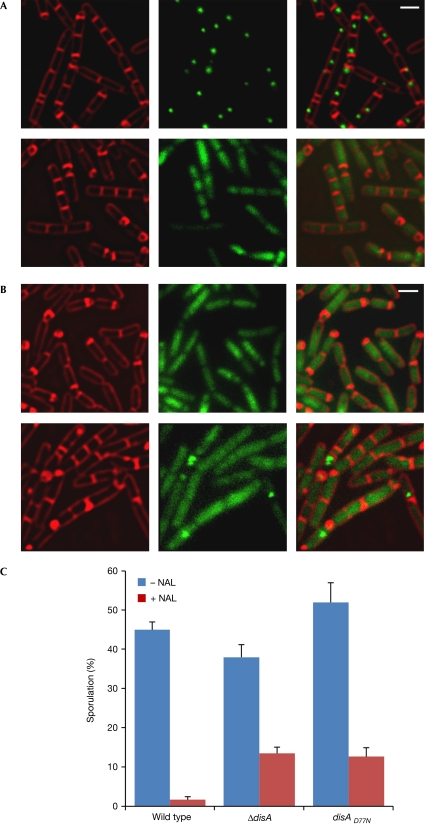
Given that DisA has a DAC domain that produces c-di-AMP, we investigated the manner in which DAC activity affects DisA function in vivo. To abolish c-di-AMP synthesis, we mutated the DAC active site (D77N; Witte et al, 2008), fused the mutated allele to gfp, and introduced the construct into B. subtilis by replacing the original disA gene. The resultant strain (YA127) was subjected to sporulation, followed by fluorescence microscopy. Surprisingly, the DisAD77N-green fluorescent protein (GFP) protein failed to form the normal DisA focus; it seemed to be spread homogenously within the cytoplasmic space (Fig 3A). Supplementation of exogenous c-di-AMP promoted sporulation, but failed to restore formation of the focus (data not shown), suggesting that the molecule has to be synthesized by the protein to maintain the focal structure. To assess whether DisA lacking DAC activity can trigger the DNA damage response, sporulating YA127 cells were treated with NAL. Despite DNA damage, many YA127 cells entered sporulation, at a proportion similar to that observed with ΔdisA cells (Fig 3C). In addition, the majority of DisAD77N-GFP molecules still failed to assemble into foci on the damaged sites after NAL treatment, with only small foci observed in a few cells (Fig 3B). It is possible that these foci are derived from DisAD77N-GFP molecules that colocalize while recognizing the same lesion. Thus, c-di-AMP synthesis is required for the construction or maintenance of the DisA focus and proper execution of the checkpoint response.
Figure 3.
Diadenylate cyclase activity is required for DisA foci assembly. (A,B) MB3 (disA-gfp) and YA127 (disAD77N-gfp) cells were induced to sporulate and subjected to fluorescence microscopy. FM4-64 (red) and signal from GFP (green) are shown. (A) MB3 (upper panels) and YA127 (lower panels) cells at 1.5 h of sporulation. (B) YA127 cells at 2.5 h of sporulation in the absence (upper panels) and presence of 350 μg/ml NAL (lower panels), added at 0 h of sporulation. (C) Percentage of sporulating MB3 (disA-gfp), YA5 (ΔdisA) and Y127 (disAD77N-gfp) cells at 3.5 h of sporulation in the absence (–) or presence (+) of 350 μg/ml NAL, added at 0 h of sporulation. Cells were visualized using fluorescence microscopy, with the appearance of polar septa serving as the marker of sporulation. One representative experiment out of three independent experiments is shown. Standard deviation was calculated from the means of several fields of cells. At least 600 cells were counted for each treatment. GFP, green fluorescent protein; NAL, nalidixic acid.
YybT controls intracellular c-di-AMP levels
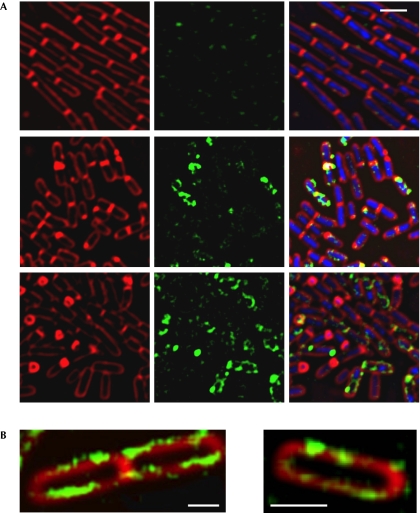
Another potential regulator of c-di-AMP is YybT, which was shown recently to hydrolyse c-di-AMP in vitro. Deletion of YybT increased survival of spores treated with DNA-damaging agents, suggesting that it antagonizes DisA (Rao et al, 2010). To investigate the way in which YybT contributes to the DisA signalling pathway, we examined its time of expression and subcellular localization. Cells harbouring yybT–gfp were induced to sporulate and followed by fluorescence microscopy (Fig 4A). At time 0, the fluorescence from YybT–GFP was faint and seemed to be mainly near the cell poles. As sporulation proceeded, there was a rise in fluorescence from YybT–GFP, with the signal localized into punctate structures around the cell circumference in the vicinity of the membrane (Fig 4A,B). This membrane association is probably mediated by the two predicted N-terminal transmembrane helices of YybT (Rao et al, 2010). The increase in YybT–GFP levels during sporulation was further corroborated by quantifying the total fluorescence intensity, and by western blot analysis (supplementary Fig S3 online).
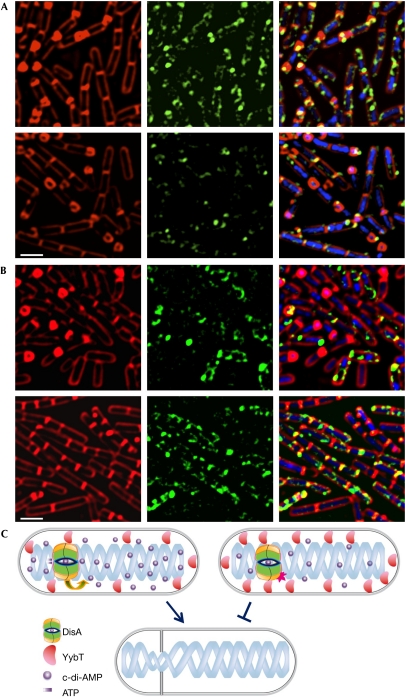
Figure 4.
YybT is expressed on entry into sporulation. (A) YA180 (yybT–gfp) cells were induced to sporulate and then subjected to fluorescence microscopy. Shown are FM4-64 (red), DAPI (blue) and signal from YybT–GFP (green) at 0 h (upper panels), 1.5 h (middle panels) and 3.5 h (lower panels) of sporulation. Images were scaled to the same intensity range. (B) Enlarged fluorescence images of YA180 (yybT–gfp) cells at 1.5 h of sporulation. Shown are FM4-64 (red) and signal from YybT–GFP (green). Scale bars, 1 μm. DAPI, 4,6-diamidino-2-phenylindole; GFP, green fluorescent protein.
The time period in which YybT–GFP expression was elevated correlated with the duration of DisA production (Bejerano-Sagie et al, 2006). However, no significant colocalization was observed between the two proteins (supplementary Fig S4 online), and dissimilarly to DisA dynamics, YybT was relatively stationary. Conversly, YybT–GFP expression was significantly decreased in a strain lacking DisA (Fig 5A; supplementary Fig S3 online), indicating that YybT production is partly dependent on levels of DisA or c-di-AMP.
Figure 5.
Components of the c-di-AMP pathway. (A) YA180 (yybT–gfp; upper panels) and YA185 (ΔdisA, yybT–gfp; lower panels) cells were induced to sporulate and then subjected to fluorescence microscopy. Shown are FM4-64 (red), DAPI (blue) and signal from YybT–GFP (green) at 3.5 h of sporulation. (B) YA180 (yybT–gfp) cells were induced to sporulate in the absence (upper panels) and presence (lower panels) of 50 ng/ml MMC. Shown are FM4-64 (red), DAPI (blue) and signal from YybT–GFP (green) at 3.5 h of sporulation. Images were scaled to the same intensity range. Scale bars, 1 μm. (C) A schematic illustration of the activity of c-di-AMP as a secondary molecule signalling DNA damage. Upper left cell: At the onset of sporulation, DisA moves within the cell, scanning the chromosome for lesions, and produces c-di-AMP. YybT localizes to the cell periphery and degrades c-di-AMP. The total concentration of c-di-AMP increases signalling sporulation to proceed (lower cell). Upper right cell: In the presence of broken DNA, DisA stalls at the site of lesion and halts c-di-AMP production. Consequently, YybT expression increases, further reducing the cellular c-di-AMP pool. The drop in c-di-AMP level inhibits sporulation (lower cell). c-di-AMP, cyclic diadenosine monophosphate; DAPI; 4,6-diamidino-2-phenylindole; DisA, DNA integrity scanning protein; GFP, green fluorescent protein; MMC, Mitomycin C.
To further elucidate whether YybT is active in the DisA checkpoint pathway, we examined YybT–GFP expression after induction of DNA damage. Cells harbouring YybT–GFP were treated with MMC during sporulation. YybT–GFP levels increased on exposure to DNA damage (Fig 5B; supplementary Fig S3 online), suggesting that YybT is involved in decreasing the levels of c-di-AMP. To substantiate that YybT regulates c-di-AMP concentrations, c-di-AMP levels were measured in ΔyybT cells, by using HPLC. A fourfold increase in c-di-AMP levels was evident in the mutant compared with wild-type cells, an increase similar to that observed in cells overproducing DisA (Fig 2C). Furthermore, YybT overexpression resulted in decreased c-di-AMP levels, to an extent similar to that seen in the absence of DisA (Fig 2C). We conclude that, similarly to DisA, YybT phosphodiesterase has a key role in altering the intracellular levels of the signalling molecule c-di-AMP, in a manner responsive to DNA integrity.
A model for c-di-AMP action as a secondary messenger
Pathways converting DNA lesions into active signals that affect cellular processes underlie genomic integrity. We uncover a primitive signalling system in B. subtilis that informs chromosomal lesions before proceeding into cellular differentiation. On the basis of our results and other recent reports (Witte et al, 2008; Rao et al, 2010), we propose a model in which broken DNA is sensed using a small molecule as a secondary messenger (Fig 5C). At the onset of sporulation, DisA molecules gather to form a focus that moves dynamically within the cells, converting pairs of ATP molecules into c-di-AMP. The focal assembly could reflect a strategy for achieving a synchronized synthesis of c-di-AMP. The latter is used to modify the activity of target proteins that enhance sporulation by affecting Spo0A. In this way, the rise in c-di-AMP level, generated by DisA, operates as a positive signal that advances sporulation. Upon encountering a lesion, the DisA focus arrests at the damaged site and halts c-di-AMP synthesis. In parallel, the production of the c-di-AMP-degrading enzyme YybT is increased. These two events lead to a rapid decline in the c-di-AMP cellular pool, which in turn modifies the activities of target proteins, culminating in a temporary block in sporulation.
The use of small molecules as messengers to report integrity of cellular processes is an efficient way to facilitate an immediate response, as they can rapidly diffuse and deliver information. The molecular targets of c-di-AMP are not known, and neither is the manner in which the molecule modifies their activity. c-di-AMP might bind to sporulation effector proteins directly and alter their conformation, thereby modifying their function. Such a strategy is used by the nucleotide GTP that modulates the activation of the transcription factor CodY in B. subtilis. GTP-bound CodY functions as a repressor of various stationary-phase and sporulation genes, thereby globally controlling a developmental transition (Ratnayake-Lecamwasam et al, 2001). Similarly, c-di-GMP was found to bind to and activate the motility break protein YcgR, causing the Escherichia coli motility motor to slow down (Ross et al, 1987; Boehm et al, 2010; Paul et al, 2010). Thus, small molecules can modulate the activity of macromolecules, triggering global changes in cellular pathways.
c-di-AMP prevalence in bacteria
The importance of c-di-AMP as a signalling molecule in bacteria is only beginning to be appreciated. A DAC domain has been identified in 275 species including gram-positive and -negative (Romling, 2008), supporting the idea that this molecule is ubiquitous and probably functions in various cellular processes. In B. subtilis, the DAC domain has been identified in two other proteins, YbbP and YojJ, with unknown functions (Romling, 2008). We found that in the absence of these proteins, the basal level of c-di-AMP observed during vegetative growth was almost undetectable (data not shown), suggesting that they affect the cellular concentration of this molecule. Recently, c-di-AMP was shown to be generated by the bacterium Listeria monocytogenes and to trigger a host innate immune response during infection (Woodward et al, 2010). Whether c-di-AMP production is restricted to bacteria, or whether it functions as a secondary messenger in other organisms will be the subject of future research.
Methods
General methods, fluorescence-microscopy methods, calculation of fluorescence levels, quantification of c-di-AMP by HPLC and mass spectrometry, and plasmid constructions are presented in the supplementary information online.
Strains. B. subtilis strains were derivatives of the wild-type strain PY79 and are listed in supplementary Table S1 online. Plasmid constructions are described in supplementary Table S2 online and in the supplementary Methods online.
Introducing c-di-AMP into living cells. A concentration of 1.4 μM c-di-AMP (BIOLOG) was incubated with 0.04 nM polyamines (Aldrich) for 20 min at 37 °C. The mixture was added to cells at 0 h of sporulation. Polyamines were added to all samples in a given experiment, as a control. Sporulation progression was unaffected by the presence of polyamines up to 4 h after addition. However, polyamines affect later stages of sporulation and reduce the number of viable spores. Polyamines enhance penetration of DNA-damaging agents; thus, when using polyamines, concentrations of DNA-damaging agents were reduced.
Extraction of c-di-AMP from living cells. A volume of 1 litre of cell culture was centrifuged at 4,000 r.p.m. for 30 min; the cell pellet was resuspended in 10ml lysis buffer (10 mM Tris pH 8, 10 mM MgCl2 and 0.5 mg/ml lysozyme) and incubated at 37 °C for 30 min. Extracts were centrifuged at 4,000 r.p.m. for 30 min and the supernatant was removed. The extraction procedure was repeated twice by resuspending the resulting pellets in 2 ml lysis buffer. The collected supernatant fluid was heated at 100 °C for 3 min and then supplemented with 100% ethanol (final concentration 70%). The supernatant was incubated for 20 min on ice and centrifuged at 7,000 r.p.m. for 10 min at 4 °C, to separate insoluble material from nucleotides. It was then incubated at −80 °C for 1 h and heat-dried at 40 °C. Pellets were resuspended in 1 ml H2Ox2, filtered (0.22 μm) and analysed by HPLC and mass spectrometry.
Supplementary information is available at EMBO reports online (http://www.emboreports.org).
Supplementary Material
Acknowledgments
We are grateful to A. Rouvinski (Hebrew University), R. Losick (Harvard University) and members of the Ben-Yehuda laboratory for discussions and comments. We thank O. Moshel (Hebrew University) for mass spectrometry analysis. This work was supported by the Human Frontier Science Program Young Investigator Award (RGY78/2007), and by the United States–Israel Binational Foundation grant (2005235) awarded to S.B.-Y.
Footnotes
The authors declare that they have no conflict of interest.
References
- Bejerano-Sagie M, Oppenheimer-Shaanan Y, Berlatzky I, Rouvinski A, Meyerovich M, Ben-Yehuda S (2006) A checkpoint protein that scans the chromosome for damage at the start of sporulation in Bacillus subtilis. Cell 125: 679–690 [DOI] [PubMed] [Google Scholar]
- Ben-Yehuda S, Rudner DZ, Losick R (2003) RacA, a bacterial protein that anchors chromosomes to the cell poles. Science 299: 532–536 [DOI] [PubMed] [Google Scholar]
- Boehm A, Kaiser M, Li H, Spangler C, Kasper CA, Ackermann M, Kaever V, Sourjik V, Roth V, Jenal U (2010) Second messenger-mediated adjustment of bacterial swimming velocity. Cell 141: 107–116 [DOI] [PubMed] [Google Scholar]
- Burkholder WF, Kurtser I, Grossman AD (2001) Replication initiation proteins regulate a developmental checkpoint in Bacillus subtilis. Cell 104: 269–279 [DOI] [PubMed] [Google Scholar]
- Cairns J (2002) A DNA damage checkpoint in Escherichia coli. DNA Repair (Amst) 1: 699–701 [DOI] [PubMed] [Google Scholar]
- Elledge SJ (1996) Cell cycle checkpoints: preventing an identity crisis. Science 274: 1664–1672 [DOI] [PubMed] [Google Scholar]
- Ireton K, Grossman AD (1992) Coupling between gene expression and DNA synthesis early during development in Bacillus subtilis. Proc Natl Acad Sci USA 89: 8808–8812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul K, Nieto V, Carlquist WC, Blair DF, Harshey RM (2010) The c-di-GMP binding protein YcgR controls flagellar motor direction and speed to affect chemotaxis by a ‘Backstop Brake’ mechanism. Mol Cell 38: 128–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piggot PJ, Hilbert DW (2004) Sporulation of Bacillus subtilis. Curr Opin Microbiol 7: 579–586 [DOI] [PubMed] [Google Scholar]
- Rao F, See RY, Zhang D, Toh DC, Ji Q, Liang ZX (2010) YybT is a signaling protein that contains a cyclic dinucleotide phosphodiesterase domain and a GGDEF domain with ATPase activity. J Biol Chem 285: 473–482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratnayake-Lecamwasam M, Serror P, Wong KW, Sonenshein AL (2001) Bacillus subtilis CodY represses early-stationary-phase genes by sensing GTP levels. Genes Dev 15: 1093–1103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhardt HC, Yaffe MB (2009) Kinases that control the cell cycle in response to DNA damage: Chk1, Chk2, and MK2. Curr Opin Cell Biol 21: 245–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romling U (2008) Great times for small molecules: c-di-AMP, a second messenger candidate in Bacteria and Archaea. Sci Signal 1: pe39. [DOI] [PubMed] [Google Scholar]
- Ross P et al. (1987) Regulation of cellulose synthesis in Acetobacter xylinum by cyclic diguanylic acid. Nature 325: 279–281 [DOI] [PubMed] [Google Scholar]
- Rowland SL, Burkholder WF, Cunningham KA, Maciejewski MW, Grossman AD, King GF (2004) Structure and mechanism of action of Sda, an inhibitor of the histidine kinases that regulate initiation of sporulation in Bacillus subtilis. Mol Cell 13: 689–701 [DOI] [PubMed] [Google Scholar]
- Weinert TA, Hartwell LH (1988) The Rad9 gene controls the cell-cycle response to dna damage in Saccharomyces cerevisiae. Science 241: 317–322 [DOI] [PubMed] [Google Scholar]
- Witte G, Hartung S, Buttner K, Hopfner KP (2008) Structural biochemistry of a bacterial checkpoint protein reveals diadenylate cyclase activity regulated by DNA recombination intermediates. Mol Cell 30: 167–178 [DOI] [PubMed] [Google Scholar]
- Woodward JJ, Iavarone AT, Portnoy DA (2010) c-di-AMP secreted by intracellular Listeria monocytogenes activates a host type I interferon response. Science 328: 1703–1705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- York K, Kenney TJ, Satola S, Moran CP Jr, Poth H, Youngman P (1992) Spo0A controls the sigma A-dependent activation of Bacillus subtilis sporulation-specific transcription unit spoIIE. J Bacteriol 174: 2648–2658 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.