Abstract
Mosquito-borne diseases such as malaria, dengue fever and filariasis cause an enormous health burden to people living in tropical and subtropical regions of the world. Despite years of intense effort to control them, many of these diseases are increasing in prevalence, geographical distribution and severity, and options to control them are limited. The transinfection of mosquitos with the maternally inherited, endosymbiotic bacteria Wolbachia is a promising new biocontrol approach. Fruit fly Wolbachia strains can invade and sustain themselves in mosquito populations, reduce adult lifespan, affect mosquito reproduction and interfere with pathogen replication. Wolbachia-infected Aedes aegypti mosquitoes have been released in areas of Australia in which outbreaks of dengue fever occur, as a prelude to the application of this technology in dengue-endemic areas of south-east Asia.
Keywords: Aedes aegypti , Chikungunya, dengue, Drosophila , Wolbachia pipientis
See Glossary for abbreviations used in this article.
Glossary.
- CI
cytoplasmic incompatibility
- CHIKV
Chikungunya virus
- CLA
cell-line adapted
- DCV
Drosophila C virus
- DENV
dengue virus
- LRIM1
leucine-rich repeat immune protein
- TEP1
thioester containing protein
Introduction
Insect-borne diseases, particularly those transmitted by mosquitoes, are among the leading causes of mortality and morbidity in humans. Malaria—caused by infection of Plasmodium protozoan parasites by the bite of anopheline mosquitoes—results in an estimated 1–2 million deaths per year, taking a dramatic toll on health and socioeconomic development in affected areas (WHO, 2008). The annual incidences of mosquito-borne diseases including dengue fever, yellow fever, Japanese encephalitis, West Nile virus, Chikungunya and lymphatic filariasis are increasing due to human travel, rapid urbanization and failures of preventative public-health measures (Adams & Kapan, 2009; Chen & Wilson, 2010; Gould & Solomon, 2008; Gubler, 2002). Dengue fever is the most important arboviral disease in humans; 40% of the population of the world in more than 100 countries is at risk of infection and an estimated 50 million–100 million cases occur annually (Guzman & Kouri, 2002; Kyle & Harris, 2008; WHO, 2009). Dengue viruses (DENV) are primarily transmitted by the infectious bite of female Aedes aegypti mosquitoes and, to a much lesser extent, Aedes albopictus (Lambrechts et al, 2010). No effective vaccines or treatments against dengue fever exist (Wilder-Smith et al, 2010), and control methods are failing to prevent the global increase in the incidence of the disease (Morrison et al, 2008). New approaches are clearly needed if these trends are to be reversed.
The use of insecticides to target mosquitoes as a means of disease control can be effective, but is often prohibitively expensive, unsustainable and environmentally undesirable. This is particularly evident for anthropophilic species such as A. aegypti, which breed in densely populated urban and semi-urban areas (Wilder-Smith & Gubler, 2008). Furthermore, repeated exposure of mosquitoes to insecticides has allowed insecticide resistance to develop, increasing the need to use more-expensive alternative compounds (WHO, 1998; Zaim & Guillet, 2002). Insecticides are normally only used for the control of dengue fever during outbreaks, and their effectiveness is questionable. Alternative approaches aimed at environmental management of mosquitoes—such as the removal of oviposition sites, the introduction of mosquito predators such as fish or copepods (Kay & Vu, 2005) and personal protection against mosquito bites (repellents and nets)—have been beneficial in some cases. However, these strategies often require constant intervention, and can be expensive and difficult to implement in urban areas.
Wolbachia pipientis and disease control
The potential application of the symbiotic bacteria Wolbachia pipientis to the control of mosquito-borne diseases has emerged as a recent addition to the arsenal of weapons against mosquitoes. It has the benefit of being more environmentally benign than insecticide-based approaches and potentially more cost effective. Wolbachia-induced cytoplasmic incompatibility (CI) was proposed as a tool for Culex mosquito control as early as 1967 (Laven, 1967) and there were trials to eradicate mosquitoes in India in the 1970s (Curtis & Adak, 1974), but although there has been some field testing, it has never been operationally implemented. In recent years, there has been a resurgence of interest in Wolbachia as a means by which to control insect-transmitted diseases.
Wolbachia—an initially obscure α-proteobacterium, first identified in the ovaries of Culex mosquitoes in 1924 (Hertig & Wolbach, 1924)—is probably the most-common known endosymbiotic microbe in the biosphere. It is thought to infect up to 76% of the estimated 2 million–5 million insect species on Earth (Hilgenboecker et al, 2008; Jeyaprakash & Hoy, 2000). The success of these small (0.5–1μm), intracellular bacteria has been attributed to their ability to induce a series of reproductive distortions in their hosts to increase the reproductive success of infected females, thus enhancing the maternal transmission of Wolbachia (Werren et al, 2008). These traits include transforming genotypic males into phenotypic females, modifying male sperm so that females cannot produce progeny unless they mate with a male infected with the same strain of Wolbachia, or inducing the parthenogenetic reproduction of females (Stouthamer et al, 1999). Wolbachia can also provide direct fitness benefits to their hosts by affecting nutrition and development (Brownlie et al, 2009; Hosokawa et al, 2010), influencing fecundity (Aleksandrov et al, 2007) or oogenesis (Dedeine et al, 2001) and providing resistance to pathogens (Bian et al, 2010; Glaser & Meola, 2010; Hedges et al, 2008; Kambris et al, 2010; Moreira et al, 2009a; Osborne et al, 2009; Panteleev et al, 2007; Teixeira et al, 2008). Interestingly, some Wolbachia strains seem to have lost the ability to synchronize their replication with the host cell, and can dramatically reduce the lifespan of their hosts. One Wolbachia strain (wMelPop) that shortens the lifespan of adult Drosophila by up to 50% was discovered from experiments on fruit-fly lifespan mutants (Min & Benzer, 1997). This strain—also named ‘popcorn’, due to its ability to over-replicate and fill the brain tissues of infected flies—has been proposed as a potential tool for the control of mosquito-borne diseases, as it also reduces the longevity of adult female mosquitoes (Brownstein et al, 2003; McMeniman et al, 2009; Sinkins & O'Neill, 2000).
Although mosquito-borne pathogens can differ intrinsically—for example, malaria is caused by a protozoan parasite, whereas dengue fever is caused by a single-stranded RNA virus—their transmission is always influenced by the age of the mosquito. The reason for this is relatively simple: pathogens need to replicate in the body of the mosquito before reaching the salivary glands, in order to be successfully transmitted into a new human host by a bite. This period of development within the mosquito is called the extrinsic incubation period (EIP). Consequently, only female mosquitoes that are older than the EIP—usually 10–14 days for dengue fever (Salazar et al, 2007)—are vectors of epidemiological importance. Thus, disease-control approaches that aim to reduce mosquito lifespan have the potential to decrease disease transmission (Brownstein et al, 2003; Cook et al, 2008; Rasgon et al, 2003; Sinkins & O'Neill, 2000). Using wMelPop Wolbachia for disease control involves transferring this life-shortening Wolbachia strain into mosquito populations to remove the older individuals, which are the ones able to transmit the pathogen, from the population. The efficient maintenance and spread of the Wolbachia infection into field populations is crucial to the success of this strategy. Parallel approaches to reduce mosquito lifespan are also being developed, such as the use of spores from entomopathogenic fungi such as Beauveria bassiana in mosquito traps (Blanford et al, 2005; Darbro & Thomas, 2009; Thomas & Read, 2007) or delayed-acting insecticides (Read & Thomas, 2009).
Wolbachia transfer into A. aegypti mosquitoes
Although Wolbachia infections are relatively common in mosquitoes (Kittayapong et al, 2000; Ricci et al, 2002) including Culex pipiens (Yen & Barr, 1973), C. quinquefasciatus, Aedes fluviatilis (Moreira et al, 2009a) and A. albopictus (Sinkins et al, 1995), the main vectors for dengue fever (A. aegypti) and malaria (Anopheles spp.) are not naturally infected by Wolbachia. Approaches that use Wolbachia for the control of diseases transmitted by uninfected, naive insects rely on the successful establishment of stable Wolbachia infections, usually by embryonic microinjection of Wolbachia-infected cytoplasm or Wolbachia purified from infected insect hosts. To create stably transinfected lines, embryo injections must target the region near the pole cells in pre-blastoderm embryos to incorporate Wolbachia into the developing germline and favour the transmission of Wolbachia to offspring. Several Wolbachia strains have been transferred across sometimes phylogenetically distant insects (Table 1) and, importantly, the phenotypes induced by these strains in their native hosts are generally also expressed in the newly infected hosts.
Table 1. Wolbachia strains transinfected into mosquitoes and induced phenotypes.
| Strain | Original host | Transinfected host | Phenotype in new host | Reference |
|---|---|---|---|---|
| wMel | Drosophila melanogaster, then Aedes albopictus cell line | Aedes aegypti | CI, DENV interference | (T. Walker et al, unpublished data) |
| wMelPop-CLA | Drosophila melanogaster, then Aedes albopictus cell line | Aedes aegypti | CI, life shorteningBlood-feeding alterationBendy proboscisPlasmodium, DENV, CHIKV interferenceIncreased metabolismIncreased activityInhibition of filarial nematodes | (McMeniman et al, 2009)(Turley et al, 2009)(Moreira et al, 2009b)(Moreira et al, 2009a)(Evans et al, 2009)(Kambris et al, 2009) |
| wAlbB | Aedes albopictus | Aedes aegypti | CI DENV interference | (Xi et al, 2005)(Bian et al, 2010) |
| wAlbA, B | Aedes albopictus | Aedes aegypti | Partial CI | (Ruang-Areerate & Kittayapong, 2006) |
| wRi | Drosophila simulans | Aedes albopictus | CI | (Xi et al, 2006) |
| wMelPop | Drosophila melanogaster | Aedes albopictus | CI, life shortening, embryo mortality | (Suh et al, 2009) |
| wPip | Culex pipiens | Aedes albopictus | CI, lower hatch rate, reduced fecundity | (Calvitti et al, 2010) |
CI, cytoplasmic incompatibility; CHIKV, Chikungunya virus; DENV, dengue virus.
Wolbachia transinfection experiments are more likely to be successful when the donor and recipient organisms are closely related. In line with this, the transfer of wMelPop from its natural host, Drosophila melanogaster, into the dengue fever vector A. aegypti was achieved in our laboratory after Wolbachia was first maintained by continuous passage in A. albopictus in vitro cell culture for almost 4 years (McMeniman et al, 2008; Fig 1). Wolbachia adapted to a mosquito intracellular environment, facilitating transfection in vivo. After microinjection of thousands of A. aegypti embryos, two stable wMelPop-CLA (cell-line-adapted) lines with maternal transmission rates of approximately 100% were generated (McMeniman et al, 2009; Fig 1). wMelPop-CLA-infected mosquitoes showed an approximately 50% reduction in adult lifespan, compared with their uninfected counterparts (McMeniman et al, 2009; Fig 2). The halving of adult mosquito lifespan and the high Wolbachia maternal transmission rates were also maintained in more genetically diverse outbred mosquitoes, and larval nutrition did not affect the life-shortening ability of the wMelPop-CLA strain (Yeap et al, 2010). The wMelPop-CLA infection is widespread in A. aegypti tissues, with high bacterial densities in the head (brain and ommatidia), thorax (salivary glands, muscle) and abdomen (fat tissue, reproductive tissues and Malphigian tubules; Moreira et al, 2009a; Fig 3). Wide distribution across tissues has been found in other transinfected mosquitoes, such as A. aegypti infected with the wAlbB strain from A. albopictus (Bian et al, 2010). By using quantitative PCR and western blot analyses, this strain was also found in reproductive tissues, midgut, muscles and heads, in both native A. albopictus (Dobson et al, 1999) and the transfected A. aegypti (Bian et al, 2010), although the densities are not as high as those found in A. aegypti infected with wMelPop-CLA.
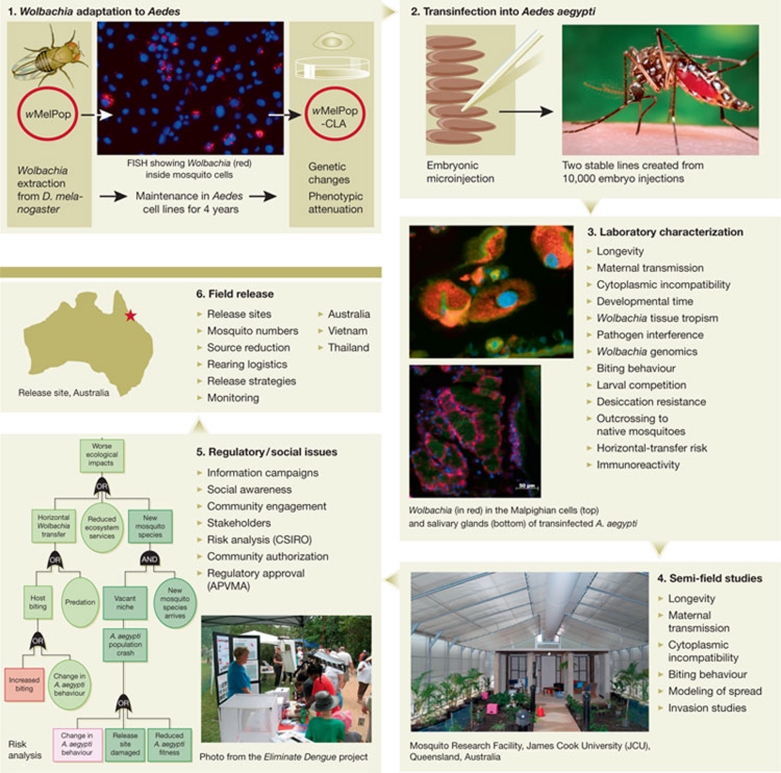
Figure 1.
From the generation of Wolbachia-infected mosquitoes to their release for the control of dengue fever in Australia. (1) wMelPop Wolbachia were extracted from their native D. melanogaster host and maintained in Aedes cell lines for 4 years. During this time, genetic changes and phenotypic adaptation occurred (McMeniman et al, 2008; I. Iturbe-Ormaetxe and J. Brownlie, unpublished data). (2) After injecting more than 10,000 A. aegypti embryos with purified Wolbachia, two stable transinfected mosquito lines were generated (McMeniman et al, 2009). (3) Transinfected mosquitoes were characterized for Wolbachia-induced phenotypes, including ability to block pathogen replication (Moreira et al, 2009a). (4) Studies were conducted in secure cages at James Cook University, Cairns, Australia, to determine the ability of transinfected mosquitoes to infect natural populations under semi-natural conditions. (5) Extensive community engagement and information campaigns (McNaughton et al, 2010), together with risk analysis (Murphy et al, 2010) preceded the granting of regulatory approval from the Australian government for the open release of the mosquitoes into the environment. (6) The release of A. aegypti mosquitoes transinfected with wMel Wolbachia started in January 2011 in two townships near to Cairns, Queensland, Australia and will take place for 12 weeks. This will be followed by extensive monitoring of the invasion of Wolbachia into field populations of A. aegypti, and the effect of this on dengue-fever transmission, before further releases in Vietnam (Jeffery et al, 2009) and Thailand. APVMA, Australian Pesticides and Veterinary Medicines Authority; CSIRO, Commonwealth Scientific and Industrial Research Organisation.
Figure 2.
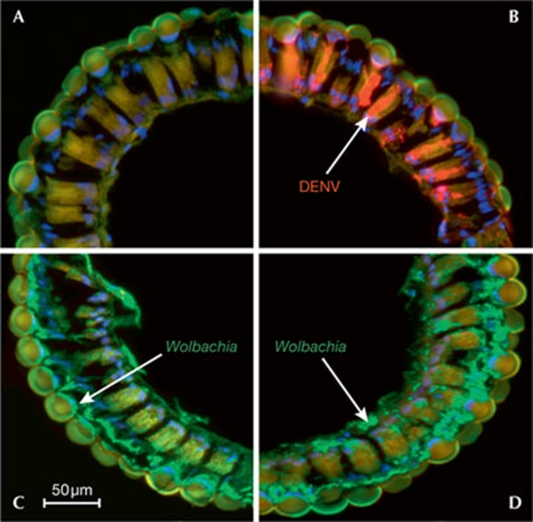
Immunofluorescence staining of Aedes aegypti mosquito compound eyes (ommatidia). wMelPop-CLA Wolbachia is shown in green, DENV in red and DNA in blue. (A) Ommatidia section of a control, uninfected mosquito (no DENV, no Wolbachia). (B) Ommatidia of a Wolbachia-free mosquito, 14 days post-infection with DENV (seen in red). (C) Ommatidia of a Wolbachia-transinfected A. aegypti mosquito. Scale bar, 50 μm. (D) Ommatidia of a Wolbachia-transinfected A. aegypti mosquito, 14 days post-infection with DENV; DENV levels are dramatically reduced by the presence of Wolbachia and no DENV signal is detectable (Figure modified from Moreira et al, 2009a, with permission). CLA, cell-line adapted; DENV, Dengue virus.
Figure 3.
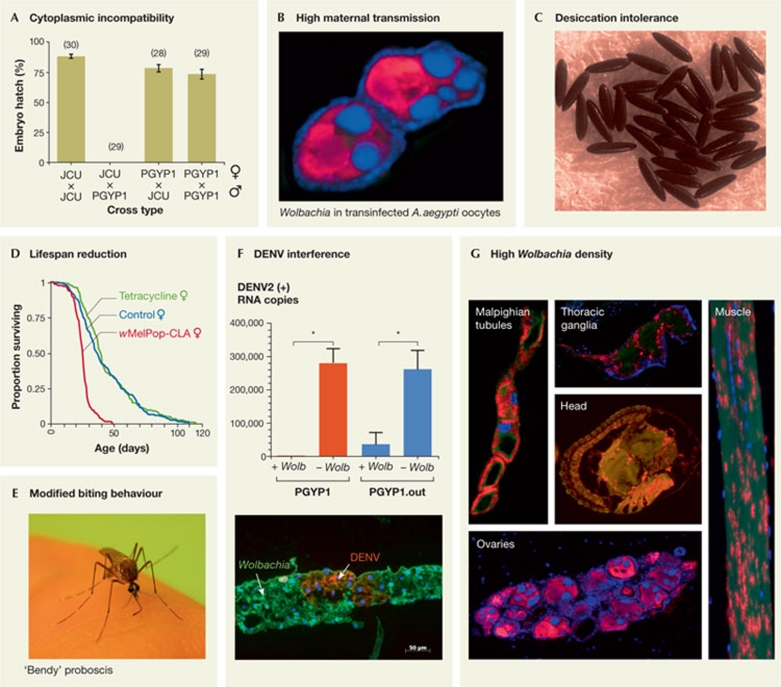
Phenotypes induced by the wMelPop-CLA strain in transinfected Aepes aegypti mosquitoes. (A) 100% cytoplasmic incompatibility. Crosses between Wolbachia-infected males (PGYP1) and uninfected females (JCU) produce no offspring, whereas every other crossing combination produces viable embryos (Figure taken from McMeniman et al, 2009, with permission). (B) Transmission to almost 100% of the mosquito offspring, as suggested by the high levels of Wolbachia (in red) in the oocytes of infected females, shown by FISH (McMeniman et al, 2009; Moreira et al, 2009a). (C) Dramatic reduction of A. aegypti egg viability (McMeniman et al, 2011). (D) Reduction of adult mosquito lifespan by 50%, compared with wild-type mosquitoes or uninfected counterparts after tetracycline curing of the Wolbachia infection (Figure taken from McMeniman et al, 2009, with permission). (E) Alteration of biting behaviour and reduction of blood-feeding success in ageing A. aegypti mosquitoes. ‘Shaky’ and ‘bendy’ proboscis phenotypes are commonly observed (Turley et al, 2009; Moreira et al, 2009b). (F) Inhibition of DENV replication, in both inbred (PGYP1) and outbred (PGYP1.out) mosquitoes. The bottom panel shows the cellular exclusion of Wolbachia and DENV in fat tissue of some Wolbachia-infected mosquitoes 14 days post-DENV injection (Figures taken from Moreira et al, 2009a, with permission). (G) Widespread tissue infection in A. aegypti, including Malpighian tubules, thoracic ganglia, thoracic muscle, ovaries, heads and salivary glands (Fig 1) as shown by FISH (Wolbachia stained in red, DNA stained in blue; Moreira et al, 2009a; T. Walker et al, unpublished data). CLA, cell-line adapted; DENV, Dengue virus; FISH, flourescence in situ hybridization.
Wolbachia interference with viruses and parasites
A key element in the use of Wolbachia for the control of insect-borne disease has been the discovery that some Wolbachia strains can interfere with insect viruses in Drosophila and human pathogens in mosquitoes. Wolbachia strains can protect Drosophila flies from RNA viruses such as Drosophila C (DCV), Cricket paralysis, Flock House and Nora viruses (Hedges et al, 2008; Osborne et al, 2009; Teixeira et al, 2008), West Nile virus (Glaser & Meola, 2010), as well as the fungus Beauveria bassiana (Panteleev et al, 2007). Interestingly, the presence of Wolbachia interferes with a wider range of pathogens in transinfected mosquitoes including nematodes and bacteria (Kambris et al, 2009), viruses such as DENV (Fig 2,3F) and Chikungunya (CHIKV; Bian et al, 2010; Moreira et al, 2009a), as well as the avian and rodent malaria parasites Plasmodium gallinaceum (Moreira et al, 2009a) and P. berghei (Kambris et al, 2010). Natural Wolbachia strains that infect mosquitoes have also been shown to induce resistance to viruses—as in C. quinquefasciatus mosquitoes, that are resistant to West Nile virus (Glaser & Meola, 2010)—although this resistance seems less pronounced in comparison to transinfected Wolbachia strains such as wMelPop-CLA (Moreira et al, 2009a).
The mechanisms by which some Wolbachia strains interfere with a variety of pathogens remain unclear. One hypothesis is that pathogen interference is partly mediated by the induction of antimicrobial peptides and pre-activation of the innate immune response in the insect (Kambris et al, 2010; Kambris et al, 2009; Moreira et al, 2009a). The presence of wMelPop-CLA Wolbachia in A. aegypti induced the expression of several immune effector molecules, including cecropin, defensin, thio-ester containing proteins and C-type lectins (Moreira et al, 2009a). When the wMelPop strain was transiently injected into adult Anopheles gambiae, several immune genes were upregulated, as shown by whole-genome arrays (Kambris et al, 2009), resulting in the inhibition of Plasmodium development (Kambris et al, 2010). Some of these genes, in particular LRIM1 and TEP1, have been found to inhibit Plasmodium development by interfering with the opsonization pathway (Blandin et al, 2004; Povelones et al, 2009). Indirect supporting evidence for the role of the immune system in pathogen suppression was reported by Kokoza and colleagues, who showed that the co-expression of two antimicrobial peptides (cecropin A and defensin A) in transgenic A. aegypti induces resistance to infection with the bacterial pathogen Pseudomonas aeruginosa, and reduces the number of P. gallinaceum oocysts, completely blocking transmission of this avian malaria parasite to naive chickens (Kokoza et al, 2010). Furthermore, Wolbachia infections in A. aegypti and Drosophila have been shown to increase haemolymph melanization (Thomas et al, 2010), a key constituent of the insect innate immune system that is involved in the encapsulation of foreign bodies and parasites (Carton & Nappi, 1997; Theopold et al, 2004).
Wolbachia-mediated pathogen interference is unlikely to result solely from an upregulation of the insect host immune system. Wolbachia infection provides protection against DENV in mosquito cell lines, even though these cells lack whole-organism or tissue-specific immunity (Frentiu et al, 2010). Similarly, the Wolbachia strain wRi does not induce an immune response in its native D. simulans hosts (Bourtzis et al, 2000), and yet confers protection against DCV in these flies (Osborne et al, 2009). However, wRi induces an immune response in transinfected D. melanogaster cell lines (Xi et al, 2008), and mosquitoes artificially infected with Wolbachia also show upregulation of the immune system (Kambris et al, 2010; Kambris et al, 2009; Moreira et al, 2009a). Given that immune response to Wolbachia infection has been observed only in hosts artificially infected with new strains, such responses might not be due to the Wolbachia infection, but instead to an unusual effect of the new host–symbiont combination, such as elevated, unnatural Wolbachia densities (McGraw et al, 2002).
The observed interference of some Wolbachia strains with DENV replication and dissemination could also be caused by direct competition for cellular resources. This seems to be a plausible explanation given (i) the high density of the wMelPop-CLA Wolbachia infection in most A. aegypti tissues, such as muscle, fat, nervous tissue, salivary glands and Malpighian tubules (Fig 3G); (ii) the exclusion between Wolbachia and DENV in the fat tissue of some mosquitoes that contain both microorganisms (Moreira et al, 2009a; Fig 3F); and (iii) the reliance of DENV replication on host fatty-acid synthesis (Heaton et al, 2010; Heaton & Randall, 2010), as fatty acids might be sequestered by Wolbachia. In agreement with this hypothesis, Wolbachia-mediated viral interference seems to depend on bacterial loads. In Drosophila, strains that grow at higher densities such as wMel and wRi provide high levels of protection, whereas low-density strains such as wNo and wHa provide no protection against insect viruses (Osborne et al, 2009). Furthermore, transinfected Wolbachia strains in A. aegypti mosquitoes that grow to high densities—such as wMelPop-CLA—provide almost complete protection from pathogens (Moreira et al, 2009a). Variability in tissue density and localization is common across strains, and probably influences the effects on the host, as well as pathogen interference. For example, some Aedes species such as A. fluviatilis and A. albopictus can transmit Plasmodium and DENV/CHIKV, respectively, despite being naturally infected with Wolbachia, although they are not typically important epidemic vectors (Lambrechts et al, 2010). A. fluviatilis is infected with the wFlu strain of Wolbachia (Moreira et al, 2009a), but the tissue distribution and density of this strain is limited compared with the wMel/wMelPop-CLA infections, showing more similarity to the superinfected Wolbachia strains present in A. albopictus (I. Iturbe-Ormaetxe, unpublished data). Lower Wolbachia densities in these species or a preference of these Wolbachia strains for reproductive tissues instead of gut, salivary glands or fat tissue—which DENV or Plasmodium parasites infect (Moreira et al, 2009a)—could explain the differences in vector competence. Wolbachia can also coexist with Japanese encephalitis viruses in Armigeres mosquitoes (Tsai et al, 2006), but whether this coexistence is related to density or tissue tropism is yet to be determined. The specific tissues that Wolbachia must infect and the role of tissue-specific Wolbachia density in pathogen interference also remain unknown.
Molecular genetics of Wolbachia
The molecular mechanisms underlying the reproductive phenotypes and pathogen interference of Wolbachia remain mostly unknown. Particular effort has been devoted to elucidating the mechanisms for CI, the main driving force for Wolbachia population invasion in transinfected mosquitoes. Among the effectors proposed to induce CI in Drosophila and mosquitoes, ankyrin (ANK) domain proteins have received special attention (Duron et al, 2007; Iturbe-Ormaetxe et al, 2005; Sinkins et al, 2005; Walker et al, 2007). Despite being relatively rare in prokaryotes (Sedgwick & Smerdon, 1999), ANK genes are particularly abundant in Wolbachia genomes (Klasson et al, 2009b; Walker et al, 2007; Wu et al, 2004) and are known to be involved in protein–protein interactions in many systems, including closely related Anaplasma spp. (Caturegli et al, 2000; Ijdo et al, 2007; Park et al, 2004) and Ehrlichia spp. (Rikihisa & Lin, 2010; Zhu et al, 2009). ANK repeats are found in proteins involved in cell-cycle regulation, in transcriptional regulators, toxins and cyclin-dependent kinase inhibitors (Al-Khodor et al, 2010; Bork, 1993; Michaely & Bennett, 1992; Sedgwick & Smerdon, 1999). Orthologue ANK proteins from closely related Wolbachia strains that induce different phenotypes in their hosts can vary greatly in their architecture, number of ANK repeats and presence of transmembrane domains. They can also be absent or truncated in some strains (Iturbe-Ormaetxe et al, 2005). This variability probably affects the affinity, specificity, localization, expression and function of these ANK proteins and makes them good candidates to be mediators in reproductive phenotypes. Wolbachia is an unculturable, untransformable bacterium and Yamada and colleagues therefore used an alternative approach to genetically test several candidate Wolbachia genes (Yamada et al, 2011). Transgenic Drosophila flies were created that expressed either a single candidate Wolbachia ANK gene or complemented these genes in Drosophila strains that were infected with a non-CI-inducing Wolbachia strain lacking the ANK gene to be tested. Both the expression of single ANK genes in Wolbachia-uninfected flies and the complementation of a single ANK gene in Drosophila infected with a non-CI Wolbachia strain were unable to mimic the CI phenotype, indicating that the expression of single ANK genes is not enough to induce CI, and additional factors must be involved (Yamada et al, 2011).
The WO Wolbachia bacteriophage, which infects up to 90% of insect Wolbachia strains (Bordenstein & Wernegreen, 2004; Gavotte et al, 2004) has also been suggested to induce CI in insects, particularly as viral filtrates obtained from Wolbachia extracts are able to induce CI in Nasonia wasps (Williams et al, 1993). A series of WO-encoded genes, such as the virulence factor VrlC, have been implicated in Wolbachia pathogenicity (Kent & Bordenstein, 2010), due to the correlation between sequence variation in these genes and the induction of CI in C. pipiens mosquitoes (Duron et al, 2006). However, no correlation was found between the presence of phage capsid genes and the induction of CI in Culex mosquitoes (Gavotte et al, 2007). Bordenstein and colleagues proposed a model that links high Wolbachia-phage density with lower Wolbachia density and less CI (Bordenstein et al, 2006). At the cellular level, CI has been studied in Drosophila and the wasp Nasonia (Serbus et al, 2008), in which inhibition of Cdk1 activation, and chromosome segregation and condensation defects lead to delayed nuclear-envelope breakdown (Tram et al, 2003; Tram & Sullivan, 2002). Wolbachia also impairs H3.3/H4 histone accumulation in the male pronucleus, which ultimately leads to defects in transcription regulation in the host nuclei (Landmann et al, 2009).
However, determining the molecular basis of these phenotypic effects has been hampered by two difficulties: the obligate symbiotic nature of Wolbachia makes it impossible to grow these bacteria in cell-free culture, and there is no genetic-transformation technology available that would make genetic testing possible. On the other hand, the sequencing of several complete Wolbachia genomes (Foster et al, 2005; Klasson et al, 2009a; Klasson et al, 2008; Salzberg et al, 2005; Salzberg et al, 2009; Wu et al, 2004) has been an important advance in the field, and several more are being sequenced or annotated (Iturbe-Ormaetxe et al, 2011; Werren et al, 2008).
Comparative genomic studies between the closely related wMel (Wu et al, 2004) and wMelPop genomes revealed a series of genetic differences, including an approximately 150 Kb inversion, several insertions/deletions (including some ANK repeats and mobile elements), and about 200 single nucleotide polymorphisms, which might be responsible for the over-replication phenotype and the ability of wMelPop to shorten lifespan (Riegler et al, 2005; I. Iturbe-Ormaetxe and J. Brownlie, unpublished data). Interestingly, when the wMelPop strain was sequenced almost 4 years after adaptation to mosquito cells (wMelPop-CLA), we found that the genome had quickly evolved during cell culture through a series of deletion and insertion events that could be sequentially characterized over time (McMeniman et al, 2008; McMeniman et al, 2009; I. Iturbe-Ormaetxe and J. Brownlie, unpublished data). As a result, several wMelPop-CLA genes were inactivated, compared with the original wMelPop strain from flies. These genetic differences could explain the attenuation of the over-replication and the life-shortening phenotype that was observed after the wMelPop-CLA strain was transferred back into flies following its maintenance in cell culture (McMeniman et al, 2008). We are sequencing other closely related strains to identify the genetic basis for these phenotypes, in particular the over-replication and life-shortening phenotypes. However, the aforementioned lack of genetic-transformation technologies for Wolbachia will make functional genetic studies challenging.
Wolbachia invasion of mosquito populations
Wolbachia-infected female mosquitoes must transmit the bacteria to their progeny at a high frequency for it to spread successfully and invade uninfected insect populations. The wMelPop-CLA strain is transmitted from mother to offspring at almost 100% efficiency in transinfected A. aegypti mosquitoes (McMeniman et al, 2009). A nearly perfect maternal transmission rate is predicted from fluorescence in situ hybridization and immunolocalization studies, which show extremely high Wolbachia densities in ovaries of wMelPop-CLA-infected female mosquitoes (Fig 3B), ensuring the transfer of the infection to the next generation. Males are a dead end for Wolbachia, which is completely absent from mature sperm and seminal fluids.
Another important requirement is that Wolbachia spreads into uninfected populations through the induction of reproductive distortions that favour infected females. The wMelPop-CLA strain induces very high (almost 100%) CI rates in transinfected A. aegypti mosquitoes (Fig 3A; McMeniman et al, 2009). Maternal transmission and CI give infected females a reproductive advantage, as uninfected females produce no offspring when they mate with infected males. The ability of Wolbachia-infected A. aegypti to spread in semi-natural conditions has been tested in secure, outdoor mosquito cages in Australia (Fig 1.4; Ritchie et al, 2011); Wolbachia can spread to high frequencies approaching fixation in interbreeding, caged populations of mosquitoes in 3 months, when initial frequencies are two Wolbachia-infected mosquitos for every wild-type mosquito (T. Walker et al, unpublished data).
Fitness of Wolbachia-infected mosquitoes
Wolbachia-infected mosquitoes can only spread and invade uninfected mosquito populations if the fitness cost of infection is less than the fitness advantage that CI provides for the infection to spread. Pathogen protection might also provide a fitness advantage to Wolbachia-infected mosquitoes that will assist their spread in the field. Apart from the reduction in lifespan, some of the fitness effects induced by the wMelPop-CLA infection in A. aegypti include an increase of metabolic rate and activity in the mosquito (Evans et al, 2009), and a fecundity cost. The latter is detected as a steady reduction in hatch rates after the first gonothropic cycle, probably due to an impaired ability to feed as the mosquitoes age (Turley et al, 2009). Another significant effect of wMelPop-CLA infection in A. aegypti is the reduction of egg survival during periods of embryonic quiescence (Fig 3; McMeniman & O'Neill, 2010). This might be a desired control mechanism for population suppression in areas with pronounced wet/dry seasonality, by preventing the next generation of mosquitoes from hatching after the dry season.
The ability of mosquitoes infected with wMelPop-CLA to feed on human hosts has been tested by looking at the volume of blood they have ingested, their ability to probe successfully, and other aspects of their biting behaviour (Moreira et al, 2009b; Turley et al, 2009). Wolbachia does not affect the response time of mosquitoes to humans, but its presence reduces the number and size of blood meals taken. wMelPop-CLA Wolbachia also induced behavioural changes in old mosquitoes termed ‘shaky’ or ‘bendy’, in which the proboscis bends and is unable to pierce the skin; 65% of 35-day-old insects showed the bendy phenotype (Fig 3E; Turley et al, 2009). Wolbachia-infected A. aegypti produce smaller volumes of saliva, which contain the same levels of the anti-platelet-aggregation enzyme, apyrase, as uninfected mosquitoes (Moreira et al, 2009b).
Despite the ability of the wMelPop-CLA strain to induce strong CI and interfere with DENV replication in transinfected A. aegypti mosquitoes, the fitness effects produced in its host might be counterproductive to, or even completely block, the establishment of this strain in natural populations of mosquitoes (Turelli, 2010). Alternative, less-virulent strains might therefore be required.
In Drosophila, viral interference is induced by several Wolbachia strains that are closely related to wMelPop (Hedges et al, 2008; Osborne et al, 2009) suggesting non-life-shortening strains with more desirable invasion characteristics would also affect transmission of dengue fever. For example, the wMel strain naturally infects wild populations of D. melanogaster worldwide (Riegler et al, 2005), suggesting a high potential for it to invade mosquito populations. We have recently established wMel-infected A. aegypti lines that have lower fitness costs, but still significantly interfere with dengue (T. Walker et al, unpublished data). Strains such as this might be preferable to the wMelPop-CLA strain for deployment in the field.
From the lab to the field
The phenotypic effects of Wolbachia on laboratory A. aegypti colonies need to be tested in natural conditions and in mosquitoes with the same genetic backgrounds as those in the intended release area. Out-crossing laboratory-reared wMelPop-CLA-infected mosquitoes with Australian wild-stocks has revealed that the dengue-fever interference, life-shortening and fitness indicators are maintained (Moreira et al, 2009a; Yeap et al, 2010). Large, semi-natural, purpose-built cages are being used to determine the ability of Wolbachia strains to invade uninfected mosquito populations.
A trial release of Wolbachia-infected A. aegypti is taking place in two localities in north Queensland, Australia, during the 2011 wet season, with regulatory approval from the Australian government and strong support from the community (Fig 1; McNaughton et al, 2010). Risk assessments have concluded that there is a negligible risk of the release of Wolbachia-infected A. aegypti resulting in more harm than that caused by naturally occurring A. aegypti over a 30-year period (Murphy et al, 2010). We have also addressed several safety concerns about releasing Wolbachia-infected mosquitoes into the field. There is no evidence for the transfer of Wolbachia to humans by mosquito bites (Popovici et al, 2010). Lateral transfer of Wolbachia to non-target species is unlikely as Wolbachia are maternally transmitted, and horizontal transfer has only been reported on rare occasions (Heath et al, 1999; Huigens et al, 2004). There is also no evidence of Wolbachia transfer to mosquito predators such as spiders and geckos (Popovici et al, 2010). There is potential for horizontal transfer of Wolbachia DNA into mosquito genomes (Fenn et al, 2006; Hotopp et al, 2007; Klasson et al, 2009a; Kondo et al, 2002; McNulty et al, 2010; Nikoh et al, 2008; Woolfit et al, 2009), but such cases take place over evolutionary timescales and are extremely rare. Furthermore, the consequences of such transfer are unlikely to increase the risk of adverse events associated with the open release of Wolbachia-infected mosquitoes.
Future perspectives
The ability of some Wolbachia strains to reduce the lifespan of A. aegypti, invade mosquito populations through the induction of CI and, in particular, interfere with the replication of a variety of pathogens, has placed this bacterium at the frontline of new approaches targeting mosquito-borne diseases in an environmentally friendly manner. The release of Wolbachia-infected, dengue-refractory mosquitoes in Australia in 2011 will create the basis for the implementation of this strategy in Vietnam, Thailand and possibly other countries in which the magnitude of dengue fever is higher and the ecology and breeding habits of mosquitoes—particularly in large urban areas—make control challenging. This Wolbachia-based, biocontrol approach could be applied to invasive mosquito species, such as A. albopictus, which has recently become a secondary dengue fever vector in Asia and has rapidly spread in the USA and large parts of Europe and Africa (Knudsen et al, 1996; Lambrechts et al, 2010). A. albopictus is also a vector for CHIKV in these countries (Bonilauri et al, 2008; Pages et al, 2009).
One main goal for the Wolbachia-based biocontrol approach to mosquito-borne-disease control is to transfer Wolbachia into anopheline mosquitoes, the most-common vectors of human malaria. The ability to maintain Wolbachia in Anopheles cell lines (McMeniman et al, 2008; Rasgon et al, 2006a; Rasgon et al, 2006b) and the successful establishment of transient somatic wMelPop infections in Anopheles mosquitoes (Jin et al, 2009; Kambris et al, 2010)—which inhibit Plasmodium development by activating the mosquito immune response (Kambris et al, 2010)—are promising advances that suggest stable transinfection might be possible.
A full list of Wolbachia literature and resources can be found at the Wolbachia website (http://www.wolbachia.sols.uq.edu.au) and information about the field release of Wolbachia-infected mosquitoes for dengue fever control can be found at (http://www.eliminatedengue.com).
Acknowledgments
We thank L. Moreira for helpful and constructive comments on the manuscript. This work was supported by grants from the Foundation for the National Institutes of Health through the Grand Challenges in Global Health Initiative of the Bill & Melinda Gates Foundation, the National Health and Medical Research Council of Australia, the Australian Research Council and the National and International Research Alliances programme of the Queensland state government.
Footnotes
The authors declare that they have no conflict of interest.
References
- Adams B, Kapan DD (2009) Man bites mosquito: understanding the contribution of human movement to vector-borne disease dynamics. PLoS ONE 4: e6763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Khodor S, Price CT, Kalia A, Abu Kwaik Y (2010) Functional diversity of ankyrin repeats in microbial proteins. Trends Microbiol 18: 132–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aleksandrov ID, Aleksandrova MV, Goriacheva II, Roshchina NV, Shaikevich EV, Zakharov IA (2007) Removing endosymbiotic Wolbachia specifically decreases lifespan of females and competitiveness in a laboratory strain of Drosophila melanogaster. Genetika 43: 1372–1378 [PubMed] [Google Scholar]
- Bian G, Xu Y, Lu P, Xie Y, Xi Z (2010) The endosymbiotic bacterium Wolbachia induces resistance to dengue virus in Aedes aegypti. PLoS Pathog 6: e1000833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blandin S, Shiao SH, Moita LF, Janse CJ, Waters AP, Kafatos FC, Levashina EA (2004) Complement-like protein TEP1 is a determinant of vectorial capacity in the malaria vector Anopheles gambiae. Cell 116: 661–670 [DOI] [PubMed] [Google Scholar]
- Blanford S, Chan BH, Jenkins N, Sim D, Turner RJ, Read AF, Thomas MB (2005) Fungal pathogen reduces potential for malaria transmission. Science 308: 1638–1641 [DOI] [PubMed] [Google Scholar]
- Bonilauri P et al. (2008) Chikungunya virus in Aedes albopictus, Italy. Emerg Infect Dis 14: 852–854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordenstein SR, Marshall ML, Fry AJ, Kim U, Wernegreen JJ (2006) The tripartite associations between bacteriophage, Wolbachia, and arthropods. PLoS Pathogens 2: e43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordenstein SR, Wernegreen JJ (2004) Bacteriophage flux in endosymbionts (wolbachia): infection frequency, lateral transfer, and recombination rates. Mol Biol Evol 21: 1981–1991 [DOI] [PubMed] [Google Scholar]
- Bork P (1993) Hundreds of ankyrin-like repeats in functionally diverse proteins: mobile modules that cross phyla horizontally? Proteins 17: 363–374 [DOI] [PubMed] [Google Scholar]
- Bourtzis K, Pettigrew MM, O'Neill SL (2000) Wolbachia neither induces nor suppresses transcripts encoding antimicrobial peptides. Insect Mol Biol 9: 635–639 [DOI] [PubMed] [Google Scholar]
- Brownlie JC, Cass BN, Riegler M, Witsenburg JJ, Iturbe-Ormaetxe I, McGraw EA, O'Neill SL (2009) Evidence for metabolic provisioning by a common invertebrate endosymbiont, Wolbachia pipientis, during periods of nutritional stress. PLoS Pathog 5: e1000368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownstein JS, Hett E, O'Neill SL (2003) The potential of virulent Wolbachia to modulate disease transmission by insects. J Invertebr Pathol 84: 24–29 [DOI] [PubMed] [Google Scholar]
- Calvitti M, Moretti R, Lampazzi E, Bellini R, Dobson SL (2010) Characterization of a new Aedes albopictus (Diptera: Culicidae)-Wolbachia pipientis (Rickettsiales: Rickettsiaceae) symbiotic association generated by artificial transfer of the wPip strain from Culex pipiens (Diptera: Culicidae). J Med Entomol 47: 179–187 [DOI] [PubMed] [Google Scholar]
- Carton Y, Nappi AJ (1997) Drosophila cellular immunity against parasitoids. Parasitol Today 13: 218–227 [DOI] [PubMed] [Google Scholar]
- Caturegli P, Asanovich KM, Walls JJ, Bakken JS, Madigan JE, Popov VL, Dumler JS (2000) ankA: an Ehrlichia phagocytophila group gene encoding a cytoplasmic protein antigen with ankyrin repeats. Infect Immun 68: 5277–5283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen LH, Wilson ME (2010) Dengue and chikungunya infections in travelers. Curr Opin Infect Dis 23: 438–444 [DOI] [PubMed] [Google Scholar]
- Cook PE, McMeniman CJ, O'Neill SL (2008) Modifying insect population age structure to control vector-borne disease. Adv Exp Med Biol 627: 126–140 [DOI] [PubMed] [Google Scholar]
- Curtis CF, Adak T (1974) Population replacement in Culex fatigens by means of cytoplasmic incompatibility. Laboratory experiments with non-overlapping generations. Bull World Health Organ 51: 249–255 [PMC free article] [PubMed] [Google Scholar]
- Darbro JM, Thomas MB (2009) Spore persistence and likelihood of aeroallergenicity of entomopathogenic fungi used for mosquito control. Am J Trop Med Hyg 80: 992–997 [PubMed] [Google Scholar]
- Dedeine F, Vavre F, Fleury F, Loppin B, Hochberg ME, Bouletreau M (2001) Removing symbiotic Wolbachia bacteria specifically inhibits oogenesis in a parasitic wasp. Proc Natl Acad Sci USA 98: 6247–6252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobson SL, Bourtzis K, Braig HR, Jones BF, Zhou W, Rousset F, O'Neill SL (1999) Wolbachia infections are distributed throughout insect somatic and germ line tissues. Insect Biochem Mol Biol 29: 153–160 [DOI] [PubMed] [Google Scholar]
- Duron O, Bernard C, Unal S, Berthomieu A, Berticat C, Weill M (2006) Tracking factors modulating cytoplasmic incompatibilities in the mosquito Culex pipiens. Mol Ecol 15: 3061–3071 [DOI] [PubMed] [Google Scholar]
- Duron O, Boureux A, Echaubard P, Berthomieu A, Berticat C, Fort P, Weill M (2007) Variability and expression of ankyrin domain genes in Wolbachia variants infecting the mosquito Culex pipiens. J Bacteriol 189: 4442–4448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans O, Caragata EP, McMeniman CJ, Woolfit M, Green DC, Williams CR, Franklin CE, O'Neill SL, McGraw EA (2009) Increased locomotor activity and metabolism of Aedes aegypti infected with a life-shortening strain of Wolbachia pipientis. J Exp Biol 212: 1436–1441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenn K, Conlon C, Jones M, Quail MA, Holroyd NE, Parkhill J, Blaxter M (2006) Phylogenetic relationships of the Wolbachia of nematodes and arthropods. PLoS Pathog 2: e94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster J et al. (2005) The Wolbachia genome of Brugia malayi: endosymbiont evolution within a human pathogenic nematode. PLoS Biol 3: e121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frentiu FD, Robinson J, Young PR, McGraw EA, O'Neill SL (2010) Wolbachia-mediated resistance to dengue virus infection and death at the cellular level. PLoS ONE 5: e13398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavotte L, Henri H, Stouthamer R, Charif D, Charlat S, Bouletreau M, Vavre F (2007) A survey of the bacteriophage WO in the endosymbiotic bacteria Wolbachia. Mol Biol Evol 24: 427–435 [DOI] [PubMed] [Google Scholar]
- Gavotte L, Vavre F, Henri H, Ravallec M, Stouthamer R, Bouletreau M (2004) Diversity, distribution and specificity of WO phage infection in Wolbachia of four insect species. Insect Mol Biol 13: 147–153 [DOI] [PubMed] [Google Scholar]
- Glaser RL, Meola MA (2010) The native Wolbachia endosymbionts of Drosophila melanogaster and Culex quinquefasciatus increase host resistance to West Nile virus infection. PLoS ONE 5: e11977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould EA, Solomon T (2008) Pathogenic flaviviruses. Lancet 371: 500–509 [DOI] [PubMed] [Google Scholar]
- Gubler DJ (2002) Epidemic dengue/dengue hemorrhagic fever as a public health, social and economic problem in the 21st century. Trends Microbiol 10: 100–103 [DOI] [PubMed] [Google Scholar]
- Guzman MG, Kouri G (2002) Dengue: an update. Lancet Infect Dis 2: 33–42 [DOI] [PubMed] [Google Scholar]
- Heath BD, Butcher RDJ, Whitfield WGF, Hubbard SF (1999) Horizontal transfer of Wolbachia between phylogenetically distant insect species by a naturally occurring mechanism. Curr Biology 9: 313–316 [DOI] [PubMed] [Google Scholar]
- Heaton NS, Perera R, Berger KL, Khadka S, Lacount DJ, Kuhn RJ, Randall G (2010) Dengue virus nonstructural protein 3 redistributes fatty acid synthase to sites of viral replication and increases cellular fatty acid synthesis. Proc Natl Acad Sci USA 107: 17345–17350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton NS, Randall G (2010) Dengue virus-induced autophagy regulates lipid metabolism. Cell Host Microbe 8: 422–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedges LM, Brownlie JC, O'Neill SL, Johnson KN (2008) Wolbachia and virus protection in insects. Science 322: 702. [DOI] [PubMed] [Google Scholar]
- Hertig M, Wolbach SB (1924) Studies of Rickettsia-like microorganisms in insects. J Med Res 44: 329–374 [PMC free article] [PubMed] [Google Scholar]
- Hilgenboecker K, Hammerstein P, Schlattmann P, Telschow A, Werren JH (2008) How many species are infected with Wolbachia?—A statistical analysis of current data. FEMS Microbiol Lett 281: 215–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosokawa T, Koga R, Kikuchi Y, Meng XY, Fukatsu T (2010) Wolbachia as a bacteriocyte-associated nutritional mutualist. Proc Natl Acad Sci USA 107: 769–774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotopp JC et al. (2007) Widespread lateral gene transfer from intracellular bacteria to multicellular eukaryotes. Science 317: 1753–1756 [DOI] [PubMed] [Google Scholar]
- Huigens ME, de Almeida RP, Boons PA, Luck RF, Stouthamer R (2004) Natural interspecific and intraspecific horizontal transfer of parthenogenesis-inducing Wolbachia in Trichogramma wasps. Proc Royal Soc Lond B Biol Sci 271: 509–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ijdo JW, Carlson AC, Kennedy EL (2007) Anaplasma phagocytophilum AnkA is tyrosine-phosphorylated at EPIYA motifs and recruits SHP-1 during early infection. Cell Microbiol 9: 1284–1296 [DOI] [PubMed] [Google Scholar]
- Iturbe-Ormaetxe I, Burke GR, Riegler M, O'Neill SL (2005) Distribution, expression, and motif variability of ankyrin domain genes in Wolbachia pipientis. J Bacteriol 187: 5136–5145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iturbe-Ormaetxe I, Woolfit M, Rances E, Duplouy A, O'Neill SL (2011) A simple protocol to obtain highly pure Wolbachia endosymbiont DNA for genome sequencing. J Microbiol Methods 84: 134–136 [DOI] [PubMed] [Google Scholar]
- Jeffery JA, Thi Yen N, Nam VS, Nghia le T, Hoffmann AA, Kay BH, Ryan PA (2009) Characterizing the Aedes aegypti population in a Vietnamese village in preparation for a Wolbachia-based mosquito control strategy to eliminate dengue. PLoS Negl Trop Dis 3: e552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeyaprakash A, Hoy MA (2000) Long PCR improves Wolbachia DNA amplification: wsp sequences found in 76% of sixty-three arthropod species. Insect Molec Biol 9: 393–405 [DOI] [PubMed] [Google Scholar]
- Jin C, Ren X, Rasgon JL (2009) The virulent Wolbachia strain wMelPop efficiently establishes somatic infections in the malaria vector Anopheles gambiae. Appl Environ Microbiol 75: 3373–3376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kambris Z, Blagborough AM, Pinto SB, Blagrove MS, Godfray HC, Sinden RE, Sinkins SP (2010) Wolbachia stimulates immune gene expression and inhibits plasmodium development in Anopheles gambiae. PLoS Pathog 6: e1001143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kambris Z, Cook PE, Phuc HK, Sinkins SP (2009) Immune activation by life-shortening Wolbachia and reduced filarial competence in mosquitoes. Science 326: 134–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay B, Vu SN (2005) New strategy against Aedes aegypti in Vietnam. Lancet 365: 613–617 [DOI] [PubMed] [Google Scholar]
- Kent BN, Bordenstein SR (2010) Phage WO of Wolbachia: lambda of the endosymbiont world. Trends Microbiol 18: 173–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittayapong P, Baisley KJ, Baimai V, O'Neill SL (2000) Distribution and diversity of Wolbachia infections in Southeast Asian mosquitoes (Diptera: Culicidae). J Med Entomol 37: 340–345 [DOI] [PubMed] [Google Scholar]
- Klasson L et al. (2008) Genome evolution of Wolbachia strain wPip from the Culex pipiens group. Mol Biol Evol 25: 1877–1887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klasson L, Kambris Z, Cook PE, Walker T, Sinkins SP (2009a) Horizontal gene transfer between Wolbachia and the mosquito Aedes aegypti. BMC Genomics 10: 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klasson L et al. (2009b) The mosaic genome structure of the Wolbachia wRi strain infecting Drosophila simulans. Proc Natl Acad Sci USA 106: 5725–5730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen AB, Romi R, Majori G (1996) Occurrence and spread in Italy of Aedes albopictus, with implications for its introduction into other parts of Europe. J Am Mosq Control Assoc 12: 177–183 [PubMed] [Google Scholar]
- Kokoza V, Ahmed A, Woon Shin S, Okafor N, Zou Z, Raikhel AS (2010) Blocking of Plasmodium transmission by cooperative action of Cecropin A and Defensin A in transgenic Aedes aegypti mosquitoes. Proc Natl Acad Sci USA 107: 8111–8116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo N, Nikoh N, Ijichi N, Shimada M, Fukatsu T (2002) Genome fragment of Wolbachia endosymbiont transferred to X chromosome of host insect. Proc Natl Acad Sci USA 99: 14280–14285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyle JL, Harris E (2008) Global spread and persistence of dengue. Annu Rev Microbiol 62: 71–92 [DOI] [PubMed] [Google Scholar]
- Lambrechts L, Scott TW, Gubler DJ (2010) Consequences of the expanding global distribution of Aedes albopictus for dengue virus transmission. PLoS Negl Trop Dis 4: e646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landmann F, Orsi GA, Loppin B, Sullivan W (2009) Wolbachia-mediated cytoplasmic incompatibility is associated with impaired histone deposition in the male pronucleus. PLoS Pathog 5: e1000343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laven H (1967) Eradication of Culex pipiens fatigans through cytoplasmic incompatibility. Nature 216: 383–384 [DOI] [PubMed] [Google Scholar]
- McGraw EA, Merritt DJ, Droller JN, O'Neill SL (2002) Wolbachia density and virulence attenuation after transfer into a novel host. Proc Natl Acad Sci USA 99: 2918–2923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMeniman CJ, Hughes GL, O'Neill SL (2011) A Wolbachia symbiont in Aedes aegypti disrupts mosquito egg development to a greater extent when mosquitoes feed on nonhuman versus human blood. J Med Entomol 48: 76–84 [DOI] [PubMed] [Google Scholar]
- McMeniman CJ, Lane AM, Fong AW, Voronin DA, Iturbe-Ormaetxe I, Yamada R, McGraw EA, O'Neill SL (2008) Host adaptation of a Wolbachia strain after long-term serial passage in mosquito cell lines. Appl Environ Microbiol 74: 6963–6969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMeniman CJ, Lane RV, Cass BN, Fong AW, Sidhu M, Wang YF, O'Neill SL (2009) Stable introduction of a life-shortening Wolbachia infection into the mosquito Aedes aegypti. Science 323: 141–144 [DOI] [PubMed] [Google Scholar]
- McMeniman CJ, O'Neill SL (2010) A virulent Wolbachia infection decreases the viability of the dengue vector Aedes aegypti during periods of embryonic quiescence. PLoS Negl Trop Dis 4: e748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNaughton D, Clough A, Johnson P, Ritchie S, O'Neill S (2010) Beyond the ‘back yard’: Lay knowledge about Aedes aegypti in northern Australia and its implications for policy and practice. Acta Trop 116: 74–80 [DOI] [PubMed] [Google Scholar]
- McNulty SN et al. (2010) Endosymbiont DNA in endobacteria-free filarial nematodes indicates ancient horizontal genetic transfer. PLoS ONE 5: e11029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaely P, Bennett V (1992) The ANK repeat: a ubiquitous motif involved in macromolecular recognition. Trends Cell Biol 2: 127–129 [DOI] [PubMed] [Google Scholar]
- Min KT, Benzer S (1997) Wolbachia, normally a symbiont of Drosophila, can be virulent, causing degeneration and early death. Proc Natl Acad Sci USA 94: 10792–10796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira LA et al. (2009a) A Wolbachia symbiont in Aedes aegypti limits infection with dengue, Chikungunya, and Plasmodium. Cell 139: 1268–1278 [DOI] [PubMed] [Google Scholar]
- Moreira LA, Saig E, Turley AP, Ribeiro JM, O'Neill SL, McGraw EA (2009b) Human probing behavior of Aedes aegypti when infected with a life-shortening strain of Wolbachia. PLoS Negl Trop Dis 3: e568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison AC, Zielinski-Gutierrez E, Scott TW, Rosenberg R (2008) Defining challenges and proposing solutions for control of the virus vector Aedes aegypti. PLoS Med 5: e68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy B, Jansen C, Murray J, De Barro PJ. (2010) Risk Analysis on the Australian Release of Aedes aegypti (L.) (Diptera: Culicidae) Containing Wolbachia. CSIRO [Google Scholar]
- Nikoh N, Tanaka K, Shibata F, Kondo N, Hizume M, Shimada M, Fukatsu T (2008) Wolbachia genome integrated in an insect chromosome: evolution and fate of laterally transferred endosymbiont genes. Genome Res 18: 272–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne SE, Leong YS, O'Neill SL, Johnson KN (2009) Variation in antiviral protection mediated by different Wolbachia strains in Drosophila simulans. PLoS Pathog 5: e1000656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pages F, Peyrefitte CN, Mve MT, Jarjaval F, Brisse S, Iteman I, Gravier P, Tolou H, Nkoghe D, Grandadam M (2009) Aedes albopictus mosquito: the main vector of the 2007 Chikungunya outbreak in Gabon. PLoS ONE 4: e4691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panteleev D, Goriacheva II, Andrianov BV, Reznik NL, Lazebnyi OE, Kulikov AM (2007) The endosymbiotic bacterium Wolbachia enhances the nonspecific resistance to insect pathogens and alters behavior of Drosophila melanogaster. Genetika 43: 1277–1280 [PubMed] [Google Scholar]
- Park J, Kim KJ, Choi KS, Grab DJ, Dumler JS (2004) Anaplasma phagocytophilum AnkA binds to granulocyte DNA and nuclear proteins. Cell Microbiol 6: 743–751 [DOI] [PubMed] [Google Scholar]
- Popovici J, Moreira LA, Poinsignon A, Iturbe-Ormaetxe I, McNaughton D, O'Neill SL (2010) Assessing key safety concerns of a Wolbachia-based strategy to control dengue transmission by Aedes mosquitoes. Mem Inst Oswaldo Cruz 105: 957–964 [DOI] [PubMed] [Google Scholar]
- Povelones M, Waterhouse RM, Kafatos FC, Christophides GK (2009) Leucine-rich repeat protein complex activates mosquito complement in defense against Plasmodium parasites. Science 324: 258–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasgon JL, Gamston CE, Ren X (2006a) Survival of Wolbachia pipientis in cell-free medium. Appl Environ Microbiol 72: 6934–6937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasgon JL, Ren X, Petridis M (2006b) Can Anopheles gambiae be infected with Wolbachia pipientis? Insights from an in vitro system. Appl Environ Microbiol 72: 7718–7722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasgon JL, Styer LM, Scott TW (2003) Wolbachia-induced mortality as a mechanism to modulate pathogen transmission by vector arthropods. J Med Entomol 40: 125–132 [DOI] [PubMed] [Google Scholar]
- Read AF, Thomas MB (2009) Microbiology. Mosquitoes cut short. Science 323: 51–52 [DOI] [PubMed] [Google Scholar]
- Ricci I, Cancrini G, Gabrielli S, D'Amelio S, Favi G (2002) Searching for Wolbachia (Rickettsiales: Rickettsiceae) in mosquitoes (Diptera: Culicidae): large polymerase chain reaction survey and new identifications. J Med Entomol 39: 562–567 [DOI] [PubMed] [Google Scholar]
- Riegler M, Sidhu M, Miller WJ, O'Neill SL (2005) Evidence for a global Wolbachia replacement in Drosophila melanogaster. Curr Biology 15: 1428–1433 [DOI] [PubMed] [Google Scholar]
- Rikihisa Y, Lin M (2010) Anaplasma phagocytophilum and Ehrlichia chaffeensis type IV secretion and Ank proteins. Curr Opin Microbiol 13: 59–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie SA, Johnson PH, Freeman AJ, Odell RG, Graham N, Dejong PA, Standfield GW, Sale RW, O'Neill SL (2011) A secure semi-field system for the study of Aedes aegypti. PLoS Negl Trop Dis 5: e988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruang-Areerate T, Kittayapong P (2006) Wolbachia transinfection in Aedes aegypti: a potential gene driver of dengue vectors. Proc Natl Acad Sci USA 103: 12534–12539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar MI, Richardson JH, Sanchez-Vargas I, Olson KE, Beaty BJ (2007) Dengue virus type 2: replication and tropisms in orally infected Aedes aegypti mosquitoes. BMC Microbiol 7: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzberg SL, Hotopp JC, Delcher AL, Pop M, Smith DR, Eisen MB, Nelson WC (2005) Serendipitous discovery of Wolbachia genomes in multiple Drosophila species. Genome Biol 6: R23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzberg SL, Puiu D, Sommer DD, Nene V, Lee NH (2009) Genome sequence of the Wolbachia endosymbiont of Culex quinquefasciatus JHB. J Bacteriol 191: 1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedgwick SG, Smerdon SJ (1999) The ankyrin repeat: a diversity of interactions on a common structural framework. Trends Biochem Sci 24: 311–316 [DOI] [PubMed] [Google Scholar]
- Serbus LR, Casper-Lindley C, Landmann F, Sullivan W (2008) The genetics and cell biology of Wolbachia-host interactions. Annu Rev Genet 42: 683–707 [DOI] [PubMed] [Google Scholar]
- Sinkins SP, Braig HR, O'Neill SL (1995) Wolbachia pipientis: bacterial density and unidirectional cytoplasmic incompatibility between infected populations of Aedes albopictus. Exp Parasitol 81: 284–291 [DOI] [PubMed] [Google Scholar]
- Sinkins SP, O'Neill SL (2000) Wolbachia as a Vehicle to Modify Insect Populations. Boca Raton, FL, USA: CRC Press [Google Scholar]
- Sinkins SP, Walker T, Lynd AR, Steven AR, Makepeace BL, Godfray HC, Parkhill J (2005) Wolbachia variability and host effects on crossing type in Culex mosquitoes. Nature 436: 257–260 [DOI] [PubMed] [Google Scholar]
- Stouthamer R, Breeuwer JA, Hurst GD (1999) Wolbachia pipientis: microbial manipulator of arthropod reproduction. Annu Rev Microbiol 53: 71–102 [DOI] [PubMed] [Google Scholar]
- Suh E, Mercer DR, Fu Y, Dobson SL (2009) Pathogenicity of life-shortening Wolbachia in Aedes albopictus after transfer from Drosophila melanogaster. Appl Environ Microbiol 75: 7783–7788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira L, Ferreira A, Ashburner M (2008) The bacterial symbiont Wolbachia induces resistance to RNA viral infections in Drosophila melanogaster. PLoS Biol 6: e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theopold U, Schmidt O, Soderhall K, Dushay MS (2004) Coagulation in arthropods: defence, wound closure and healing. Trends Immunol 25: 289–294 [DOI] [PubMed] [Google Scholar]
- Thomas MB, Read AF (2007) Can fungal biopesticides control malaria? Nat Rev Microbiol 5: 377–383 [DOI] [PubMed] [Google Scholar]
- Thomas P, Kenny N, Eyles D, Moreira LA, O'Neill SL, Asgari S (2010) Infection with the wMel and wMelPop strains of Wolbachia leads to higher levels of melanization in the hemolymph of Drosophila melanogaster, Drosophila simulans and Aedes aegypti. Dev Comp Immunol 35: 360–365 [DOI] [PubMed] [Google Scholar]
- Tram U, Ferree PM, Sullivan W (2003) Identification of Wolbachia–host interacting factors through cytological analysis. Microbes Infect 5: 999–1011 [DOI] [PubMed] [Google Scholar]
- Tram U, Sullivan W (2002) Role of delayed nuclear envelope breakdown and mitosis in Wolbachia-induced cytoplasmic incompatibility. Science 296: 1124–1126 [DOI] [PubMed] [Google Scholar]
- Tsai KH, Huang CG, Wu WJ, Chuang CK, Lin CC, Chen WJ (2006) Parallel infection of Japanese encephalitis virus and Wolbachia within cells of mosquito salivary glands. J Med Entomol 43: 752–756 [DOI] [PubMed] [Google Scholar]
- Turelli M (2010) Cytoplasmic incompatibility in populations with overlapping generations. Evolution 64: 232–241 [DOI] [PubMed] [Google Scholar]
- Turley AP, Moreira LA, O'Neill SL, McGraw EA (2009) Wolbachia infection reduces blood-feeding success in the dengue fever mosquito, Aedes aegypti. PLoS Negl Trop Dis 3: e516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker T, Klasson L, Sebaihia M, Sanders MJ, Thomson NR, Parkhill J, Sinkins SP (2007) Ankyrin repeat domain-encoding genes in the wPip strain of Wolbachia from the Culex pipiens group. BMC Biol 5: 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werren JH, Baldo L, Clark ME (2008) Wolbachia: master manipulators of invertebrate biology. Nat Rev Microbiol 6: 741–751 [DOI] [PubMed] [Google Scholar]
- WHO (1998) Test Procedures for Insecticide Resistance Monitoring in Malaria Vectors, Bio-Efficacy and Persistence of Insecticides on Treated Surfaces. Report of the WHO Informal Consultation on WHO/HQ (document WHO/CDS/CPC/MAL/98.12), 28–30 September. Geneva, Swizerland: World Health Organization [Google Scholar]
- WHO (2008) World Malaria Report 2008. World Malaria Report. Geneva, Swizerland: World Health Organization [Google Scholar]
- WHO (2009) Dengue and Dengue Hemorragic Fever. Fact Sheet, Vol 117. Geneva, Swizerland: World Health Organization [Google Scholar]
- Wilder-Smith A, Gubler DJ (2008) Geographic expansion of dengue: the impact of international travel. Med Clin North Am 92: 1377–1390 [DOI] [PubMed] [Google Scholar]
- Wilder-Smith A, Ooi EE, Vasudevan SG, Gubler DJ (2010) Update on dengue: epidemiology, virus evolution, antiviral drugs, and vaccine development. Curr Infect Dis Rep 12: 157–164 [DOI] [PubMed] [Google Scholar]
- Williams EH, Fields S, Saul Ii GB (1993) Transfer of incompatibility factors between stocks of Nasonia (=Mormoniella) vitripennis. J Invertebr Pathol 61: 206–210 [DOI] [PubMed] [Google Scholar]
- Woolfit M, Iturbe-Ormaetxe I, McGraw EA, O'Neill SL (2009) An ancient horizontal gene transfer between mosquito and the endosymbiotic bacterium Wolbachia pipientis. Mol Biol Evol 26: 367–374 [DOI] [PubMed] [Google Scholar]
- Wu M et al. (2004) Phylogenomics of the reproductive parasite Wolbachia pipientis wMel: A streamlined genome overrun by mobile genetic elements. PLoS Biol 2: 327–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi Z, Gavotte L, Xie Y, Dobson SL (2008) Genome-wide analysis of the interaction between the endosymbiotic bacterium Wolbachia and its Drosophila host. BMC Genomics 9: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi Z, Khoo CC, Dobson SL (2005) Wolbachia establishment and invasion in an Aedes aegypti laboratory population. Science 14: 326–328 [DOI] [PubMed] [Google Scholar]
- Xi ZY, Khoo CCH, Dobson SL (2006) Interspecific transfer of Wolbachia into the mosquito disease vector Aedes albopictus. Proc Royal Soc Lond B Biol Sci 273: 1317–1322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada R, Iturbe-Ormaetxe I, Brownlie JC, O'Neill SL (2011) Functional test of the influence of Wolbachia genes on cytoplasmic incompatibility expression in Drosophila melanogaster. Insect Mol Biol 20: 75–85 [DOI] [PubMed] [Google Scholar]
- Yeap HL et al. (2010) Dynamics of the “popcorn” Wolbachia infection in outbred Aedes aegypti informs prospects for mosquito vector control. Genetics 187: 583–595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen JH, Barr AR (1973) The etiological agent of cytoplasmic incompatibility in Culex pipiens. J Invertebr Pathol 22: 242–250 [DOI] [PubMed] [Google Scholar]
- Zaim M, Guillet P (2002) Alternative insecticides: an urgent need. Trends Parasitol 18: 161–163 [DOI] [PubMed] [Google Scholar]
- Zhu B, Nethery KA, Kuriakose JA, Wakeel A, Zhang X, McBride JW (2009) Nuclear translocated Ehrlichia chaffeensis ankyrin protein interacts with a specific adenine-rich motif of host promoter and intronic Alu elements. Infect Immun 77: 4243–4255 [DOI] [PMC free article] [PubMed] [Google Scholar]



 Scott L O' Neill, Iñaki Iturbe-Ormaetxe & Thomas Walker
Scott L O' Neill, Iñaki Iturbe-Ormaetxe & Thomas Walker