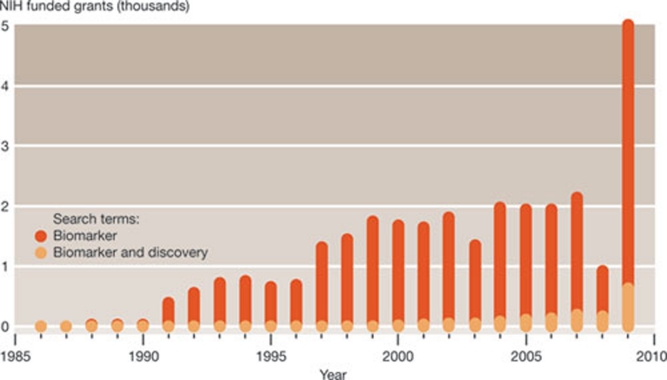
Biomarkers are considered to be the cornerstones of a preventive and personalized medicine of the future. Interest in relevant research is therefore booming, and so are investments. A recent survey of research grants awarded by the National Institutes of Health (NIH) between 1986 and 2009 revealed that almost 29,000 grants containing the term ‘biomarker’ were awarded during this period, with a dramatic increase in 2009 (Fig 1). The total money for these NIH grants in 2008 and 2009 alone was more than US$2.5 billion. The private sector is also attracted by biomarkers—regarding them as a possible solution to the paucity of R&D productivity in the pharmaceutical sector—and many biotech companies are already pursuing their use, or offer specialized screening services.
The private sector is also attracted by biomarkers—regarding them as a possible solution to the paucity of R&D productivity in the pharmaceutical sector…
Figure 1.
Number of NIH grants (1986–2009) that contained the terms ‘biomarker’ or ‘biomarker and discovery’, as derived from the NIH RePORT database (http://projectreporter.nih.gov/reporter.cfm). A similar trend is obtained when PubMed is searched for scholarly publications containing the term ‘biomarker’ over the same time-frame. Reproduced with permission from Ptolemy & Rifai (2010).
The ‘gold-rush’ for biomarkers is spurred by the potentially large benefits—both economical and societal—for those that succeed in identifying a reliable marker with great diagnostic value for disease manifestation or progression, ideally in a peripheral fluid such as blood or urine. The quest for cancer biomarkers dominates, but increasing efforts are being devoted to finding markers for other conditions, from cardiovascular risk or autoimmune diseases such as type 1 diabetes, to blood–brain barrier damage or infection-related complications such as sepsis.
Alzheimer disease is one area in which biomarker research could have a huge impact. Although a predictive test for Alzheimer disease risk is needed, no confirmed biomarkers exist. Diagnosing Alzheimer disease in its early stages remains extremely difficult, although new neuroimaging techniques and advanced software offer some hope (BBC, 2011). As the biochemical changes in the brain's extracellular fluid that indicate pathologies are expressed in the cerebrospinal fluid (CSF), the best molecular evidence comes from measuring tau protein and β-amyloid in the CSF. Yet, blood plasma could also be a source of biomarkers for neurodegenerative changes in the brain, which would make Alzheimer disease diagnosis much simpler. So far, however, things seem complicated, partly owing to the inherent difficulty in yielding an accurate classification of cases of disease and controls. For example, a blood-based protein biomarker profile that has just been released (O'Bryant et al, 2010) has minimal overlap with 18 markers that were previously reported to be able to distinguish Alzheimer disease and control subjects with close to 90% accuracy (Ray et al, 2007).
Is there a possibility of finding an Alzheimer disease-specific signature in plasma? Simon Lovestone, professor of old-age psychiatry at the Biomedical Research Centre for Mental Health at King's College (London, UK) thinks so. “Our proteomic work shows this to be the case,” he said. A study by his team used two-dimensional gel electrophoresis coupled with mass spectrometry, identifying several proteins previously implicated in the disease, including complement factor H (CFH) precursor and α-2-macroglobulin; their elevation was shown to be specific for Alzheimer disease and to correlate with disease severity (Hye et al, 2006). This and other evidence, Lovestone argues, indicates that a panel of plasma markers will probably be needed to discover and confirm Alzheimer disease, rather than a single biomarker. Moreover, these blood-based markers will not stand alone in practice, Lovestone thinks, but will be used together with CSF and imaging markers—a combination that offers added value.
Efforts to identify biomarkers of disease state are one the main drivers of proteomics research. Notwithstanding the progress made so far (Fig 2), the promise of new techniques to search the proteome for reliable biomarkers has yet to be fulfilled. An analysis conducted by Leigh Anderson from the Plasma Proteome Institute in Washington, DC, USA, on the basis of US Food and Drug Administration (FDA) approvals for protein-based assays until 2008, revealed that only 22 new tests were introduced to clinical practice in the past 15 years, and, notably, none of these were results of proteomic-based investigations (Anderson, 2010). “This rate falls far short of that needed to support projected medical needs and indicates serious deficiencies in the protein biomarker pipeline,” Anderson wrote.
Notwithstanding the progress made so far, the promise of new techniques to search the proteome for reliable biomarkers has yet to be fulfilled
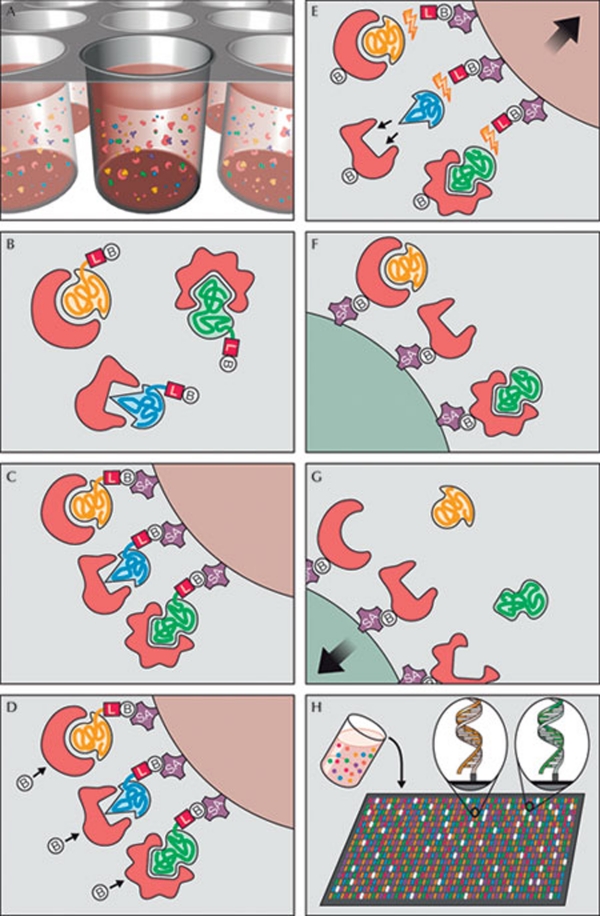
Figure 2.
Principle of multiplex SOMAmer affinity assay, a new aptamer-based proteomic technology for biomarker discovery capable of simultaneously measuring thousands of proteins from small sample volumes. (A) Aptamers (SOMAmers)—short stretches of nucleic acids that form protein-binding three-dimensional structures—and samples are mixed in 96-microwell plates and allowed to bind. Cognate and non-cognate SOMAmer-target protein complexes form. Free SOMAmer and protein are also present. (B) SOMAmer molecules (yellow, blue, and green) have unique shapes selected to bind to a specific protein. SOMAmers contain biotin (B), a photo-cleavable linker (L) and a fluorescent tag at the 5´ end. Most SOMAmers (yellow and green) bind to cognate proteins (red), but some (shown in blue) form non-cognate complexes. (C) SOMAmers are captured onto a bead coated with streptavidin (SA), which binds to biotin. Uncomplexed proteins are washed away. (D) Proteins are tagged with NHS-biotin. (E) UV light (hν) cleaves the linker and SOMAmers are released from beads, leaving biotin on the bead. Samples are challenged with an anionic competitor (dextran sulphate). Non-cognate complexes (blue SOMAmer) preferentially dissociate. (F) SOMAmer–protein complexes are captured onto new avidin-coated beads by protein biotin tag. Free SOMAmers are washed away. (G) SOMAmers are released from complexes into a high-pH solution. (H) The remaining SOMAmers are quantified by hybridization to microarray containing single-stranded DNA probes complementary to SOMAmer DNA sequence, which form a double-stranded helix. Hybridized SOMAmers are detected by fluorescent tags when the array is scanned. Figure credit: Gold et al (2010). http://creativecommons.org/licenses/by/2.5
The hurdles to be overcome are undeniably huge. Protein concentrations in blood or urine vary by several orders of magnitude, and molecules that could have informative value might be dispersed and masked by more-common proteins. Splicing and post-translational variants are adding to the molecular variety. Finally, transferring biomarker detection techniques from research to the clinical laboratory can prove difficult. Although most clinical diagnostics use single-protein biomarkers, it is likely that cracking the early signature of complex human diseases will require the detection of multiple biomarkers, and the development of complex algorithms to find specific patterns. Other variables, such as poorly standardized criteria for the selection of patients and controls, or procedures for the handling, collection, storage and processing of samples also pose problems for moving biomarker research into the clinic.
…transferring biomarker detection techniques from research to the clinical laboratory can prove difficult
“A problem in identifying biomarkers is the biological variability, which is often underestimated,” commented Harald Mischak, Chief Scientific Officer of mosaiques-diagnostics, a company based in Hannover, Germany, that specializes in proteomics services for clinical diagnosis and pharmaceutical research. “Analysis of a biomarker in a small cohort may well suggest significant association with the disease or pathology of interest. However, in fact it only shows association with a difference between the cohorts investigated, and may well not at all be associated with disease.” Mischak believes that this problem can be partly addressed by using multi-centred sampling and the mandatory verification of results in an independent cohort. He recalled the case of urinary proteomics to identify disease-associated biomarkers that have been verified in several independent cohorts; these are already being used in patient evaluation and clinical trials, and are being evaluated by regulatory agencies for qualification as biomarkers (Alkhalaf et al, 2010; Mischak et al, 2010). “What is still substantially funded in Europe is the discovery of ‘potential biomarkers’. What actually is required is the application of potential biomarkers in large, multicentric trials to demonstrate their benefit. Unfortunately, by far less studies with such aims are funded, for a variety of reasons,” Mischak said.
The quest for new blood-based biomarkers is not limited to proteins. Circulating microRNAs (miRNAs)—non-coding RNAs that inhibit messenger RNA translation or induce its degradation—have emerged as a new class of potential biomarkers. A team led by Maurizio C. Capogrossi at the Istituto Dermopatico dell'Immacolata–IRCCS in Rome, Italy, in collaboration with investigators from the Centro Cardiologico Monzino–IRCCS in Milan, examined miRNA plasma levels in humans with acute myocardial infarction and in a mouse model (D'Alessandra et al, 2010). They found that levels of miR-1, miR-133a, miR-133b and miR-499-5p increased a few hours after the onset of myocardial-infarction symptoms in both humans and mice, and after five days had returned to normal. In humans, miR-1, miR-133a and miR-133b reached their peak levels before plasma troponin I, which is considered an excellent biomarker for cardiac injury, demonstrating that selected miRNAs are sensitive markers of cardiac ischaemic damage. “Since the initial description of circulating miRNAs in pregnant women in 2008, a number of studies have shown that miRNAs may represent novel biomarkers of a variety of diseases,” Capogrossi commented. “To date it is still too early to say that one miRNA, or a miRNA signature characterized by changes in the blood level of several miRNAs, provides higher diagnostic accuracy than standard diagnostic procedures for any disease. Time will tell.” Nevertheless, he said that it is noteworthy that, in myocardial infarction, the miRNA release process seems to be selective, resulting in miRNAs differentiated from the commonly used biomarkers of myocardial infarction—such as troponins and creatine kinase—that are released by necrotic cells on disruption of the plasma membrane. “Whether this major difference will make them better diagnostic tools remains to be established,” Capogrossi cautioned.
A different approach was taken to identify genes selectively expressed in psychiatric disorders, for which there are to date no clinical blood tests. “Given the complex nature of these disorders, the current reliance on a person's self-reported symptoms and the clinician's impression of the patient after interview is a rate-limiting step in delivering the best possible care,” said Alexander Niculescu from the Indiana University School of Medicine in Indianapolis, Indiana USA. His team has identified genetic markers for bipolar affective disorder and blood RNA biomarkers for mood and psychotic disorders, all of which have predictive ability in independent cohorts (Le-Niculescu et al, 2009; Patel et al, 2010; Kurian et al, 2011). “We are working hard to increase the accuracy of these tests, and integrate them with other clinical measures into algorithms and scores that can inform diagnosis, treatment and preventive interventions,” he said. “We think such approaches will revolutionize psychiatry, medicine, and society at large.”
Whether it is proteins, genes or RNA, more action is needed to nurture the clinical use of biomarkers, and thus leverage their economic value. “I see the big gap between the discovery and scientific qualification of a biomarker, and the requirements of the regulatory agencies as a big obstacle to actually implementing biomarkers in clinical use for the benefit of the patients. This gap must be closed,” Mischak said. “Ideally, regulatory agencies should be involved in the study planning to help them understand the new technologies, and at the same time to ensure that data are produced that allow regulatory qualification and, if appropriate, clinical implementation.”
Whether it is proteins, genes or RNA, more action is needed to nurture the clinical use of biomarkers, and thus leverage their economic value
The pharmaceutical sector is also gearing up to enter the biomarker market, which is forecasted to expand in the next few years, especially for cancer. Much of the economic value of biomarkers is linked to the concept of ‘companion diagnostics’—the idea that new drugs will come with a companion test to identify variations in drug metabolism and predict individual responses. So far, however, progress to the market has been slow for biomarkers, and new business models are under scrutiny. “Diagnostics are considered low-value-added by health-care payers in comparison with therapeutics. Moreover, evaluating the health and economic benefits of novel diagnostic tests has proven difficult. Firms are thus unwilling to invest heavily in the development of diagnostic biomarkers unless they are associated with the prescription of a particular drug which captures value for the test, as is the case in pharmacogenomics or theranostics,” explains a report from the biotechnology division of the Organization for Economic Cooperation and Development (OECD, 2010). “If there is a push toward requiring the generation of data for the clinical evaluation of diagnostic tests, the experts predict that will entail a shift in the diagnostic business model towards one closer to that of therapeutics: fewer high quality products make it to market but will need to be reimbursed at rates that better reflect their value in health care.”
As the scientific, regulatory and business challenges to bringing diagnostic and safety (Sidebar A) biomarkers to the clinic and market are overwhelming for any single stakeholder—including government, industry, academia, providers and patient advocates—collaboration is necessary. Positive examples of this exist, and might provide paradigms for the future. The Biomarkers Consortium, a public–private biomedical research partnership managed by the Foundation for NIH (FNIH) in Bethesda (Maryland, USA) is fostering pre-competitive collaboration—sharing early stages of research that benefit all—as a feasible approach to biomarker qualification. The first completed project of the Biomarkers Consortium analysed data from clinical trials conducted by four pharmaceutical companies and demonstrated that adiponectin has potential utility as a predictor of metabolic responses to peroxisome proliferator-activated receptor (PPAR) agonists in individuals with type 2 diabetes (Wagner et al, 2010).
As the scientific, regulatory and business challenges to bringing diagnostic and safety biomarkers to the clinic and market are overwhelming for any single stakeholder […] collaboration is necessary
Sidebar A | Biomarkers for drug safety.
In addition to being indicators of normal biological processes and disease conditions, biomarkers can also measure drug response or the effectiveness of a medical treatment. As the cost of developing drugs has sky-rocketed in recent years, thereby reducing the number of new drug approvals, finding safety biomarkers might provide a more focused and productive strategy for drug development. “From an industry standpoint, drug-induced toxicity is a serious issue, killing 30% of compounds overall, from leads in the pre-clinic all the way to marketed products. The availability of better pre-clinical toxicity biomarkers thus remains a key strategic goal,” commented an editorial in Nature Biotechnology introducing a series of articles on biomarker qualification (Anon, 2010).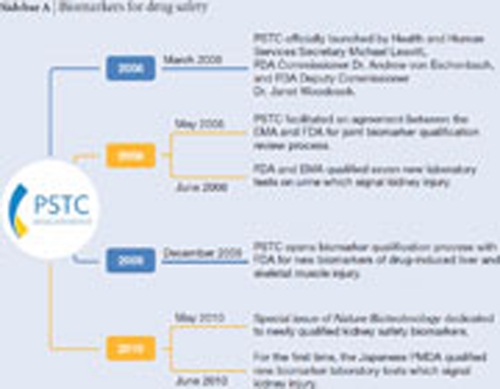
With this in mind, the FDA has recently developed the biomarkers qualification process, a new regulatory process to smooth the path of integrating new biomarkers into therapies; the European Medicines Evaluation Agency (EMEA) and the Pharmaceuticals and Medical Devices Agency in Japan are acting in a similar manner (Goodsaid & Mendrick, 2010). Urinary biomarkers of kidney toxicity have been the first to initiate and test the process. Following the submission of data by the Predictive Safety Testing Consortium (PSTC; see Figure)—a public–private partnership led by the non-profit Critical Path Institute—seven new urinary markers for nephrotoxicity have been reviewed and qualified in animal models through a joint FDA–EMEA effort (EMEA, 2009).
Experts from academia, government and industry are often called to interact to improve the development of biomarkers to determine drug safety. During a workshop on the topic, organized by the Institute of Medicine of the US National Academies of Science in Washington, DC, USA, one of the issues identified as affecting the development and use of biomarkers to detect drug toxicity was understanding the mechanisms of action of the drugs. “Although a biomarker can provide predictive information based solely on the association between its intensity and organ toxicity or other outcomes, biomarkers have their greatest value when they unveil a mechanism that can be understood so the drug can be altered to avoid the toxicity. The same is true when biomarkers reveal mechanisms of benefit. Yet regardless of whether such mechanistic insights are gained, reliable information that can distinguish who is at risk and who will benefit is valuable. And the discovery of a predictive biomarker can lead to further research on the association between that biomarker and an outcome,” (Olson et al, 2009).
“Biomarkers represent a particularly fertile area for collaboration, given the resources (data, expertise, financial) and challenges required to develop and qualify them. Consensus across multiple sectors is highly desirable, and public–private consortia are an ideal approach,” said Jenna Mills, communications manager for FNIH. “Recent advances, including the implementation of network models and large-scale consortia, highlight the increasing role that pre-competitive collaboration is assuming within biomedical research and drug development. Data-sharing projects can be completed and answer many questions that would otherwise be impossible to resolve using the data sets of each individual company alone.” Nine more projects, including four clinical trials, are ongoing, focusing on cancer, metabolic disorders and neurological disorders. “This model of biomarker validation fosters future research which addresses medical needs, aids regulatory authorities with making informed decisions, and allows all stakeholders involved to align their priorities in order to ultimately benefit the public,” said Mills. In addition, the Biomarkers Consortium is about to launch two clinical trials aimed at the qualification of new kidney safety biomarkers in collaboration with the Predictive Safety Testing Consortium (PSTC), expanding on the pre-clinical work of the PSTC nephrotoxicity working group (Sidebar A).
This vision is a change from the traditional model of drug development. Nevertheless, despite the potholes remaining to be avoided or filled, it seems to be the road ahead.
Footnotes
The author declares that he has no conflict of interest.
References
- Alkhalaf A et al. (2010) Multicentric validation of proteomic biomarkers in urine specific for diabetic nephropathy. PLoS ONE 5: e13421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson NL (2010) The clinical plasma proteome: a survey of clinical assays for proteins in plasma and serum. Clin Chem 56: 177–185 [DOI] [PubMed] [Google Scholar]
- Anon (2010) Biomarkers on a roll. Nat Biotechnol 28: 431. [DOI] [PubMed] [Google Scholar]
- BBC (2011) New system uses brain scans to spot early Alzheimer's. 7 Mar. http://www.bbc.co.uk/news/health-12653306
- D'Alessandra Y et al. (2010) Circulating microRNAs are new and sensitive biomarkers of myocardial infarction. Eur Heart J 31: 2765–2773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EMEA (2009) Final conclusions on the pilot joint EMEA/FDA VXDS experience on qualification of nephrotoxicity biomarkers. London, UK: European Medicines Agency http://www.ema.europa.eu/docs/en_GB/document_library/Regulatory_and_procedural_guideline/2009/10/WC500004205.pdf
- Gold L et al. (2010) Aptamer-based multiplexed proteomic technology for biomarker discovery. PLoS ONE 5: e15004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodsaid FM, Mendrick DL (2010) Translational medicine and the value of biomarker qualification. Sci Transl Med 2: 47ps44. [DOI] [PubMed] [Google Scholar]
- Hye A et al. (2006) Proteome-based plasma biomarkers for Alzheimer's disease. Brain 129: 3042–3050 [DOI] [PubMed] [Google Scholar]
- Kurian SM et al. (2011) Identification of blood biomarkers for psychosis using convergent functional genomics. Mol Psychiatry 16: 37–58 [DOI] [PubMed] [Google Scholar]
- Le-Niculescu H, Kurian SM, Yehyawi N, Dike C, Patel SD, Edenberg HJ, Tsuang MT, Salomon DR, Nurnberger JI Jr, Niculescu AB (2009) Identifying blood biomarkers for mood disorders using convergent functional genomics. Mol Psychiatry 14: 156–174 [DOI] [PubMed] [Google Scholar]
- Mischak H et al. (2010) Recommendations for biomarker identification and qualification in clinical proteomics. Sci Transl Med 2: 46ps42. [DOI] [PubMed] [Google Scholar]
- O'Bryant SE, Xiao G, Barber R, Reisch J, Doody R, Fairchild T, Adams P, Waring S, Diaz-Arrastia R, Texas Alzheimers Research Consortium (2010) A serum protein-based algorithm for the detection of Alzheimer disease. Arch Neurol 67: 1077–1081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- OECD (2010) Biomarkers and Targeted Therapies. Paris, France: Organization for Economic Cooperation and Development. http://www.oecd.org/document/48/0,3746,en_2649_34537_39405168_1_1_1_1,00.html
- Olson S, Robinson S, Giffin R (2009) Accelerating the development of biomarkers for drug safety: workshop summary. Washington, DC, USA: National Academies Press [PubMed] [Google Scholar]
- Patel SD, Le-Niculescu H, Koller DL, Green SD, Lahiri DK, McMahon FJ, Nurnberger JI Jr, Niculescu AB 3rd (2010) Coming to grips with complex disorders: genetic risk prediction in bipolar disorder using panels of genes identified through convergent functional genomics. Am J Med Genet B Neuropsychiatr Genet 153B: 850–877 [DOI] [PubMed] [Google Scholar]
- Ptolemy AS, Rifai N (2010) What is a biomarker? Research investments and lack of clinical integration necessitate a review of biomarker terminology and validation schema. Scand J Clin Lab Invest 70: 6–14 [DOI] [PubMed] [Google Scholar]
- Ray S et al. (2007) Classification and prediction of clinical Alzheimer's diagnosis based on plasma signaling proteins. Nat Med 13: 1359–1362 [DOI] [PubMed] [Google Scholar]
- Wagner JA et al. (2010) The Biomarkers Consortium: practice and pitfalls of open-source precompetitive collaboration. Clin Pharmacol Ther 87: 539–542 [DOI] [PubMed] [Google Scholar]