Abstract
Oxidative stress, associated with either normal metabolism or disease conditions, affects many cellular activities. Most of our knowledge in this field is derived from fully differentiated cells. Embryonic stem cells (ESCs) have attracted enormous attention for their potential applications in cell therapy, but little is known about how the unique properties of ESCs are affected by oxidative stress. We have investigated the effects of oxidative stress induced by H2O2 on several cellular activities of mouse ESCs. Like differentiated cells, ESCs are sensitive to H2O2-induced apoptosis when continuously exposed to H2O2 at the concentrations above 150 μM. However, unlike differentiated cells, ESCs are resistant to oxidative stress induced senescence. This is demonstrated by the results that when subjected to a short-term sublethal concentration and duration of H2O2 treatment, fibroblasts enter the senescent state with enlarged flattened cell morphology concurrent with increased expression of senescence marker p21. On the contrary, ESCs neither show any sign of senescence nor express p21. Instead, ESCs enter a transient cell cycle arrest state, but they have remarkable recovery capacity to resume the normal cell proliferation rate without losing the ability of self-renewal and pluripotency. Our results further revealed that H2O2 inhibits cell adhesion and the expression of cyclin D1, which are early events proceeding apoptosis and cell cycle arrest. In conclusion, our data suggest that ESCs are sensitive to H2O2 toxicity, but may have unique mechanisms that prevent H2O2-induced senescence and protect self-renewal capacity.
Introduction
Normal cellular activities require a balanced redox environment. Disturbances in the normal redox state from deregulated metabolism or other cellular damage may result in oxidative stress, which is considered to be a major cause of aging and age-related diseases and affects numerous other cellular processes [1]. A certain amount of reactive oxygen species (ROS), such as O2− and H2O2, is generated during normal cellular metabolism [2,3]. Normally, these ROS are metabolized and tightly controlled in the cell. However, excess ROS can be generated from deregulated metabolism or other cellular damage and results in oxidative stress. Therefore, the production of ROS is an inevitable consequence of cellular activity associated with normal metabolism as well as diseased states.
ROS have traditionally been considered as toxic by-products of cellular metabolism. It is now recognized that they have broad impacts on various cellular events and act as signaling molecules [3,4]. Although different cell types may have different tolerance levels to oxidative stress, cells usually undergo apoptotic cell death when exposed to high level of ROS [5], or they may enter into senescent state when the ROS level is sublethal [6]. Cellular senescence is defined as a state where cells lose their ability to divide due to irreversible growth arrest. Depending on cell type and physiological state, the cell doubling time may vary, but senescence is an unavoidable event for primary cells in culture. It is believed that senescence under normal culture conditions is caused by telomere shortening during continuous proliferation, a phenomenon known as replicative senescence. Under various stress conditions, such as oxidative stress, DNA damage, or oncogenic mutation, cells can enter senescence state without telomere shortening. This type of senescence is known as stress-induced premature senescence. Senescent cells are characterized by their enlarged flattened cell bodies. They do not divide but can be viable for a long period of time. At the molecular level, increased expression of cell cycle inhibitors, including p21waf1 (p21), p19ARF (p19), and p16 INK4A (p16), is believed to be at least partly responsible for the onset of senescent state [7]. It is proposed that oxidative stress-induced apoptosis and senescence may act as mechanisms that protect the organism from further damages such as malignant transformation [8,9]. However, recent studies have indicated that appropriate levels of ROS generated within the cells may act as signaling molecules that regulate a wide range of cellular processes depending on cell types, such as cellular defense [2], and vascular functions [4,10], differentiation, and proliferation [11–13]. Most of the studies related to oxidative stress in the literature have used fully differentiated primary cells or cell lines. Embryonic stem cells (ESCs) have attracted enormous attention in recent years due to their ability to differentiate to various types of cells, which may be eventually used as the sources for cell-based therapy. While it is exciting to recognize the potential benefit, currently we have limited knowledge of basic physiology of this unique cell type. Much research in the field has been focused on the investigation of ESC differentiation and pluripotency, largely driven by their potential therapeutic applications. However, a fundamentally important issue yet to be addressed is how ESCs protect themselves from damage caused by various forms of stress. The medical application of ESC-derived cells with damage or mutation could be detrimental to patients. ESCs are derived from the inner cell mass of an early stage embryo known as a blastocyst, which is in the pre-implantation stage during embryogenesis. Under in vivo condition, ESCs are exposed to a hypoxic environment of the uterine cavity. At this stage, ESCs have only a few mitochondria for ATP production [14]. It has been recently shown that during ESC differentiation, the number of mitochondria increases. Once cells start to produce large amounts of ATP through oxidative phosphorylation, ROS will be produced as by-products. Thus, oxidative stress could be an important factor that affects in vitro ESC propagation and differentiation [14]. Therefore, understanding the response of ESCs to oxidative stress will be important for proper maintenance of ESC pluripotency and for directed differentiation of specific cell lineages for clinical applications. Under proper culture conditions, ESCs have unlimited capacity for self-renewal. It is reported that ESCs can self-renew continuously for 2 years in culture [15]. Apparently, they do not undergo replicative senescence under the conditions optimized for the maintenance of pluripotency. ESCs have an unusual cell cycle control mechanism to allow rapid cell division, which does not follow the conventional roles of cell cycle regulation [16]. When induced, they can differentiate into all types of cells derived from the 3 germ layers, a property known as pluripotency [17–19]. These unique properties of ESCs constitute the basis of embryonic development. It is imperative that they must be equipped with effective cellular defense mechanisms to deal with various cellular insults and to ensure normal growth and development. Indeed, recent studies suggested that stress defense in human ESCs is superior to that of differentiated cells as demonstrated by their DNA repair capacity [20], which is down-regulated during differentiation [21]. Nonetheless, when the damages reach the degree that is beyond repair, apoptotic cell death of ESCs may be a safeguard mechanism to avoid potential developmental abnormalities, as illustrated in the cases of ESC apoptosis induced by DNA damage agents and UV irradiation [22,23]. How ESCs respond to different levels of oxidative stress is largely unknown. It is conceivable that ESCs may share some common defense mechanisms with fully differentiated cells. However, considering the fundamental differences in the regulatory systems between the 2 types of cells, it is most likely that ESCs must have some unique systems to cope with oxidative stress. Using well-established in vitro cell culture models that investigate H2O2-induced oxidative stress, we examined the effects of H2O2-induced oxidative stress applied at different levels and durations on ESC proliferation, apoptosis, senescence, and the self-renewal.
Materials and Methods
ESC culture and cell treatment
Generation of ESCs has been previously described [24,25]. They were cultured in DMEM containing 15% fetal bovine serum (FBS) and 1,000 U/mL leukemia inhibitory factor (LIF) and were routinely maintained in cell culture dishes coated with 0.1% gelatin at 37°C in a humidified atmosphere at 5% CO2 as previously described [26]. Mouse embryonic fibroblasts (MEFs, purchased from Millipore) were cultured in the same medium for ESCs without LIF.
Oxidative stress was induced by exposing cells to H2O2 (Sigma, St. Louis, MO) added to the medium. Two formulas of treatments were used: (1) cells were treated with different concentrations of H2O2 for the entire course of experiment to determine the cell tolerance to H2O2 toxicity (long-term treatment) and (2) cells were briefly treated with 150 μM for 2 h (short-term treatment). The medium was then changed to fresh medium to allow cells to recover. This treatment was sublethal and was designed to assess the effects of H2O2 on different cellular activities. Seeded cells were usually cultured for 12 to 48 h before treatment. Cell density was controlled at 30%–60% confluence at the time of treatment or otherwise the numbers of cells initially seeded on the culture dishes were specified in individual experiments.
Embryoid bodies (EB) formation was performed by suspending ESCs in bacterial culture dishes (1 × 105 cells/mL) in which ESCs clumped and formed EBs as previously described [26]. After incubation for 24 h, the medium was changed to LIF-free ESC medium containing 15% FBS and was refreshed every other day. EBs were cultured for 10 days.
Cell viability, proliferation, and cell cycle analysis
Cell viability and proliferation were determined by toluidine blue cell staining method [27]. Cells treated under various conditions were fixed with 3% paraformaldehyde for 30 min, washed with phosphate buffered saline (PBS), and then stained with 1% toluidine blue. The wells were washed extensively with water. Then, the dye was extracted with 2% sodium dodecyl sulfate (SDS). The absorbance at 630 nm, which correlates with the number of viable cells and was used as an indirect measurement of cell number, was measured with a microtiter plate reader. Cell cycle was analyzed by flow cytometry as we previously described [27]. In brief, cells were released from the culture dishes with trypsin, washed with PBS containing 1% FBS, and then fixed with 80% ethanol at 4°C for 15 min. Fixed cells were stained with 20 μg/mL propidium iodide (PI) in PBS buffer containing 1% FBS, 0.05% Triton X-100, and 50 μg/mL RNase. After 30 min incubation at room temperature, the percentage of cells in different phases of the cell cycle was determined by DNA content (PI intensity) with an Accuri C6 flow cytometer. The population of G1, S, and G2/M phase cells was determined with the CFlow software.
Apoptosis analysis
Apoptosis was determined by cell morphology, nuclear/DNA fragmentation, and annexin-V labeling. Control cells and H2O2-treated cells were stained with 10 μM Hoechst 33285 and examined under a microscope. Apoptotic cells are characterized by disintegrated cell bodies and fragmented nuclei. Apoptosis was further confirmed and quantified with annexin-V–fluorescein isothiocyanate (FITC) apoptosis staining. After treatment with H2O2, both floating cells in the medium and the cells attached to the culture dishes were collected and combined. After being washed twice with cold PBS, cells were resuspended in annexin-binding buffer, and incubated with annexin-V–FITC according to the manufacturer's instructions (BioLegend, San Diego, CA). Apoptotic cells-labeled annexin-V–FITC were identified and quantified with an Accuri C6 flow cytometer.
Cell adhesion analysis
The cell adhesion assay was carried out according to the procedures previously described [27]. To analyze the effect of H2O2 on cells already attached, cells were cultured for 24 h before H2O2 treatment. To analyze the effect of H2O2 on the initial cell adhesion, H2O2 was added to the culture medium at the same time when cells were seeded. After incubating for different time periods as indicated, non-adherent cells in the medium were removed. Cells attached to the culture dishes were measured by toluidine blue staining method.
Self-renewal and pluripotency analysis
ESCs were seeded in 6-well dishes (5 × 103 cells/well) and incubated for 2 days to allow for the formation of small colonies. They were treated with 150 μM H2O2 for 2 h (short term) as previously defined. The cells were then cultured in normal medium for additional 5 days. Undifferentiated ESCs form compact colonies and express alkaline phosphatase (AP) and pluripotency markers [28]. AP expression was determined with a Fast Red TR salt™ staining kit (Sigma) according to the manufacturer's instruction. The expression levels of pluripotency markers, Oct4, Sox2, and Nanog, were determined by western blot and by real-time RT-qPCR.
Senescence analysis
Senescence was determined by morphological criteria and by cellular/biochemical marker analysis. ESCs were cultured and treated with H2O2 under the same conditions as described for self-renewal and pluripotency analysis. MEFs (∼50% confluence) were used in a parallel experiment as a positive control. At the end of the experiment, the cells were fixed with 3% paraformaldehyde for 30 min, washed with PBS, and stained with a β-galactosidase (SA-β-gal) detecting kit (Sigma) according to the manufacturer's instruction and examined under a phase contrast microscope. Senescent cells were identified by their enlarged/flattened cell bodies and expression of SA-β-gal, a widely used senescent cell marker [29]. Senescence was further assessed by the expression level of cell cycle inhibitors, p21, p19, and p16, as biochemical senescence markers by quantitative real-time PCR (RT-qPCR).
RNA extraction, reverse transcription, and polymerase chain reaction (RT-PCR)
Total RNA was extracted using Tri-reagent (Sigma). cDNA was prepared by M-MLV reverse transcriptase. The specificity of the PCR was determined by the dissociation curve and confirmed by agarose gel electrophoresis. Quantitative real-time PCR (RT-qPCR) was performed using SYBR green jumpstart Taq ready mix on a MX3000PTM Real-time PCR system (Stratagene, La Jolla, CA) as previously reported [26]. Sequences of the primer sets are as follows (F, forward; R, reverse):
β-actin, F: 5′-CATGTACGTAGCCATCCAGGC-3′, R: 5′-CTCTTTGATGTCACGCACGAT-3′; Cyclin D1, F: 5′-CAGAAGTGCGAAGAGGAGGTC-3′, R: 5′-TCATCTTAGAGGCCACGAACAT-3′; Cyclin A, F: 5′-CAGTCACAGGACAGAGCTG G-3′, R: 5′-GGGCATGTTGTGGCGCTTTG-3′; Cyclin E, F: 5′-CCTCCAAAGTTGCACCAGTTTGC-3′, R: 5′-GACACACTTCTCTATGTCGCACC-3′; p19, F: 5′-ATGCTGGATTGCAGAGCAGTA-3′, R: 5′-ACGGGGCACATTATTTTTAGTCT-3′; p16, F: 5′-CGCAGGTTCTTGGTCACTGT-3′, R: 5′-TGTTCACGAAAGCCAGAGCG-3′; p21, F: 5′-CGAGAACGGTGGAACTTTGAC-3′, R: 5′-CAGGGCTCAGGTAGACCTTG-3′; Oct4, F: 5′-AGTTGGCGTGGAGACTTTGC-3′, R: 5′-CAGGGCTTTCATGTCCTGG-3′; Sox2, F: 5′-GACAGCTACGCGCACATGA-3′, R: 5′-GGTGCATCGGTTGCATCTG-3′; Nanog, F: 5′-TTGCTTACAAGGGTCTGCTACT-3′, R: 5′-ACTGGTAGAAGAATCAGGGCT-3′.
The mRNA level from qRT-PCR was calculated using the comparative Ct method [30]. β-Actin mRNA was used as a calibrator for the calculation of relative mRNA levels of the tested genes.
Cell lysate preparation and western blot analysis
ESCs were lysed in M-PER mammalian cell protein extraction buffer (Pierce, Rockford, IL) supplemented with a cocktail of protease inhibitors. After being kept on ice for 30 min, the extracts were centrifuged at 15,000g for 15 min at 4°C. The supernatant was designated as the cell lysate and was subjected to SDS-PAGE. Western blot analysis was carried out as previously described [31].
Statistical analysis
Statistical analysis was performed using a 2-tailed unpaired Student's t-test. Differences are considered statistically significant when P < 0.05.
Results
The effects of H2O2 on ESC viability and apoptosis
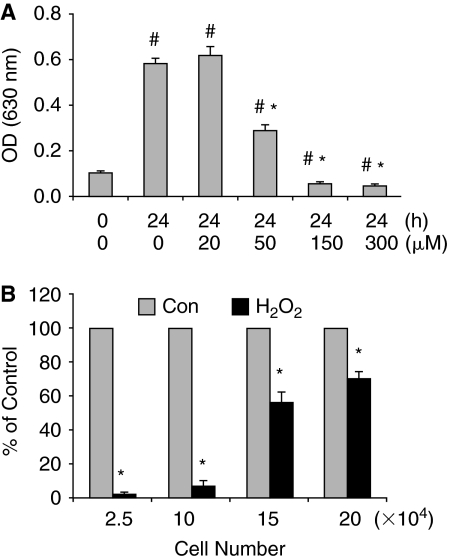
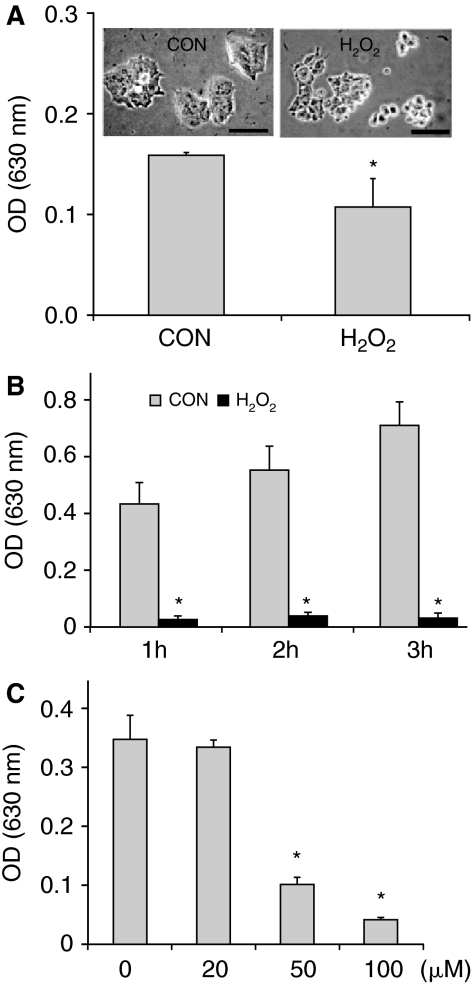
H2O2 is an intermediate product of cellular metabolism and one of the major sources of intracellular ROS. Therefore, it is widely used in the study of oxidative stress in cell culture models [32]. To test how ESCs respond to H2O2, a set of experiments with different strengths and durations of oxidative stress induced by H2O2 were performed. We first tested the effects of H2O2 on cell proliferation and viability on ESCs at different concentrations ranging from 20 to 300 μM. The number of viable cells was determined after 24 h treatment. As shown in Figure 1A, H2O2 at 20 μM did not affect cell proliferation while 50 μM H2O2 showed significant inhibitory effect. At the concentrations above 150 μM, H2O2 not only inhibited cell proliferation, but also caused cell death since the remaining cells after treatment were less than initially seeded (Fig. 1A). The above experiment was conducted at the cell density of ∼30% confluence. We noticed that the effect of H2O2 on ESCs is inversely related to cell density. At higher densities, the cells were more resistant to the effect of H2O2 (Fig. 1B), similar to the finding reported by Wiese et al. in differentiated cells [33].
FIG. 1.
Effect of H2O2 on cell proliferation and viability. (A) Effect of H2O2 concentrations. Embryonic stem cells (ESCs) (2.5 × 104/well) were seeded in 24-well culture plates. After incubation for 24 h to allow cells for attachment, cells were exposed to different concentrations of H2O2 continuously for 24 h. Time zero (0 h) represents the time point when H2O2 was added. The absorbance at 630 nm, which correlates with the number of attached cells, was measured after toluidine blue staining. The results are mean ± SD from a representative experiment carried out in triplicate. The experiment was performed at least 3 times with similar results. #P < 0.05, compared with the value of 0 h/0 μM H2O2 sample; *P < 0.001, compared with the value of 24 h/0 μM H2O2 sample. (B) Effect of H2O2 is affected by cell density. Different number of cells was seeded in each well of 24-well culture plates. The cells were treated with 150 μM H2O2 for 24 h. The cell number in the control experiment (Con) of each group was taken as 100%. Results are mean ± standard deviation (SD) of 3 independent experiments. *P < 0.05, compared with the value of the control group.
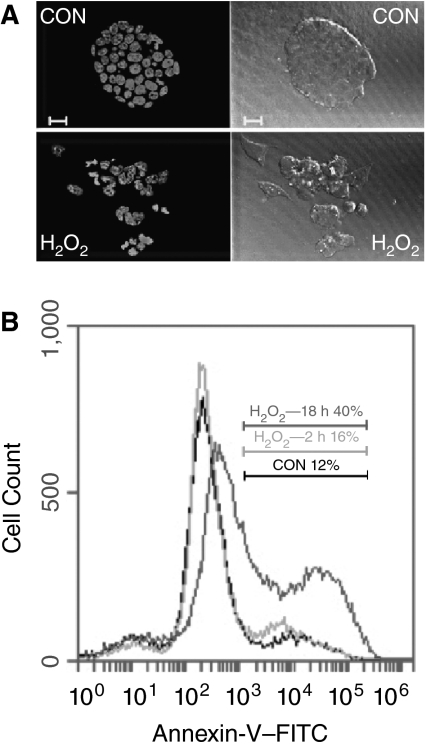
We speculated that the reduced cell number by H2O2 at the concentrations above 50 μM could be due to at least 2 mechanisms: induction of apoptosis and/or inhibition of cell proliferation. To test this hypothesis, we first conducted apoptosis analysis. In the control experiment, ESCs grew as compact colonies. Individual cells can be identified by their round intact nuclei stained with Hoechst 33285, a DNA-binding dye that gives a bright blue color under a fluorescent microscope. The cells treated with 150 μM and 300 μM H2O2 showed characteristics of apoptosis, disrupted cell bodies and fragmented nuclei (Fig. 2A), while the integrity of colonies and the nuclei was not affected by H2O2 below 50 μM (data not shown). Apoptosis was confirmed and quantified by annexin-V cell staining. Phosphatidylserine translocation from the inner to the outer plasma membrane is an early marker of plasma membrane alteration in apoptotic cells. Annexin-V binds to phosphatidylserine with high affinity and thus is commonly used as an indication of early apoptosis. As shown in Figure 2B, 150 μM H2O2 induced slight increase of annexin-V-positive cells at 2 h treatment. The apoptotic cells increased to 40% after 18 h incubation. The 300 μM H2O2 caused apoptosis in a similar pattern but with stronger effect (data not shown). These results demonstrated that apoptotic cell death is a major factor that contributed the reduced number shown in Figure 1 at the concentrations of 150 and 300 μM H2O2.
FIG. 2.
H2O2-induced apoptosis. (A) Nuclear fragmentation analysis. Embryonic stem cells (ESCs) treated with 150 μM H2O2 for 24 h were stained with Hoechst 33285 and examined and photographed with a LSM 510 confocal microscope (scale bar unit = 20 μm). Left panels show the nuclear staining. Right panels illustrate the colony morphology. Apoptotic cells induced by H2O2 are indicated by the fragmented nuclei and disrupted colonies. (B) Apoptosis analysis by annexin-V–FITC labeling. ESCs were treated with 150 μM H2O2 for 2 and 18 h. The cells collected from medium and the culture dishes were combined, labeled with annexin-V–FITC, and analyzed by flow cytometry. The area and percentage of annexin-V-positive cells (earlier apoptotic cells) in control experiment (CON) and H2O2-treated cells are indicated in the overlaid flow cytometry profiles.
Short-term treatment of ESCs with H2O2 induced transient cell cycle arrest
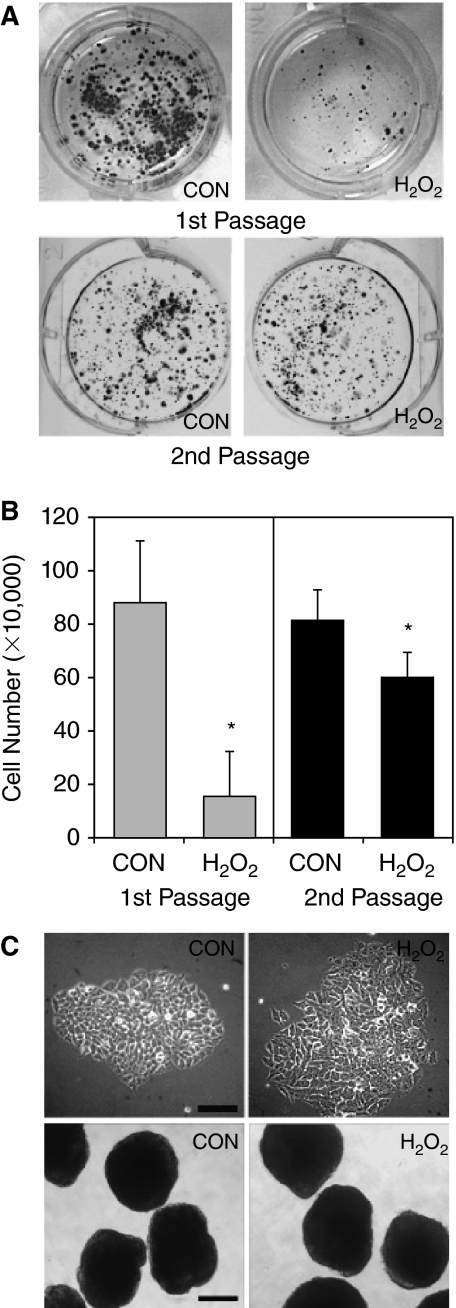
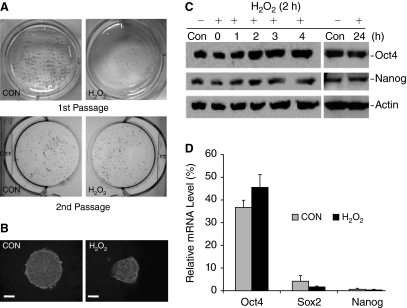
In somatic cells, it has been reported that a short-term treatment with 150 μM H2O2 for 2 h represents a sublethal condition that is known to cause cell cycle arrest and senescence [34,35]. Therefore, we investigated the response of ESCs to H2O2-induced oxidative stress under this condition. After the cells were treated with 150 μM H2O2 for 2 h, H2O2 was removed by changing medium to allow cells for recovery for 4 days. The resulting cells were designated as first passage cells. In comparison with control, H2O2 treatment resulted in significantly reduced number of colonies (Fig. 3A, first passage). To investigate if the short-term oxidative stress has a long-term impact on ESC proliferation, the control cells and cells treated with H2O2 (first passage cells) were released by trypsin. Equal numbers of cells were seeded onto new cell culture dishes and cultured under the normal culture conditions for 4 days (designated as second passage cells). Interestingly, the numbers of colonies derived from the control and H2O2-treated group were nearly the same (Fig. 3A, second passage). To quantitatively measure the cell number of first and second passage cells, the control and treated cells were released from the dishes and counted. As shown in Figure 3B, the initial H2O2 treatment significantly reduced cell number in the first passage, but cells survived oxidative stress can recover and show a growth rate close to that of control cells in the second passage. Furthermore, the colonies formed from second passage of treated cells displayed morphology of undifferentiated ESCs and were able to form EBs with size and shape similar to those derived from control cells (Fig. 3C).
FIG. 3.
Embryonic stem cells (ESCs) have remarkable recovery capacity from H2O2-inhibited cell proliferation. (A) Effect of H2O2 on colony formation. ESCs were seeded on 6-well dish and were treated with 150 μM H2O2 for 2 h. The cells were then cultured in fresh medium for 4 days. The cells were fixed and stained with toluidine blue staining and photographed with a digital camera (first passage). In parallel experiments, the control cells and treated cells were released by trypsin. The same numbers of cells were replated on to new culture dishes and cultured for 4 days in the normal culture medium without further treatment. The cells were fixed and visualized after toluidine blue staining (second passage). (B) Effect of H2O2 on cell proliferation. To quantitatively determine the effect of H2O2 on cell proliferation, control cells and H2O2 treated cells as shown in (A) were released by typsin and counted with a hemacytometer. The results are means ± SD of 3 independent experiments. *P < 0.05. (C) Cell morphology and embryoid bodies (EB) formation. Upper panels show the morphology of second passage cells (as shown in A but without toluidine blue staining) at higher magnification under a phase contrast microscope. Scale bar unit =100 μm. Lower panels show the size and morphology of EBs formed from second passage cells. Scale bar unit = 300 μm.
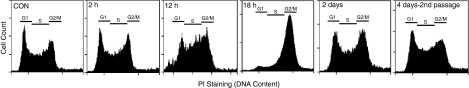
Since treatment with 150 μM H2O2 for 2 h only induced a slight increase of apoptosis (Fig. 2B), a likely explanation for the observations described in Figure 3 is that H2O2 may cause a transient but strong inhibitory effect on ESC proliferation. To test this hypothesis, we performed cell cycle analysis by flow cytometry. A unique characteristic of ESCs is that they proliferate very actively with a short period of the G1 phase and large population in the S phase of their mitotic cycle [25]. Figure 4 shows cell cycle profiles of untreated cells (CON) and cells at different times post short-term H2O2 treatment. At 2 h, the distributions of the cells at different phases of the cell cycle were similar to that of control cells. However, there was an apparent accumulation of cells at the G2/M phase at 12 h, which become the majority of cell population of the cell cycle by 18 h. This is a pattern of G2/M arrest in ESCs similar to that caused by nocodazol [36]. After culture for 2 days, the cell cycle profile significantly recovered. The second passage cells derived from initial H2O2 (as defined in Fig. 3) exhibited cell cycle profile similar to that of untreated cells (Fig. 5, 4-day second passage cells). These results are well-correlated with the H2O2-induced transient cell proliferation inhibition and subsequent recovery as described in Figure 3.
FIG. 4.
H2O2 induces transient cell cycle arrest of embryonic stem cells (ESCs). ESCs were seeded on 6-well dish (50% confluence) and were treated with 150 μM H2O2 for 2 h. The cells were then cultured in fresh medium for the times indicated. Control cells (CON) represent cells without H2O2 treatment. In parallel experiments, cells cultured for 3 days post H2O2 treatment were released by trypsin and replated on a new dish. The cells cultured for 4 days were designated as 4-day second passage cells. The cells were fixed with 80% ethanol, stained with PI, and subjected to flow cytometry analysis. The distribution areas of cells at G1, S, and G2/M phases were indicated by bars.
FIG. 5.
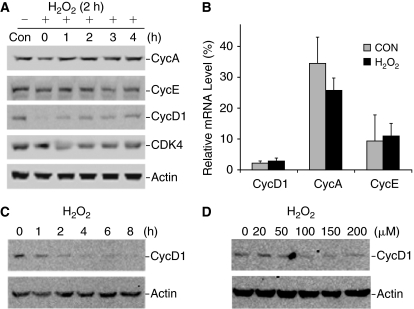
Effect of H2O2 on the expression of cell cycle proteins. (A) Embryonic stem cells (ESCs) were treated with 150 μM H2O2 treatment for 2 h. The medium was then changed to fresh medium (designated as 0 h). Cells were then incubated for the times indicated. The expression levels of cyclins and CDK4 were detected by western blot. (B) The mRNA level of cyclins was determined by RT-qPCR in cells from second passage of control and H2O2-treated cells (as defined in Fig. 3). The results are means ± SD of 3 independent experiments. The difference is not significant between controls and treated groups. (C) ESCs were subjected to 150 μM H2O2 continuously for the times indicated. (D) ESCs were treated with different concentrations of H2O2 treatment for 2 h. Cyclins and CDK4 were detected with their specific antibodies (Santa Cruz Biotechnology, Santa Cruz, CA). β-Actin was used as a control for protein loading in western blot analysis.
The effects of H2O2 on cell cycle regulator expression in ESCs
To investigate the molecular mechanism that led to H2O2-induced cell cycle arrest, we examined the expression of several cell cycle regulators. The cells were subjected to 150 μM H2O2 treatment for 2 h. The medium was then changed to fresh medium. This time was designated as the starting point (0 h) for cell recovery from oxidative stress. As shown in Figure 5A, the expression of cyclin D1 was significantly reduced at the time point right after H2O2 treatment while cyclin A, cyclin E, and CDK4 did not seem to be affected (Fig. 5A, 0 h). However, cyclin D1 started to recover immediately following the removal of H2O2. At 4 h incubation after treatment, cyclin D1 level nearly reached the level before treatment (Fig. 5A, 4 h). No difference was detected in the expression of cyclin D1, cyclin A, and cyclin E in control and H2O2-treated second passage cells (Fig. 5B) (as previously defined in Fig. 3). It is interesting to note that H2O2-induced decrease of cyclin D1 could not recover if the cells were continuously exposed to H2O2 (Fig. 5C). The dose–response analysis indicated that the effect of H2O2 on the cyclin D1 down-regulation was apparent at the concentrations above 50 μM (Fig. 5D).
Anti-adhesion induced by H2O2 is an early event preceding cell cycle arrest and apoptosis
Untreated cells were firmly attached to the cell culture dishes when they were incubated for 24 h after seeding. We noticed that cells were roundup and had a tendency to detach when treated with 150 μM H2O2 (Fig. 6A, inset). This observation indicated that H2O2 has anti-adhesive effect, which was quantitatively determined by measuring the adherent cells after removing the medium that contained detached cells. As shown in Figure 6A (graph), H2O2 treatment for 2 h resulted in 20%–30% cell loss (from cell detachment). The cells survived the treatment (attached cells) could recover to normal morphology upon removal of H2O2. The anti-adhesive effect of H2O2 was dramatic to the initial cell attachment when H2O2 was added at the same time when cells were seeded. As demonstrated in Figure 6B, in the absence of H2O2 (CON), the number of attached cells increased over time during a time course of 3 h incubation. However, 150 μM H2O2 profoundly inhibited ESC adhesion. At the end of a 3 h period of incubation, only a few cells attached to the cell culture dish while the rest of cells existed as clumps in the medium. Prolonged incubation did not improve cell adhesion. To test if cells can recover from H2O2 treatment, H2O2 was removed from medium by centrifugation. However, cells were still unable to attach when they were resuspended in fresh medium and reseeded on to a new dish, indicating that H2O2 treatment under this condition resulted in the cell damage to the extent that cells were no longer able to recover. Apparently, cells in suspension were much more susceptible to H2O2 toxicity than attached cells. The anti-adhesive effect of H2O2 was concentration-dependent (Fig. 6C), correlating with its effect on cell viability (Fig. 1A). These results indicated that the anti-adhesive effect is likely an early event that contributes to H2O2-induced cell cycle arrest and apoptosis.
FIG. 6.
Effect of H2O2 on cell morphology and cell adhesion. (A) Effect of H2O2 on cell morphology and adhesion of attached cells. Embryonic stem cells (ESCs) were cultured for 24 h before they were treated with 150 μM H2O2. Inset shows the morphology of control cells and cells that were treated with 150 μM H2O2 for 2 h. Cells were photographed under a phase contrast microscope (scale bar = 50 μm). The graph represents cell number of attached cells after removal of detached cells in the medium. Cell number was indirectly measured by OD 630 nm after toluidine blue staining. (B) Effect of H2O2 on initial cell adhesion. H2O2 was added to the medium to the final concentration of 150 μM at the time when ESCs were seeded. The graph shows the relative cells number in the absence (CON) or in the presence of H2O2 incubated for the indicated times. (C) Effect of different concentrations of H2O2 on the initial cell adhesion. ESCs were seeded in the absence of H2O2 (control, 0 μM) or in the presence of different concentrations of H2O2 and incubated for 2 h. Results are means ± SD of 3 independent experiments with duplicate assay. *P < 0.05, compared with the value of the control group.
Short-term H2O2-induced oxidative stress does not affect ESC self-renewal and pluripotency
Self-renewal and pluripotency of mouse ESCs are maintained by LIF presented in the cell culture medium. ESCs grow in compact colonies and express AP, which is commonly used as a marker to indicate the undifferentiated state of ESCs. Since detection of AP activity requires its accumulation in colonies that are older than 4-day culture and contain at least 30 cells [28], we examined the effect of short-term H2O2 treatment on AP expression using cells that had been cultured for 4 days post H2O2 treatment (first passage cells as described in Fig. 3). In comparison with control cells, the number of AP-positive colonies was reduced in H2O2-treated group (Fig. 7A, red colonies, first passage), proportional to the reduced total number of cells identified by toluidine blue staining (Fig. 3A, blue colonies, first passage). However, after replated on new cell culture dishes, the numbers of AP-positive colonies derived from control and H2O2-treated cells were similar (Fig. 7A, red colonies, second passage). The AP-positive colonies were further analyzed under a microscope at higher magnification. The overall colony morphology and the AP level were not apparently affected by H2O2 since the intensity of AP staining of individual colonies was similar in control cells and treated cells (Fig. 7B). It should be pointed out that, in addition to the typical compact colonies formed from undifferentiated ESCs, some flattened spreading colonies formed from spontaneous differentiating cells with decreased AP staining were also detected in both treated and control groups, but there was no significant difference between 2 groups.
FIG. 7.
H2O2 treatment does not affect embryonic stem cells (ESCs) pluripotency and self-renewal. (A) ESCs were seeded on 6-well dish and were treated with 150 μM H2O2 for 2 h. The cells were then cultured in fresh medium for 4 days (first passage). In parallel experiments, the control cells and treated cells and were released by trypsin. Same numbers of cells were replated on to new culture dishes and cultured for 4 days in the normal culture medium without further treatment (second passage). Undifferentiated ESCs were identified by alkaline phosphatase (AP) expression. The AP-positive colonies are shown by their red color. (B) Representative colonies formed from control cells and H2O2-treated cells (first passage) were examined and photographed under a phase contrast microscope (scale bar unit = 100 μm). (C) Western blot analysis of Oct4 and Nanog expression after the cells were treated with H2O2 under the conditions specified. β-Actin was used as a control for protein loading. (D) The mRNA level of pluripotency markers determined by RT-qPCR in cells from second passage of control and H2O2-treated cells. The results are means ± SD of 3 independent experiments. The difference is not significant between controls and treated groups.
It is well-recognized that Oct4, Sox2, and Nanog, known as pluripotency markers, are the major genes that are responsible for the maintenance of ESC self-renewal and pluripotency [17–19]. As shown in Figure 7C, Oct4 and Nanog are abundantly expressed in ESCs as detected by western blot analysis with their specific antibodies (Santa Cruz Biotechnology, Santa Cruz, CA), but short-term exposure of ESCs to H2O2 did not alter their expression since similar levels of Oct4 and Nanog were detected in control cells and H2O2-treated cells in a time course up to 24 h post-treatment. At the mRNA level determined by RT-qPCR, Oct4, Sox2, and Nanog were also comparable in the second passage of control and H2O2-treated cells (Fig. 7C). Together, these results indicate that short-term H2O2-induced oxidative stress does not compromise ESC self-renewal and pluripotency.
ESCs are resistant to H2O2-induced senescence
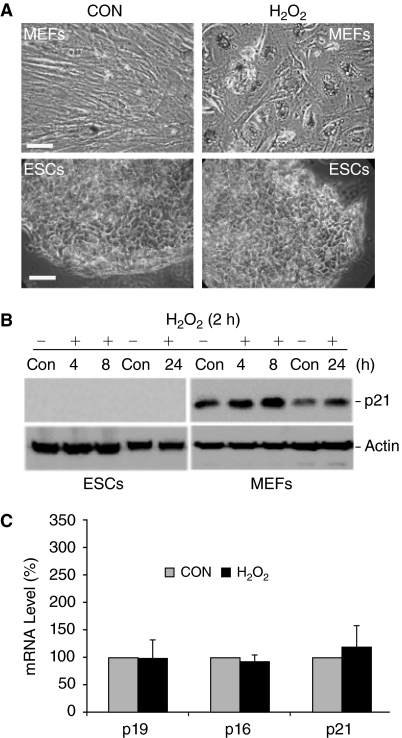
As mentioned earlier, the short-term oxidative stress induced by 150 μM H2O2 for 2 h treatment was an experimental condition that was designed to induce senescence in somatic cells. As expected, this treatment resulted in about 80% of MEFs becoming senescent (Fig. 8A). A key feature of proliferative MEFs is that they have elongated cell bodies (Fig. 8A, CON). The cells treated with H2O2 entered a permanent senescent state with characteristic of enlarged and flattened cell bodies (Fig. 8A, H2O2). Senescent MEFs were readily detected at 5–7 days post-treatment. They did not divide, but were viable for a long period of time and expressed β-galactosidase (β-Gal) (Fig. 8A, H2O2, blue color). On the contrary, ESCs treated with H2O2 showed neither senescent morphology nor β-Gal expression under the similar experimental conditions, indicating that they are resistant to H2O2-induced senescence (Fig. 8A, ESCs). To confirm this conclusion, we further analyzed the expression of p21, a commonly used biochemical senescent marker expressed at the onset of senescence [7]. Its expression was readily detectable in untreated MEFs, which was up-regulated by H2O2 treatment. The expression level of p21 remained elevated in all H2O2-treated cells during a time course up to 24 h, but it was undetectable in either control or H2O2-treated ESCs at any time point tested (Fig. 8B). The ESCs shown in Figure 8A were 5-day-old ESC colonies post H2O2 treatment. Although the mRNA of 3 senescence markers of p21, p19, and p16 was detectable by RT-qPCR in these cells, there was no detectable change in the mRNA level of all 3 genes between control and H2O2-treated cells (Fig. 8C). Similar results were obtained in second passage cells after H2O2 treated. These results further support the conclusion that the condition effectively inducing senescence of MEFs was unable to do so in ESCs.
FIG. 8.
Embryonic stem cells (ESCs) are resistant to H2O2-induced senescence. (A) H2O2 induces senescence of mouse embryonic fibroblasts (MEFs) but not ESCs. Cells were treated with 150 μM H2O2 for 2 h. The cells were then cultured in fresh medium. MEFs at day 10 and ESCs at day 5 post H2O2 treatment were fixed. Senescent cells are indicated by their enlarged cell bodies and the expression of β-galactosidase (β-Gal). Cells were examined and photographed under a phase contrast microscope (scale bar unit = 50 μm). (B) Western blot analysis of p21 expression. Cells were treated with 150 μM H2O2 for 2 h. The cells were then cultured in fresh medium for the times indicated. The expression of p21 was detected with its specific antibodies. β-Actin was used as a control for protein loading. (C) Analysis of the expression of senescence marker genes in ESCs. The mRNA of each gene was determined by RT-qPCR from ESCs described in (A) and normalized to β-actin mRNA. Results are means ± SD of 3 independent experiments. The difference is not significant between controls and treated groups.
Discussion
It has been known that different types of cells have different responses and tolerance to H2O2-induced oxidative stress [2]. For instance, in fibroblasts, low concentrations of H2O2 (<10 μM) stimulate cell proliferation; intermediate concentrations (∼150 μM) cause growth arrest or senescence; and high levels of H2O2 (>400 μM) induce rapid apoptosis [13]. Similar results were reported in vascular smooth muscle cells [10]. It should be pointed out that the “high” and “low” levels of oxidative stress induced by H2O2 are relative measurement defined by different investigators and they may vary depending on cell types and experimental conditions. Our results show that ESC proliferation and viability displayed sensitivity to H2O2 comparable to those reported for fibroblasts and vascular cells. Furthermore, H2O2-induced ESC death is significantly affected by cell density at the time of cell treatment, as reported in a previous study demonstrating an inverse relationship between H2O2 toxicity and cell confluence in somatic cells [33].
Our data obtained from different analyses suggest that the cytotoxicity of H2O2 at the concentrations above 50 μM is attributed to H2O2-induced anti-adhesion, apoptosis, and inhibition of cell proliferation, or combination of all 3 events. An accurate assessment of each of these factors' contribution to the observed result is difficult, since they could be interrelated events. Nevertheless, it is clear that continuous long-term exposure of ESCs to H2O2 (>150 μM) causes apoptosis while a short-term sublethal H2O2 treatment induces a transient cell cycle arrest. In both cases, the anti-adhesion effect is the prominent events preceding H2O2-induced apoptosis and cell cycle arrest. Since treatment with 150 μM H2O2 for 2 h caused significant cell detachment, we believe that this factor together with the subsequent cell cycle arrest is the major cause leading to the reduced cell number under this condition. An important finding is that cells survived the brief H2O2 treatment have remarkable recovery capacity since they showed similar cell proliferation rate, morphology, and the expression levels of cell cycle regulators to the control cells after several days' culture in fresh medium. It is known that mitotic somatic cells spend most of their time in G1 phase and their progression to the S phase is largely controlled by cyclin-dependent kinases (CDK) whose activity is regulated by various cyclins. Although expressed in ESCs, several major CDK–cyclin control complexes, such as Cdk4/cyclin D, seem to exhibit little or no regulatory activity. Instead, division of ESCs is driven by the Cdk2/cyclin A/E pathway that is constitutively active throughout the cell cycle [16]. This may explain the short G1 period and account for the unusual cycle control mechanism that allows for rapid ESC division. We speculated that H2O2 treatment may affect the expression of these cell cycle regulators. We indeed found that the expression of cyclin D1 was significantly down-regulated, but the expression of cyclin A, cyclin E, and CDK4 was not significantly affected. The recovery of cyclin D1 upon the removal of H2O2 correlates well with the recovery of cell proliferation rate. It has been reported that similar treatment of fibroblasts induces a transient cell cycle arrest, which is attributed to the marked down-regulation of cyclin D1 [37]. Interestingly, our result in ESCs resembled these observations; however, the contribution of cyclin D1 down-regulation to the cell cycle arrest is uncertain according to the above-mentioned hypothesis that cyclin D1 does not play a major role in the control of ESC cycle. Therefore, the molecular mechanisms that account for H2O2-inhibited proliferation of ESCs remain to be investigated.
Although the signaling mechanisms that mediate the effects of H2O2 have been intensively investigated in somatic cells [3,38], we are not aware of studies describing the anti-adhesion effect of H2O2 as reported here. Clearly, it is an early event that directly causes cell detachment and may indirectly affect cell cycle progression and apoptosis in ESCs. It is well-known that interference of cell adhesion significantly affects cell proliferation and causes apoptosis of anchorage-dependent cells [39]. The molecular mechanism behind our observation in ESCs remains to be investigated. However, a recent study by Yang et al. [40] indicates that H2O2 affects the structure and function of the cell membrane through interacting with lipid rafts. It will be interesting to see if this might be related to the anti-adhesion effect of H2O2 on ESCs.
It is recently proposed that ESCs have defense systems against stresses that are superior to those of differentiated cells [15,20]. However, our results, and those reported by others [41], show that ESCs do not display higher tolerance to H2O2 cytotoxicity than other cells since they undergo apoptosis at comparable concentrations of H2O2 [33]. However, in support of the aforementioned hypothesis, our results suggest that the self-renewal capacity of ESCs is protected from H2O2-induced oxidative stress. This is demonstrated by the fact that the expression levels of AP and the pluripotency genes are not compromised. Furthermore, cells recovered from short-term H2O2 stress are able to resume high cell proliferation rate and can form EB (a property of undifferentiated ESCs) similar to control cells. Together, these results indicate that the short-term oxidative stress does not have apparent impact on pluripotency and self-renewal capacity of ESCs. Likewise, ESCs are also protected from H2O2-induced senescence. This is a fundamentally different character of ESCs from fibroblasts. Oxidative stress up-regulates the expression of several cell cycle inhibitors, including p21waf1, p19ARF, and p16INK4A, which have been associated with the onset of senescence [7]. As demonstrated in this study, short-term H2O2 oxidative stress effectively induces MEF senescence concurrent with elevated expression of p21. The fact that none of these senescent markers was induced by H2O2 may explain, at least partly, the resistance of ESCs to senescence although the molecular mechanisms remain to be identified.
In summary, the data described in this study reveal some similarities as well as differences between fully differentiated somatic cells and ESCs in response to oxidative stress. Like differentiated cells, ESCs show comparable sensitivity to H2O2 toxicity, but unlike differentiated cells that are susceptible to oxidative stress-induced senescence, ESCs survived a short-term oxidative stress can recover from cell cycle arrest without entering senescent state or losing their self-renewal capacity. These properties may have important implications for ESC physiology; at the level of oxidative stress beyond the capacity of the cell to handle, apoptosis may be the best way to avoid transmission of mutation or other damages to the new cells, on the other hand, ESCs may have effective mechanisms to avoid entering premature senescence and to preserve self-renewal capacity at certain levels of oxidative stress encountered during embryogenesis. Combination of both mechanisms may ensure the integrity of ESCs and normal development of the organism. However, it should be emphasized that the resistance of ESCs to H2O2-induced oxidative stress is defined under our specified experimental conditions. It is conceivable that the pluripotency and proliferative capacity of ESCs could be affected under different conditions. In this aspect, a rational experiment will be testing the long-term exposure of ESCs to low concentrations of H2O2 (20–50 μM). It is particularly interesting to note that a recent study [42] has found that Oct4 is phosphorylated in response to H2O2-induced oxidative stress in ESCs. It is proposed that Oct4 is a stress responsive gene that may play important roles in the regulation of oxidative response in ESCs through transcription [42]. We are currently investigating how the expression level and transcriptional activity of Oct4 are affected by oxidative stress under different experimental conditions.
Acknowledgments
We thank Dr. Christopher Gabel for providing mouse ESCs. We also thank Baobin Kang for microscopy analysis and Mississippi Functional Genomics Network for the use of the facility. This work was supported by NIH grant HL082731 (Y-L.G.)
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Halliwell B. Oxidative stress and cancer: have we moved forward? Biochem J. 2007;401:1–11. doi: 10.1042/BJ20061131. [DOI] [PubMed] [Google Scholar]
- 2.Yoon SO. Yun CH. Chung AS. Dose effect of oxidative stress on signal transduction in aging. Mech Ageing Dev. 2002;123:1597–1604. doi: 10.1016/s0047-6374(02)00095-7. [DOI] [PubMed] [Google Scholar]
- 3.Griendling KK. Sorescu D. Lassègue B. Ushio-Fukai M. Modulation of protein kinase activity and gene expression by reactive oxygen species and their role in vascular physiology and pathophysiology. Arterioscler Thromb Vasc Biol. 2000;20:2175–2183. doi: 10.1161/01.atv.20.10.2175. [DOI] [PubMed] [Google Scholar]
- 4.Rhee SG. Kang SW. Jeong W. Chang TS. Yang KS. Woo HA. Intracellular messenger function of hydrogen peroxide and its regulation by peroxiredoxins. Curr Opin Cell Biol. 2005;17:183–189. doi: 10.1016/j.ceb.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 5.Cao C. Lu S. Kivlin R. Wallin B. Card E. Bagdasarian A. Tamakloe T. Chu WM. Guan KL. Wan Y. AMP-activated protein kinase contributes to UV- and H2O2-induced apoptosis in human skin keratinocytes. J Biol Chem. 2008;283:28897–28908. doi: 10.1074/jbc.M804144200. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 6.Lu T. Finkel T. Free radicals and senescence. Exp Cell Res. 2008;314:1918–1922. doi: 10.1016/j.yexcr.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ben-Porath I. Weinberg RA. The signals and pathways activating cellular senescence. Int J Biochem Cell Biol. 2005;37:961–976. doi: 10.1016/j.biocel.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 8.Sun P. Yoshizuka N. New L. Moser BA. Li Y. Liao R. Xie C. Chen J. Deng Q. Yamout M. Dong MQ. Frangou CG. Yates JR., III Wright PE. Han J. PRAK is essential for ras-induced senescence and tumor suppression. Cell. 2007;128:295–308. doi: 10.1016/j.cell.2006.11.050. [DOI] [PubMed] [Google Scholar]
- 9.Dolado I. Swat A. Ajenjo N. De Vita G. Cuadrado A. Nebreda AR. p38alpha MAP kinase as a sensor of reactive oxygen species in tumorigenesis. Cancer Cell. 2007;11:191–205. doi: 10.1016/j.ccr.2006.12.013. [DOI] [PubMed] [Google Scholar]
- 10.Taniyama Y. Griendling KK. Reactive oxygen species in the vasculature: molecular and cellular mechanisms. Hypertension. 2003;42:1075–1081. doi: 10.1161/01.HYP.0000100443.09293.4F. [DOI] [PubMed] [Google Scholar]
- 11.Li J. Stouffs M. Serrander L. Banfi B. Bettiol E. Charnay Y. Steger K. Krause KH. Jaconi ME. The NADPH oxidase NOX4 drives cardiac differentiation: Role in regulating cardiac transcription factors and MAP kinase activation. Mol Biol Cell. 2006;17:3978–3988. doi: 10.1091/mbc.E05-06-0532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sauer H. Wartenberg M. Reactive oxygen species as signaling molecules in cardiovascular differentiation of embryonic stem cells and tumor-induced angiogenesis. Antioxid Redox Signal. 2005;7:1423–1434. doi: 10.1089/ars.2005.7.1423. [DOI] [PubMed] [Google Scholar]
- 13.Kim BY. Han MJ. Chung AS. Effects of reactive oxygen species on proliferation of Chinese hamster lung fibroblast (V79) cells. Free Radic Biol Med. 2001;30:686–698. doi: 10.1016/s0891-5849(00)00514-1. [DOI] [PubMed] [Google Scholar]
- 14.Cho YM. Kwon S. Pak YK. Seol HW. Choi YM. Park do J. Park KS. Lee HK. Dynamic changes in mitochondrial biogenesis and antioxidant enzymes during the spontaneous differentiation of human embryonic stem cells. Biochem Biophys Res Commun. 2006;348:1472–1478. doi: 10.1016/j.bbrc.2006.08.020. [DOI] [PubMed] [Google Scholar]
- 15.Zeng X. Human embryonic stem cells: mechanisms to escape replicative senescence? Stem Cell Rev. 2007;3:270–279. doi: 10.1007/s12015-007-9005-x. [DOI] [PubMed] [Google Scholar]
- 16.Takumi M. Mattson MP. Rao MS. Cellular lifespan and senescence signaling in embryonic stem cells. Aging Cell. 2008;3:333–343. doi: 10.1111/j.1474-9728.2004.00134.x. [DOI] [PubMed] [Google Scholar]
- 17.Niwa H. How is pluripotency determined and maintained? Development. 2007;134:635–646. doi: 10.1242/dev.02787. [DOI] [PubMed] [Google Scholar]
- 18.Wernig M. Meissner A. Foreman R. Brambrink T. Ku M. Hochedlinger K. Bernstein BE. Jaenisch R. In vitro reprogramming of fibroblasts into a pluripotent ES-cell-like state. Nature. 2007;448:318–324. doi: 10.1038/nature05944. [DOI] [PubMed] [Google Scholar]
- 19.Yu J. Vodyanik MA. Smuga-Otto K. Antosiewicz-Bourget J. Frane JL. Tian S. Nie J. Jonsdottir GA. Ruotti V. Stewart R. Slukvin II. Thomson JA. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 20.Maynard S. Swistowska AM. Lee JW. Liu Y. Liu ST. Da Cruz AB. Rao M. de Souza-Pinto NC. Zeng X. Bohr VA. Human embryonic stem cells have enhanced repair of multiple forms of DNA damage. Stem Cells. 2008;26:2266–2274. doi: 10.1634/stemcells.2007-1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saretzki G. Walter T. Atkinson S. Passos JF. Bareth B. Keith WN. Stewart R. Hoare S. Stojkovic M. Armstrong L. von Zglinicki T. Lako M. Downregulation of multiple stress defense mechanisms during differentiation of human embryonic stem cells. Stem Cells. 2008;26:455–464. doi: 10.1634/stemcells.2007-0628. [DOI] [PubMed] [Google Scholar]
- 22.Nishitai G. Shimizu N. Negishi T. Kishimoto H. Nakagawa K. Kitagawa D. Watanabe T. Momose H. Ohata S. Tanemura S. Asaka S. Kubota J. Saito R. Yoshida H. Mak TW. Wada T. Penninger JM. Azuma N. Nishina H. Katada T. Stress induces mitochondria-mediated apoptosis independent of SAPK/JNK activation in embryonic stem cells. J Biol Chem. 2004;279:1621–1626. doi: 10.1074/jbc.M310335200. [DOI] [PubMed] [Google Scholar]
- 23.Stambrook PJ. An ageing question: do embryonic stem cells protect their genomes? Mech Ageing Dev. 2007;128:31–35. doi: 10.1016/j.mad.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 24.Allen M. Svensson L. Roach M. Hambor J. McNeish J. Gabel CA. Deficiency of the stress kinase p38alpha results in embryonic lethality: characterization of the kinase dependence of stress responses of enzyme-deficient embryonic stem cells. J Exp Med. 2000;191:859–870. doi: 10.1084/jem.191.5.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roach ML. Stock JL. Byrum R. Koller BH. McNeish JD. A new embryonic stem cell line from DBA/1lacJ mice allows genetic modification in a murine model of human inflammation. Exp Cell Res. 1995;221:520–525. doi: 10.1006/excr.1995.1403. [DOI] [PubMed] [Google Scholar]
- 26.Guo YL. Ye J. Huang F. p38alpha MAP kinase-deficient mouse embryonic stem cells can differentiate to endothelial cells, smooth muscle cells, and neurons. Dev Dyn. 2007;236:3383–3392. doi: 10.1002/dvdy.21374. [DOI] [PubMed] [Google Scholar]
- 27.Guo YL. Yang B. Altered cell adhesion and cell viability in a p38alpha mitogen-activated protein kinase-deficient mouse embryonic stem cell line. Stem Cells Dev. 2006;15:655–664. doi: 10.1089/scd.2006.15.655. [DOI] [PubMed] [Google Scholar]
- 28.O'Connor MD. Kardel MD. Iosfina I. Youssef D. Lu M. Li MM. Vercauteren S. Nagy A. Eaves CJ. Alkaline phosphatase-positive colony formation is a sensitive, specific, and quantitative indicator of undifferentiated human embryonic stem cells. Stem Cells. 2008;26:1109–1116. doi: 10.1634/stemcells.2007-0801. [DOI] [PubMed] [Google Scholar]
- 29.Dasari A. Bartholomew JN. Volonte D. Galbiati F. Oxidative stress induces premature senescence by stimulating caveolin-1 gene transcription through p38 mitogen-activated protein kinase/Sp1-mediated activation of two GC-rich promoter elements. Cancer Res. 2006;66:10805–10814. doi: 10.1158/0008-5472.CAN-06-1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guo YL. Kang B. Han J. Williamson JR. p38beta MAP kinase protects rat mesangial cells from TNF-alpha-induced apoptosis. J Cell Biochem. 2001;82:556–565. doi: 10.1002/jcb.1180. [DOI] [PubMed] [Google Scholar]
- 32.Forman HJ. Use and abuse of exogenous H2O2 in studies of signal transduction. Free Radic Biol Med. 2007;42:926–932. doi: 10.1016/j.freeradbiomed.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wiese AG. Pacifici RE. Davies KJ. Transient adaptation of oxidative stress in mammalian cells. Arch Biochem Biophys. 1995;318:231–240. doi: 10.1006/abbi.1995.1225. [DOI] [PubMed] [Google Scholar]
- 34.Zdanov S. Debacq-Chainiaux F. Remacle J. Toussaint O. Identification of p38MAPK-dependent genes with changed transcript abundance in H2O2-induced premature senescence of IMR-90 hTERT human fibroblasts. FEBS Lett. 2006;580:6455–6463. doi: 10.1016/j.febslet.2006.10.064. [DOI] [PubMed] [Google Scholar]
- 35.Haq R. Brenton JD. Takahashi M. Finan D. Finkielsztein A. Damaraju S. Rottapel R. Zanke B. Constitutive p38HOG mitogen-activated protein kinase activation induces permanent cell cycle arrest and senescence. Cancer Res. 2002;62:5076–5082. [PubMed] [Google Scholar]
- 36.Jirmanova L. Bulavin DV. Fornace AJ., Jr Inhibition of the ATR/Chk1 pathway induces a p38-dependent s-phase delay in mouse ES cells. Cell Cycle. 2008;4:1428–1434. doi: 10.4161/cc.4.10.2055. [DOI] [PubMed] [Google Scholar]
- 37.Barnouin K. Dubuisson ML. Child ES. Fernandez de Mattos S. Glassford J. Medema RH. Mann DJ. Lam EW. H2O2 induces a transient multi-phase cell cycle arrest in mouse fibroblasts through modulating cyclin D and p21Cip1 expression. J Biol Chem. 2002;277:13761–13770. doi: 10.1074/jbc.M111123200. [DOI] [PubMed] [Google Scholar]
- 38.Frippiat C. Dewelle J. Remacle J. Toussaint O. Signal transduction in H2O2-induced senescence-like phenotype in human diploid fibroblasts. Free Radic Biol Med. 2002;33:1334–1346. doi: 10.1016/s0891-5849(02)01044-4. [DOI] [PubMed] [Google Scholar]
- 39.Frisch SM. Ruoslahti E. Integrins and anoikis. Curr Opin Cell Biol. 1997;9:701–706. doi: 10.1016/s0955-0674(97)80124-x. [DOI] [PubMed] [Google Scholar]
- 40.Yang B. Oo TN. Rizzo V. Lipid rafts mediate H2O2 prosurvival effects in cultured endothelial cells. FASEB J. 2006;20:1501–1503. doi: 10.1096/fj.05-5359fje. [DOI] [PubMed] [Google Scholar]
- 41.Kim YH. Han HJ. High-glucose-induced prostaglandin E(2) and peroxisome proliferator-activated receptor delta promote mouse embryonic stem cell proliferation. Stem Cells. 2008;26:745–755. doi: 10.1634/stemcells.2007-0786. [DOI] [PubMed] [Google Scholar]
- 42.Kang J. Gemberling M. Nakamura M. Whitby FG. Handa H. Fairbrother WG. Tantin D. A general mechanism for transcription regulation by Oct1 and Oct4 in response to genotoxic and oxidative stress. Genes Dev. 2009;23:208–222. doi: 10.1101/gad.1750709. [DOI] [PMC free article] [PubMed] [Google Scholar]