Abstract
We suggest that characterization of processes involved in differentiation of the pluripotential cardiac precursor cells in their embryonic environment will permit identifying pathways important for induction of diverse stem cells toward the cardiac phenotype. Phenotypic characteristics of cardiac cells are their contractile and electrical properties. The objective of the present study was to define whether calcium (Ca++) has a regulatory role in the pluripotential precursor cell population during commitment into cardiomyocytes. We used the chick embryo model because of ease of staging the embryos and visibility of heart development. Using the Ca++ indicator Fluo-3/acetoxymethyl and confocal microscopy, we demonstrated the existence of higher free Ca++ levels in the cardiogenic precursor cells than in neighboring cell populations outside of the heart fields. Subsequently, gastrulation stage 4/5 chick embryos were set up in modified New cultures in the medium containing either the L-type Ca channel blocker, diltiazem, or the N-type Ca channel inhibitor, 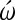 -conotoxin. The embryos were incubated for 22–24 h during which time the control embryos developed, beating looping hearts. At the end of incubation, exposure to the L-type channel blockade with diltiazem resulted in an inhibition of cardiomyogenesis in the most posterior, uncommitted, part of the heart fields. N-type channel blockade with
-conotoxin. The embryos were incubated for 22–24 h during which time the control embryos developed, beating looping hearts. At the end of incubation, exposure to the L-type channel blockade with diltiazem resulted in an inhibition of cardiomyogenesis in the most posterior, uncommitted, part of the heart fields. N-type channel blockade with 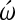 -conotoxin was less intense. Cells in the most anterior cardiogenic regions that were already committed at time of exposure continued to differentiate. Thus, regulation and maintenance of normal cytosolic Ca levels are necessary for the early steps of cardiomyocyte specification and commitment leading to differentiation.
-conotoxin was less intense. Cells in the most anterior cardiogenic regions that were already committed at time of exposure continued to differentiate. Thus, regulation and maintenance of normal cytosolic Ca levels are necessary for the early steps of cardiomyocyte specification and commitment leading to differentiation.
Introduction
Human embryonic stem cells (hESCs) display the capacity to proliferate in the undifferentiated state and, with proper environmental conditions, can differentiate into multiple cell types, including beating cardiomyocytes that display spontaneous rhythmicity [1]. The major phenotypic characteristics of beating cardiac cells, as with all cells that undergo membrane polarization/depolarization cycles, are their contractile and electrical properties. The objective of this study was to determine whether calcium (Ca) regulation is involved in the differentiation of the embryonic cardiac precursor population into cardiomyocytes. We suggest that defining the processes and factors involved in differentiation of the pluripotential cardiac precursor cells in their normal embryonic development will provide critical pathways for selective induction of diverse stem cells toward the cardiac phenotype.
In differentiated, mature cardiomyocytes, Ca++ entry across the sarcolemma is tightly coupled to Ca++ release from the sarcoplasmic reticulum (SR). The coupling between excitation and contraction in adult cardiomyocytes occurs by myocyte membrane depolarization followed by opening of the voltage-gated L-type Ca channels. This results in a Ca++ influx through the sarcolemma. This Ca influx activates the Ca release channels, the ryanodine receptors, localized in the SR and thereby induces the Ca-induced Ca release. The transient increase of intracellular Ca allows Ca by binding to contractile elements to generate force and movement. Cellular mechanisms also exist to prevent a cumulative gain of cytosolic Ca++ and for contractile apparatus relaxation: The SR Ca-ATPase (SERCA) pumps Ca ions into the SR and the Na-Ca exchanger transports Ca out of the cell into the extracellular milieu. Ultrastructural characteristics of the cardiomyocyte facilitating Ca++ handling include cell membrane invaginations, known as the t-tubule network, and the SR. In the chick embryo this ultrastructural cellular network is usually considered to begin forming around day 2–3 of myocardial development, well after the first heartbeats are detectable around 33 h after fertilization. In the embryonic day 12 beating mouse heart, evidence was presented that cytosolic Ca++ signals are locally released by 3-dimensional SR-like structures containing SR Ca++ uptake ATPases (SERCA) and Ca++ release channels (ryanodine receptors) localized at regular intervals throughout the cytosol [2]. It was suggested the local cytosolic Ca++ releases are necessary for the initial heartbeats during the embryonic phase of heart development until terminal differentiation after birth [2]. At the earlier pluripotential precursor stages, intracellular Ca stores most likely relate to the cell membrane, organelles, protein, and buffer-bound Ca. However, one cannot discount that SR-like structures or organelles may be beginning to form. This has not been analyzed.
Differentiation of hESC-derived cardiomyocytes in culture shows development of spontaneous beating areas within embryoid bodies. The electrophysiological properties were shown to be similar to mature differentiated cells in regard to ion channels and ionic currents [3,4]. The hESC-derived cardiomyocytes expressed a large sodium current density and the action potential was shortened by L-type Ca channel blockade. We previously demonstrated that the Na/K-ATPase, that is, the sodium pump, plays an important role in maintaining normal ionic balances during differentiation of cardiomyocytes [5]. Inhibition of the sodium pump is generally accepted to affect the activity of the Na-Ca exchanger 1; we and others provided evidence that the Na-Ca exchanger 1 drives the initial embryonic heartbeats, as well as disrupts normal myofibrillogenesis [6,7]. A close association appears to exist during cardiomyocyte differentiation among Ca fluxes, differentiation of the contractile apparatus, and maintenance of ionic balances. Given the potential of induced pluripotential stem cells and hESCs to regenerate tissue in diseased hearts, more precise mechanistic understanding of the normal embryonic processes should aid in recapitulating cardiomyocyte differentiation and in high enough cell numbers for ultimate clinical application in heart diseases. We here provide evidence that cytoplasmic Ca++ is important already early in embryogenesis in the specification and commitment steps of the cardiac precursor cell population leading to differentiation, before it becomes necessary for excitation–contraction coupling.
Materials and Methods
Chick embryos
Chick embryos were incubated in the lab in a G.Q.F. Manufacturing Co. egg incubator until embryos were between Hamburger and Hamilton (HH) stages 4–8 [8]. Embryos were removed from the yolk and set up in modified New cultures as previously described [9]. Chick embryos were then set up as described below for determination of free Ca in cardiac precursor cells within the cardiogenic crescent and for determination of effects of L-type and N-type Ca channel blockers on cardiac cell myofibrillogenesis as a marker for differentiation.
Determination of normal levels of free Ca++ in the precursor cells of the cardiogenic crescent
HH stage 7 embryos were preloaded with 5 μM Fluo-3/acetoxymethyl ester, a Ca ion indicator that provides a sensitive method for identifying intracellular sites of Ca mobilization (Molecular Probes). The indicator was made up in dimethylsulfoxide (0.2% final concentration) according to manufacturer's guidelines. Fluo-3 has an excitation optimum at 500 nm and is thus suitable for confocal laser scanning microscopy. To facilitate permeability into the embryo, 0.02% pluronic F-127 (Molecular Probes) was added to the Fluo-3 solution. Preloading had no apparent affect on the embryos in the subsequent 1 h incubation period at 37°C, as embryos continued to develop as ascertained by continuing head-fold development. After preloading, the embryos were rinsed in Simm's Balanced Salt Solution (SBSS) to remove any dye nonspecifically associated with extracellular membranes. The embryos were placed in a perfusion chamber on a slide and covered with a glass coverslip resting on filter supports to prevent flattening of the embryo and to permit optimal resolution of the heart fields.
The 4-chambered heart arises from the first and second heart fields. The first heart field gives rise to the left ventricle, and the second field, Islet-1 expressing cells, gives rise to part of the right ventricle and outflow region. Both fields are specified during gastrulation [10]. It is only the cardiac precursor cells of the first heart field that differentiate first and are most likely the cells that we analyzed in this study. Many similar pathways are active during differentiation of the second heart field later in development.
Laser confocal scanning microscopy of cytosolic Ca++ in cardiac precursor cells in embryonic heart regions
The Fluo-3/acetoxymethyl preloaded embryo was scanned using a Leica Confocal Laser Scanning microscope equipped with an argon laser and fluorescein filter. The posterior part, the most uncommitted, of the left-heart-forming field was scanned. This is a region where the cardiac precursor cells are beginning to undergo specification [11]. The embryo within the perfusion chamber was stimulated by perfusion from the left side of the incubation chamber with high Ca++ medium (5 mM) or acetylcholine (5 μM) diffusing across the heart fields. Both factors resulted in similar outcomes of intracellular cytosolic Ca++ release. Acetylcholine accentuates release of intracellular Ca++ stores via the muscarinic receptor G-protein-activated inositol 1,4,5-triphosphate producing pathway.
L-type and N-type Ca channel blockade
HH stage 3+ to 5 chick embryos were removed from the yolk and set up in modified New cultures with 2:2:1 culture medium [9] containing either the L-type Ca channel blocker, diltiazem (2 μM), or the N-type Ca channel inhibitor, 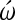 -conotoxin (4 μM). Although in the adult, N-type Ca channels are found primarily at presynaptic vesicles, it was tested whether during gastrulation both channels may be present. Doses were chosen that corresponded to those used in similar studies using cell cultures or embryos at later stages [12,13] and resulted in embryonic viability. Embryos were incubated for 22–24 h in the presence of the Ca channel blockers at which time control embryonic hearts in 2:2:1 medium only, displayed normal looping. The embryos were removed, rinsed well, fixed in 3.3% paraformaldehyde, and processed for immunohistochemistry using routine methods previously described [14]. MF-20 antibody (purchased from Developmental Studies Hybridoma Bank, Iowa University) was used to localize sarcomeric myosin heavy chain as a marker for cardiac cell differentiation.
-conotoxin (4 μM). Although in the adult, N-type Ca channels are found primarily at presynaptic vesicles, it was tested whether during gastrulation both channels may be present. Doses were chosen that corresponded to those used in similar studies using cell cultures or embryos at later stages [12,13] and resulted in embryonic viability. Embryos were incubated for 22–24 h in the presence of the Ca channel blockers at which time control embryonic hearts in 2:2:1 medium only, displayed normal looping. The embryos were removed, rinsed well, fixed in 3.3% paraformaldehyde, and processed for immunohistochemistry using routine methods previously described [14]. MF-20 antibody (purchased from Developmental Studies Hybridoma Bank, Iowa University) was used to localize sarcomeric myosin heavy chain as a marker for cardiac cell differentiation.
Microscopy
A Nikon Optiophot Fluorescence Microscope was used to analyze the immunostained sections and digitized images were acquired with a Princeton Max cooled CCD camera.
Results
Fluo-3 observation of increased intracellular Ca++ stores in the cardiac precursor population in the avian embryonic cardiogenic crescent
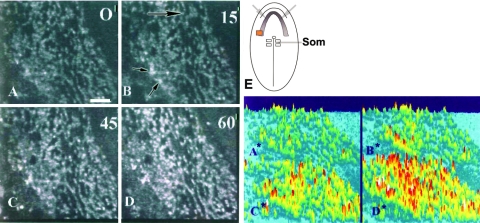
At HH stage 7 the primary heart field displays a gradient of differentiation with more differentiated cells present anteriorly to cells undergoing specification in the posterior part of the cardiogenic field [15]. In our current study we focused on the undifferentiated region in the posterior part of the cardiogenic crescent (Fig. 1; see also Fig. 4). The live, Fluo-3-preloaded, chick embryonic posterior part of the heart field is displayed at time 0, that is, preaddition to stimulation with 10 μL of acetylcholine or exogenous Ca++. Normal background autofluorescence is detectable (Fig. 1A). Fifteen seconds after addition of exogenous Ca/acetylcholine on the left side of the perfusion chamber, an area of higher Ca++ begins to appear in cells in the left side of the heart field in response to the diffusion wave (Fig. 1B). Forty-five seconds later, most of the cells reveal a higher Fluo-3 fluorescence intensity indicative of the release of Ca++ levels (Fig. 1C). After 1 min poststimulation, most cells reveal a high fluorescence indicative of cytosolic-free Ca ions that have been released from intracellular stores. As can be seen, this release of Ca from intracellular stores is specific for cells within the heart-forming region. The cells surrounding the cardiogenic area did not show increased fluorescent signal. Panels A*–D* represent color thresholding of intensity levels for the gray-scale images of Fluo-3 signal: light blue is background; dark blue cells are within autofluorescing regions of the heart field; yellow is low level intensity; red, increasing intensity; and white, high intensity levels.
FIG. 1.
Free Ca++ in cardiac progenitor cells with release from intracellular stores after exogenous Ca++ or acetylcholine stimulation. Same undifferentiated part of the cardiogenic crescent is shown in all panels over time, as diffusion front of exogenous Ca++ moves from left to right across the heart field (see long arrow in panel B). (A) Background autofluorescence at 0 time, that is, prestimulation. (B–D) Increasing levels of Ca++ seen with scans every 15 s up to 60 s poststimulation. Small black arrows in (B) point to free Ca++ ion fluorescence beginning to appear. Corresponding panels (A*–D*) are shown on right with intensity levels color coded: light blue, background; dark blue, autofluorescing cells within cardiogenic crescent; green, lowest level of signal intensity; yellow, low intensity; red, higher intensity levels; white, highest levels of free calcium (Ca). (E) A diagram of a Hamburger and Hamilton (HH) stage 7 chick embryo as used in these studies is shown for reference. The differentiating anterior parts of the heart fields (gray gradient) are defined by light gray arrows. An orange box marks the posterior, undifferentiated part of the left cardiac field that was monitored in this analysis. Som, somites near primitive streak.
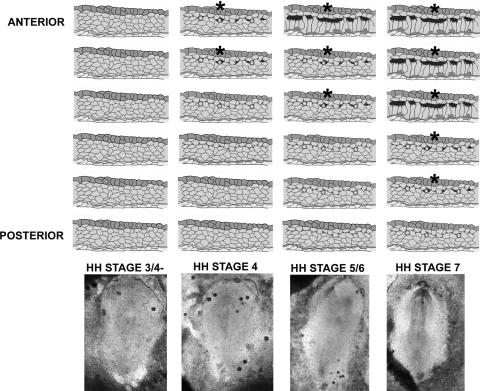
FIG. 4.
Temporal changes represented diagrammatically along the anterior to posterior (A/P) axis in the cardiac progenitor cells of the primary heart fields leading to the differentiated phenotype. HH stage 3/4−: Mesenchymal cells in the mesoderm (light grey) remain pluripotential. These cells are sensitive to Ca++ blockade. HH stage 4: Cells in the anterior most regions of the field are committed and begin the cell sorting process. The sorting cells are now refractory to Ca++ blockade (asterisks). More posteriorly cells are being specified toward the cardiac lineage by expressing β-catenin and N-cadherin to define the future dorsal boundary of the cardiac compartment [21,22,35]. Cell sorting has not yet been initiated and cells remain sensitive to Ca++ blockade. HH stage 5/6: Cells in the anterior most regions have now sorted out to form the epithelial, polarized, differentiating cardiac compartment, and cells just more posterior are initiating steps toward sorting (asterisks), leading to pericardial coelom formation (black). These cells are refractory to Ca++ blockade. More posterior cells are just beginning to commit and are becoming specified along an A/P gradient. These posterior cells remain sensitive to the blockade. HH stage 7: Most of the primary heart field is now becoming committed anteriorly and cells are in the process of cell sorting, except for the cells in the most posterior part of the heart field. Bottom row shows representative embryos for each stage depicted.
Ca channel blockage inhibits cardiac precursor cell differentiation
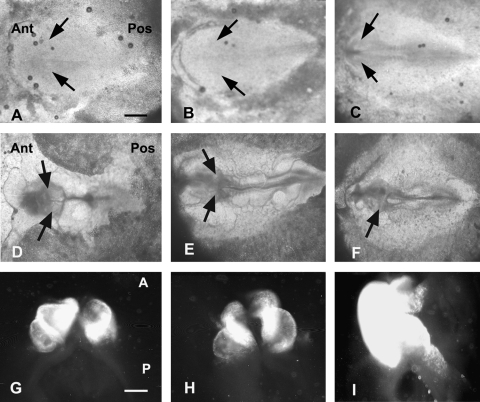
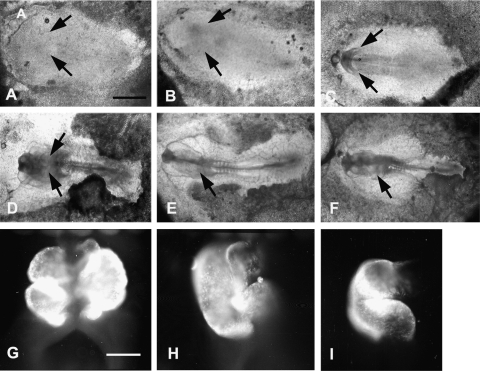
We were primarily interested in determining whether blockade of either the L-type or the N-type Ca channels during early heart development will affect normal cardiomyocyte differentiation. We found this to be the case. More severe inhibition of cardiomyogenesis was effected with the L-type channel inhibitor diltiazem (Fig. 2) than with inhibition of the N-type channel by ω′-conotoxin (Fig. 3). HH stage 4 and 5 embryos incubated in the presence of diltiazem (Fig. 2A, B) displayed cardiabifida with 2 shortened heart tubes of differentiated cardiomyocytes. The tube length of the stage 4 diltiazem-exposed heart (Fig. 2C, F) was shorter than in those embryos exposed at stage 5 (Fig. 2D, G). By stage 8 (Fig. 2E, 4 somites), the Ca-related processes have been completed, and cardiac cell differentiation is no longer affected for most of the length of the heart, except for the outflow and inflow regions of the heart where differentiation is still ongoing (Fig. 2E, H). The N-type channel blocker ω′-conotoxin also inhibited cardiomyogenesis, but to a lesser degree than L-type channel blockade (Fig. 3A–I). Cells that did differentiate with both channel inhibitor exposure were found in the most anterior part of the heart field that apparently were already committed to differentiate at time of exposure (diagrammatically depicted in Fig. 4). With later timing of Ca-channel block, the population of committed cells increases and severity of the effect decreases until commitment of progenitor cells is completed.
FIG. 2.
Inhibition of cardiac cell differentiation in chick embryos using the L-type Ca channel blocker diltiazem at HH stage 4 and stage 8 embryos [8]. (A–C) Stage 4, 5, and 8 embryos at time 0 of incubation in diltiazem. Black arrows point to bilateral heart-forming fields. (D–F). Same above embryos at the end of 22–24 h incubation period in the presence of diltazem. Black arrows point to cardiac tissue shown in panels G, H and I. (G–I) Differentiated cardiac tissue at the end of incubation period is shown in the above embryos by immunofluorescent localization of sarcomeric myosin heavy chain using MF20 monoclonal antibody. Magnification bar in (A) = 500 μm (same for B–F); magnification bar in (G) = 300 μm (same for H and I).
FIG. 3.
Inhibition of cardiac cell differentiation in chick embryos using the N-type Ca channel blocker 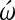 -conotoxin at HH stage 4 and stage 7+ embryos [8]. (A–C) Stage 4, 5, and 7+ embryos at time 0 of incubation in ω′-conotoxin. Black arrows point to bilateral heart-forming fields. (D–F) Same above embryos at the end of the 22–24 h incubation period in the presence of
-conotoxin at HH stage 4 and stage 7+ embryos [8]. (A–C) Stage 4, 5, and 7+ embryos at time 0 of incubation in ω′-conotoxin. Black arrows point to bilateral heart-forming fields. (D–F) Same above embryos at the end of the 22–24 h incubation period in the presence of 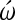 -conotoxin. Black arrows point to cardiac tissue shown in panels G, H and I. (G–I) Differentiated cardiac tissue at end of incubation period is shown in the above embryos by immunofluorescent localization of sarcomeric myosin heavy chain using MF20 monoclonal antibody. Magnification bar in (A) = 700 μm (same for B–F); magnification bar in (G) = 350 μm (same for H and I).
-conotoxin. Black arrows point to cardiac tissue shown in panels G, H and I. (G–I) Differentiated cardiac tissue at end of incubation period is shown in the above embryos by immunofluorescent localization of sarcomeric myosin heavy chain using MF20 monoclonal antibody. Magnification bar in (A) = 700 μm (same for B–F); magnification bar in (G) = 350 μm (same for H and I).
Discussion
In recent years research on ionic currents in the embryonic mammalian heart has related to studies of differentiated cardiomyocytes of the tubular or 4-chambered heart or of differentiated ventricular cells in culture [2,16,17]. Many studies have been directed at Ca handling and ion channels in hESC-derived cardiomyocytes [1,3,18,19]. Ca involvement at stages of cell specification and commitment stages of the cardiac progenitor cells in vivo has received less attention. Ca-handling capability had been demonstrated previously in the gastrulating chick embryo. The presence of muscarinic cholinergic receptors was shown already at the time of germ layer formation [20]. Here we demonstrate that the cardiac progenitor cells within the cardiogenic crescent respond to exogenous Ca++ or to acetylcholine stimulation, as the diffusion front of the stimulants moves across the heart-forming field, and that intracellular Ca++ stores are released in cardiogenic cells in contrast to cells outside of the heart-forming field. L-type and N-type Ca channels are both present at the time of induction of cardiomyogenesis, with the L-type channel being predominant: blockade of the L-type Ca channel at stages 4 and 5 by diltiazem inhibited cardiac cell differentiation, except in the more anterior cells where commitment and differentiation had already commenced (diagrammatically represented in Fig. 4). Cardiac phenotypic differentiation characterized by evidence of an electrical potential and myofibrillognesis is initiated after the ventral mesoderm cells sort out to form a distinct epithelial, polarized, cardiac compartment. Sorting out process is depicted in Fig. 4 with asterisks. We suggest that these are the stages of permanent commitment when the cardiac cell sorting process forms a distinct compartment and epithelialization is completed (asterisks). These cells are now refractory to Ca++ blockade and continue to differentiate even in the presence of the inhibitors. This means that Ca++-mediated pathways must be involved in regulating the specification and early commitment processes of cardiomyogenesis that most likely are associated with N-cadherin/β-catenin-mediated cell adhesion [21,22]. After commitment to differentiate, the role of Ca++ shifts from a morphogenetic regulatory one to a functional effector role. Most developmental processes and regulatory pathways tend to be well conserved among the early vertebrates. It is suggested that our results would relate to the mouse embryo as well at similar stages, approximately between 6.5 and 7 days of gestation. Cultured mouse embryos exposed to L-type channel blockade at later stages than we addressed here, embryonic day 7.5 and older, displayed abnormal cardiac looping, gene expression, and organ development that may relate to effects also on later differentiation, possibly of the second heart field, as well as heart function [23,24]. Similarly, Ca channel blockers also were shown in cultured postimplantation rat embryos to induce morphological anomalies of the heart, head, neural tube, and forelimb [25].
The adverse effects of Ca channel blockade are similar to the effects that we reported previously with lithium ion exposure and with exogenous Wnt3A exposure [26]. All 3 branches of Wnt signaling pathways may be involved in heart organogenesis. The 3 branches include the canonical Wnt (Wnt/β-catenin) pathway, the noncanonical (β-catenin independent pathway) or planar cell polarity pathway involved in cell polarization, and the Wnt/Ca++ pathway. Lithium inhibits glycogen synthase kinase 3, mimicking canonical Wnt signaling, to result in β-catenin stabilization and translocation into the nucleus to turn on target genes [27]. The involvement of canonical Wnt signaling pathway is important in early cardiogenesis and is biphasic [28,29]. Wnt signaling early in gastrulation promotes cardiac differentiation [29]. If Wnt signaling, however, is potentiated into stages 4 and 5 of gastrulation, as by exogenous Wnt3A or LiCl exposure, it becomes inhibitory to cardiomyogenesis [26]. Normally, in the embryo Dickkopf-1 antagonizes canonical Wnt signaling to initiate differentiation [30]. Downstream of canonical Wnt signaling, phosphatidylinositol kinases and phosphoinositide metabolism are regulated and act as second messengers [31]. The cardiac cell migration and polarization [21] steps appear to be modulated by Wnt 11 [32], a noncanonical Wnt. The present results suggest that a relationship also may exist with the third branch of Wnt signaling in the cardiac progenitor cell population via the Wnt-Ca++ pathway.
It was reported in the gastrulating chick embryo that stimulation of muscarinic acetylcholine receptors and the accompanying release of intracellular Ca++ stores was also accompanied by rapid phosphatidylphosphate (IP3) formation. With long-term LiCl exposure, the accumulation of IP3 was elevated to as much as 264% of control [20]. Thus, Ca handling appears coupled to the phosphoinositol second-messenger pathway of Wnt signaling, thereby augmenting the Wnt-Ca++ and/or noncanonical Wnt 11 signaling pathways important in cell migration and cardiogenesis [33]. It has been demonstrated in Xenopus embryos that Dishevelled (Dsh) plays an important role in signal transduction in Wnt-β-catenin and in the Wnt-mediated planar cell polarity pathways. Most importantly, for the purpose of this study, Dsh also activates effectors of the Wnt-Ca++ pathway relating to regulating Ca++ flux, protein kinase C and Ca/calmodulin-dependent protein kinase II [34]. Taken together, the present study and the roles of the different Wnt signaling pathways suggest the Wnt-Ca++ signaling pathway may regulate Ca fluxes to affect cardiac progenitor cell differentiation. If normal Ca levels are disrupted during cardiac induction, cardiac progenitor cell differentiation is inhibited. Once cells are committed and differentiation is initiated, Ca channel blockade affects only the role of Ca++ in cardiac contractility.
Acknowledgments
These studies were initiated at the University of Medicine and Dentistry of New Jersey with support (to K.K.L.) from NIH grant HL48746 and an American Heart Association Grant-in-Aid; at USF partial support was obtained from the Mason Endowment and NIH grant HL67306.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Kehat I. Kenyagin-Karsenti D. Snir M. Segev H. Amit M. Gepstein A. Livne E. Binah O. Itskovitz-Eldor J. Gepstein L. Human embryonic stem cells can differentiate into myocytes with structural and functional properties of cardiomyocytes. J Clin Invest. 2001;108:407–414. doi: 10.1172/JCI12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Korhonen T. Rapila R. Ronkainen VP. Koivumaki JT. Tavi P. Local Ca2+ releases enable rapid heart rates in developing cardiomyocytes. J Physiol. 2010;588:1407–1417. doi: 10.1113/jphysiol.2009.185173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Satin J. Itzhaki I. Rapoport S. Schroder EA. Izu L. Arbel G. Beyar R. Balke CW. Schiller J. Gepstein L. Calcium handling in human embryonic stem cell-derived cardiomyocytes. Stem Cells. 2008;26:1961–1972. doi: 10.1634/stemcells.2007-0591. [DOI] [PubMed] [Google Scholar]
- 4.Sedam O. Dolnikov K. Zeevi-Levin N. Leibovich N. Amit M. Itskovitz-Eldor J. Binah O. 1,4,5-Inositol triphosphate-operated intracellular Ca2+ stores and angiotensin-II/endothelin-1 signaling pathway are functional in human embryonic stem cell-derived cardiomyocytes. Stem Cells. 2008;26:3130–3138. doi: 10.1634/stemcells.2008-0777. [DOI] [PubMed] [Google Scholar]
- 5.Linask KK. Gui YH. Inhibitory effects of ouabain on early heart development and cardiomyogenesis in the chick embryo. Dev Dynamics. 1995;203:93–105. doi: 10.1002/aja.1002030110. [DOI] [PubMed] [Google Scholar]
- 6.Linask KK. Han MD. Artman M. Ludwig CA. Sodium-calcium exchanger (NCX-1) and calcium modulation. NCX protein expression patterns and regulation of early heart development. Dev Dynamics. 2001;221:249–264. doi: 10.1002/dvdy.1131. [DOI] [PubMed] [Google Scholar]
- 7.Koushik SV. Wang J. Rogers R. Moskophidis D. Lambert NA. Creazzo RL. Conway SJ. Targeted inactivation of the sodium-calcium exchanger (Ncx1) results in the lack of a heartbeat and abnormal myofibrillar organization. FASEB J. 2001;15:1209–1211. doi: 10.1096/fj.00-0696fje. [DOI] [PubMed] [Google Scholar]
- 8.Hamburger V. Hamilton H. Series of embryonic chicken growth. J Morphology. 1951;88:49–92. [PubMed] [Google Scholar]
- 9.Linask KK. Lash JW. A role for fibronectin in the migration of avian precardiac cells. I. Dose dependent effects of fibronectin antibody. Dev Biol. 1988;129:315–323. doi: 10.1016/0012-1606(88)90378-8. [DOI] [PubMed] [Google Scholar]
- 10.Cai CL. Liang X. Shi Y. Chu PH. Pfaff SL. Chen J. Evans S. Isl1 identifies a cardiac progenitor population that proliferates prior to differentiation and contributes a majority of cells to the heart. Dev Cell. 2003;5:877–889. doi: 10.1016/s1534-5807(03)00363-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Linask K. Regulation of heart morphology: current molecular and cellular perspectives on the coordinated emergence of cardiac form and function. Birth Defects Res C. 2003;69:14–24. doi: 10.1002/bdrc.10004. [DOI] [PubMed] [Google Scholar]
- 12.Bixby JL. Grunwald GB. Bookman RJ. Ca influx and neurite growth in response to purified N-cadherin and laminin. J Cell Biol. 1994;127:1461–1475. doi: 10.1083/jcb.127.5.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lampidis T. Kolonias D. Savaraj N. Rubin R. Cardiostimulatory and antiarrhythmic activity of tubulin-binding agents. Proc Natl Acad Sci U S A. 1992;89:1256–1260. doi: 10.1073/pnas.89.4.1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Linask KK. Tsuda T. Application of plastic embedding for sectioning whole-mount immunostained early vertebrate embryos. In: Tuan RS, editor; CW Lo., editor. Developmental Biology Protocols. Humana; Totowa, NJ: 2000. pp. 165–173. [DOI] [PubMed] [Google Scholar]
- 15.Linask KK. Regulation of heart morphology: current molecular and cellular perspectives on the coordinated emergence of cardiac form and function. Birth Defects Res C Embryo Today. 2003;69:14–24. doi: 10.1002/bdrc.10004. [DOI] [PubMed] [Google Scholar]
- 16.deDiego C. Chen F. Xie L. Dave A. Thu M. Rongey C. Weiss J. Valderrabano M. Cardiac alternans in embryonic mouse ventricles. Am J Physiol Heart Circ Physiol. 2008;294:H433–H440. doi: 10.1152/ajpheart.01165.2007. [DOI] [PubMed] [Google Scholar]
- 17.Koivumaki JT. Takalo J. Korhonen T. Tavi P. Weckstrom M. Modelling sarcoplasmic reticulum calcium ATPase and its regulation in cardiac myocytes. Philos Transact A Math Phys Eng Sci. 2009;367:2181–2202. doi: 10.1098/rsta.2008.0304. [DOI] [PubMed] [Google Scholar]
- 18.Itzhaki I. Schiller J. Beyar R. Satin J. Gepstein L. Calcium handling in embryonic stem cell-derived cardiac myocytes: of mice and men. Ann N Y Acad Sci. 2006;1080:207–215. doi: 10.1196/annals.1380.017. [DOI] [PubMed] [Google Scholar]
- 19.Passier R. Mummery C. Origin and use of embryonic and adult stem cells in differentiation and tissue repair. Cardiovasc Res. 2003;58:324–335. doi: 10.1016/s0008-6363(02)00770-8. [DOI] [PubMed] [Google Scholar]
- 20.Laasberg T. Ca2+-mobilizing receptors of gastrulating chick embryo. Comp Biochem Physiol. 1990;97C:9–12. doi: 10.1016/0742-8413(90)90164-5. [DOI] [PubMed] [Google Scholar]
- 21.Linask KK. N-cadherin localization in early heart development and polar expression of Na, K-ATPase, and integrin during pericardial coelom formation and epithelialization of the differentiating myocardium. Dev Biol. 1992;151:213–224. doi: 10.1016/0012-1606(92)90228-9. [DOI] [PubMed] [Google Scholar]
- 22.Linask KK. Knudsen KA. Gui YH. N-cadherin-catenin interaction: necessary component of cardiac cell compartmentalization during early vertebrate heart development. Dev Biol. 1997;185:148–164. doi: 10.1006/dbio.1997.8570. [DOI] [PubMed] [Google Scholar]
- 23.Porter GJ. Makuck R. Rivkees S. Intracellular calcium plays an essential role in cardiac development. Dev Dyn. 2003;227:280–290. doi: 10.1002/dvdy.10307. [DOI] [PubMed] [Google Scholar]
- 24.Hove JR. Koster RW. Forouhar AS. Acevedo-Bolton G. Fraser SE. Gharib M. Intracardiac fluid forces are an essential epigenetic factor for embryonic cardiogenesis. Nature. 2003;142:172–177. doi: 10.1038/nature01282. [DOI] [PubMed] [Google Scholar]
- 25.Stein G. Srivastava M. Merker H. Neubert D. Effects of calcium channel blockers on the development of early rat postimplantation embryos in culture. Arch Toxicol. 1990;64:623–638. doi: 10.1007/BF01974690. [DOI] [PubMed] [Google Scholar]
- 26.Manisastry SM. Han M. Linask KK. Early temporal-specific responses and differential sensitivity to lithium and Wnt-3A exposure during heart development. Dev Dyn. 2006;235:2160–2174. doi: 10.1002/dvdy.20878. [DOI] [PubMed] [Google Scholar]
- 27.Klein PS. Melton DA. A molecular mechanism for the effect of lithium on development. Proc Natl Acad Sci U S A. 1996;93:8455–8459. doi: 10.1073/pnas.93.16.8455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kwon C. Cordes KR. Srivastava D. Wnt/beta-catenin signaling acts at multiple developmental stages to promote mammalian cardiogenesis. Cell Cycle. 2008;7:3815–3818. doi: 10.4161/cc.7.24.7189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ueno S. Weidinger G. Osugi T. Kohn AD. Golob JL. Pabon L. Reinecke H. Moon RT. Murry CE. Biphasic role for Wnt/beta-catenin signaling in cardiac specification in zebrafish and embryonic stem cells. Proc Natl Acad Sci U S A. 2007;104:9685–9690. doi: 10.1073/pnas.0702859104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Foley AC. Mercola M. Heart induction by Wnt antagonists depends on the homeodomain transcription factor Hex. Genes Dev. 2005;19:387–396. doi: 10.1101/gad.1279405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qin Y. Lin L. Pan W. Wu D. Regulation of phosphatidylinositol kinases and metabolism by Wnt3A and Dvl. J Biol Chem. 2009;284:22544–22548. doi: 10.1074/jbc.M109.014399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nagy II. Railo A. Rapila R. Hast T. Sormunen R. Tavi P. Rasanen J. Vainio SJ. Wnt-11 signalling controls ventricular myocardium development by patterning N-cadherin and beta-catenin expression. Cardiovasc Res. 2010;85:100–109. doi: 10.1093/cvr/cvp254. [DOI] [PubMed] [Google Scholar]
- 33.Pandur P. Lasche M. Eisenberg L. Kuhl M. Wnt-11 activation of a non-canonical Wnt signaling pathway is required for cardiogenesis. Nature. 2002;418:636–641. doi: 10.1038/nature00921. [DOI] [PubMed] [Google Scholar]
- 34.Sheldahl LC. Slusarski DC. Pandur P. Miller JR. Kuhl M. Moon RT. Dishevelled activates Ca2+ flux, PKC, and CamKII in vertebrate embryos. J Cell Biol. 2003;161:769–777. doi: 10.1083/jcb.200211094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Linask K. Gui YH. Rasheed R. Kwon L. Pattern development during pericardial coelom formation and specification of the cardiomyocyte cell population by N-cadherin and the Drosophila armadillo protein homologue in the early chick embryo. Mol Biol Cell. 1992;3(Suppl.):206A. [Google Scholar]