Abstract
Hematopoietic differentiation of embryonic stem (ES) cells can be enhanced by co-culture with stromal cells derived from hematopoietic tissues and by overexpression of the transcription factor HOXB4. In this study, we compare the hematopoietic inductive effects of stromal cell lines derived from different subregions of the embryonic aorta-gonad-mesonephros tissue with the commonly used OP9 stromal cell line and with HOXB4 activation. We show that stromal cell lines derived from the aorta and surrounding mesenchyme (AM) act at an earlier stage of the differentiation process compared with the commonly used OP9 stromal cells. AM stromal cells were able to promote the further differentiation of isolated brachyury-GFP+ mesodermal cells into hematopoietic progenitors, whereas the OP9 stromal cells could not support the differentiation of these cells. Co-culture and analyses of individual embryoid bodies support the hypothesis that the AM stromal cell lines could enhance the de novo production of hematopoietic progenitors, lending support to the idea that AM stromal cells might act on prehematopoietic mesoderm. The induction level observed for AM stromal cells was comparable to HOXB4 activation, but no additive effect was observed when these 2 inductive strategies were combined. Addition of a γ-secretase inhibitor reduced the inductive effects of both the stromal cell line and HOXB4, providing clues to possible shared molecular mechanisms.
Introduction
Directed differentiation of embryonic stem (ES) cells is a valuable model system to study the specification and regulation of hematopoietic stem and progenitor cells and could also provide a source of therapeutic cells [1–4]. The production of mature blood cells, including erythrocytes, myeloid, and lymphoid lineages as well as multilineage progenitors, from both mouse and human ES cells has been achieved using a range of culture protocols; including embryoid body (EB) formation, co-culture with stroma and strategies involving chemically defined conditions [5–13]. However, the development of a robust protocol for the production of definitive hematopoietic stem cells (HSCs) capable of long-term reconstitution in vivo has been more challenging [14–20]. The most successful strategy for generating repopulating HSCs from mouse ES cells to date involves overexpression of HOXB4 and CDX4, followed by co-culture on the M-CSF-deficient OP9 stromal cell line [21–23]. HSCs derived from ES cells in this way display a unique surface phenotype representative of a developmentally immature cell with characteristics of both embryonic and mature HSCs [22]. HOXB4 overexpression can also enhance hematopoietic differentiation of human ES cells [24,25], but although OP9 stromal cells can augment in vitro hematopoietic colony formation [26] and support the survival of HOXB4-overexpressing cells, neither of these strategies resulted in the production of hES-derived HSCs capable of long-term reconstitution in vivo [25,27].
During embryonic development, hematopoiesis is initiated in the yolk sac by the appearance of primitive hematopoietic cells [28,29]. This transient wave of primitive hematopoiesis is followed by definitive hematopoietic progenitors and HSCs, representing long-term adult repopulating cells, which appear and mature in several embryonic sites [30–34]. HSCs arise de novo in the aorta-gonad-mesonephros (AGM) region during embryonic development, suggesting that this region would be a potent source of signals that promote and maintain hematopoietic stem and progenitor cells [35,36]. Several studies have demonstrated that stromal cell lines derived from this region can support both adult and embryonic HSCs and can facilitate the production of definitive transplantable HSCs from more immature precursors within the yolk sac and para-aortic splanchnopleures [37–42]. We have previously shown that primary E10.5 AGM explants and AGM-derived stromal cell lines significantly enhance hematopoietic differentiation of both mouse and human ES cells [43,44]. Indeed, the co-culture of hES cells on one of the stromal cell lines (AM20.1B4) derived from the aorta-mesonephros subregion has provided a promising approach for the production of transplantable HSCs from human ES cells [44]. We set out to define more clearly the mechanism of action of the AGM-derived stromal cells, to assess whether HOXB4 overexpression could further enhance their inductive properties and to explore the cellular targets and molecular mechanisms underlying these inductive strategies. The identification and detailed understanding of strategies that can enhance the hematopoietic differentiation of ES cells without the need for genetic manipulation would provide an easier route to clinical application.
Materials and Methods
ES cells
CGR8 ES cells, 7a-GFP ES cells [45], Bry-201 ES cells [46], and HOXB4-ERT2 hormone inducible ES cell clones (Jackson et al., manuscript in preparation) were maintained in their undifferentiated state as described previously [43].
Stromal cell lines
Clonal stromal cell lines were derived from the aorta-mesonephros (AM) or urogenital ridge (UG) subregions of E10-11 AGM tissue or from E11 fetal livers as described in references [39,40]. AM and UG-derived clonal stromal cell lines (AM20.1B4, AM20.1A4, UG26.1B6, UG26.2D3) carried the SV40 large T antigen and were maintained at the permissive temperature of 33°C. The AM14.1C4 and EL08.1D2 cell lines carried the Ly-6E.1-lacZ transgene. All the embryo-derived cell lines were maintained on gelatin-coated flasks in medium comprising 50% myelocult long-term culture medium M5300 (Stem Cell Technologies, Vancouver, BC), 40% alpha-minimal essential medium (Invitrogen, Carlsbad, CA) with 10% fetal calf serum (FCS; Sigma, St. Louis, MO), supplemented with 1 mM l-glutamine (Gibco, Grand Island, NY) and 0.05 mM 2-mercaptoethanol (Sigma). The OP9 stromal cell line derived from newborn calvaria of M-CSF deficient mice [7] was maintained at 37°C in 80% alpha-minimal essential medium (MEM) with 20% FCS, supplemented with 2 mM l-glutamine and 0.1 mM 2-mercaptoethanol.
ES cell differentiation strategies
Embryoid bodies (EBs) were prepared in hanging drops by aliquoting 10 μL droplets of ES medium (including leukemia inhibitory factor, LIF) containing 300 ES cells onto the upturned lid of a Petri dish [16,43]. EB aggregates were harvested after 2 days and differentiation was initiated in suspension culture for 1 day in ES medium without LIF, before plating EBs onto confluent γ-irradiated (30 Gy) stromal cell layers. In the case of HOXB4-ERT2 ES cells, 800 nM tamoxifen was added to differentiation cultures on Days 1, 3, and 5 of EB differentiation, marking the time after LIF withdrawal. Differentiation cultures were washed with phosphate-buffered saline (PBS) and cell suspensions obtained by enzymatic digestion with dispase solution [PBS containing 1.2 U/mL dispase II (Roche Diagnostics, Indianapolis, IN) and 70 μg/mL DNAseI (Sigma)] for 45 min at 37°C. Dispase activity was neutralized with ES medium (4× volume) and single cell suspensions were obtained by gently passing samples through a 23-gauge needle.
Single EBs were co-cultured in 24-well plates directly on gelatin or on irradiated stromal layers. In noncontact cultures, EBs were placed in transwell inserts (transparent Greiner Bio-one 24 well ThinCert-tissue culture inserts, membrane pore size 0.4 μm, pore density 2 × 106 cm−2). Individual EBs were picked manually into 100 μL dispase solution and incubated for 45 min at 37°C in 96 well plates, then dissociated into a single cell suspension by gentle pipetting. Dispase activity was neutralized with ES medium (no LIF) before the cells were assessed for hematopoietic activity.
To obtain brachyury+ or − cells for co-culture, Bry-201 ES cells were prepared in high density suspension cultures (3 × 104 cells/mL of ES medium without LIF) to promote spontaneous EB formation. Cell suspensions were placed in sterile bacteriological grade Petri dishes and incubated at 37°C (humidified 5% CO2 atmosphere), medium was replaced every 2 days. After 4 days of differentiation, the EBs were dissociated in dispase solution and brachyury-GFP+ or brachyury-GFP− cells were isolated by fluorescence activated cell sorting (FACS). 1 × 105 sorted cells were co-cultured per 25 cm2 flask of irradiated stromal cells for a further 6 days of differentiation.
Distinguishing between stromal and ES cell lines: normalization of data
When co-cultures were analyzed in hematopoietic colony assays the input cell population contained both ES cells and stromal cells. It was therefore necessary to distinguish between stromal cells and ES cells to calculate the frequency of hematopoietic colony forming units (CFUs) within a defined number of input ES cells. In the majority of experiments ES cells that expressed eGFP constitutively (7a-GFP ES cells) were used and the proportion of ES cells present in the mixed population was determined by flow cytometry. When using ES cell lines such as Bry-201, CGR8, and HOXB4-ERT2 that did not express a constitutive marker gene, confluent stromal layers were stained with Vybrant DiD cell labeling solution (Invitrogen, Carlsbad, CA) prior to irradiation and co-culture. Briefly, stromal layers were washed with PBS, stained with 1:250 dilution Vybrant DiD solution in PBS for 20 min at 37°C then washed 3 times with PBS. DiD stained stromal cells could be readily detected by flow cytometry (FL-3/FL-4 channel), thus allowing the proportion of DiD negative ES-derived cells to be accurately determined. This strategy facilitated accurate measurement of the proportion of brachyury-GFP positive ES-derived cells present in stromal co-cultures.
Hematopoietic colony forming assays
Methylcellulose-based hematopoietic colony assays and CFU-A assays were performed as described previously [43]. Statistical significance was determined by t-tests for parametric or nonparametric data sets.
Flow cytometry
Flow cytometric analyses were carried out on a BD FACSCalibur flow cytometer (488 nm and 633 nm lasers) with CellQuest software. FACS was performed on a FACS Vantage SE cell sorter (Becton Dickinson, dual output 351 nm/488 nm and 633 nm lasers) using FACSDiva software. Cells were incubated with optimal concentrations of anti-mouse monoclonal antibodies for 20 min at 4°C. Unbound primary antibody was removed by washing twice with PBS. Streptavidin-phycoerythrin (PE) or streptavidin-allophycocyanin (APC) conjugated secondary antibodies were used to detect biotinylated primary antibodies. Data for 1 × 105 live cells were acquired and electronic gates were set using unstained cells, cells stained with secondary antibody alone, single stains and biological controls. Directly conjugated or biotinylated antimouse monoclonal antibodies against cKit, CD45, Gr-1, CD11b, B220, CD106 (VCAM-1), CD34, CD31, streptavidin-APC, and streptavidin-PE were purchased from Caltag; CD150 was from Biolegend; Sca-1, CD49d, Ter119, Flk1, CD41, CD48, and CD244 monoclonal antibodies were purchased from BD.
Gamma-secretase inhibition
To inhibit signaling by the γ-secretase pathway, co-cultures were setup as described above and the γ-secretase inhibitor (cat. no. 565771; Calbiochem, San Diego, CA) was added at a final concentration of 4 μM in ES medium (no LIF). The inhibitor was added to co-cultures between Days 4 and 6 of EB differentiation. The dimethyl-sulphoxide (DMSO) diluent was used at the equivalent concentration as a control for these experiments.
Transfection and immunohistochemistry of COS7 cells
COS7 cells were transiently transfected with a pCAGHOX-B4ERT2IP construct using Lipofectamine 2000 (Invitrogen, Carlsbad, CA). After confirmation by western blotting that the expected size fusion protein was produced (data not shown), cells were treated with 800 nM tamoxifen (4OHT) (Sigma, St. Louis, MO), fixed with 4% paraformaldehyde for 10 min, washed twice in PBS then permeabilized for 5 min in 0.1% Triton X-100. HOXB4 protein was detected using an I12 rat anti-HOXB4 antibody primary antibody (1/100; Developmental Studies Hybridoma Bank, University of Iowa) and a FITC-labeled goat antirat Ig secondary antibody (1/100; Santa Cruz Biotechnology, Santa Cruz, CA). Nuclei were visualized using 10 mL DAPI (Roche Diagnostics, Indianapolis, IN).
Quantitative reverse transcription (RT)-polymerase chain reaction (PCR)
Total RNA was extracted using RNeasy Mini Kit (Qiagen, Valencia, CA) and cDNA prepared by reverse transcription using SuperScript™ III First-strand Synthesis SuperMix (Invitrogen, Carlsbad, CA). Quantitative real-time RT-PCR was performed on an ABI 7500FAST qPCR machine using TaqMan PCR chemistry under universal conditions (Applied Biosystems, Foster City, CA). Samples were analyzed in triplicate, using HPRT as the endogenous control. Relative quantitation was calculated using the deltaCT method in ABI SDS1.4 software and data are presented as fold change in gene expression relative to an internal calibrator. Primers: Hey1 (Forward: 5′-GCA GGA GGG AAA GGT TAT TTT GA-3′; Reverse: 5′-CGA AAC CCC AAA CTC CGA TAG-3′. Probe: 5′-CGC CCT GGC TAT GG-3′): HPRT (forward 5′-GCT CGA GAT GTC ATG AAG GAG A; reverse 5′-AAA GAA CTT ATA GCC CCC CTT GA; probe CCA TCA CAT TGT GGC CCT CTG TGT G).
Results
Hematopoietic differentiation is enhanced by co-culture with stromal cell lines derived from the aorta and surrounding mesenchyme of the AGM region
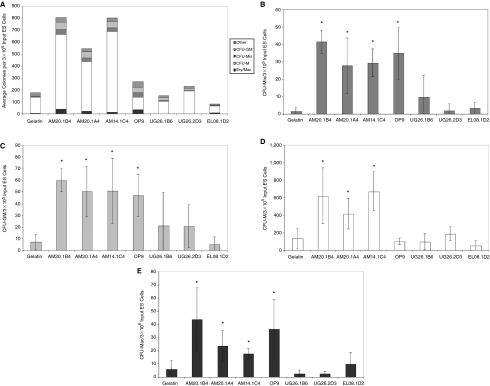
A significant increase in hematopoietic colony formation was observed when ES cells were differentiated in co-culture with 3 independently derived stromal cell lines isolated from the aorta-mesenchyme (AM) of the AGM, but not from 2 stromal cell lines derived from the urogenital ridges (UG) nor from a cell line derived from the fetal liver (Fig. 1A–1E). The induction level of the AM cell lines was comparable to the commonly used OP9 cell line. Importantly, the induction of hematopoietic activity in AGM-derived stromal cells was independent of the expression of the large T antigen: AM20-1B4, AM20.1A4 and UG26.1B6 were derived from tgA58 transgenic embryos, whereas the AM14.1C4 and EL08.1D2 were derived from the transgenic mouse strain carrying the Ly-6E.1-LacZ transgene. The number of multipotent CFU-Mix colonies that were generated after co-culture with AM20.1B4, AM20.1A4, and AM14.1C4 stromal cells was statistically comparable to the number generated in OP9 co-cultures and significantly higher than control cultures (Fig. 1B). On average, the frequency of CFU-Mix was 22-, 15-, 16-, and 18-fold higher than controls in AM20.1B4, AM20.1A4, AM14.1C4, and OP9 co-cultures, respectively. The number of CFU-GM and erythroid/macrophage colonies generated in AM co-cultures was also statistically comparable to that in OP9 cultures and enhanced compared to controls (Fig. 1C and 1E). CFU-M colony numbers were enhanced in AM co-cultures, but not in OP9 co-cultures, which is consistent with the fact that the OP9 stromal cells do not express functional M-CSF [7] (Fig. 1D).
FIG. 1.
Induction of hematopoietic differentiation by co-culture with stromal cell lines. The total number of hematopoietic colonies (A) and the number of specific colony types including CFU-Mix (B), CFU-GM (C), CFU-M (D), and Ery/Mac (E) per 3 × 105 embryonic stem (ES)-derived cells (7a-GFP ESC) in control gelatin cultures or after co-culture on stromal cell lines to 6 days differentiation. Data in A represent the mean of between 3 and 11 independent experiments, whereas data in B–D represent the mean (±SD) of 3 representative experiments, *P < 0.05 compared to gelatin controls. To ensure the entire hematopoietic output was represented in A, remaining colonies such as definitive erythroid and CFU-mast were categorized as “other.”
Increased expression of hematopoietic markers by ES cells differentiating on AM stromal lines
Flow cytometric analysis revealed that the frequency of ES-derived cells expressing hematopoietic surface antigens in AM co-cultures was comparable to OP9 co-cultures and significantly higher than controls (Table 1).It has been reported that ES-derived HSCs are CD150+ and heterogeneous for CD48 expression [22] and that in murine adult bone marrow and fetal liver, HSCs are enriched in the CD150+ CD48− CD244− cell fraction [22,47–49]. In enhancing stromal co-cultures, there was an increase in the proportion of CD150+ ES-derived cells, which did not co-express CD48 (Table 1).No expression of CD244 was detected, suggesting that CD150+CD48−CD244− cells were present. In particular, OP9 and AM20.1A4 co-cultures yielded an average of 13% and 15% CD150+ (CD48−CD244−] ES-derived cells, respectively. An increase in cKit+Sca-1+ EB-derived cells in AM co-cultures was also observed, though it was not determined if these cells were negative for lineage markers. The enhanced emergence or maturation of hematopoietic cells that express adult-type surface markers is consistent with the potential of the AGM region to maturate or expand hematopoietic stem and progenitor cells in culture. Taoudi and colleagues (2008) reported that a novel culture system involving dissociation and reaggregation of E11.5 AGM region with growth factors, can promote maturation of pre-HSCs to HSCs that acquire an adult-like surface phenotype, including cKit and Sca-1. Hematopoietic markers associated with myeloid, erythroid, and lymphoid cells were also detected on ES cells differentiating in AM and OP9 co-cultures (Table 1).The variability observed in expression of these mature markers could reflect a subtle balance between self-renewal of hematopoietic progenitors (CFU) and their terminal differentiation into mature hematopoietic cell types.
Table 1.
Expression of Hematopoietic Surface Antigens on ES-Derived Cells in Co-Culture
| |
% Positive ES-derived cells in co-culture |
||||
|---|---|---|---|---|---|
| Surface Marker | Gelatin | AM20.1B4 | AM20.1A4 | AM14.1C4 | OP9 |
| c-Kit+ | 3.95 ± 5.0 | 14.9 ±16.7 | 18.4 | 16.9 | 9.8 ± 8.0 |
| Sca-1+ | 1.5 ± 3.1 | 6.7 ± 8.6 | 21.6 | 8.2 | 5.0 ± 8.5 |
| c-Kit+ Sca-1+ | 0.05 ± 0.1 | 2.6 ± 2.3 | 5.8 | 2.7 | 2.9 ± 4.8 |
| CD150+ | 0. 9 ± 0.2 | 2.0 ± 1.4 | 14.8 ± 20 | 3.7 ± 5.0 | 12.8 ± 3.5 |
| CD150+CD48+ | 0.1 ± 0.06 | 0.9 ± 0.8 | 0.8 ± 0.6 | 0.3 ± 0.3 | 1.6 ± 1.0 |
| CD45+ | 1.6 ± 1.6 | 7.2 ± 10.2 | 9.6 ± 4.8 | 7.0 ± 0.3 | 11.0 ± 9.3 |
| Gr-1+ | 0.8 ± 1.2 | 6.1 ± 9.5 | 2.2 ± 1.1 | 1.4 ± 0.1 | 4.4 ± 3.2 |
| CD11b+ | 1.5 ± 1.5 | 7.5 ± 11.0 | 15.1 ± 14.0 | 2.3 ± 1.3 | 5.1 ± 2.7 |
| B220+ | 2.9 ± 6.0 | 3.6 ± 3.0 | 17.0 ± 12.1 | 4.4 ± 0.7 | 4.8 ± 4.7 |
| Ter119+ | 0.5 ± 0.6 | 2.9 ± 5.0 | 2.4 ± 1.3 | 2.1 ± 2.3 | 1.6 ± 2.2 |
The proportion of ES-derived cells (assessed by constitutive eGFP expression in 7a-GFP ES cells) in co-cultures expressing hematopoietic surface markers after culture on gelatin or on stromal cells to 10 days differentiation. Data represent the mean (±SD) of between 1 and 9 independent experiments. Results for UG and EL co-cultures were comparable with gelatin controls (data not shown).
AM-derived and OP9 stromal cell lines act after mesoderm specification
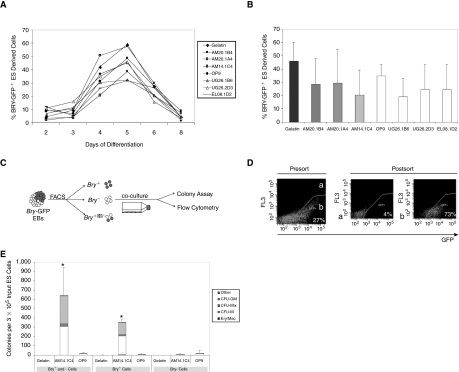
ES cells carrying the mesodermal brachyury-eGFP reporter gene (Bry-201 [46]) were used to assess whether the hematopoietic inductive effects of the AM and OP9 stromal cells lines could be explained by a differential effect on mesoderm formation. We first confirmed that the growth rates of ES-derived cells in the different co-cultures were comparable (Supplementary Fig. 1; Supplementary materials available online at www.liebertonline.com/scd) and that the AM stromal cell co-culture induced hematopoietic activity in the Bry-201 ES cells (Supplementary Fig. 2; Supplementary materials available online at www.liebertonline.com/scd). Flow cytometric analysis of eGFP expression showed no significant differences in the kinetics of brachyury expression, with the peak of brachyury- eGFP expression at Day 5 of differentiation in all culture conditions (Fig. 2A). The timing of our peak of brachyury-GFP expression is delayed by 1 day compared to that previously described (Fehling et al., 2003) which likely reflects differences in differentiation conditions.[46] There was no significant correlation (r2 = 0.027) between the hematopoietic activity (Supplementary Fig. 2) and the proportion of brachyury+ cells (Fig. 2B), indicating that the AM and OP9 co-cultures mediate their hematopoietic inductive effects on ES cells after mesoderm specification. Our data also show that the noninducing UG and EL stroma cell lines do not have a negative effect on mesoderm formation.
FIG. 2.
Stromal cell lines mediate their effects after brachyury expression in embryoid bodies (EBs). (A) Percentage of brachyury-eGFP expressing cells in Bry-201 EBs (Days 2–8) differentiated under the different co-culture conditions, P > 0.05 between conditions (1 representative experiment of 3 is shown). (B) Proportion of brachyury-eGFP expressing embryonic stem (ES) cells in co-cultures at Day 5 (P > 0.2 when comparing each stromal co-culture to gelatin controls, n = 3 experiments). (C) Experimental strategy: brachyury-eGFP positive and negative cells were isolated by fluorescence-activated cell sorter (FACS) from 4-day-old suspension EBs and then co-cultured for a further 6 days before assessing hematopoietic activity. (D) Representative example of FACS profile of unsorted and sorted populations. (E) Number of hematopoietic colonies produced from brachyury-eGFP positive and negative populations after co-culture on gelatin, AM14.1C4 or OP9 stromal layers for 6 days (*P < 0.02 compared to corresponding gelatin and OP9 co-cultures).
Brachyury+, but not Brachyury−, cells respond to the hematopoietic inductive effects of the AM stromal cell lines
To further define the stage of induction within the AM/EB co-cultures, brachyury-eGFP+ and brachyury−eGFP− cells were isolated from Day 4 EBs then co-cultured on AM14.1C4 and OP9 stromal cells (Fig. 2C and 2D). The production of multilineage hematopoietic colonies provided evidence that brachyury+, but not brachyury−, cells responded to the AM14.1C4 stroma (Fig. 2E). These data confirm that the hematopoietic activity, not surprisingly, arises from a brachyury+ population and support the hypothesis that the inductive effect of the AM14.1C4 stromal line acts on differentiating ES cells after the expression of brachyury. By contrast, differentiation of sorted populations on gelatin or on OP9 stromal cells resulted low hematopoietic colony formation, suggesting that hematopoietic differentiation of the sorted cells was not supported in these conditions. Flow cytometric analysis confirmed that ES-derived cells were indeed present in all conditions and that the survival/proliferation of the cells was not significantly different in the OP9 condition compared to AM. We observed that co-culture of an equal mixture of brachyury+ and brachyury− cells on AM14.1C4 cells resulted in a level of hematopoietic activity comparable to the level observed when twice as many brachyury+ cells were co-cultured alone, suggesting that ES-derived brachyury− cells provide additional hematopoietic support within the co-culture niche (Fig. 2E).
AM20.1B4 stromal cells have a proliferative and inductive effect on hematopoietic differentiation
The differentiation system used in this study involved the initial production of EBs that were subsequently co-cultured on stromal cell lines with the final hematopoietic output being assayed on the disaggregated cultures. We considered that the enhanced hematopoietic colony formation observed after co-culture could either be the result of an increase in the number of hematopoietic progenitors generated within each EB or an increase in the number of EBs with associated hematopoietic activity. The former possibility might indicate that the AM-derived stromal cell lines could mediate their enhancing activities by inducing prehematopoietic mesoderm cells to a hematopoietic fate, whereas the latter might be explained by an increase in the proliferation of hematopoietic progenitors that arise spontaneously in differentiating cultures. To distinguish between these possibilities, we assayed the number of hematopoietic colonies produced from individually co-cultured EBs. As the growth rate of cells under the different co-culture conditions were statistically comparable, the number of hematopoietic colonies produced from EBs in each assay dish could be directly compared [43] (Supplementary Fig. 1). When compared to control cultures and noninducing co-cultures, AM20.1B4 co-culture resulted in significantly more EBs that had associated hematopoietic activity and more hematopoietic colonies were detected in each EB compared to controls (Table 2).This suggests that the AM20.1B4 stromal line had both an inductive and a proliferative effect on ES-derived hematopoietic progenitors (Table 2).The analysis of single EBs cultured in transwell cultures showed that hematopoietic activity was significantly reduced in EBs when contact with AM20.1B4 was prevented, indicating that both the inductive and proliferative effect was dependent on cell contact (Table 2).
Table 2.
Analysis of Single EBs Differentiated in Co-Culture
| Co-culture of single EBs | % EBs with hematopoietic activity | Hematopoietic colonies per EB | |
|---|---|---|---|
| 6 days differentiation | |||
| Gelatin | Contact (n = 207) | 52.7 | 3.2 ± 6.9 |
| Noncontact (n = 24) | 37.5 | 4.5 ± 8.9 | |
| AM20.1B4 | Contact (n = 201) | 64.2* | 16.8 ± 33.7* |
| Noncontact (n = 24) | 16.7† | 0.6 ± 2.2† | |
| UG26.1B6 | Contact (n = 47) | 42.6 | 1.7 ± 3.7 |
| Noncontact (n = 24) | 20.8 | 0.4 ± 1.1 | |
| EL08.1D2 | Contact (n = 48) | 27.1* | 0.4 ± 0.7* |
| Noncontact (n = 24) | 29.2 | 0.5 ± 0.9 | |
| 10 days differentiation | |||
| Gelatin | Contact (n = 48) | 68.3 | 11.1 ± 12.2 |
| Noncontact (n = 24) | 70.8 | 10.3 ± 13.4 | |
| AM20.1B4 | Contact (n = 48) | 93.5* | 37 ± 27.9* |
| Noncontact (n = 24) | 47.8† | 11 ± 14.3† | |
| UG26.1B6 | Contact (n = 48) | 60.9 | 7.0 ± 10.6 |
| Noncontact (n = 24) | 54.2 | 8.8 ± 10.3 | |
| EL08.1D2 | Contact (n = 207) | 52.7 | 3.2 ± 6.9 |
| Noncontact (n = 24) | 37.5 | 4.5 ± 8.9 | |
The proportion of individual EBs with associated hematopoietic activity and the number of hematopoietic colonies detected within individual dissociated EBs after culture on gelatin or in direct contact with stromal cells. In noncontact conditions, contact between EBs and stromal cells was prevented by the presence of transwell inserts. The number (n) of EBs (7a-GFP) analyzed in each experiment is shown.
P < 0.004 comparison of contact cultures: stromal co-culture versus corresponding gelatin culture.
P < 0.01 comparison of noncontact versus corresponding contact culture.
Hematopoietic inductive effects of stromal cells and HOXB4 are not additive
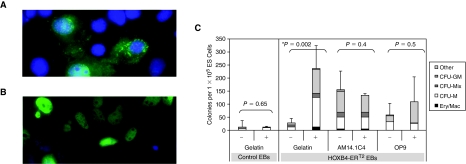
We generated ES cell lines overexpressing a tamoxifen-inducible form of HOXB4 by stable integration of a HOXB4-ERT2 fusion cDNA under control of the CAG promoter into the ES cell genome (Jackson et al., manuscript in preparation). Nuclear translocation of the HOXB4–ERT2 fusion protein was confirmed in tamoxifen-treated COS7 cells transiently transfected with the CAG-ERT2 construct by immunohistochemistry using an anti-HOXB4 antibody (Fig. 3A and 3B). Further proof of the functionality of this fusion protein is demonstrated by the observed increase in hematopoietic progenitor formation when HOXB4 was activated (Fig. 3C). There was no significant difference in the number of nonhematopoietic secondary EBs observed, confirming that neither the stromal cell culture nor HOXB4 induction had a nonspecific proliferative effect (Supplementary Fig. 3; Supplementary materials available online at www.liebertonline.com/scd). The level of induction by HOXB4 was comparable to the level in stromal cell co-culture and no additive effect was observed when the 2 induction strategies were combined (Fig. 3C). These data have led us to hypothesize that they might mediate their effect through overlapping signaling pathways. To test this hypothesis we have assessed the effects of inhibiting the γ-secretase pathway on the inductive effects of the stromal cells and HOXB4.
FIG. 3.
No additive effect when the AM stroma and HOXB4 induction strategies are combined. Cos7 cells, transiently transfected with the pCAGHOXB4ERT2IP construct, cultured in the absence (A) or the presence (B) of tamoxifen followed by immunocytochemical analysis using an anti-HOXB4 antibody (green) and counterstaining of the nucleus with DAPI (blue). (C) Number of hematopoietic colonies observed when control CGR8 embryoid bodies (EBs) and CGR8-HOXB4-ERT2 inducible EBs were cultured in the presence (+) or absence (−) of tamoxifen (added on Days 1, 3, and 5 of differentiation) and/or co-cultured with AM14.1C4 or OP9 stromal cell layers. CFU assays were setup at 6 days differentiation.
The γ-secretase inhibitor attenuates the hematopoietic inductive effects of both the stromal cell lines and HOXB4 overexpression
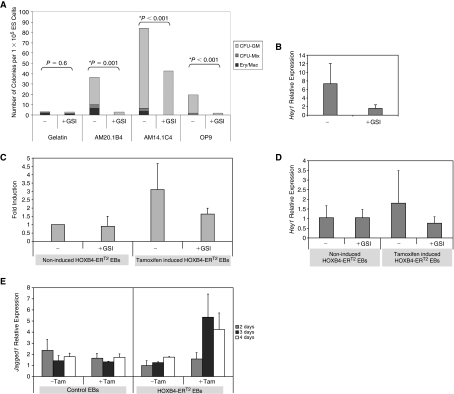
Since the inductive effects of the stroma were dependent on direct ES-stromal cell contact, we considered that the Notch pathway might play a role in this inductive effect. We therefore tested the effects of a γ-secretase inhibitor (GSI) on the inductive effects of the AM co-culture system. The Notch pathway has been reported to have a profound effect on mesoderm differentiation of ES cells [50,51]. We had shown that the stromal lines mediated their effects after the production of mesoderm, so we added the GSI inhibitor between Days 4 and 6 after the onset of brachyury expression. In 3 replicate experiments, the presence of the GSI inhibitor resulted in a significant reduction in the hematopoietic inductive effects of the stromal lines (Fig. 4A). On average, the number of multipotent hematopoietic progenitors (CFU-Mix, CFU-GM, and Ery/Mac) was reduced by 57%, 63%, and 56% in AM20.1B4, AM14.1C4, and OP9 co-cultures, respectively. No significant difference was observed in the numbers of secondary EBs detected in the presence of the inhibitor, indicating that the inhibitor did not have a general toxic effect on differentiating ES cells (Supplementary Fig. 3). Taken together, the data suggest that γ-secretase mediated signaling is required for enhancing the production of multipotent hematopoietic progenitors. Quantitative RT-PCR revealed that Hey1 gene transcript, a downstream target of Notch signaling, was significantly reduced in the presence of the γ-secretase inhibitor in this system, confirming its effectiveness in inhibiting Notch-mediated signaling (Fig. 4B).
FIG. 4.
γ-Secretase inhibition of embryoid bodies (EBs) differentiated using the 2 induction strategies. (A) Frequency of multipotent hematopoietic colonies (CFU-Mix, CFU-GM, Ery/Mac) per 1 × 105 input embryonic stem (ES)-derived cells differentiated to 6 days on gelatin, AM14.1C4 or OP9 in the presence (+) or absence (−) of γ-secretase inhibitor (GSI), added between Days 4 and 6. One representative experiment of 3 is shown. (B) Quantitative reverse transcription-polymerase chain reaction (RT-PCR) showed a significant reduction in Hey1 gene transcript in EBs in the presence of γ-secretase inhibitor (GSI). (C) γ-Secretase inhibition of HOXB4-ERT2 EBs differentiated on gelatin. Cells were either noninduced or induced with tamoxifen in the presence (+) or absence (−) of GSI, added between Days 4 and 6. The fold induction in CFU-Mix, CFU-GM, and Ery/Mac colony numbers over noninduced control HOXB4-ERT2 EBs is shown (±SD between experiments). (D) Quantitative RT-PCR showing an increase in Hey1 gene transcript after HOXB4 activation which was inhibited in the presence of the γ-secretase inhibitor (GSI). (E) Quantitative RT-PCR showing the expression pattern of Jagged1 in control CGR8 EBs or HOXB4-ERT2 EBs differentiated in the presence (+) or absence (−) of tamoxifen, added from Days 2 to 4.
To investigate whether signaling pathways mediated by γ-secretase also played a role in the induction of ES-derived hematopoiesis by HOXB4, GSI was added to HOXB4-ERT2 EBs. Addition of inhibitor between Days 4 and 6 of differentiation attenuated the hematopoietic enhancing effects of HOXB4 induction (Fig. 4C). The number of multipotent progenitors (CFU-Mix, CFU-GM, and Ery/Mac) detected in tamoxifen-induced HOXB4-ERT2 cells was reduced by ∼50% in the presence of the GSI inhibitor and a comparable reduction in expression of the Notch target, Hey1, was observed (Fig. 4C and 4D). The Notch ligand Jagged1 is expressed at higher levels after HOXB4 activation, indicating that this could contribute to the hematopoietic inductive effects of HOXB4 (Fig. 4E).
Discussion
The therapeutic application of stem cell research to the treatment of hematological disease is severely limited by our inability to generate large numbers of cells in an efficient manner. To address these limitations, we require a fuller understanding of the cellular targets and molecular mechanisms involved in the strategies which have been used to enhance hematopoietic differentiation. Furthermore, the development of strategies that avoid the need for genetic manipulation, such as overexpression of HOXB4, would provide an easier route to clinical application. We previously reported that co-culture with AGM-derived stromal cell lines significantly induced the production of hematopoietic progenitors from both mouse and human ES cells [43,44] and currently represents an efficient strategy for the production of HSCs capable of long-term reconstitution from human ES cells [44]. This study set out to compare the inductive effects of AGM stromal cell lines with OP9 and HOXB4 and to analyze the cellular targets and molecular mechanisms associated with their activity.
Stromal cell lines derived from AGM subregions have distinct effects on hematopoietic differentiation
A significant increase in hematopoietic progenitor production was observed when ES cells were differentiated in co-culture with 3 independently derived stromal cell lines isolated from the aorta-mesonephros (AM) subregion of the AGM and the level of induction was comparable to that observed with the OP9 cell line. No hematopoietic inductive effect was observed when ES cells were co-cultured with stromal lines derived from the urogenital ridge (UG) and fetal liver which contrasts with the potent supportive effects of the UG26.1B6 and EL08.1D2 cell lines on adult bone marrow derived progenitors and HSCs [37–42] and human ES cells [44]. Although the UG26.1B6 was able to promote hematopoietic colony formation from human ES cells, it did not enhance repopulating HSCs [44] and we speculate therefore that this stromal cell line exerts a proliferative effect on hematopoietic progenitors that arise spontaneously from differentiating hESCs. Our combined data on mouse and human ES cells supports the idea that the signals required to direct hematopoietic differentiation of mouse and human ES cells differ from those required for the support of adult hematopoietic stem and progenitor cells.
Co-culture of single EBs on the AM cell line resulted in an increase in both the number of EBs with associated hematopoietic activity and the amount of hematopoietic activity in each EB, whereas co-culture on cell lines derived from the UG did not differ significantly from control cultures. The fact that we observed an increase in the number of individual EBs with hematopoietic activity suggests that the AM cell line is able to induce the de novo production of hematopoietic progenitors from ES cells, as well as enhance their expansion. This interpretation is based on the assumptions that each hematopoietic colony (CFU) detected in our assay is the product of a single hematopoietic progenitor cell and that the sensitivity of the assay is such that every progenitor with colony forming potential is detected. If the number of progenitors within an EB must exceed a threshold before a colony is detected, then an increase in the proportion of EBs with CFU activity could arise if progenitors were stimulated to proliferate beyond this threshold and an increase in proliferation might be misinterpreted as an inductive effect. Nevertheless, the data presented here demonstrate the potent effects of the AM cell line on hematopoietic differentiation and are consistent with the role of the aorta subregion of the AGM in mediating de novo induction of HSCs in vivo [35,52–58]. Indeed, our data is supported by the fact that the AM stromal cell line was the most efficient in the production of HSCs from hES cells [44].
AM-derived stromal cell lines act after the differentiation of mesoderm
We have shown that AM-derived and OP9 stroma did not mediate their hematopoietic enhancing effects by promoting the numbers of cells expressing brachyury, suggesting that they act downstream of mesoderm specification. Co-culture of FACS-isolated cell populations on AM14.1C4 showed that, as expected, hematopoietic progenitors arise from brachyury+ ancestors. Our data also reveal that non-hematopoietic brachyury- ES-derived cells play a supportive role to progenitors in the co-culture microenvironment (Fig. 2). The OP9 cell line did not support the growth and/or subsequent differentiation of brachyury+ cells, suggesting that signals from this cell line act at a later stage of the differentiation process, most likely by expansion of hematopoietic progenitors.
HOXB4 overexpression did not further enhance the effects of AM-co-culture
Activation of HOXB4 resulted in an increase in hematopoietic activity comparable to that of co-culture of EBs on AM stromal cells, but combining the 2 induction strategies did not have an additive effect. Comparable results were observed with human ES cells, where no additive effect on in vitro hematopoietic colony production was observed when OP9 co-culture and HOXB4 overexpression were combined [27]. One explanation for these findings is that common molecular mechanisms might be responsible for the 2 induction strategies. The identification and analysis of such common mechanisms would lead to a fuller understanding of this complex differentiation system. To provide support for this hypothesis we tested the effects of inhibiting the γ-secretase pathway on both strategies.
γ-Secretase inhibition attenuates AM stromal cell-and HOXB4-mediated hematopoietic differentiation
The hematopoietic enhancing effects of both the AM stromal cell co-culture and HOXB4 activation were reduced when γ-secretase activity was inhibited. γ-Secretase is a protease that mediates transmembrane cleavage of target receptors to regulate intracellular signaling. Although a number of signaling pathways are mediated by γ-secretase activity, including Notch, CD44, N-cadherin, VEGFR-1, and ErbB-4 [59], our data are consistent with a number of reports implicating the Notch pathway in the hematopoietic differentiation of ES cells in vitro and in the development of the hematopoietic system in vivo [51,60]. Notch is a highly conserved signaling pathway regulating cell fate decisions and the effects of Notch activation or depletion are complex and dependent on timing, dose, and context [61]. Activation of the Notch pathway in ES cells inhibits differentiation into primitive hematopoietic cells, apparently by activating inhibitors of the Wnt pathway [60]. In contrast to its effects on primitive hematopoiesis, Notch signaling is implicated in the production of definitive hematopoietic cells in the AGM region, possibly by modulating Runx1 expression and transcriptional activity [62–64]. In our experiments, the observed decrease in hematopoietic activity in the presence of GSI suggests that definitive hematopoietic cells are produced in AM co-cultures, though this needs to be tested directly by transplantation assays, it is consistent with the production of HSCs from hESC [44]. Notch signaling has also been shown to be active in hematopoietic clusters of the midgestational dorsal aorta [63,65,66]; consistent with the potent hematopoietic inductive effects of the AM-derived stromal lines over that of the UG-derived stroma.
The hematopoietic enhancing activity of the AM stromal cells could be due to a direct effect on Notch signaling, or they might be providing support for a Notch/γ-secretase-dependent interaction between ES-derived cells. Our finding that brachyury− cells were able to support the hematopoietic differentiation of brachyury+ cells in the context of the AM co-culture is consistent with the idea that the stromal cell co-culture might also modulate an intrinsic niche within the differentiating EBs.
A number of genes associated with the Notch signaling pathway have been identified as HOXB4 target genes [67]. We show that the hematopoietic inducing effects of HOXB4, as well as the increased Hey1 expression, was attenuated by GSI and that there is a significant increase in Jagged1 expression upon HOXB4 activation. Taken together, this suggests that the hematopoietic inducing activity of HOXB4 could be mediated in part by alterations in the intrinsic ES-derived hematopoietic niche. These observations support the hypothesis that the HOXB4 and AM stromal induction strategies mediate their hematopoietic enhancing effects through common signaling pathways and that co-culture of human ES cells on AM stromal cell lines may alleviate the requirement for HOXB4 in the production of transplantable cells for therapy. We are currently confirming the role of the Notch pathway using more specific gain- and loss-of-function strategies, as well as screening for other signaling pathways that are shared between the 2 inductive strategies.
Supplementary Material
Acknowledgments
This work is funded by Leukaemia and Lymphoma Research and the University of Edinburgh College of Medicine and Veterinary Medicine (S.G.K. and C.H.). We thank Gordon Keller for the gift of the bry-GFP ES cell line and Dr. Sandrine Prost and Professor Elaine Dzierzak for valuable comments and critical reading of the manuscript.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Keller G. Embryonic stem cell differentiation: emergence of a new era in biology and medicine. Genes Dev. 2005;19:1129–1155. doi: 10.1101/gad.1303605. [DOI] [PubMed] [Google Scholar]
- 2.Lu SJ. Feng Q. Park JS. Vida L. Lee BS. Strausbauch M. Wettstein PJ. Honig GR. Lanza R. Biologic properties and enucleation of red blood cells from human embryonic stem cells. Blood. 2008;112:4475–4484. doi: 10.1182/blood-2008-05-157198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Evans MJ. Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292:154–156. doi: 10.1038/292154a0. [DOI] [PubMed] [Google Scholar]
- 4.Martin GR. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc Natl Acad Sci USA. 1981;78:7634–7638. doi: 10.1073/pnas.78.12.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doetschman TC. Eistetter H. Katz M. Schmidt W. Kemler R. The in vitro development of blastocyst-derived embryonic stem cell lines: formation of visceral yolk sac, blood islands and myocardium. J Embryol Exp Morphol. 1985;87:27–45. [PubMed] [Google Scholar]
- 6.Keller G. Kennedy M. Papayannopoulou T. Wiles MV. Hematopoietic commitment during embryonic stem cell differentiation in culture. Mol Cell Biol. 1993;13:473–486. doi: 10.1128/mcb.13.1.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nakano T. Kodama H. Honjo T. Generation of lymphohematopoietic cells from embryonic stem cells in culture. Science. 1994;265:1098–1101. doi: 10.1126/science.8066449. [DOI] [PubMed] [Google Scholar]
- 8.Nakayama N. Lee J. Chiu L. Vascular endothelial growth factor synergistically enhances bone morphogenetic protein-4-dependent lymphohematopoietic cell generation from embryonic stem cells in vitro. Blood. 2000;95:2275–2283. [PubMed] [Google Scholar]
- 9.Park C. Afrikanova I. Chung YS. Zhang WJ. Arentson E. Fong Gh G. Rosendahl A. Choi K. A hierarchical order of factors in the generation of FLK1- and SCL-expressing hematopoietic and endothelial progenitors from embryonic stem cells. Development. 2004;131:2749–2762. doi: 10.1242/dev.01130. [DOI] [PubMed] [Google Scholar]
- 10.Pearson S. Sroczynska P. Lacaud G. Kouskoff V. The stepwise specification of embryonic stem cells to hematopoietic fate is driven by sequential exposure to Bmp4, activin A, bFGF and VEGF. Development. 2008;135:1525–1535. doi: 10.1242/dev.011767. [DOI] [PubMed] [Google Scholar]
- 11.Purpura KA. Morin J. Zandstra PW. Analysis of the temporal and concentration-dependent effects of BMP-4, VEGF, and TPO on development of embryonic stem cell-derived mesoderm and blood progenitors in a defined, serum-free media. Exp Hematol. 2008;36:1186–1198. doi: 10.1016/j.exphem.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 12.Wiles MV. Keller G. Multiple hematopoietic lineages develop from embryonic stem (ES) cells in culture. Development. 1991;111:259–267. doi: 10.1242/dev.111.2.259. [DOI] [PubMed] [Google Scholar]
- 13.Chadwick K. Wang L. Li L. Menendez P. Murdoch B. Rouleau A. Bhatia M. Cytokines and BMP-4 promote hematopoietic differentiation of human embryonic stem cells. Blood. 2003;102:906–915. doi: 10.1182/blood-2003-03-0832. [DOI] [PubMed] [Google Scholar]
- 14.Burt RK. Verda L. Kim DA. Oyama Y. Luo K. Link C. Embryonic stem cells as an alternate marrow donor source: engraftment without graft-versus-host disease. J Exp Med. 2004;199:895–904. doi: 10.1084/jem.20031916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gutierrez-Ramos JC. Palacios R. In vitro differentiation of embryonic stem cells into lymphocyte precursors able to generate T and B lymphocytes in vivo. Proc Natl Acad Sci USA. 1992;89:9171–9175. doi: 10.1073/pnas.89.19.9171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hole N. Graham GJ. Menzel U. Ansell JD. A limited temporal window for the derivation of multilineage repopulating hematopoietic progenitors during embryonal stem cell differentiation invitro. Blood. 1996;88:1266–1276. [PubMed] [Google Scholar]
- 17.Miyagi T. Takeno M. Nagafuchi H. Takahashi M. Suzuki N. Flk1+ cells derived from mouse embryonic stem cells reconstitute hematopoiesis in vivo in SCID mice. Exp Hematol. 2002;30:1444–1453. doi: 10.1016/s0301-472x(02)00961-x. [DOI] [PubMed] [Google Scholar]
- 18.Müller AM. Dzierzak EA. ES cells have only a limited lymphopoietic potential after adoptive transfer into mouse recipients. Development. 1993;118:1343–1351. doi: 10.1242/dev.118.4.1343. [DOI] [PubMed] [Google Scholar]
- 19.Palacios R. Golunski E. Samaridis J. In vitro generation of hematopoietic stem cells from an embryonic stem cell line. Proc Natl Acad Sci USA. 1995;92:7530–7534. doi: 10.1073/pnas.92.16.7530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Potocnik AJ. Nielsen PJ. Eichmann K. In vitro generation of lymphoid precursors from embryonic stem cells. EMBO J. 1994;13:5274–5283. doi: 10.1002/j.1460-2075.1994.tb06861.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kyba M. Perlingeiro RC. Daley GQ. HoxB4 confers definitive lymphoid-myeloid engraftment potential on embryonic stem cell and yolk sac hematopoietic progenitors. Cell. 2002;109:29–37. doi: 10.1016/s0092-8674(02)00680-3. [DOI] [PubMed] [Google Scholar]
- 22.McKinney-Freeman SL. Naveiras O. Yates F. Loewer S. Philitas M. Curran M. Park PJ. Daley GQ. Surface antigen phenotypes of hematopoietic stem cells from embryos and murine embryonic stem cells. Blood. 2009;114:268–278. doi: 10.1182/blood-2008-12-193888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Y. Yates F. Naveiras O. Ernst P. Daley GQ. Embryonic stem cell-derived hematopoietic stem cells. Proc Natl Acad Sci USA. 2005;102:19081–19086. doi: 10.1073/pnas.0506127102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bowles KM. Vallier L. Smith JR. Alexander MR. Pedersen RA. HOXB4 overexpression promotes hematopoietic development by human embryonic stem cells. Stem Cells. 2006;24:1359–1369. doi: 10.1634/stemcells.2005-0210. [DOI] [PubMed] [Google Scholar]
- 25.Wang L. Menendez P. Shojaei F. Li L. Mazurier F. Dick JE. Cerdan C. Levac K. Bhatia M. Generation of hematopoietic repopulating cells from human embryonic stem cells independent of ectopic HOXB4 expression. J Exp Med. 2005;201:1603–1614. doi: 10.1084/jem.20041888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vodyanik MA. Bork JA. Thomson JA. Slukvin II. Human embryonic stem cell-derived CD34+ cells: efficient production in the coculture with OP9 stromal cells and analysis of lymphohematopoietic potential. Blood. 2005;105:617–626. doi: 10.1182/blood-2004-04-1649. [DOI] [PubMed] [Google Scholar]
- 27.Ji J. Vijayaragavan K. Bosse M. Menendez P. Weisel K. Bhatia M. OP9 stroma augments survival of hematopoietic precursors and progenitors during hematopoietic differentiation from human embryonic stem cells. Stem Cells. 2008;26:2485–2495. doi: 10.1634/stemcells.2008-0642. [DOI] [PubMed] [Google Scholar]
- 28.Moore MA. Metcalf D. Ontogeny of the haemopoietic system: yolk sac origin of in vivo and in vitro colony forming cells in the developing mouse embryo. Br J Haematol. 1970;18:279–296. doi: 10.1111/j.1365-2141.1970.tb01443.x. [DOI] [PubMed] [Google Scholar]
- 29.Palis J. Yoder MC. Yolk-sac hematopoiesis: the first blood cells of mouse and man. Exp Hematol. 2001;29:927–936. doi: 10.1016/s0301-472x(01)00669-5. [DOI] [PubMed] [Google Scholar]
- 30.Cumano A. Dieterlen-Lievre F. Godin I. Lymphoid potential, probed before circulation in mouse, is restricted to caudal intraembryonic splanchnopleura. Cell. 1996;86:907–916. doi: 10.1016/s0092-8674(00)80166-x. [DOI] [PubMed] [Google Scholar]
- 31.Godin IE. Garcia-Porrero JA. Coutinho A. Dieterlen-Lièvre F. Marcos MA. Para-aortic splanchnopleura from early mouse embryos contains B1a cell progenitors. Nature. 1993;364:67–70. doi: 10.1038/364067a0. [DOI] [PubMed] [Google Scholar]
- 32.Kumaravelu P. Hook L. Morrison AM. Ure J. Zhao S. Zuyev S. Ansell J. Medvinsky A. Quantitative developmental anatomy of definitive haematopoietic stem cells/long-term repopulating units (HSC/RUs): role of the aorta-gonad-mesonephros (AGM) region and the yolk sac in colonisation of the mouse embryonic liver. Development. 2002;129:4891–4899. doi: 10.1242/dev.129.21.4891. [DOI] [PubMed] [Google Scholar]
- 33.Müller AM. Medvinsky A. Strouboulis J. Grosveld F. Dzierzak E. Development of hematopoietic stem cell activity in the mouse embryo. Immunity. 1994;1:291–301. doi: 10.1016/1074-7613(94)90081-7. [DOI] [PubMed] [Google Scholar]
- 34.Ottersbach K. Dzierzak E. The murine placenta contains hematopoietic stem cells within the vascular labyrinth region. Dev Cell. 2005;8:377–387. doi: 10.1016/j.devcel.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 35.Medvinsky A. Dzierzak E. Definitive hematopoiesis is autonomously initiated by the AGM region. Cell. 1996;86:897–906. doi: 10.1016/s0092-8674(00)80165-8. [DOI] [PubMed] [Google Scholar]
- 36.Medvinsky AL. Samoylina NL. Müller AM. Dzierzak EA. An early pre-liver intraembryonic source of CFU-S in the developing mouse. Nature. 1993;364:64–67. doi: 10.1038/364064a0. [DOI] [PubMed] [Google Scholar]
- 37.Matsuoka S. Tsuji K. Hisakawa H. Mj Xu. Ebihara Y. Ishii T. Sugiyama D. Manabe A. Tanaka R. Ikeda Y. Asano S. Nakahata T. Generation of definitive hematopoietic stem cells from murine early yolk sac and paraaortic splanchnopleures by aorta-gonad-mesonephros region-derived stromal cells. Blood. 2001;98:6–12. doi: 10.1182/blood.v98.1.6. [DOI] [PubMed] [Google Scholar]
- 38.Ohneda O. Fennie C. Zheng Z. Donahue C. La H. Villacorta R. Cairns B. Lasky LA. Hematopoietic stem cell maintenance and differentiation are supported by embryonic aorta-gonad-mesonephros region-derived endothelium. Blood. 1998;92:908–919. [PubMed] [Google Scholar]
- 39.Oostendorp RA. Harvey KN. Kusadasi N. de Bruijn MF. Saris C. Ploemacher RE. Medvinsky AL. Dzierzak EA. Stromal cell lines from mouse aorta-gonads-mesonephros sub-regions are potent supporters of hematopoietic stem cell activity. Blood. 2002;99:1183–1189. doi: 10.1182/blood.v99.4.1183. [DOI] [PubMed] [Google Scholar]
- 40.Oostendorp RA. Medvinsky AJ. Kusadasi N. Nakayama N. Harvey K. Orelio C. Ottersbach K. Covey T. Ploemacher RE. Saris C. Dzierzak E. Embryonal subregion-derived stromal cell lines from novel temperature-sensitive SV40 T antigen transgenic mice support hematopoiesis. J Cell Sci. 2002;115(Pt 10):2099–2108. doi: 10.1242/jcs.115.10.2099. [DOI] [PubMed] [Google Scholar]
- 41.Weisel KC. Gao Y. Shieh JH. Moore MA. Stromal cell lines from the aorta-gonado-mesonephros region are potent supporters of murine and human hematopoiesis. Exp Hematol. 2006;34:1505–1516. doi: 10.1016/j.exphem.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 42.Xu MJ. Tsuji K. Ueda T. Mukouyama YS. Hara T. Yang FC. Ebihara Y. Matsuoka S. Manabe A. Kikuchi A. Ito M. Miyajima A. Nakahata T. Stimulation of mouse and human primitive hematopoiesis by murine embryonic aorta-gonad-mesonephros-derived stromal cell lines. Blood. 1998;92:2032–2040. [PubMed] [Google Scholar]
- 43.Krassowska A. Gordon-Keylock S. Samuel K. Gilchrist D. Dzierzak E. Oostendorp R. Forrester LM. Ansell JD. Promotion of haematopoietic activity in embryonic stem cells by the aorta-gonad-mesonephros microenvironment. Exp Cell Res. 2006;312:3595–3603. doi: 10.1016/j.yexcr.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 44.Ledran MH. Krassowska A. Armstrong L. Dimmick I. Renström J. Lang R. Yung S. Santibanez-Coref M. Dzierzak E. Stojkovic M. Oostendorp RA. Forrester L. Lako M. Efficient hematopoietic differentiation of human embryonic stem cells on stromal cells derived from hematopoietic niches. Cell Stem Cell. 2008;3:85–98. doi: 10.1016/j.stem.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 45.Gilchrist DS. Ure J. Hook L. Medvinsky A. Labeling of hematopoietic stem and progenitor cells in novel activatable EGFP reporter mice. Genesis. 2003;36:168–176. doi: 10.1002/gene.10209. [DOI] [PubMed] [Google Scholar]
- 46.Fehling HJ. Lacaud G. Kubo A. Kennedy M. Robertson S. Keller G. Kouskoff V. Tracking mesoderm induction and its specification to the hemangioblast during embryonic stem cell differentiation. Development. 2003;130:4217–4227. doi: 10.1242/dev.00589. [DOI] [PubMed] [Google Scholar]
- 47.Kiel MJ. Yilmaz OH. Iwashita T. Yilmaz OH. Terhorst C. Morrison SJ. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121:1109–1121. doi: 10.1016/j.cell.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 48.Kim I. He S. Yilmaz OH. Kiel MJ. Morrison SJ. Enhanced purification of fetal liver hematopoietic stem cells using SLAM family receptors. Blood. 2006;108:737–744. doi: 10.1182/blood-2005-10-4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yilmaz OH. Kiel MJ. Morrison SJ. SLAM family markers are conserved among hematopoietic stem cells from old and reconstituted mice and markedly increase their purity. Blood. 2006;107:924–930. doi: 10.1182/blood-2005-05-2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lowell S. Benchoua A. Heavey B. Smith AG. Notch promotes neural lineage entry by pluripotent embryonic stem cells. PLoS Biol. 2006;4:e121. doi: 10.1371/journal.pbio.0040121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schroeder T. Meier-Stiegen F. Schwanbeck R. Eilken H. Nishikawa S. Häsler R. Schreiber S. Bornkamm GW. Nishikawa S. Just U. Activated Notch1 alters differentiation of embryonic stem cells into mesodermal cell lineages at multiple stages of development. Mech Dev. 2006;123:570–579. doi: 10.1016/j.mod.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 52.de Bruijn MF. Ma X. Robin C. Ottersbach K. Sanchez MJ. Dzierzak E. Hematopoietic stem cells localize to the endothelial cell layer in the midgestation mouse aorta. Immunity. 2002;16:673–683. doi: 10.1016/s1074-7613(02)00313-8. [DOI] [PubMed] [Google Scholar]
- 53.de Bruijn MF. Peeters MC. Luteijn T. Visser P. Speck NA. Dzierzak E. CFU-S(11) activity does not localize solely with the aorta in the aorta-gonad-mesonephros region. Blood. 2000;96:2902–2904. [PubMed] [Google Scholar]
- 54.de Bruijn MF. Speck NA. Peeters MC. Dzierzak E. Definitive hematopoietic stem cells first develop within the major arterial regions of the mouse embryo. EMBO J. 2000;19:2465–2474. doi: 10.1093/emboj/19.11.2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.North T. Gu TL. Stacy T. Wang Q. Howard L. Binder M. Marín-Padilla M. Speck NA. Cbfa2 is required for the formation of intra-aortic hematopoietic clusters. Development. 1999;126:2563–2575. doi: 10.1242/dev.126.11.2563. [DOI] [PubMed] [Google Scholar]
- 56.North TE. de Bruijn MF. Stacy T. Talebian L. Lind E. Robin C. Binder M. Dzierzak E. Speck NA. Runx1 expression marks long-term repopulating hematopoietic stem cells in the midgestation mouse embryo. Immunity. 2002;16:661–672. doi: 10.1016/s1074-7613(02)00296-0. [DOI] [PubMed] [Google Scholar]
- 57.Taoudi S. Gonneau C. Moore K. Sheridan JM. Blackburn CC. Taylor E. Medvinsky A. Extensive hematopoietic stem cell generation in the AGM region via maturation of VE-cadherin+CD45+ pre-definitive HSCs. Cell Stem Cell. 2008;3:99–108. doi: 10.1016/j.stem.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 58.Taoudi S. Medvinsky A. Functional identification of the hematopoietic stem cell niche in the ventral domain of the embryonic dorsal aorta. Proc Natl Acad Sci USA. 2007;104:9399–9403. doi: 10.1073/pnas.0700984104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Boulton ME. Cai J. Grant MB. gamma-Secretase: a multifaceted regulator of angiogenesis. J Cell Mol Med. 2008;12:781–795. doi: 10.1111/j.1582-4934.2008.00274.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cheng X. Huber TL. Chen VC. Gadue P. Keller GM. Numb mediates the interaction between Wnt and Notch to modulate primitive erythropoietic specification from the hemangioblast. Development. 2008;135:3447–3458. doi: 10.1242/dev.025916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schwanbeck R. Schroeder T. Henning K. Kohlhof H. Rieber N. Erfurth ML. Just U. Notch signaling in embryonic and adult myelopoiesis. Cells Tissues Organs (Print) 2008;188:91–102. doi: 10.1159/000113531. [DOI] [PubMed] [Google Scholar]
- 62.Burns CE. Traver D. Mayhall E. Shepard JL. Zon LI. Hematopoietic stem cell fate is established by the Notch-Runx pathway. Genes Dev. 2005;19:2331–2342. doi: 10.1101/gad.1337005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kumano K. Chiba S. Kunisato A. Sata M. Saito T. Nakagami-Yamaguchi E. Yamaguchi T. Masuda S. Shimizu K. Takahashi T. Ogawa S. Hamada Y. Hirai H. Notch1 but not Notch2 is essential for generating hematopoietic stem cells from endothelial cells. Immunity. 2003;18:699–711. doi: 10.1016/s1074-7613(03)00117-1. [DOI] [PubMed] [Google Scholar]
- 64.Nakagawa M. Ichikawa M. Kumano K. Goyama S. Kawazu M. Asai T. Ogawa S. Kurokawa M. Chiba S. AML1/Runx1 rescues Notch1-null mutation-induced deficiency of para-aortic splanchnopleural hematopoiesis. Blood. 2006;108:3329–3334. doi: 10.1182/blood-2006-04-019570. [DOI] [PubMed] [Google Scholar]
- 65.Robert-Moreno A. Espinosa L. de la Pompa JL. Bigas A. RBPjkappa-dependent Notch function regulates Gata2 and is essential for the formation of intra-embryonic hematopoietic cells. Development. 2005;132:1117–1126. doi: 10.1242/dev.01660. [DOI] [PubMed] [Google Scholar]
- 66.Robert-Moreno A. Guiu J. Ruiz-Herguido C. López ME. Inglés-Esteve J. Riera L. Tipping A. Enver T. Dzierzak E. Gridley T. Espinosa L. Bigas A. Impaired embryonic haematopoiesis yet normal arterial development in the absence of the Notch ligand Jagged1. EMBO J. 2008;27:1886–1895. doi: 10.1038/emboj.2008.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schiedlmeier B. Santos AC. Ribeiro A. Moncaut N. Lesinski D. Auer H. Kornacker K. Ostertag W. Baum C. Mallo M. Klump H. HOXB4's road map to stem cell expansion. Proc Natl Acad Sci USA. 2007;104:16952–16957. doi: 10.1073/pnas.0703082104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.