Abstract
Fibroblast growth factor (FGF) and FGF receptor (FGFR) are expressed in various cells including endothelial progenitor cells and hematopoietic cells. The interaction between FGF and FGFR is associated with the proliferation, migration, and survival of these cells. In this report, we examined the effects of FGFR2 signaling on hematopoiesis in immature hematopoietic cells, using mutant mice in which a constitutively active form of FGFR2 mutant was caused to be overexpressed by the Tie2 promoter (FGFR2 Tg mice). Under normal conditions, hematopoiesis of FGFR2 Tg mice and wild type (Wt) mice do not differ significantly, except for the weight and cell numbers of the thymus. However, the c-kit+Sca-1+lineage− bone marrow cells (BMCs) of FGFR2 Tg mice facilitate the formation of colony-forming units of culture. When these BMCs were transplanted into the recipient bone marrow (intra-bone marrow–bone marrow transplantation), there was better reconstitution of donor hematopoietic cells. In the in vitro experiment, the c-kit+Sca-1+lineage− BMCs from FGFR2 Tg mice showed fewer apoptotic cells than those from Wt mice. These results suggest that the antiapoptotic effect of FGFR2 signaling facilitates the hematopoiesis of FGFR2 Tg mice.
Introduction
Fibroblast growth factor (FGF) belongs to a family of heparin-binding polypeptides and shows multiple functions, including effects on cell proliferation, differentiation, survival, and motility [1,2]. Twenty-four members of the FGF family have been identified, ranging in molecular mass from 17 to 34 kDa and share 13%–71% amino acid identity [3,4]. To date, 4 kinds of FGF receptors (FGFR) have been reported [5]. Since the expression of FGFR is widely distributed on various cells, FGF signaling plays an important role in development and morphogenesis as well as in physiological and pathological situations such as wound healing, neovascularization, tumor growth, and tumor progression [6–8]. FGF signaling also facilitates hematopoiesis. FGF acts not only directly on the hematopoietic stem cells (HSCs) and immature hematopoietic progenitor cells (IHPCs) but also indirectly on them through bone marrow stromal cells [9–11].
Tie2 is a receptor tyrosine kinase expressed in both HSCs/IHPCs and endothelial cells. The interaction of Tie2 with its ligand, angiopoietin-1 (Ang-1), induces the cobblestone formation of HSCs in vitro and maintains the long-term repopulating activity of HSCs in vivo [12–14]. Arai et al. suggested that the Tie2/Ang-1 signaling pathway plays a critical role in maintaining HSCs in a quiescent state in the bone-marrow niche [14,15].
Recently, we established mutant mice in which a constitutively active form of FGFR2 mutant was caused to be overexpressed using the Tie2 promoter (FGFR2 Tg mice) [16]. The mice showed decreased infarct size and improved cardiac performance compared with wild type (Wt) mice when acute cardiac ischemia was induced [16].
In the present study, we show that the c-kit+Sca-1+lineage− bone marrow cells (BMCs) from FGFR2 Tg mice facilitate the formation of day-14 colony-forming units of culture (CFU-C), and that these BMCs facilitate better engraftment of donor hematopoietic cells than those from Wt mice by intra-bone marrow–bone marrow transplantation (IBM-BMT).
Materials and Methods
Mice
FGFR2 Tg mice were prepared as described previously [16]. C57BL/6 mice congenic for the ly5 locus (B6-ly5.1 mice) were bred and maintained at the animal center of Kansai Medical University (Moriguchi City, Osaka, Japan). The background mice of the FGFR2 Tg mice are C57BL/6 mice (B6 mice) (ly5.2). Therefore, B6 mice were purchased from Japan SLC Inc. and used as Wt mice.
Reagents
Fluorescein isothiocyanate (FITC)-conjugated anti-CD45.1 (ly5.1) antibody (Ab), FITC-conjugated anti-c-kit Ab, FITC-conjugated anti-Mac-1 Ab, phycoerythrin (PE)-conjugated anti-CD3 Ab, PE-conjugated anti-Gr-1 Ab, PE-conjugated anti-B220 Ab, PE-conjugated anti-Sca-1 Ab, PE-conjugated anti-CD45 Ab, biotin-conjugated anti-CD45.2 (ly5.2) Ab, biotin-conjugated anti-B220 Ab, biotin-conjugated anti-Mac-1 Ab, biotin-conjugated anti-Ter119 Ab, biotin-conjugated anti-Gr-1 Ab, biotin-conjugated anti-CD3 Ab, biotin-conjugated anti-NK1.1 Ab, biotin-conjugated anti-CD11c Ab, Per-CP Cy5.5-conjugated anti-CD3 Ab, antiproliferating cell nuclear antigen (PCNA) Ab, and Per-CP Cy5.5-conjugated avidin were purchased from BD Biosciences. Ab and allophycocyanin (APC)-conjugated anti-B220 Ab was obtained from Caltag, and PE-conjugated anti-Tie2 Ab was obtained from eBioscience. Annexin V-FITC apoptosis detection kit, containing FITC-conjugated annexin V, was obtained from BioVision. Tetracolor-one was obtained from Nacalai tesque. A permeabilization reagent, IntraPrep™, was obtained from Immunotech phosphatidyl inositol (PI)-3-kinase inhibitor (LY294002) was obtained from Sigma Chemical Co.
Detection of mRNA of FGFR2 by reverse transcription (RT)-polymerase chain reaction and real-time RT-polymerase chain reaction
RNA preparation from the BMCs of Wt mice or FGFR2 Tg mice, cDNA synthesis, and polymerase chain reaction (PCR) were carried out as described previously [17]. Primers for the detection of mRNAs in this experiment were glyceraldehyde-3-phosphate dehydrogenase (G3PDH) (Toyobo) and FGFR2 [16]. PCR products were separated on a 1.2% agarose gel (Gibco BRL) and viewed by ethidium bromide (Nakarai) staining.
We also performed real-time PCR using cDNA with OPTICON2 (MJ Research), QuantiTect SYBR Green PCR kit (Qiagen), GAPDH-specific primers (Qiagen), and the primers for FGFR2 [16] to estimate the expression of FGFR2 mRNA accurately, as described previously [18].
Analyses of peripheral blood and organs
Blood samples obtained from 8- to 13-week-old FGFR2 Tg mice and Wt mice were analyzed using an automated hematology analyzer (SE 9000; Sysmex). The individual mice organs were weighed and fixed with 15% buffered formalin (Wako) for microscopical examination. A single-cell suspension was prepared from the spleen, bone marrow, and thymus of the mice. The cells were then stained with FITC-conjugated anti-Mac-1 Ab, PE-conjugated anti-Gr-1 Ab, Per-CP Cy5.5-conjugated anti-CD3 Ab, and APC-conjugated anti-B220 Ab; FITC-conjugated anti-CD8 Ab, PE-conjugated anti-CD4 Ab, and PerCP Cy5.5-conjugated anti-CD3 Ab; or FITC-conjugated anti-NK1.1 Ab. The stained cells were analyzed using the Becton Dickinson LSR flow cytometer (Becton Dickinson).
Preparation of BMCs
BMCs were harvested from the femoral and tibial bones of 8- to 12-week-old FGFR2 Tg mice (ly5.2) or Wt mice (ly5.2) and suspended in phosphate-buffered saline (PBS). The BMCs were then filtered through a 70-μm nylon wool mesh (Becton Dickinson Labware) and centrifuged at 1,500 rpm for 7 min at 4°C. After centrifugation, the BMCs were resuspended in PBS.
Preparation of lineage+ and c-kit+Sca-1+lineage− BMCs
BMCs from Wt mice or FGFR2 Tg mice were incubated with a biotin-conjugated cocktail of lineage antibodies including anti-Mac-1, anti-Gr-1, anti-NK1.1, anti-B220, anti-Ter119, anti-CD3, and anti-CD11c. The cells were then treated with magnetic beads conjugated with streptavidin (Dynabeads M-280; Dynal AS). The positively selected cells were used as lineage+ BMCs, and the negatively selected cells were incubated with FITC-conjugated c-kit Ab, PE-conjugated Sca-1 Ab, and Red PE-Cy5-conjugated streptavidin. The c-kit+Sca-1+lineage− BMCs were isolated using a fluorescence-activated cell sorter (FACS) (EPICS ALTRA; Beckman Coulter).
CFU-C assays
The colony-forming ability of the BMCs (CFU-C) was assayed as described previously [19]. Briefly, whole BMCs (104 cells/mL/well) or c-kit+Sca-1+lineage− BMCs (50 cells/mL/well) were plated in Petri dishes (Becton Dickinson) in 10 mL of Methocult GF H3434 (StemCell Technologies, Inc.). The colonies were counted 7 and 14 days later.
Examination of long-term facilitation after IBM-BMT
Recipient mice (ly5.1-B6) were exposed to 137Cs gamma irradiation at 0.96 Gy/min. The exposure dose was 8.0 Gy. The c-kit+Sca-1+lineage− BMCs were injected directly into the bone marrow cavity of the tibial bones of the recipient mice (IBM-BMT), as previously described [20]. Briefly, the region from the groin to the knee joint was shaved, and an ∼5-mm incision was made on the thigh. The knee was flexed to 90°, the proximal side of the tibia was drawn to the anterior, and a 26-gauge needle was inserted into the bone marrow cavity. Using a microsyringe (50 μL; Hamilton Company), the donor c-kit+Sca-1+lineage− BMCs (3 × 104 cells/10 μL) were injected via the needle into the bone marrow cavity.
Blood was collected from recipient mice at 4 weeks and 6 months after IBM-BMT, and the ly5 (CD45) haplotypes of the leukocytes were then analyzed by flow cytometry. In this analysis, blood was suspended in PBS supplemented with 2% fetal bovine serum and incubated with FITC-conjugated anti-CD45.1 (ly5.1) Ab; PE-conjugated anti-Gr-1, anti-CD3, anti-B220, or anti-CD45 Abs; and biotin-conjugated anti-CD45.2 (ly5.2) Ab, followed by staining with Red PE-Cy5-avidin. The stained cells were analyzed by FACScan (Becton Dickinson).
Assay for live cells, proliferating cells, and apoptotic cells
BMCs (2 × 106/mL) from Wt mice and FGFR2 Tg mice were stained with FITC-conjugated anti-c-kit Ab; PE-conjugated anti-Sca-1 Ab; and biotin-conjugated lineage antibodies, including anti-CD3, anti-NK1.1, anti-Gr-1, anti-Mac-1, anti-Ter119, anti-CD11c, and biotin-labeled anti-B220, followed by staining with PE-Cy5.5-coupled avidin. The c-kit+Sca-1+lineage− BMCs were collected by a cell sorter. Sorted cells were cultured in a CO2 incubator for 1 day, and the numbers of live cells, proliferating cells, and apoptotic cells were estimated. Tetracolor-one was used to determine the number of live cells.
The percentage of proliferating cells in c-kit+Sca-1+lineage− BMCs was determined by detecting the cells expressing PCNA. The cultured cells were stained with PE-labeled anti-c-kit Ab; APC-labeled anti-Sca-1 Ab; biotin-conjugated lineage antibodies, including anti-Gr-1, anti-Mac-1, anti-CD3, anti-B220, anti-NK1.1, and anti-CD11c, followed by PerCP Cy5.5-avidin. Next, the cells were fixed with reagent 1 of IntraPrep for 15 min at room temperature. After washing the cells, they were incubated in reagent 2 of IntraPrep and permeabilized for 5 min at room temperature. FITC-conjugated anti-PCNA Ab or isotype-matched control was then added to the cells with reagent 2. After incubation for 20 min, the cells were washed, resuspended in PBS, and analyzed by FACSCaliber (BD Biosciences).
Apoptotic cells were detected using the annexin V-FITC apoptosis detection kit. The cultured cells were stained with FITC-conjugated annexin V; PE-conjugated anti-c-kit Ab; APC-conjugated anti-Sca-1 Ab; biotin-conjugated lineage antibodies, such as anti-Gr-1, anti-Mac-1, anti-CD3, anti-B220, anti-NK1.1, and anti-CD11c in the annexin buffer; followed by PerCP Cy5.5-avidin. The stained cells were analyzed by FACSCaliber. In the culture using PI-3-kinase inhibitor, BMCs (2 × 106/mL) were cultured with or without the inhibitor (LY294002) for 12 h and the percentage of apoptotic cells in the c-kit+Sca-1+lineage− BMCs calculated.
Statistical analyses
Differences between groups were evaluated by the Student's t-test. P values <0.05 were considered to be statistically significant.
Results
Expression of FGFR2 mRNA
FGFR2 expression in the heart, aorta, lungs, and endothelial cells of FGFR2 Tg mice was higher than in Wt mice, as previously described [16]. Since this study aimed to examine the effects of overexpression of FGFR2 on the immature hematopoietic cells expressing Tie2, we first examined the mRNA expression of BMCs in both types of mice. As shown in Fig. 1A and B, mRNA expression of FGFR2 in the BMCs of FGFR2 Tg mice was higher than in Wt mice. Next, we studied mRNA expression of FGFR2 in the lineage+ cells and c-kit+Sca-1+lineage− cells obtained from the bone marrow of Wt mice and FGFR2 Tg mice. As shown in Fig. 1C, mRNA of FGFR2 was more highly expressed in the c-kit+Sca-1+lineage− BMCs than in the lineage+ BMCs in FGFR Tg mice. In Wt mice, mRNA of FGFR2 was expressed in c-kit+Sca-1+lineage− BMCs but not in lineage+ BMCs. These results suggest that FGFR2 is highly expressed not only in the heart, aorta, lungs, and endothelial cells but also in the BMCs of FGFR2 Tg mice, and that FGFR2 is more highly expressed in immature hematopoietic cells than in lineage+ cells of both Wt and FGFR2 Tg mice.
FIG. 1.
mRNA expression of FGFR2 in BMCs of FGFR Tg mice and Wt mice. RNA was extracted from the BMCs of mice, followed by preparation of cDNA. RT-PCR (A) and real-time RT-PCR (B) were performed using the cDNA, as described in the Materials and Methods section. We obtained lineage+ cells and purified the c-kit+Sca-1+lineage− cells from the BMCs of Wt mice and FGFR2 Tg mice using magnetic beads and a cell sorter, as described in the Materials and Methods section. RT-PCR was performed to detect the mRNA expression of FGFR2 in lineage+ BMCs and c-kit+Sca-1+lineage− BMCs (C). *, p < 0.05. BMCs, bone marrow cells; FGFR, fibroblast growth factor receptor; PCR, polymerase chain reaction; Wt, wild type.
Hematopoiesis in FGFR2 Tg mice under normal conditions
To compare the hematopoietic function of FGFR2 Tg mice and normal mice (Wt mice), we first analyzed complete blood counts (CBCs), including white blood cell (WBC) numbers, populations of WBCs, red blood cell numbers, and platelet numbers using SE 9000. There were no significant differences in the CBCs of FGFR2 Tg mice and Wt mice (data not shown). We also examined the weights of the spleen, liver, thymus, kidneys, and heart, and the percentages of CD4+ T cells, CD8+ T cells, B cells, NK cells, Mac-1+ cells, and Gr-1+ cells in the spleen and the thymus. There were no significant differences in the cell populations or weights of these organs, except for the thymus. The thymus in FGFR2 Tg mice was significantly heavier than in Wt mice (88.3 ± 8.1 mg vs. 56.3 ± 14.8 mg). However, there were no significant differences in the populations of CD4−CD8−, CD4+CD8−, CD4−CD8+, or CD4+CD8+ cells between Wt and FGFR2 Tg mice. These results suggest that normal hematopoiesis occurs in FGFR2 Tg mice under normal conditions.
CFU-C assays
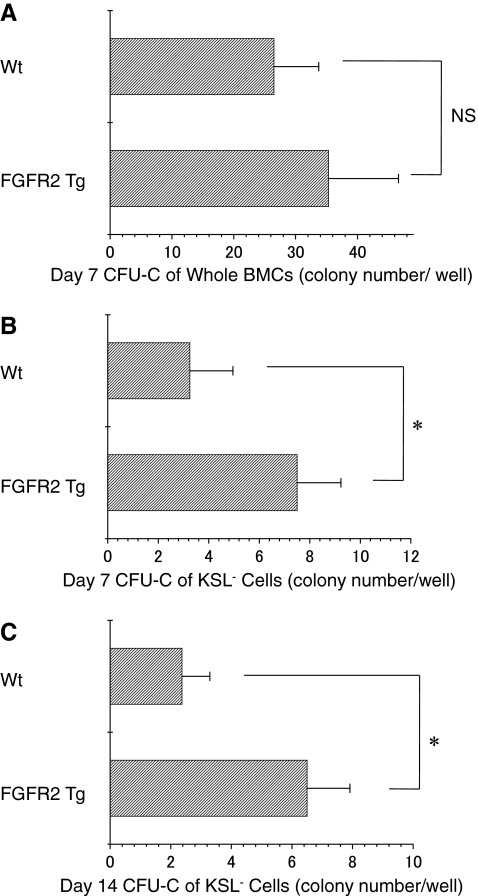
The hematopoietic function of FGFR2 Tg mice in comparison with Wt mice was examined using the day-7 CFU-C assays, because this assay shows the hematopoietic activity of hematopoietic progenitors [21]. The day-7 CFU-C assays using whole BMCs showed greater number of colonies in FGFR2 Tg mice than in Wt mice (Fig. 2A). However, there was statistically no significant difference in CFU-C counts between Wt and FGFR2 Tg mice. Therefore, the assays were performed with the c-kit+Sca-1+lineage− BMCs from Wt mice or FGFR2 Tg mice purified using magnetic beads and a cell sorter, because the c-kit+Sca-1+lineage− BMCs are very immature HPCs and HSCs [22].As shown in Fig. 2B and C, the numbers of day-7 and day-14 CFU-Cs in FGFR2 Tg mice were significantly higher than in Wt mice. These results suggest that hematopoiesis in FGFR2 Tg mice is regulated normally under normal conditions, but that c-kit+Sca-1+lineage− BMCs in FGFR Tg mice show high hematogenic function when rapid hematopoiesis is required.
FIG. 2.
The c-kit+Sca-1+lineage− BMCs of FGFR2 Tg mice facilitate the formation of CFU-C. BMCs prepared from Wt mice and FGFR2 Tg mice, as described in the Materials and Methods section, were used to perform the day-7 CFU-C assays (n = 4) (A). The c-kit+Sca-1+lineage− cells were prepared from the BMCs of Wt mice and FGFR2 Tg mice using magnetic beads and a fluorescence-activated cell sorter. The day-7 and day-14 CFU-C assays were carried out using these cells, as described in the Materials and Methods section (n = 7) (B, C). BMCs, bone marrow cells; CFU-C, colony-forming units of culture; FGFR, fibroblast growth factor receptor; Wt, wild type; KSL, c-kit+Sca-1+lineage−; NS, not significant. *, p < 0.05.
The percentages of c-kit+Sca-1+lineage− BMCs in Wt and FGFR2 Tg mice were 0.119 ± 0.017 and 0.126 ± 0.012, respectively, consistent with previous data [22]. The number of day-7 CFU-C using whole BMCs in 1 leg was 5,565 ± 1,519 in Wt mice and 7,067 ± 2,262 in FGFR2 Tg mice. The numbers of day-7 CFU-C using c-kit+Sca-1+lineage− BMCs in 1 leg were 3,962 ± 2,081 and 9,469 ± 2,187, while the values for day-14 CFU-C using c-kit+Sca-1+lineage− BMCs in 1 leg were 2,786 ± 1,160 and 8,116 ± 1,909 in Wt mice and FGFR2 Tg mice, respectively. Comparing CFU-C in 1 leg, the numbers were greater in FGFR2 Tg than in Wt mice.
Reconstitution of donor hematopoietic cells after IBM-BMT
Next, we examined the hematopoietic function of c-kit+Sca-1+lineage− BMCs from FGFR2 Tg mice in vivo. Irradiated B6-ly5.1 mice were transplanted with c-kit+Sca-1+lineage− BMCs from FGFR2 Tg mice or Wt mice by IBM-BMT. Peripheral blood was collected from the recipient mice 4 weeks later. Mice transplanted with the c-kit+Sca-1+lineage− BMCs from FGFR2 Tg mice showed a higher reconstitution of donor-type hematopoietic cells, as shown in Fig. 3. Donor hematopoietic cells were dominant not only in total WBCs (CD45+ cells) but also in various lineages in the peripheral blood. A similar result was obtained when the mice were analyzed 6 months after IBM-BMT (data not shown).
FIG. 3.
The c-kit+Sca-1+lineage− BMCs from FGFR2 Tg mice facilitate dominant donor hematopoiesis. c-kit+Sca-1+lineage− cells were prepared from the BMCs of Wt mice and FGFR2 Tg mice using magnetic beads and a fluorescence-activated cell sorter, and transplanted into irradiated B6-ly5.1 mice. One month after the transplantation, peripheral blood was obtained from the mice. Donor hematopoietic cells were detected with expression of both CD45.2 (ly5.2) and CD45. Percentages of donor nuclear cells and various donor-derived lineage cells were examined in the peripheral blood. n = 4. Representative data are shown from 2 independent experiments. BMCs, bone marrow cells; FGFR, fibroblast growth factor receptor; Wt, wild type. *, p < 0.05.
Reduction of apoptosis in c-kit+Sca-1+lineage− BMCs in FGFR2 Tg mice
We examined the mechanisms underlying the better reconstitution of the hematopoietic cells of FGFR2 Tg mice. First, we cultured purified c-kit+Sca-1+lineage− BMCs in RPMI 1640 containing 10% fetal calf serum for 24 h and estimated the number of live cells using tetracolor-one, since the chromogenic reaction of tetracolor-one positively correlates with cell numbers [23]. As shown in Fig. 4A, the number of live cells in FGFR2 Tg mice was significantly higher than in Wt mice, suggesting that FGFR2 signaling can accelerate cell proliferation, or can suppress apoptosis, or both. The percentages of proliferative cells and apoptotic cells in c-kit+Sca-1+lineage− BMCs were determined 1 day after culture. Proliferative cells were detected by examining the cells expressing PCNA, since PCNA is reported to express in proliferating cells [24]. Apoptotic cells were detected using annexin V, which can bind to the phosphatidylserine expressed on the surface of apoptotic cells [25]. As shown in Fig. 4B, the c-kit+Sca-1+lineage− BMCs from FGFR2 Tg mice showed more PCNA+ cells than those from Wt mice. However, there was statistically no significant difference in PCNA+ cells between FGFR2 Tg mice and Wt mice. In contrast, there were significantly fewer apoptotic c-kit+Sca-1+lineage− BMCs in FGFR2 Tg mice than in Wt mice, which suggests that the c-kit+Sca-1+lineage− BMCs of FGFR2 mice are resistant to apoptosis, resulting in better hematopoietic activity compared with those of Wt mice. We also cultured the BMCs of FGFR2 mice with or without PI-3-kinase inhibitor for 12 h and analyzed the percentages of apoptotic cells. The percentages of apoptotic cells in the c-kit+Sca-1+lineage− BMCs increased in the culture condition with the PI-3-kinase inhibitor, suggesting that this inhibitor can disable the function of FGFR2 signaling, as we previously described [16].
FIG. 4.
The c-kit+Sca-1+lineage− BMCs from FGFR2 Tg mice are more resistant to apoptosis than those from Wt mice. (A) c-kit+Sca-1+lineage− cells were prepared from the BMCs of Wt mice and FGFR2 Tg mice using magnetic beads and a fluorescence-activated cell sorter. The cells were cultured for 24 h and the number of live cells examined with tetracolor-one. (B, C) BMCs from Wt mice and FGFR2 Tg mice were cultured for 24 h. c-kit/Sca-1/Lin/PCNA (B) or c-kit/Sca-1/Lin/annexin V (C) were stained to examine the expression of PCNA (B) or annexin V (C) in c-kit+Sca-1+lineage− BMCs. BMCs of Wt mice and FGFR2 Tg mice were also cultured with or without PI-3-kinase (LY294002, 25 μM) for 12 h. The percentages of annexin V+ cells were determined to detect apoptotic cellsin the c-kit+Sca-1+lineage− BMCs. Representative data are shown from 2 independent experiments. n = 4. BMCs, bone marrow cells; FGFR, fibroblast growth factor receptor; PCNA, proliferating cell nuclear antigen; Wt, wild type; PI, phosphatidyl inositol. *, p < 0.05.
Discussion
In this study, we have shown that the c-kit+Sca-1+lineage− BMCs of FGFR2 Tg mice have greater resistance to apoptosis and thereby facilitate hematopoiesis after IBM-BMT.
IBM-BMT has various benefits compared with conventional BMT. For instance, IBM-BMT can facilitate donor hematopoiesis and reduce the severity of graft-versus-host disease [20,26–28], based on which it has recently been attempted in humans [29]. The interaction of HSCs and stromal cells is crucial for the maintenance and proliferation of hematopoiesis [30,31]. The stromal cells form a niche that an support and regulate the maintenance, proliferation, and differentiation of HSCs. IBM injection of donor BMCs is an ideal method for setting them within the niche, and was therefore performed here.
In the present study, there were no significant differences in the CBCs, percentages of respective fractions of the peripheral blood, cell numbers of the spleen, cell numbers of the BM, or populations of the BM between Wt mice and FGFR2 Tg mice, even though FGFR2 was constitutively activated in the cells expressing Tie2, which is expressed on immature hematopoietic cells. This suggests that hematopoiesis in FGFR2 Tg mice is regulated normally. In the day-7 CFU-C assays, whole BMCs from FGFR2 Tg mice formed greater number of colonies, but there were no significant differences between the BMCs of Wt mice and FGFR2 Tg mice (Fig. 2).The c-kit+Sca-1+lineage− BMCs from FGFR2 Tg mice formed significantly more colonies (Fig. 3) and also showed better hematopoietic reconstitution ability (data not shown) than those from Wt mice. These results suggest that the c-kit+Sca-1+lineage− BMCs from FGFR2 Tg mice have better reconstitution capacity not only in the short term but also in the long term. The proliferation assay and the assay for apoptosis revealed that the c-kit+Sca-1+lineage− BMCs of FGFR2 Tg mice are more resistant to apoptosis than those of Wt mice.
This study also showed that signaling through PI-3-kinase is crucial for the antiapoptotic effects of FGFR2 Tg mice, since these effects were suppressed by the PI-3-kinase inhibitor LY294002, as we previously described [16]. The effects of FGFR on cell proliferation are controversial. It has been reported that FGFR can transmit the signal for cell proliferation via the mitogen-activated protein (MAP)-kinase pathway but can transmit negative signals for cell proliferation through Sprouty, which inhibits the FGFR-stimulated rat sarcoma/microtubule affinity regulating kinase (Ras/MARK) pathway [32,33]. Consistent with the above results, this suggests that the mechanisms underlying the resistance to apoptosis in BMCs are similar to those in endothelial cells of FGFR2 Tg mice.
Our data suggest that the overexpression of FGFR2 is useful for BMT. However, its application in human beings requires a more efficient method for the transfection of the FGFR2 gene construct to the HSCs safely and securely. We have shown that the expression of FGFR2 in c-kit+Sca-1+lineage− BMCs is higher than in lineage+ BMCs in FGFR2 Tg mice, and that FGFR2 is expressed in c-kit+Sca-1+lineage− BMCs even in Wt mice. Thus, the stimulation of FGFR2 has some effect on the c-kit+Sca-1+lineage− BMCs even in normal mice. It has been reported that FGFs can sustain the proliferation of hematopoietic progenitor cells, maintaining their primitive phenotype [34,35], and can induce myelopoiesis [36], megakaryocytopoiesis [37], and erythropoiesis [38]. Therefore, if the expression of FGFR2 on HSCs can be upregulated in one way or another, FGFs could be used more effectively to accelerate hematopoiesis.
Tie2 is expressed not only on endothelial cells but also on c-kit+Sca-1+lineage− BMCs [14]. In the FGFR2 Tg mice, the promoter of Tie2 regulates the expression of FGFR2: the c-kit+Sca-1+lineage− BMCs expressing Tie2 produce constitutively activated FGFR2. Even though the percentages of Tie2+ cells in the c-kit+Sca-1+lineage− BMCs of Wt mice and FGFR2 Tg mice did not differ significantly, the c-kit+Sca-1+lineage− BMCs from FGFR2 Tg mice were better facilitated than those from Wt mice or the whole BMCs from FGFR2 Tg mice. This could be attributed to the expression of FGFR2, which suppresses apoptosis, resulting in better reconstitution of c-kit+Sca-1+lineage− BMCs of FGFR2 Tg mice than those of Wt mice.
In this study, we have shown that the c-kit+Sca-1+lineage− BMCs of FGFR2 mice are more resistant to apoptosis than those of Wt mice. However, hematopoiesis in FGFR2 Tg mice is normally regulated under normal conditions. When rapid hematopoiesis is required, the c-kit+Sca-1+lineage− BMCs of FGFR2 Tg mice show high hematogenic function, resulting in rapid recovery of donor hematopoietic cells after BMT. Since these phenomena are convenient and suitable for BMT, an efficient and safe method to transfect the FGFR2 gene construct to HSCs would be highly desirable.
Acknowledgments
We thank Ms. Y. Tokuyama, Ms. K. Hayashi, and Ms. A. Kitajima for their expert technical assistance, and also Mr. Hilary Eastwick-Field and Ms. K. Ando for helping with the preparation of this manuscript. This study was supported by grants from the “21st Century COE Program” of the Ministry of Education, Culture, Sports, Science and Technology of Japan; the Department of Transplantation for Regeneration Therapy (sponsored by Otsuka Pharmaceutical Co., Ltd.); the Molecular Medical Science Institute, Otsuka Pharmaceutical Co., Ltd. (Tokyo, Japan); and Japan Immunoresearch Laboratories Co., Ltd. (Takasaki, Gunnma, Japan); a Grant-in-Aid for Scientific Research (C) 21590447; and an award from Dr. Toshiko Kitanishi.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Basilico C. Moscatelli D. The FGF family of growth factors and oncogenes. Adv Cancer Res. 1992;59:115–165. doi: 10.1016/s0065-230x(08)60305-x. [DOI] [PubMed] [Google Scholar]
- 2.Baird A. Fibroblast growth factors: activities and significance of non-neurotrophin neurotrophic growth factors. Curr Opin Neurobiol. 1994;4:78–86. doi: 10.1016/0959-4388(94)90035-3. [DOI] [PubMed] [Google Scholar]
- 3.Krejci P. Prochazkova J. Bryja V. Kozubik A. Wilcox WR. Molecular pathology of the fibroblast growth factor family. Hum Mutat. 2009;30:1245–1255. doi: 10.1002/humu.21067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fischer S. Draper BW. Neumann CJ. The zebrafish fgf24 mutant identifies an additional level of Fgf signaling involved in vertebrate forelimb initiation. Development. 2003;130:3515–3524. doi: 10.1242/dev.00537. [DOI] [PubMed] [Google Scholar]
- 5.Böttcher RT. Niehrs C. Fibroblast growth factor signaling during early vertebrate development. Endocr Rev. 2005;26:63–77. doi: 10.1210/er.2003-0040. [DOI] [PubMed] [Google Scholar]
- 6.Braun S. auf dem Keller U. Steiling H. Werner S. Fibroblast growth factors in epithelial repair and cytoprotection. Philos Trans R Soc Lond B Biol Sci. 2004;359:753–757. doi: 10.1098/rstb.2004.1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ziche M. Donnini S. Morbidelli L. Development of new drugs in angiogenesis. Curr Drug Targets. 2004;5:485–493. doi: 10.2174/1389450043345371. [DOI] [PubMed] [Google Scholar]
- 8.Powers CJ. McLeskey SW. Wellstein A. Fibroblast growth factors, their receptors and signaling. Endocr Relat Cancer. 2000;7:165–197. doi: 10.1677/erc.0.0070165. [DOI] [PubMed] [Google Scholar]
- 9.Wilson EL. Rifkin DB. Kelly F. Hannocks MJ. Gabrilove JL. Basic fibroblast growth factor stimulates myelopoiesis in long-term human bone marrow cultures. Blood. 1991;77:954–960. [PubMed] [Google Scholar]
- 10.Kashiwakura I. Takahashi TA. Fibroblast growth factor and ex vivo expansion of hematopoietic progenitor cells. Leuk Lymphoma. 2005;46:329–333. doi: 10.1080/10428190400019958. [DOI] [PubMed] [Google Scholar]
- 11.Allouche M. Bikfalvi A. The role of fibroblast growth factor-2 (FGF-2) in hematopoiesis. Prog Growth Factor Res. 1995;6:35–48. doi: 10.1016/0955-2235(95)00041-0. [DOI] [PubMed] [Google Scholar]
- 12.Dumont DJ. Yamaguchi TP. Conlon RA. Rossant J. Breitman ML. tek, a novel tyrosine kinase gene located on mouse chromosome 4, is expressed in endothelial cells and their presumptive precursors. Oncogene. 1992;7:1471–1480. [PubMed] [Google Scholar]
- 13.Sato TN. Qin Y. Kozak CA. Audus KL. Tie-1 and tie-2 define another class of putative receptor tyrosine kinase genes expressed in early embryonic vascular system. Proc Natl Acad Sci U S A. 1993;90:9355–9358. doi: 10.1073/pnas.90.20.9355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arai F. Hirao A. Ohmura M. Sato H. Matsuoka S. Takubo K. Ito K. Koh GY. Suda T. Tie2/angiopoietin-1 signaling regulates hematopoietic stem cell quiescence in the bone marrow niche. Cell. 2004;118:149–161. doi: 10.1016/j.cell.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 15.Arai F. Suda T. Maintenance of quiescent hematopoietic stem cells in the osteoblastic niche. Ann N Y Acad Sci. 2007;1106:41–53. doi: 10.1196/annals.1392.005. [DOI] [PubMed] [Google Scholar]
- 16.Matsunaga S. Okigaki M. Takeda M. Matsui A. Honsho S. Katsume A. Kishita E. Jishan C. Kurihara T. Adachi Y. Mansukhani A. Kobara M. Matoba Y. Tatsumi T. Matsubara H. Endothelium-targeted overexpression of constitutively active FGF receptor induces cardioprotection in mice myocardial infarction. J Mol Cell Cardiol. 2009;46:663–673. doi: 10.1016/j.yjmcc.2009.01.015. [DOI] [PubMed] [Google Scholar]
- 17.Adachi Y. Taketani S. Oyaizu H. Ikebukuro K. Tokunaga R. Ikehara S. Apoptosis of colorectal adenocarcinoma induced by 5-FU and/or IFN-gamma through caspase 3 and caspase 8. Int J Oncol. 1999;15:1191–1196. doi: 10.3892/ijo.15.6.1191. [DOI] [PubMed] [Google Scholar]
- 18.Koike-Kiriyama N. Adachi Y. Minamino K. Iwasaki M. Nakano K. Koike K. Yamada H. Mukaide H. Shigematsu A. Mizokami T. Matsumura M. Ikehara S. Human cord blood cells can differentiate into retinal nerve cells. Acta Neurobiol Exp. 2007;67:359–365. doi: 10.55782/ane-2007-1653. [DOI] [PubMed] [Google Scholar]
- 19.Sugihara A. Adachi Y. Inaba M. Hisha H. Sugiura K. Miyashima S. Amoh Y. Taketani S. Oyaizu H. Ikebukuro K. Kawamura M. Genba H. Horio T. Ikehara S. Age-dependent abnormalities of hematopoietic stem cells in (NZW x BXSB)F1 mice. Stem Cells. 1999;17:357–365. doi: 10.1002/stem.170357. [DOI] [PubMed] [Google Scholar]
- 20.Kushida T. Inaba M. Hisha H. Ichioka N. Esumi T. Ogawa R. Iida H. Ikehara S. Intra-bone marrow injection of allogeneic bone marrow cells: a powerful new strategy for treatment of intractable autoimmune diseases in MRL/lpr mice. Blood. 2001;97:3292–3299. doi: 10.1182/blood.v97.10.3292. [DOI] [PubMed] [Google Scholar]
- 21.Dexter TM. Spooncer E. Simmons P. Allen TD. Long-term marrow culture: an overview of techniques and experience. Kroc Found Ser. 1984;18:57–96. [PubMed] [Google Scholar]
- 22.Okada S. Nakauchi H. Nagayoshi K. Nishikawa S. Miura Y. Suda T. In vivo and in vitro stem cell function of c-kit- and Sca-1-positive murine hematopoietic cells. Blood. 1992;80:3044–3050. [PubMed] [Google Scholar]
- 23.Yamamoto O. Hamada T. Tokui N. Sasaguri Y. Comparison of three in vitro assay systems used for assessing cytotoxic effect of heavy metals on cultured human keratinocytes. J UOEH. 2001;23:35–44. doi: 10.7888/juoeh.23.35. [DOI] [PubMed] [Google Scholar]
- 24.Garcia RL. Coltrera MD. Gown AM. Analysis of proliferative grade using anti-PCNA/cyclin monoclonal antibodies in fixed, embedded tissues. Comparison with flow cytometric analysis. Am J Pathol. 1989;134:733–739. [PMC free article] [PubMed] [Google Scholar]
- 25.van Engeland M. Nieland LJ. Ramaekers FC. Schutte B. Reutelingsperger CP. Annexin V-affinity assay: a review on an apoptosis detection system based on phosphatidylserine exposure. Cytometry. 1998;31:1–9. doi: 10.1002/(sici)1097-0320(19980101)31:1<1::aid-cyto1>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 26.Ikehara S. New strategies for BMT, organ transplantation, and regeneration therapy. Hematology. 2003;8:77–81. doi: 10.1080/1024533031000084240. [DOI] [PubMed] [Google Scholar]
- 27.Ikebukuro K. Adachi Y. Suzuki Y. Iwasaki M. Nakano K. Koike Y. Mukaide H. Yamada Y. Fujimoto S. Seino Y. Oyaizu H. Shigematsu A. Kiriyama N. Hamada Y. Kamiyama Y. Ikehara S. Synergistic effects of injection of bone marrow cells into both portal vein and bone marrow on tolerance induction in transplantation of allogeneic pancreatic islets. Bone Marrow Transplant. 2006;38:657–664. doi: 10.1038/sj.bmt.1705500. [DOI] [PubMed] [Google Scholar]
- 28.Suzuki Y. Adachi Y. Minamino K. Zhang Y. Iwasaki M. Nakano K. Koike Y. Ikehara S. A new strategy for treatment of malignant tumor: intra-bone marrow-bone marrow transplantation plus CD4- donor lymphocyte infusion. Stem Cells. 2005;23:365–370. doi: 10.1634/stemcells.2004-0258. [DOI] [PubMed] [Google Scholar]
- 29.Frassoni F. Gualandi F. Podestà M. Raiola AM. Ibatici A. Piaggio G. Sessarego M. Sessarego N. Gobbi M. Sacchi N. Labopin M. Bacigalupo A. Direct intrabone transplant of unrelated cord-blood cells in acute leukaemia: a phase I/II study. Lancet Oncol. 2008;9:831–839. doi: 10.1016/S1470-2045(08)70180-3. [DOI] [PubMed] [Google Scholar]
- 30.Mauch P. Greenberger JS. Botnick L. Hannon E. Hellman S. Evidence for structured variation in self-renewal capacity within long-term bone marrow cultures. Proc Natl Acad Sci U S A. 1980;77:2927–2930. doi: 10.1073/pnas.77.5.2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kiel MJ. Morrison SJ. Uncertainty in the niches that maintain haematopoietic stem cells. Nat Rev Immunol. 2008;8:290–301. doi: 10.1038/nri2279. [DOI] [PubMed] [Google Scholar]
- 32.LaVallee TM. Prudovsky IA. McMahon GA. Hu X. Maciag T. Activation of the MAP kinase pathway by FGF-1 correlates with cell proliferation induction while activation of the Src pathway correlates with migration. J Cell Biol. 1998;141:1647–1658. doi: 10.1083/jcb.141.7.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lao DH. Yusoff P. Chandramouli S. Philp RJ. Fong CW. Jackson RA. Saw TY. Yu CY. Guy GR. Direct binding of PP2A to Sprouty2 and phosphorylation changes are a prerequisite for ERK inhibition downstream of fibroblast growth factor receptor stimulation. J Biol Chem. 2007;282:9117–9126. doi: 10.1074/jbc.M607563200. [DOI] [PubMed] [Google Scholar]
- 34.Allouche M. Bikfalvi A. The role of fibroblast growth factor-2 (FGF-2) in hematopoiesis. Prog Growth Factor Res. 1995;6:35–48. doi: 10.1016/0955-2235(95)00041-0. [DOI] [PubMed] [Google Scholar]
- 35.Allouche M. Bayard F. Clamens S. Fillola G. Sié P. Amalric F. Expression of basic fibroblast growth factor (bFGF) and FGF-receptors in human leukemic cells. Leukemia. 1995;9:77–86. [PubMed] [Google Scholar]
- 36.Gabrilove JL. White K. Rahman Z. Wilson EL. Stem cell factor and basic fibroblast growth factor are synergistic in augmenting committed myeloid progenitor cell growth. Blood. 1994;83:907–910. [PubMed] [Google Scholar]
- 37.Chen QS. Wang ZY. Han ZC. Enhanced growth of megakaryocyte colonies in culture in the presence of heparin and fibroblast growth factor. Int J Hematol. 1999;70:155–162. [PubMed] [Google Scholar]
- 38.Koritschoner NP. Bartůnĕk P. Knespel S. Blendinger G. Zenke M. The fibroblast growth factor receptor FGFR-4 acts as a ligand dependent modulator of erythroid cell proliferation. Oncogene. 1999;18:5904–5914. doi: 10.1038/sj.onc.1202979. [DOI] [PubMed] [Google Scholar]