Abstract
The difficulty in long-term expansion of mesenchymal stem cells (MSCs) using standard culture systems without the loss of their stem cell properties suggests that a critical feature of their microenvironment necessary for retention of stem cell properties is absent in these culture systems. We report here the reconstitution of a native extracellular matrix (ECM) made by human marrow cells ex vivo, which consists of at least collagen types I and III, fibronectin, small leucine-rich proteoglycans such as biglycan and decorin, and major components of basement membrane such as the large molecular weight proteoglycan perlecan and laminin. Expansion of human MSCs on this ECM strongly promoted their proliferation, retained their stem cell properties with a low level of reactive oxygen species (ROS), and substantially increased their response to BMP-2. The quality of the expanded cells following each passage was further tested by an in vivo transplantation assay. The results showed that MSCs expanded on the ECM for multiple passages still retained the same capacity for skeletogenesis. In contrast, the bone formation capacity of cells expanded on plastic was dramatically diminished after 6–7 passages. These findings suggest that the marrow stromal cell-derived ECM is a promising matrix for expanding large-scale highly functional MSCs for eventual use in stem cell-based therapy. Moreover, this system should also be invaluable for establishment of a unique tissue-specific ECM, which will facilitate control of the fate of MSCs for therapeutic applications.
Introduction
Bone marrow mesenchymal stem cells (MSCs) are able to not only replicate to produce identical daughter stem cells, but also differentiate into many distinct cell types including osteoblasts, hematopoiesis-supporting stromal cells, adipocytes, chondrocytes, myocytes, and endothelial cells [1–3]. Because of these capabilities, MSCs are involved in tissue regeneration throughout life, and are potentially useful for the treatment of many degenerative diseases [4–6]. However, a major bottleneck in clinical application of MSCs has been their limited number, because they are rare in the primary tissue (∼0.001%) [7]. With extensive passaging, MSCs often lose multilineage differentiation potential [8–11]. Moreover, it has been reported that expansion of human and mouse MSCs is accompanied by cellular senescence and outgrowth of transformed cells, though transformation is less frequent in cultured human MSCs [12–16]. Taken together, these observations suggest that a critical feature of the marrow microenvironment that facilitates retention of stem cell properties is absent in these in vitro culture systems.
Studies in other regenerating tissues (such as hair follicle stem cells, hematopoietic stem cells, and intestinal stem cells) have led to the concept that a tissue-specific microenvironment or niche maintains stem cell self-renewal capacity while facilitating production of mature progeny, and that the extracellular matrix (ECM) constitutes an important component of the niche [17,18]. Recently, we reported that the ECM prepared from mouse marrow stromal cells significantly promoted proliferation of mouse MSCs, preserved their stem cell properties, and enhanced their capacity for skeletogenesis [19]. One potential mechanism for the retention of MSC properties may be the ability of the ECM to sequester endogenously produced growth-promoting factors such as BMP-2 [19].
In order to obtain the applicability of results for eventual therapeutic application, we have extensively investigated the ability of a cell-free human bone marrow-derived ECM to support human MSC behavior and displayed a comprehensive picture of the changes wrought by the ECM. The present study strongly suggests that the ECM-based culture system provides an ideal environment for large-scale expansion of highly functional MSCs for eventual use in stem cell-based therapy.
Materials and Methods
Cells
Freshly isolated human bone marrow mononuclear cells obtained from 20- to 30-year-old donors were purchased from ALLCELLS (Emeryville, CA), and grown on tissue culture plastic at an initial seeding of 3 × 105 cells/cm2 until 70% confluence (2–3 weeks) in the expansion medium [α-MEM (Life Technologies, Grand Island, NY), glutamine (2 mM), penicillin (100 U/mL), streptomycin (100 μg/mL; Biofluids, Rockville, MD), and 15 % preselected fetal bovine serum (FBS; Becton Dickinson, Franklin Lakes, NJ)]. After washing with phosphate-buffered saline (PBS) to remove non-adherent cells, the adherent cells, considered as passage 1, were detached by trypsin treatment (0.02% for 2 min at 37°C), and collected for storage or directly used for the establishment of ECM or the investigation of the behavior of MSCs maintained on the various substrata.
Preparation of cell-free ECM from cultured bone marrow cells, and tissue culture plates coated with fibronectin or collagen type I
A standard procedure based on our previous studies was utilized [19]. Cells from passages 1 or 2 were seeded onto tissue culture plastic at 1 × 104 cells/cm2, and cultured for 15 days. The medium was changed every 3–4 days, and ascorbic acid (50 μM) was added during the final 8 days of culture. After extensive washing with PBS, cells were removed by incubation with 0.5% Triton X-100 containing 20 mM NH4OH in PBS for 5 min at room temperature. After washing with PBS 4 times, PBS containing 50 μg/mL gentamicin and 0.25 μg/mL fungizone was added to the plates, which were stored at 4°C for up to 4 months. Tissue culture plates coated with fibronectin or collagen type I were prepared as previously described [20].
Scanning electron microscopy (SEM)
Cultures seeded onto coverslips coated with or without the ECM were washed 3 times with PBS and fixed with 2% glutaraldehyde in 0.1 M sodium cacodylate buffer (pH 7.2) for 1 h and then transferred to 0.1 M cacodylate buffer solution. The specimens were dehydrated in ascending concentrations of ethanol (from 70% to 100%). After dehydration, the coverslips were attached to a stub and sputtered with gold–palladium. The specimens were examined using an EVO-50EP SEM manufactured by Carl-Zeiss SMT.
Immunohistochemistry
Stromal cell-derived ECM was fixed for 30 min with 4% formaldehyde in PBS at room temperature, washed with PBS, and blocked with 5% normal goat serum containing 0.1% BSA in PBS for 1 h. The matrices were then incubated with the relevant primary antibodies (1:10 dilution; Santa Cruz Biotechnology, Santa Cruz, CA) in 2% goat serum for 2 h. Non-specific isotype IgG (1:10 dilution) was used as a negative control. After washing with PBS, samples were incubated with the appropriate FITC-conjugated second antibody and washed. Specimens were mounted using DAPI containing mounting medium (Vector Laboratories, Burlingame, CA), and visualized using a FV500 Fluoview Confocal Microscope equipped with image analysis software to quantify fluorescence intensity in a given region of interest.
Determination of colony-forming unit fibroblasts (CFU-F), osteoblasts (CFU-OB), and adipocytes (CFU-AD)
Freshly isolated human bone marrow mononuclear cells obtained from 20- to 30-year-old donors were plated into 6-well plates uncoated or coated with the indicated matrices at 3 × 104 cells/cm2, incubated for 4 h at 37°C, and washed twice with PBS to remove non-adherent cells. Then, the cells were cultured to generate CFU-F colonies in the expansion medium. After 14 days of culture, CFU-F colonies were visualized with crystal violet staining. To assess CFU-OB colony formation, CFU-F colonies were maintained for an additional 25 days in osteoblast differentiation medium [expansion medium supplemented with 10−7 M dexamethasone (Sigma) and 10−4 M l-ascorbate-2-phosphate (Wako Chemicals, Richmond, VA)]. The CFU-OB colonies were detected by von Kossa staining. To assess CFU-AD colony formation, CFU-F colonies were maintained for an additional 10 days in adipogenic medium (DMEM containing 10% FBS, 0.5 mM IBMX, 10−6 M dexamethasone, 10 μM insulin, 200 μM indomethacin) [21]. CFU-AD colonies were visualized with Oil Red O staining. Average size and intensity of CFU-F and CFU-AD colonies were quantified using the NIH ImageJ program. Osteocalcin secretion in the supernatant collected from the primary CFU-OB assay before von Kossa staining was measured using Metra Osteocalcin EIA kit (QUIDEL Corporation, San Diego, CA) following the manufacturer's instructions.
MSC self-renewal was determined by the replication assay as described previously [19,22]. Basically, MSCs were subcultured on ECM or plastic for serial passages, and colony assay was performed separately on plastic following each passage. Since freshly isolated human bone marrow cells maintained on the ECM proliferated considerably faster (∼10 days for the cells reached to confluence) than those grown on plastic (∼20 days for the cells reached to confluence), comparative replication assays could not be carried out at the same time following the primary culture. Thus, we used the pre-cultured cells on plastic (passage 2 or 3) as the starting cell population. Aliquots (2 × 105 cells) of passage 3 (P3) human bone marrow cells, which we also used to determine the initial numbers of CFUs including CFU-F, CFU-AD, and CFU-OB, were seeded onto 100-mm plastic or plastic coated with the ECM. After 7 days of culture (70%–90% confluent, P4), the cells were detached from the various substrata, counted, and then reseeded on plastic separately for determination of CFUs. The remaining P4 cells were replated onto 100-mm plastic or plastic coated with the ECM at the same starting density of 2 × 105 cells. After 7 days of culture (P5), the cells were detached and CFUs determined. Subsequent serial passages were obtained by repeating the same procedure as with P4. The number of CFUs following each passage was determined as previously described [19]. MSC replication was expressed by the fold change in CFUs during the expansion [total number of CFUs obtained from P(n) divided by total number of CFUs obtained from P(n−1), where n is the number of passages].
Flow cytometry
Single-cell suspensions (1–2 × 106) were incubated in 100 μL of diluted anti-SSEA-4 antibodies (10 μg/mL; R&D Systems, Minneapolis, MN) for 30 min at 4°C. The stained cells were washed twice in staining buffer (PBS containing 5% FCS and 0.01% sodium azide) and incubated in 20 μg/mL of FITC-conjugated goat anti-mouse IgG for 20 min at 4°C. The cells were then washed twice with staining buffer and either immediately analyzed or fixed with 1% paraformaldehyde in PBS and analyzed within 96 h using a Becton Dickinson FACStarplus flow cytometer with 10,000 events, collected for each sample and the percentage of positively stained cells determined from fluorescence-activated cell sorting (FACS). Cells were stained with isotype IgG as a negative control. To access MSCs enriched in SSEA-4+ cell population, both SSEA-4+ and SSEA-4− cells were sorted separately from primary human bone marrow cell culture.
Measurements of intracellular reactive oxygen species (ROS) and telomerase activity
Intracellular reactive oxygen species (ROS) generation was measured with 2′,7′-dichlorodihydrofluorescein diacetate (H2DCFDA) using a ROS Assay Kit (Invitrogen, Eugene, Oregon) following the manufacturer's recommendations. ROS levels were expressed as arbitrary units (AU) of DCF fluorescence per 105 cells. Telomerase activity was measured using the quantitative telomerase detection kit (Allied Biotech, Inc., Twinsburg, OH) according to manufacturer's instructions. A breast cancer cell line (MDA231) served as a positive control and human red blood cells were used as a negative control. Experiments were performed in triplicate, and telomerase levels were expressed as attomoles (amoles) per 2 × 105 cells.
Quantification of osteocalcin and bone sialoprotein gene expression in response to BMP-2
Human bone marrow cells (passage 2) were cultured on plastic with or without the ECM in the expansion medium for 10 days, and then cultured in osteoblast differentiation medium with 2% FBS overnight and treated with BMP-2 in various doses for 3 days. Total RNA was extracted and reverse-transcribed using a High Capacity cDNA Archive Kit (Applied Biosystems, Foster City, CA). The transcripts of interest were amplified from cDNA by real-time PCR using TaqMan Universal PCR Master Mix and Assay Demand or Assay by Design primer and probe sets (Applied Biosystems, Foster City, CA). Amplification and detection were carried out with an ABI 7500 Real-Time PCR System (Applied Biosystems). Gene expression was quantified by subtracting the GAPDH threshold cycle (Ct) value from the Ct value of the gene of interest, and expressed as 2−ΔCt.
Microarray and data analysis
SSEA-4+ cells were isolated from primary human bone marrow cell culture using FACS sorting and cultured separately on plastic or the ECM in the expansion medium (α-MEM containing 15% FCS) for 12 days. The total RNA was isolated using UltraspecTM RNA (Biotecx, Houston, TX) according to the manufacturer's protocol. RNA was quantitated by measuring ultraviolet absorption at 260 nm and adjusted to 1 μg/μL with RNAse-free water.
In this experiment, RNA samples were collected separately from the cultured cells obtained from 15 different donors (20–30-year-old) purchased from ALLCELLS (Emeryville, CA). We used the “subpooling” approach whereby 3 subsets of RNA samples within each “Plastic” or “ECM” group were made, each subset comprising RNA pooled from 5 individuals for subsequent hybridization on one chip. This pooling strategy effectively normalizes interindividual noise while still retaining enough statistical power to identify most genes whose expression has changed during expansion of MSCs on the ECM versus plastic [23,24].
After pooling, RNA was sent to Genome Explorations (www.genome-explorations.com). There, RNA was converted to DNA and the labeled cRNA was prepared, which were hybridized onto Affymetrix Human Genome U133 Plus 2.0 chips. The chips were scanned, and data were pre-analyzed using Affymetrix MAS 5.0. Gene expression levels on “Plastic” chips (Plastic-A, Plastic-B, and Plastic-C) were compared with levels on “ECM” chips (ECM-A, ECM-B, and ECM-C) to determine expression differences between “Plastic” and “ECM” groups using the statistical program Significance Analysis of Microarrays (SAM) at a false discovery rate of 1%.
After preselecting genes with differential expression, advanced analysis including hierarchical clustering, functional classification, and reconstruction of biological pathways were performed using the software GeneSpring™ from Silicon Genetics (Redwood City, CA), and Gene Ontology (GO), a public database [25]. The genes highly associated with the functional groups were determined by Fisher's Exact Test [26], and then organized into virtual pathways using PathwayAssist 3.0 (http://www.ariadnegenomics.com) based on literature references. In order to further demonstrate the similarity with all published stem cell gene expression profiles, Gene Set Enrichment Analysis (GSEA) was used to examine a variety of data sets from the NCBI GEO database that are enriched with the same genes as expressed in our MSC gene set [27,28].
In vivo bone formation
Human bone marrow cells were cultured for 7 days on plastic or ECM for 10 passages. Following each passage, the cells (1 × 106) were loaded into a transplantation vehicle [hydroxyapatite/tricalcium phosphate (HA/TCP) ceramic powder (Zimmer Inc, Warsaw, IN), or Gelfoam (Pfizer, New York)] and transplanted subcutaneously into the dorsal surface of 10-week-old immunodeficient beige mice (NIHbg-nu-xid, Harlan Sprague Dawley, Indianapolis, IN), as previously described [29]. Three transplants were made for each pre-culture system, harvested after 8 weeks, fixed in 10% phosphate-buffered formalin at 4°C for 24 h, decalcified with 10% EDTA (pH 8.0) at room temperature for 1–2 weeks, and then embedded in paraffin. Each ossicle was bisected, and each half sectioned at 10 μm thickness at 100 μm intervals. A total of 9 hematoxylin–eosin (H&E) stained sections were used for quantification. The extent of new bone formation in the implants was histomorphometrically determined as areas measured by using ImageJ analysis software (NIH Image).
Statistical analysis
All data are presented as mean ± standard deviation calculated, with n = 3 or 6, depending on the experiments. Statistical analyses were done by using Student's t-test or one-way ANOVA with significance at P < 0.05. All the results were reproduced in at least 3 independent experiments.
Results
Preparation of a marrow stromal cell-derived ECM
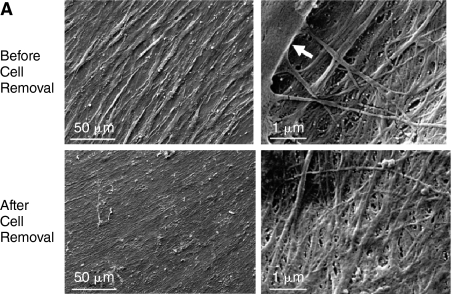
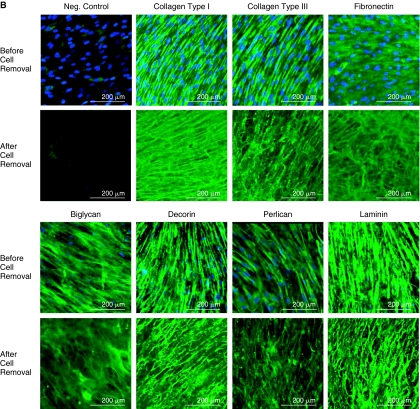
Scanning electron microscopy (SEM) revealed that stromal cells cultured from human bone marrow elaborated a fibrillar ECM (Fig. 1A). The effect of cell extraction on specific components of ECM was examined by comparing the localization of collagen types I and III, fibronectin, biglycan, decorin, perlecan, and laminin in the marrow cell-derived ECM before and after cell extraction using immuno-confocal microscopy for semiquantitative visualization (Fig. 1B). These proteins were selected because of their importance in mediating growth factors binding to the ECM and possible role in controlling MSC behavior. Collagen types I and III clearly showed a directional alignment and orientation, different from other ECM components examined, which exhibited a random distribution. Interestingly, the ECM made by the cultured stromal cells contained an abundant amount of laminin, a major component of basement membrane. Confocal microscopic analysis indicated that the ECM was ∼20 μm thick (data not shown). Cells (blue) were absent following extraction, but the protein composition of the ECM was well preserved as indicated by retention of immunostaining for all of the proteins examined.
FIG. 1.
Characteristics of human marrow stromal cell-derived extracellular matrix (ECM). (A) Scanning electron microscopy (SEM) images of stromal cell-derived ECM before and after cell removal. Left panels: low magnification; and right panels: high magnification. The structure of the ECM appeared to be similar before and after cell removal. The arrow denotes a cell. (B) Confocal fluorescence images showing localization of collagen types I and III, fibronectin, biglycan, decorin, perlecan, and laminin in the ECM elaborated by human bone marrow stromal cells before and after cell removal. The distribution of cells was visualized with DAPI staining (blue), and matrix proteins by immunofluorescence (green).
Marrow stromal cell-derived ECM enhances colony formation of human MSCs
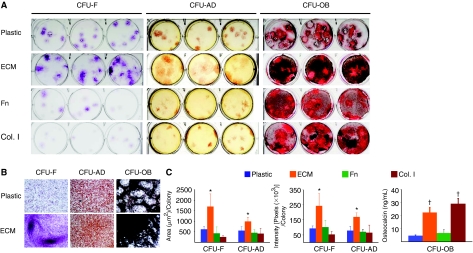
MSCs were detected and quantified by their ability to form a colony of fibroblastic cells [18]. These colony-forming cells, termed colony-forming unit fibroblasts (CFU-F), are comprised of MSCs as well as the transit amplifying progeny of MSCs [22]. The ability of MSCs to differentiate into adipocytes or osteoblasts in response to specific differentiation medium was examined by measuring CFU-adipocytes (CFU-AD) and CFU-osteoblasts (CFU-OB), respectively. When cultured on marrow stromal cell-derived ECM, MSCs developed larger and denser CFU-F, CFU-AD, and CFU-OB than those cultured on tissue culture plastic, or plastic pre-coated with fibronectin or collagen type I (Fig. 2A). Microscopic analysis revealed that CFU-F, CFU-AD, and CFU-OB on the ECM contained more methyl violet-stained fibroblast-like cells, more Oil Red O-stained adipocytes (shown in red), and more von Kossa staining for mineral (shown in dark), respectively, as compared to those on plastic (Fig. 2B). ImageJ-based histomorphometry was used to estimate the average size (number of pixels) and density (pixel intensity) of CFU-F and CFU-AD, and osteocalcin secretion in medium was measured for CFU-OB. The 2- to 4-fold increases were seen in the size and density of CFU-F and CFU-AD cultures on the ECM, as compared to the other matrices (Fig. 2C, left and middle panels). Osteocalcin protein secreted by CFU-OB cultured on the ECM or on collagen type I-coated plastic was ∼4-fold greater than those cultured on the uncoated or fibronectin-coated plastic (Fig. 2C, right panel). However, there was no significant difference in the levels of osteocalcin produced by CFU-OB developed on the ECM versus collagen type I-coated plastic.
FIG. 2.
Stromal cell-derived extracellular matrix (ECM) enhances human mesenchymal stem cells (MSCs) in colony formation. (A) The appearance of CFU-F, CFU-AD, and CFU-OB colonies generated on the various substrata. Freshly isolated human bone marrow mononuclear cells were placed into uncoated plastic (Plastic), or plastic coated with a cell-free ECM (ECM), fibronectin (Fn), or collagen type I (Col. I) at 3 × 105 cells per 10 cm2 area. After 24 h of incubation, non-adherent cells were removed and cultures maintained in α-MEM containing 15% FBS. After 14 days of culture, CFU-F colonies were visualized with crystal violet staining. CFU-OB colonies were generated by cells cultured in osteoblast differentiation medium. After 25 days of culture, CFU-OB colonies were visualized with von Kossa staining. CFU-adipocytes (CFU-AD) colonies were formed by cells cultured in adipogenic medium. After 10 days of culture, CFU-AD colonies were visualized with Oil Red O staining. (B) Microscopic views of CFU-F, CFU-AD, and CFU-OB colonies formed on plastic or on the ECM. Original magnification: 100×. (C) Quantification of average size and intensity per colony formed on the various substrata using the ImageJ program. Osteocalcin secretion in supernatant collected from CFU-OB was measured using a Metra Osteocalcin EIA kit (QUIDEL Corporation, San Diego, CA). *P < 0.05, n = 3 vs. plastic or plastic coated with fibronectin (Fn) or collagen type I (Col. I). †P < 0.05, n = 3 vs. plastic or plastic coated with Fn.
Marrow stromal cell-derived ECM promotes human MSC proliferation and suppresses reactive oxygen species (ROS)
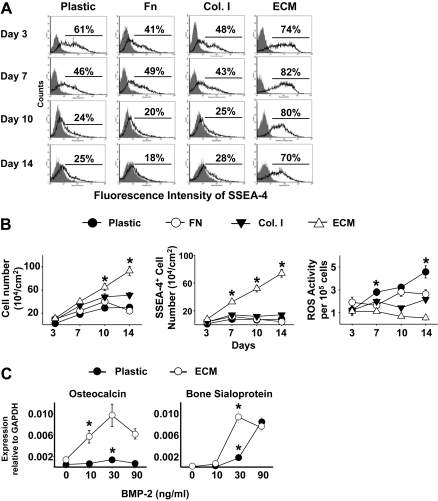
Human bone marrow cells (passage 2) were seeded onto plastic with or without ECM, or onto plastic coated with fibronectin or collagen type I at ∼5,000 cells/cm2, and maintained in the expansion medium for up to 14 days. The proportion of human MSCs in the cultures after 3, 7, 10, and 14 days was determined by flow cytometric analysis, on the basis of positive staining for SSEA-4 (Stage-specific Embryonic Antigen-4), which was originally identified as an early embryonic glycolipid antigen [30], but also shown to identify human MSCs from bone marrow [31]. We found that the percentage of SSEA-4+ cells progressively decreased during 14 days of culture on plastic, and on plastic coated with either fibronectin or collagen type I (Fig. 3A). In contrast, the ECM retained 70%–82% of SSEA-4+ cells during the entire 14 days of culture. The number of cells grown on plastic, or plastic coated with either fibronectin or collagen type I, reached a plateau at day 10, while the number of cells grown on ECM continued to increase during 14 days of culture (Fig. 3B, left panel). More importantly, the increase in the number of SSEA-4+ cells was ∼7- to 10-fold more when cells were cultured on ECM than on other substrata at day 14 of cultures (Fig. 3B, middle panel). Strikingly, the intracellular level of reactive oxygen species (ROS) was significantly lower in cells maintained on ECM than in cells maintained on other substrata (Fig. 3B, right panel).
FIG. 3.
Stromal cell-derived extracellular matrix (ECM) promotes human mesenchymal stem cell (MSC) proliferation and suppresses reactive oxygen species (ROS) formation. (A) Flow cytometric analysis of SSEA-4 expression by human MSCs from passage 2. Single-cell suspensions derived from cultures on uncoated plastic (Plastic), a cell-free ECM, or fibronectin (Fn), or collagen type I (Col. I) for various days were analyzed by fluorescence-activated cell sorting (FACS). Cells stained with primary nonspecific antibody (isotype, IgG) served as negative controls (gray peaks). (B) SSEA-4 and ROS analysis. Other cell aliquots were used to determine cell number (left panel), the number of SSEA-4+ cells (middle panel), and ROS content (right panel) expressed as arbitrary units (AU) of DCF fluorescence per 105 cells. *P < 0.05, n = 3 vs. plastic, plastic coated with fibronectin (Fn) or collagen type I (Col. I) at the same time point. (C) Enhanced BMP-2 responsiveness of MSCs cultured on ECM. Cells were cultured on ECM or uncoated plastic (Plastic) or plastic coated with fibronectin (Fn) or collagen type I (Col. I) in the expansion medium for 10 days, and then cultured in osteoblast differentiation medium with 2% fetal bovine serum (FBS) overnight and then treated for 3 days with varying doses of BMP-2, as indicated. Gene expression of osteocalcin and bone sialoprotein was determined by quantitative RT-PCR (TaqMan). n = 3; *P < 0.05, value at the lowest dose needed for the stimulation vs. vehicle control.
We next examined whether cells grown on ECM retained their osteoblastogenic response to BMP-2 stimulation. BMP-2 was added at day 10 of culture when ECM and plastic with or without pre-coating fibronectin or collagen type I retained ∼80% or ∼24% of SSEA-4+ cells, respectively (Fig. 3A). The cells on the ECM required as little as 10 ng/mL BMP-2 to stimulate osteocalcin expression with a ∼5-fold increase, reaching a peak with a ∼25-fold increase when the dose was increased to 30 ng/mL (Fig. 3C). In contrast, the cells cultured on plastic or plastic coated with fibronectin or collagen type I required 30 ng/mL BMP-2 to stimulate osteocalcin expression, exhibiting a small peak with a ∼5- to 10-fold increase (Fig. 3C). The patterns of bone sialoprotein expression in response to BMP-2 were very similar to those of osteocalcin when cells were maintained on the ECM versus plastic or plastic coated with fibronectin or collagen type I. The levels of bone sialoprotein expressed by cells maintained on the ECM were ∼6- to 8-fold higher than those maintained on plastic as well as plastic coated with fibronectin or collagen type I when treated with 30 ng/mL BMP-2 (Fig. 3C).
Marrow stromal cell-derived ECM retains SSEA-4+ cells and enrich colony-forming cells
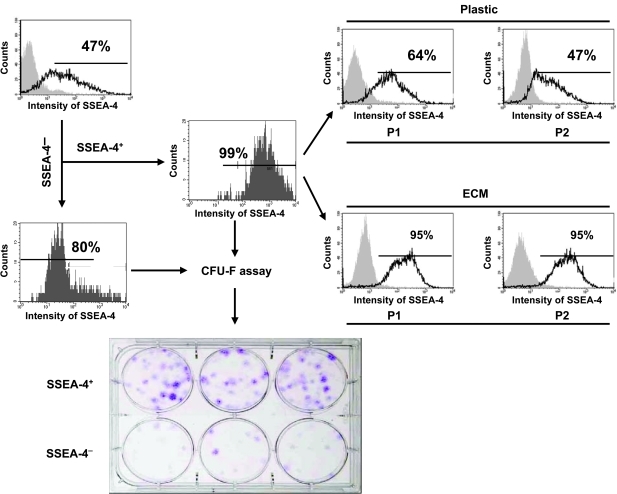
To further access MSCs enriched in SSEA-4+ cell population, we sorted both SSEA-4+ cells and SSEA-4− cells from primary human bone marrow cell culture using FACS. In this case, over 99% positive cells, and 80% negative cells were obtained separately (Fig. 4). Then CFU-F assays were performed to determine the frequency of CFU-F in both the SSEA-4+ cell population and the SSEA-4− cell population. The results suggested that the number of CFU-F in the SSEA-4+ cell population was at least 5- to 6-fold greater than that in the SSEA-4− cell population. A few of the CFU-F shown in the SSEA-4− cell population could have been generated from contaminated SSEA-4+ cells (the sorted SSEA-4− cell population contained ∼20% SSEA+ cells). To identify how SSEA-4+ cells were retained on ECM versus tissue culture plastic (Plastic), we cultured the purified SSEA-4+ cells on either ECM or tissue culture plastic up to 2 passages, and then analyzed SSEA-4+ cells by FACS following each passage. It was found that ECM retained ∼95% SSEA-4+ cells, whereas SSEA-4+ cells maintained on plastic dropped to ∼50% over 2 passages (Fig. 4). However, the majority of SSEA-4− cells failed to grow on either ECM or plastic, which was consistent with the previous observation reported by Gang et al. [31].
FIG. 4.
Marrow stromal cell-derived extracellular matrix (ECM) retains SSEA-4+ cells and enriches colony-forming cells. Freshly isolated human bone marrow mononuclear cells were cultured on tissue culture plastic at an initial seeding 3 × 105 cells/cm2 until 70% confluence (2–3 weeks) in the expansion medium. After removal of non-adherent cells, the cultured bone marrow adherent cells were detached and stained with a specific antibody against SSEA-4. SSEA-4+ cells and SSEA-4− cells were sorted using fluorescence-activated cell sorting (FACS). CFU-F assay was performed to determine the frequency of CFU-F in the sorted SSEA-4+ and SSEA-4− cell populations. In addition, SSEA-4+ cells were subcultured on either ECM or tissue culture plastic (Plastic) for 2 passages (P1 and P2). SSEA-4 expression was analyzed by FACS following each passage. For a negative control (gray peak), cells were stained with primary non-specific antibody (isotype). Simultaneously, the sorted SSEA-4+ or SSEA-4− cells were placed onto tissue culture plastic at 300 cells per well (∼10 cm2 area) in triplicate and cultured for 14 days in 3 mL α-MEM containing 15% FBS. CFU-F colonies were then visualized with crystal violet staining.
A gene expression signature of human MSCs maintained on the ECM
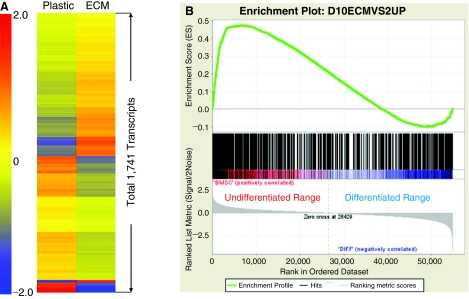
To comprehensively demonstrate how different are MSCs maintained on ECM versus plastic, we compared global patterns of gene expression in human MSCs (pre-purified SSEA-4+ cells) cultured on plastic (Plastic) versus on stromal cell-derived ECM (ECM) in the expansion medium for 12 days. We identified 1,741 transcripts either up- or down-regulated in cells cultured on ECM versus on plastic using the statistical program Significance Analysis of Microarrays (SAM) at a false discovery rate of 1% (Fig. 5A). Then, 1,741 transcripts were classified based on their biological function using the Gene Ontology (GO) database. Strikingly, based on the lowest P value, the top 3 clusters mapped by these 1,741 genes were associated with cell division (cell cycle), chromosome part, and cell movement (cytoskeleton), respectively (Table 1).Furthermore, 721 up-regulated transcripts were separated from the 1,741 transcripts, and analyzed for statistically significant enrichment of human MSCs gene expression pattern [datasetsGSE10315(http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE10315)] found in public gene sets from the NCBI GEO database using the software Gene Set Enrichment Analysis (GSEA), as previously described [28]. Figure 5B shows that this 721 gene set was highly enriched in genes related to undifferentiated human MSCs with a Normalized Enrichment Score (NES) of 1.76 and a Family Wise-error Rate (FWER) P value of 0.016, as compared to human MSCs treated with BMP-2. This strongly suggested that the genes expressed by cells maintained on the ECM were most likely the undifferentiated MSC gene set when compared with differentiated MSCs induced by BMP-2 treatment. We were not able to examine gene expression profiles of SSEA-4− cells because these cells failed to grow.
FIG. 5.
Global gene expression patterns for human mesenchymal stem cells (MSCs) cultured on extracellular matrix (ECM) compared to cells cultured on plastic. (A) Gene expression signatures of human MSCs maintained on plastic versus ECM for 12 days. They are presented by hierarchical clustering of 1,741 transcripts that were significantly up- or down-regulated by the ECM as compared to plastic. Color bar represents the range of expression levels indicated by log2 scale. (B) Enrichment plot of the 721 up-regulated transcripts on the ECM. The majority of this gene set was overrepresented within a ranked list of genes expressed by undifferentiated bone marrow stem cell (BMSC), shown in red. Normalized enrichment score (NES) was 1.76 [Actual ES divided by Mean (ESs against all permutations of the dataset)]; and a Family Wise-error Rate (FWER) P value was 0.016, which estimates the probability that the normalized enrichment score represents a false positive finding.
Table 1.
Functional Annotation Clustering (Gene Ontology)
| Count | P value | |
|---|---|---|
| Annotation cluster 1 | Enrichment score: 23.86 | |
| Cell cycle process | 141 | 4.7E-30 |
| Cell cycle | 157 | 5.2E-30 |
| Mitosis | 70 | 8.1E-28 |
| M phase of mitotic cell cycle | 70 | 1.5E-27 |
| Mitotic cell cycle | 81 | 5.8E-26 |
| Cell division | 64 | 4.7E-22 |
| Regulation of cell cycle | 88 | 2.3E-15 |
| Annotation cluster 2 | Enrichment score: 12.84 | |
| Chromosome, pericentric region | 29 | 7.5E-15 |
| Chromosome | 68 | 5.3E-13 |
| Chromosomal part | 61 | 7.6E-13 |
| Annotation cluster 3 | Enrichment score: 10.78 | |
| Microtubule cytoskeleton | 77 | 2.7E-16 |
| Intracellular non-membrane-bound organelle | 219 | 7.7E-15 |
| Microtubule | 49 | 2.4E-12 |
| Cytoskeleton | 140 | 2.6E-12 |
| Microtubule-based movement | 66 | 7.4E-8 |
| Cytoskeleton-dependent intracellular transport | 27 | 4.8E-7 |
Culture of MSCs on marrow stromal cell-derived ECM promotes self-renewal and retention of multipotentiality
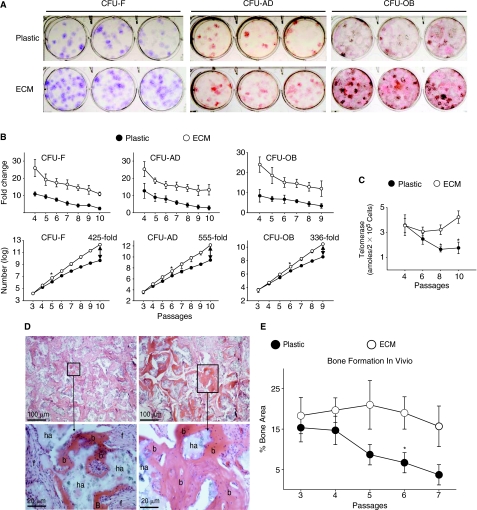
Self-renewal of MSCs was determined using a replating assay in which the increase in colony-forming cells following 7 days of pre-culture of MSCs was quantified [22]. Because the previous experiments suggested that the effects of tissue culture plastic with and without coatings of purified collagen I or fibronectin on MSC colony formation and proliferation were similar, the following comparisons were only performed between the ECM and the uncoated plastic. Figure 6A shows an example of colony formation generated by cells expanded on plastic or the ECM after 7 passages, and clearly demonstrated that the number of colonies on plastic was lower than that on the ECM. Next, the changes in replication of CFUs following serial passages were determined. Our results showed that the replication of MSCs expanded on plastic was initially lower than that of MSCs expanded on the ECM. Following serial passages, the replicative activity of the MSCs rapidly decreased when the cells were expanded on plastic as compared to those on ECM (Fig. 6B, upper panels). When the accumulation of colony-forming cells following serial passages was determined, the increase in the numbers of CFU-F, CFU-AD, and CFU-OB were ∼425-fold, 555-fold, and 336-fold greater after 9 to 10 passages, respectively, when cells were expanded on ECM compared to plastic (Fig. 6B).
FIG. 6.
Stromal cell-derived extracellular matrix (ECM) promotes replication of CFUs, and retains the ability of mesenchymal stem cells (MSCs) to form skeletal tissue in vivo. (A) Appearance of CFU-F, CFU-AD, and CFU-OB assayed after 7 passages of expansion on plastic or ECM. (B) Cell replication. Upper panels: replication of colony-forming cells expanded on the ECM versus plastic, expressed as fold changes in number of colonies with increasing passage number. The replicative activity of MSCs maintained on the ECM was significantly higher (P < 0.05) than those of MSCs maintained on plastic at all time points. Lower panels: growth kinetics of colony-forming cells (log scale) expanded on ECM versus plastic with increasing passage number. *P < 0.05, value at the earliest passage when cells expanded on ECM showed increased colony-forming activity versus plastic. (C) Telomerase activity in cells expanded on ECM versus plastic with increasing passage number. *P < 0.05 (by ANOVA), ECM versus plastic (before P8). (D) Histology of ossicle produced by implantation of P7 human bone marrow cells. While bone was formed by cells expanded on plastic (left panels), as well as by cells expanded on ECM (right panels); high magnification (lower panels) of areas selected in upper panels clearly showed more robust bone formation in the latter. b, bone; f, fibrous tissue; and ha, HA/TCP. (E) Following each passage, the cells (1 × 106) were loaded into HA/TCP ceramic powder and transplanted subcutaneously into the dorsal surface of 10-week-old immunodeficient mice. Three implants for each group were harvested at 8 weeks post-implantation. The extent of new bone formed in the implants was histomorphometrically determined as areas measured by using the ImageJ analysis software. n = 3; *P < 0.05, value at the earliest passage versus that at the passage 3 or 4. Color images available online at www.liebertonline.com/scd.
In view of the involvement of telomerase in the extension of telomere length associated with cellular life span [32], we also measured intracellular telomerase activity of expanded cells following each passage. During the entire subculturing time course, telomerase activity remained highly stable in cells maintained on the ECM, but rapidly decreased in cells maintained on plastic (Fig. 6C), as previously observed by others [33].
Next, we compared the influence of expansion on the ECM on the capacity of MSCs to form bone in vivo using a transplantation assay [29]. Following each expansion on the ECM or plastic, the cells were loaded onto a hydroxyapatite/tricalcium phosphate (HA/TCP) carrier and implanted subcutaneously into immunocompromised NIH-bg-nu-xid mice. Indeed, the amount of bone generated after 8 weeks by MSCs expanded on plastic and on the ECM was very similar before passage 4, which was ∼10%–20% of bone in the total area of the ossicle (Fig. 6D and 6E). However, the differential amount of bone formed by cells cultured on these 2 systems was exaggerated after 7 passages. Figure 6E shows that the amount of bone generated by MSCs expanded on plastic was dramatically decreased, to approximately <2% of bone in the total area of the ossicle. In contrast, MSCs expanded on ECM for 7 passages still retained their ability to form bone, generating ∼15% of bone in the total area of the ossicle (Fig. 6E).
Discussion
It is well recognized that MSCs lose their ability to self-renew as well as their multipotentiality upon long-term culture on tissue culture plastic [8,10,12,34]. Previous attempts to overcome these limitations have utilized cultures on fibronectin matrices under low oxygen tension (3%) [35] to mimic the microenvironment of the bone marrow [36], or cultures at low seeding density in low serum in the presence of growth factors [10,37]. These conditions permitted expansion of mouse and human MSCs for as many as 60 population doublings, but the full differentiation potential and cellular composition of these cell preparations remain unclear. Particularly, the ability of such cell preparations to form skeletal tissue in vivo has not been reported.
Although gene transduction of telomerase into stem cells [38] or of 4 transcription factor genes (Oct4, Sox2, c-myc, and Klf4) into somatic cells for reprogramming into pluripotent stem cells has shown promising results [39,40], this approach alters cell behavior via genetic modification, thus preventing investigation of native factors that determine stem cell fate and making these cells unpredictable for use in human therapy.
Since the ECM is an important component of the cellular niche in a tissue, supplying critical biochemical and physical signals to initiate or sustain cellular functions, we propose an alternative approach, which is to reconstitute a native tissue-specific ECM in vitro to simulate the marrow environment where MSCs are found in vivo. The studies reported herein indicate that the ECM produced by bone marrow stromal cells consists of at least collagen types I and III, fibronectin, small leucine-rich proteoglycans such as biglycan and decorin, and major components of basement membrane such as the large molecular weight proteoglycan perlecan and laminin. All these matrix proteins have been shown to be important in regulating cell adhesion, migration, proliferation, differentiation, and survival [41–44]. The source of the structural components of the ECM may be contributed by bone marrow stromal cells or adherent cells including MSCs. It is possible that these cells with other accessory cells such as hematopoietic mononuclear cells together influence properties of the ECM by secreting growth factors, cytokines, and matrix metalloproteinases that affect the biosynthetic activity of the stromal cells. Nevertheless, MSCs cultured on this ECM show remarkable promotion of proliferation, and retention of a stem cell population with a lower level of ROS, as compared to those cultured on other substrata. Interestingly, in hematopoietic stem cells, it has been reported that a high level of ROS is associated with loss of stem cell characteristics and increased differentiation as well as their apoptosis [45]. Hence, the ability of the ECM to suppress ROS may contribute to the retention of MSC characteristics.
Because MSCs are specific targets of BMP-2, which acts to induce MSC osteogenic differentiation, “true” MSCs should also respond to BMP-2 stimulation. Indeed, our data indicate that the sensitivity of MSCs to BMP-2 stimulation is dramatically increased upon maintenance on ECM, as compared to plastic. The differential sensitivity may be related to the different composition of the 2 populations of cultured cells (Fig. 3A). Efficient stimulation of MSCs with a low dose of growth factors may more closely resemble the physiological situation, suggesting that the ECM provides an optimal “home” for MSCs to retain their stem cell properties.
Furthermore, the gene expression profiles displayed a global picture to unbiasedly confirm that the ECM did restrain MSC differentiation. In addition to genes related to cell cycle and cell division, sets of genes were shown to highly relate to cytoskeleton and microtubule-based movement. These results support our observation that ECM promotes human MSC attachment and motility (data not shown). These findings encourage us to further study in great detail the regulatory relationship among genes associated with the maintenance of MSC properties in the future.
During multiple passages, MSCs cultured on ECM maintained high levels of replicative capability, accompanied by high levels of telomerase activity, compared to cells expanded on plastic. The activation of telomerase prevents telomere erosion and inhibits stem cell replicative senescence in vitro [32]. Thus, it is possible that the ECM stabilizes high levels of telomerase activity, resulting in the extension of the life span of these cells. The quality of the expanded cells following each passage was further tested by an in vivo transplantation assay. Our studies showed that MSCs expanded on the ECM for multiple passages still retained the ability to form a relatively large volume of bone tissue. In contrast, the bone formation capacity of cells expanded on plastic was dramatically diminished after 6–7 passages. These findings suggest that culture of human bone marrow cells on such ECM may be useful for large-scale enrichment of MSCs without the need for extensive subculturing or passaging.
The ECM modulates the activity of growth factors by controlling proteolytic activation of latent factors as in the case of TGF-β [46], and by sequestering factors such as PDGF and BMPs [19,47]. ECM proteins also interact with receptors to regulate binding of the cognate ligand, as occurs in the case of the EGF receptor [48]. Each of these mechanisms may contribute to the maintenance and expansion of MSCs when cultured on the stromal cell-derived ECM. In this study, we also observed that MSCs grown on the ECM underwent directional migration along the orientation of the ECM fibers with a decreased frequency of cell–cell contact, whereas MSCs grown on plastic showed random migration (data not shown). It will be interesting to further explore whether the differential patterns of cell migration are critical to influence MSC self-renewal.
In conclusion, we propose that the multilineage differentiation potential of MSCs is controlled by their interactions with a tissue-specific microenvironment or niche consisting of ECM proteins associated with growth factors. To reproduce the intricate and highly ordered nature of the ECM with synthetic or purified components is a challenging undertaking. Thus, for the purpose of reconstituting an optimal microenvironment for MSCs in vitro, we have prepared an ECM produced by bone marrow stromal cells. The ECM-based culture system described herein appears to provide an ideal environment for the large-scale expansion of highly functional MSCs for eventual use in stem cell-based therapy. Moreover, this system should also be invaluable for investigations into whether the stromal cell-derived ECM is unique in its ability to preserve MSC properties by comparing it to ECM prepared from cells derived from different tissues such as skin, fat, and muscle. Establishment of a unique tissue-specific ECM will facilitate control of the fate of MSCs for therapeutic applications.
Acknowledgments
We thank Dr. Valerie A. Lee (UTHSCSA) for her careful review of the manuscript. This work was supported by a grant from University Research Council Grants Program at the University of Texas Health Science Center at San Antonio (XDC), and supported by the National Institutes of Health (XDC: R21 AG025466; RST: ZO1AR41131). Confocal images were generated in the Core Optical Imaging Facility that is supported by UTHSCSA, NIH-NCI P30 CA54174 (San Antonio Cancer Institute), NIH-NIA P30 AG013319 (Nathan Shock Center), and NIH-NIA P01AG19316.
Author Disclosure Statement
The authors declare that there are no conflicts of interest.
References
- 1.Prockop DJ. Marrow stromal cells as stem cells for non-hematopoietic tissues. Science. 1997;276:71–74. doi: 10.1126/science.276.5309.71. [DOI] [PubMed] [Google Scholar]
- 2.Dennis JE. Merriam A. Awadallah A. Yoo JU. Johnstone B. Caplan AI. A quadripotential mesenchymal progenitor cell isolated from the marrow of an adult mouse. J Bone Miner Res. 1999;14:700–709. doi: 10.1359/jbmr.1999.14.5.700. [DOI] [PubMed] [Google Scholar]
- 3.Ferrari G. Cusella-De AG. Coletta M. Paolucci E. Stornaiuolo A. Cossu G. Mavilio F. Muscle regeneration by bone marrow derived myogenic progenitors. Science. 1998;279:1528–1530. doi: 10.1126/science.279.5356.1528. [DOI] [PubMed] [Google Scholar]
- 4.Kassem M. Stem cells: potential therapy for age-related diseases. Ann N Y Acad Sci. 2006;1067:436–442. doi: 10.1196/annals.1354.062. [DOI] [PubMed] [Google Scholar]
- 5.Banerjee M. Bhonde RR. Autologous bone marrow transplantation/mobilization: a potential regenerative medicine for systemic degenerative disorders and healthy living. Med Hypotheses. 2007;68:1247–1251. doi: 10.1016/j.mehy.2006.09.069. [DOI] [PubMed] [Google Scholar]
- 6.Petite H. Viateau V. Bensaid W. Meunier A. De Pollak C. Bourguignon M. Oudina K. Sedel L. Guillemin G. Tissue-engineered bone regeneration. Nat Biotechnol. 2000;18:959–963. doi: 10.1038/79449. [DOI] [PubMed] [Google Scholar]
- 7.Wexler SA. Donaldson C. Denning-Kendall P. Rice C. Bradley B. Hows JM. Adult bone marrow is a rich source of human mesenchymal “stem”. cells but umbilical cord and mobilized adult blood are not. Br J Haematol. 2003;121:368–374. doi: 10.1046/j.1365-2141.2003.04284.x. [DOI] [PubMed] [Google Scholar]
- 8.Banfi A. Muraglia A. Dozin B. Mastrogiacomo M. Cancedda R. Quarto R. Proliferation kinetics and differentiation potential of ex vivo expanded human bone marrow stromal cells: Implications for their use in cell therapy. Exp Hematol. 2000;28:707–715. doi: 10.1016/s0301-472x(00)00160-0. [DOI] [PubMed] [Google Scholar]
- 9.Baksh D. Song L. Tuan RS. Adult mesenchymal stem cells: characterization differentiation, and application in cell and gene therapy. J Cell Mol Med. 2004;8:301–316. doi: 10.1111/j.1582-4934.2004.tb00320.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Izadpanah R. Kaushal D. Kriedt C. Tsien F. Patel B. Dufour J. Bunnell BA. Long-term in vitro expansion alters the biology of adult mesenchymal stem cells. Cancer Res. 2008;68:4229–4238. doi: 10.1158/0008-5472.CAN-07-5272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim J. Kang JW. Park JH. Choi Y. Choi KS. Park KD. Baek DH. Seong SK. Min HK. Kim HS. Biological characterization of long-term cultured human mesenchymal stem cells. Arch Pharm Res. 2009;32:117–126. doi: 10.1007/s12272-009-1125-1. [DOI] [PubMed] [Google Scholar]
- 12.DiGirolamo CM. Stokes D. Colter D. Phinney DG. Class R. Prockop DJ. Propagation and senescence of human marrow stromal cells in culture: a simple colony-forming assay identifies samples with the greatest potential to propagate and differentiate. Br J Haematol. 1999;107:275–281. doi: 10.1046/j.1365-2141.1999.01715.x. [DOI] [PubMed] [Google Scholar]
- 13.Rubio D. Garcia-Castro J. Martin MC. de la FR. Cigudosa JC. Lloyd AC. Bernad A. Spontaneous human adult stem cell transformation. Cancer Res. 2005;65:3035–3039. doi: 10.1158/0008-5472.CAN-04-4194. [DOI] [PubMed] [Google Scholar]
- 14.Miura M. Miura Y. Padilla-Nash HM. Molinolo AA. Fu B. Patel V. Seo BM. Sonoyama W. Zheng JJ. Baker CC. Chen W. Ried T. Shi S. Accumulated chromosomal instability in murine bone marrow mesenchymal stem cells leads to malignant transformation. Stem Cells. 2006;24:1095–1103. doi: 10.1634/stemcells.2005-0403. [DOI] [PubMed] [Google Scholar]
- 15.Rosland GV. Svendsen A. Torsvik A. Sobala E. McCormack E. Immervoll H. Mysliwietz J. Tonn JC. Goldbrunner R. Lonning PE. Bjerkvig R. Schichor C. Long-term cultures of bone marrow-derived human mesenchymal stem cells frequently undergo spontaneous malignant transformation. Cancer Res. 2009;69:5331–5339. doi: 10.1158/0008-5472.CAN-08-4630. [DOI] [PubMed] [Google Scholar]
- 16.Ksiazek K. A comprehensive review on mesenchymal stem cell growth and senescence. Rejuvenation Res. 2009;12:105–116. doi: 10.1089/rej.2009.0830. [DOI] [PubMed] [Google Scholar]
- 17.Moore KA. Lemischka IR. Stem cells and their niches. Science. 2006;311:1880–1885. doi: 10.1126/science.1110542. [DOI] [PubMed] [Google Scholar]
- 18.Fuchs E. Tumbar T. Guasch G. Socializing with the neighbors: stem cells and their niche. Cell. 2004;116:769–778. doi: 10.1016/s0092-8674(04)00255-7. [DOI] [PubMed] [Google Scholar]
- 19.Chen XD. Dusevic V. Feng JQ. Manolagas SC. Jilka RL. Extracellular matrix made by bone marrow cells facilitates expansion of marrow-derived mesenchymal progenitor cells and prevents their differentiation into osteoblasts. J Bone Miner Res. 2007;22:1943–1956. doi: 10.1359/jbmr.070725. [DOI] [PubMed] [Google Scholar]
- 20.Cukierman E. Pankov R. Stevens DR. Yamada KM. Taking cell-matrix adhesions to the third dimension. Science. 2001;294:1708–1712. doi: 10.1126/science.1064829. [DOI] [PubMed] [Google Scholar]
- 21.Zuk PA. Zhu M. Mizuno H. Huang J. Futrell JW. Katz AJ. Benhaim P. Lorenz HP. Hedrick MH. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211–228. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 22.Di Gregorio GB. Yamamoto M. Ali AA. Abe E. Roberson P. Manolagas SC. Jilka RL. Attenuation of the self-renewal of transit-amplifying osteoblast progenitors in the murine bone marrow by 17 beta-estradiol. J Clin Invest. 2001;107:803–812. doi: 10.1172/JCI11653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bakay M. Chen YW. Borup R. Zhao P. Nagaraju K. Hoffman EP. Sources of variability and effect of experimental approach on expression profiling data interpretation. BMC Bioinformatics. 2002;3:4. doi: 10.1186/1471-2105-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peng X. Wood CL. Blalock EM. Chen KC. Landfield PW. Stromberg AJ. Statistical implications of pooling RNA samples for microarray experiments. BMC Bioinformatics. 2003;4:26. doi: 10.1186/1471-2105-4-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holmes C. Brown SD. All systems GO for understanding mouse gene function. J Biol. 2004;3:20. doi: 10.1186/jbiol19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Manoli T. Gretz N. Grone HJ. Kenzelmann M. Eils R. Brors B. Group testing for pathway analysis improves comparability of different microarray datasets. Bioinformatics. 2006;22:2500–2506. doi: 10.1093/bioinformatics/btl424. [DOI] [PubMed] [Google Scholar]
- 27.Sweet-Cordero A. Mukherjee S. Subramanian A. You H. Roix JJ. Ladd-Acosta C. Mesirov J. Golub TR. Jacks T. An oncogenic KRAS2 expression signature identified by cross-species gene-expression analysis. Nat Genet. 2005;37:48–55. doi: 10.1038/ng1490. [DOI] [PubMed] [Google Scholar]
- 28.Yang W. Harris MA. Heinrich JG. Guo D. Bonewald LF. Harris SE. Gene expression signatures of a fibroblastoid preosteoblast and cuboidal osteoblast cell model compared to the MLO-Y4 osteocyte cell model. Bone. 2009;44:32–45. doi: 10.1016/j.bone.2008.08.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bi Y. Stuelten CH. Kilts T. Wadhwa S. Iozzo RV. Robey PG. Chen XD. Young MF. Extracellular matrix proteoglycans control the fate of bone marrow stromal cells. J Biol Chem. 2005;280:30481–30489. doi: 10.1074/jbc.M500573200. [DOI] [PubMed] [Google Scholar]
- 30.Kannagi R. Cochran NA. Ishigami F. Hakomori S. Andrews PW. Knowles BB. Solter D. Stage-specific embryonic antigens (SSEA-3 and −4) are epitopes of a unique globo-series ganglioside isolated from human teratocarcinoma cells. EMBO J. 1983;2:2355–2361. doi: 10.1002/j.1460-2075.1983.tb01746.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gang EJ. Bosnakovski D. Figueiredo CA. Visser JW. Perlingeiro RC. SSEA-4 identifies mesenchymal stem cells from bone marrow. Blood. 2007;109:1743–1751. doi: 10.1182/blood-2005-11-010504. [DOI] [PubMed] [Google Scholar]
- 32.Cong Y. Shay JW. Actions of human telomerase beyond telomeres. Cell Res. 2008;18:725–732. doi: 10.1038/cr.2008.74. [DOI] [PubMed] [Google Scholar]
- 33.Izadpanah R. Trygg C. Patel B. Kriedt C. Dufour J. Gimble JM. Bunnell BA. Biologic properties of mesenchymal stem cells derived from bone marrow and adipose tissue. J Cell Biochem. 2006;99:1285–1297. doi: 10.1002/jcb.20904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McCulloch CA. Strugurescu M. Hughes F. Melcher AH. Aubin JE. Osteogenic progenitor cells in rat bone marrow stromal populations exhibit self-renewal in culture. Blood. 1991;77:1906–1911. [PubMed] [Google Scholar]
- 35.D'Ippolito G. Diabira S. Howard GA. Roos BA. Schiller PC. Low oxygen tension inhibits osteogenic differentiation and enhances stemness of human MIAMI cells. Bone. 2006;39:513–522. doi: 10.1016/j.bone.2006.02.061. [DOI] [PubMed] [Google Scholar]
- 36.Chow DC. Wenning LA. Miller WM. Papoutsakis ET. Modeling pO(2) distributions in the bone marrow hematopoietic compartment. I. Krogh's model. Biophys J. 2001;81:675–684. doi: 10.1016/S0006-3495(01)75732-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peister A. Mellad JA. Larson BL. Hall BM. Gibson LF. Prockop DJ. Adult stem cells from bone marrow (MSCs) isolated from different strains of inbred mice vary in surface epitopes rates of proliferation and differentiation potential. Blood. 2004;103:1662–1668. doi: 10.1182/blood-2003-09-3070. [DOI] [PubMed] [Google Scholar]
- 38.Gronthos S. Chen S. Wang CY. Robey PG. Shi S. Telomerase accelerates osteogenesis of bone marrow stromal stem cells by upregulation of CBFA1, osterix, and osteocalcin. J Bone Miner Res. 2003;18:716–722. doi: 10.1359/jbmr.2003.18.4.716. [DOI] [PubMed] [Google Scholar]
- 39.Takahashi K. Tanabe K. Ohnuki M. Narita M. Ichisaka T. Tomoda K. Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 40.Yu J. Vodyanik MA. Smuga-Otto K. ntosiewicz-Bourget J. Frane JL. Tian S. Nie J. Jonsdottir GA. Ruotti V. Stewart R. Slukvin II. Thomson JA. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 41.Yamaguchi Y. Mann DM. Ruoslahti E. Negative regulation of transforming growth factor-beta by the proteoglycan decorin. Nature. 1990;346:281–284. doi: 10.1038/346281a0. [DOI] [PubMed] [Google Scholar]
- 42.Chen XD. Fisher LW. Robey PG. Young MF. The small leucine-rich proteoglycan biglycan modulates BMP-4-induced osteoblast differentiation. FASEB J. 2004;18:948–958. doi: 10.1096/fj.03-0899com. [DOI] [PubMed] [Google Scholar]
- 43.Ahmed N. Riley C. Rice G. Quinn M. Role of integrin receptors for fibronectin, collagen and laminin in the regulation of ovarian carcinoma functions in response to a matrix microenvironment. Clin Exp Metastasis. 2005;22:391–402. doi: 10.1007/s10585-005-1262-y. [DOI] [PubMed] [Google Scholar]
- 44.Philp D. Chen SS. Fitzgerald W. Orenstein J. Margolis L. Kleinman HK. Complex extracellular matrices promote tissue-specific stem cell differentiation. Stem Cells. 2005;23:288–296. doi: 10.1634/stemcells.2002-0109. [DOI] [PubMed] [Google Scholar]
- 45.Tothova Z. Kollipara R. Huntly BJ. Lee BH. Castrillon DH. Cullen DE. McDowell EP. Lazo-Kallanian S. Williams IR. Sears C. Armstrong SA. Passegue E. DePinho RA. Gilliland DG. FoxOs are critical mediators of hematopoietic stem cell resistance to physiologic oxidative stress. Cell. 2007;128:325–339. doi: 10.1016/j.cell.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 46.Dallas SL. Rosser JL. Mundy GR. Bonewald LF. Proteolysis of latent transforming growth factor-beta (TGF-beta)-binding protein-1 by osteoclasts. A cellular mechanism for release of TGF-beta from bone matrix. J Biol Chem. 2002;277:21352–21360. doi: 10.1074/jbc.M111663200. [DOI] [PubMed] [Google Scholar]
- 47.N Nili. Cheema AN. Giordano FJ. Barolet AW. Babaei S. Hickey R. Eskandarian MR. Smeets M. Butany J. Pasterkamp G. Strauss BH. Decorin inhibition of PDGF-stimulated vascular smooth muscle cell function: potential mechanism for inhibition of intimal hyperplasia after balloon angioplasty. Am J Pathol. 2003;163:869–878. doi: 10.1016/S0002-9440(10)63447-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Santra M. Reed CC. Iozzo RV. Decorin binds to a narrow region of the epidermal growth factor (EGF) receptor, partially overlapping but distinct from the EGF-binding epitope. J Biol Chem. 2002;277:35671–35681. doi: 10.1074/jbc.M205317200. [DOI] [PubMed] [Google Scholar]