Abstract
The use of embryonic stem cells (ESCs) for regenerative medicine has generated increased attention due to the favorable attributes of these cells; namely, they are pluripotent and possess long-term self-renewal capacity. The initial aims of the present study were: (i) to use stirred suspension bioreactors to expand and differentiate ESCs into osteogenic and chondrogenic cell types and (ii) to explore if these ESC-derived cells influenced skeletal healing in an in vivo fracture model. We show that differentiation protocols used in static culture are insufficient when applied directly to suspension culture bioreactors. Moreover, when bioreactor-differentiated cells are transplanted into a burr-hole defect in bone, severe disruption of the bone architecture was noted at the fracture site, as determined by microcomputed tomography (microCT) imaging and histopathology. Further characterization of the bioreactor-differentiated cultures revealed that a subpopulation of cells in the resulting aggregates expressed the pluripotency marker Oct-4 in the nucleus. Nuclear Oct-4 expression persisted even after 30 days of culture in the absence of leukemia inhibitory factor (LIF). Remarkably, and unlike ESCs differentiated into skeletal cell types in static cultures, bioreactor-differentiated aggregates implanted subcutaneously into SCID mice formed teratomas. The development of effective ESC differentiation protocols for suspension bioreactors will require a more complete understanding of the environmental conditions within these culture systems and the influence that these conditions have on the regulation of pluripotency and differentiation in ESCs.
Introduction
Tissue-engineered bone constructs represent a promising treatment alternative for degenerative diseases and injuries of the skeletal system. Importantly, this approach mitigates the problem of donor site morbidity and overcomes the problem of donor tissue availability—the 2 greatest limitations of therapeutic bone grafting [1]. Embryonic stem cells (ESCs) are a promising source of cells for tissue engineering and cell therapies as they are pluripotent and have the capacity for long-term self-renewal. The practical implementation of ESC therapy requires a controlled and reproducible system for expanding and differentiating ESCs in culture. Recently, we described a bioprocess wherein undifferentiated murine ESCs were expanded in stirred suspension culture bioreactors as aggregates [2], while retaining high proportions of pluripotent cells over extended passaging in the bioreactors [3]. Similar bioreactor expansion protocols have also been presented by others [4].
The differentiation of ESCs into skeletal cell types, specifically chondrocytes and osteoblasts, has most commonly been induced in static culture systems [5–10]. In these studies, osteogenic differentiation of ESCs was achieved through treatment with ascorbic acid, β-glycerophosphate, and either dexamethasone or vitamin D3. Chondrogenic differentiation has been achieved by exposing ESCs to TGF-β1, BMP-2, insulin, and ascorbic acid over specific time periods [6]. The influence of certain members of the TGF-β growth factor family on the chondrogenic differentiation of ESCs was also demonstrated by Kramer et al. [7]. Similar media supplements have been combined with the micromass culture technique to further promote the chondrogenic differentiation [11]. These studies did not incorporate mechanical loading, which some have shown can influence stem cell differentiation [12].
In suspension bioreactors, the osteogenic differentiation of murine ESCs has relied on the use of microcarriers and/or encapsulation. For example, Randle et al. [13] encapsulated murine ESCs in a hydrogel to induce their differentiation into osteoblasts in a high-aspect-ratio vessel (HARV). Also, Tielens et al. [14] seeded mouse ESCs onto biodegradable microcarriers and subsequently encapsulated the cell-coated microcarriers in a photo-catalyzed polymer for osteogenic differentiation in suspension bioreactors. Again, in these studies, as the cells were encapsulated, they were not exposed to mechanical forces (ie, hydrodynamic shear) during differentiation. To our knowledge, there have been no published reports on the chondrogenic differentiation of ESCs in suspension bioreactors.
An important consideration for the use of ESC-derived cells in transplantation studies is the risk of spontaneous differentiation of the cells in vivo, which may lead to the tumor formation. For example, Wakitani et al. [15] showed that undifferentiated ESCs injected directly into a knee formed teratomas that, by 8 weeks post-injection, completely destroyed the joint. Hence, precautions must be taken to avoid the transplantation of undifferentiated ESCs. Some approaches include driving complete and specific differentiation of ESCs using extracellular signals (chemical, mechanical, matrix), and/or purifying ESC cultures using physical (cell sorting) or biological (antibiotic resistance) separation methods.
Using our previous bioreactor expansion protocols as a foundation [2], the objective of the current study was to test the effectiveness of ESC differentiation into osteogenic and chondrogenic cell types in bioreactors using our previous static differentiation protocols [5,6], and to explore if these cells would promote endochondral ossification in an in vivo fracture model. For these proof-of-principle studies, we chose a stable burr-hole fracture model, similar to that presented by Uusitalo et al. [16], as it has discrete geometry and does not require external fixation (unlike critical size defect models [17–20]). Furthermore, donor ESCs were strain-matched to immunocompetent recipient (fractured) mice, which avoided complications of fracture healing and wound repair in immunocompromised mice.
Materials and Methods
ESC culture
Murine D3 ESCs were maintained in the pluripotent state on MEFs in high-glucose DMEM supplemented with 15% FBS (Invitrogen, Carlsbad, CA), 1% nonessential amino acids, 50 U/mL penicillin, and 50 μg/mL streptomycin, 0.1 mM β-mercaptoethanol, and 1,000 U/mL leukemia inhibitory factor (LIF). Pluripotent static cultures were subcultured every second day on murine embryonic fibroblast feeder cells (MEFs). Proliferation cultures of undifferentiated ESCs in stirred suspension bioreactors were performed as described [2,21].
ESC differentiation
ESCs were differentiated in 125-mL suspension bioreactors (Corning Style Spinner Flask, NDS Technologies Inc., Vineland, NJ) by inoculating 6.0 × 104 cells/mL into 100 mL of medium without LIF. An agitation rate of 100 rpm was used for all differentiation cultures, resulting in a maximum shear stress of 6.1 dyn/cm2 in the cultures [2]. Induced differentiation in static culture was carried out as described by zur Nieden et al. [5,6]. In brief, embryoid bodies (EBs) were formed through hanging drops and the EBs were transferred to static non-adherent culture on day 3 of differentiation and then transferred to static adherent culture on day 5 of differentiation. For both static and suspension bioreactor cultures, osteoblast differentiation was induced with β-glycerophosphate (10 mM), ascorbic acid (50 μg/mL), and 1,25-OH2 vitamin D3 (5 × 10−8 M; Calbiochem, San Diego, CA) from day 5 to day 30 [5]. Induction of the chondrogenic lineage in static and suspension was achieved by addition of 10 ng/mL TGF-β1 (Peprotech, Rocky Hill, NJ) on days 3–5 and 1 μg/mL insulin and 50 μg/mL ascorbic acid from day 5 to day 30. BMP-2 (10 ng/mL; Peprotech) was added from day 3 to day 30 [6]. Control cultures (spontaneous differentiation) consisted of ESCs that were cultured in ESC maintenance medium without LIF and without any additional supplementation. Unless noted, supplements were purchased at Sigma-Aldrich.
Histochemical stains
Day 30 bioreactor aggregates were stained with Alcian blue and Alizarin red. For Alcian blue staining, aggregates were fixed overnight at 4°C in 4% paraformaldehyde, and then washed 3 times in 1× PBS. The aggregates were placed in 90% ethanol for 48 h at 4°C and then stained with Alcian blue (2 mg Alcian blue (Sigma, St. Louis, MO), 0.8 mL water, 16 mL 100% ethanol, 4 mL glacial acetic acid) for 48 h at 4°C. The aggregates were rehydrated with progressively more dilute ethanol solutions (70%, 50%, 25%, 20% ethanol) and then placed in 1× PBS and visualized. For Alizarin red staining, aggregates were fixed overnight at 4°C in 4% paraformaldehyde, and then washed 3 times in 1× PBS. The aggregates were placed in 1% KOH solution for 48 h at 4°C and then stained with Alizarin red (1 mg Alizarin red, 100 mL of 1% KOH) for 48 h at 4°C. The aggregates were rinsed with 1% KOH several times and then placed in 1× PBS and visualized.
Lowry protein assay
Aliquots of aggregates were taken from the suspension cultures at the time points indicated. Media was removed after centrifugation and aggregates were extensively washed with PBS. Cells were then lysed in RIPA buffer as described [21] and assayed for their protein content with the DC protein assay kit from BioRad according to the manufacturer's instructions. Absorbance readings were taken at 750 nm and protein content was taken from a BSA standard curve.
ALP assay
Bioreactor cultures were assessed for ALP activity as described previously [21]. Protein lysates were mixed with p-nitrophenylphosphate (Sigma) and absorbance at 405 nm was measured immediately (t0). After 20 min of incubation at 37°C, the reaction was stopped with 3 N NaOH and a second absorbance reading was taken (t20). Units of ALP activity were calculated as described [5] and normalized to the total protein content of the sample.
Calcium assay
Calcium content in the aggregates from the bioreactor cultures was measured using an absorbance-based assay system. The purple substrate Arsenazo III (DCL, Toronto, Canada) was mixed with aliquots of the cell lysates and absorbance measured at 650 nm in a Benchmark Plus microplate spectrophotometer (BioRad, Hercules, CA).
Metachromatic test
Proteoglycan content in the aggregates from the differentiated cultures was determined with the DMMB assay [22]. Proteoglycans were extracted in 4 M guanidine–HCl/0.05 M sodium acetate (pH 5.8) containing 100 mM 6-aminocaproic acid, 10 mM EDTA, 5 mM benzamidine/HCl, 10 mM N-ethylmaleimide, 0.4 mM pepstatin, 1 mM PMSF, and 1 μg/mL soybean trypsin inhibitor for 48 h at 4°C. Non-completely digested cells were separated from the lysate by centrifugation. Lysates were mixed with DMMB reagent (0.16% w/v DMMB in 0.2% formic acid containing 2 mg/mL sodium formate, pH 3.5) and changes in absorption were detected at 535 nm in a spectrophotometer. The concentration of proteoglycans in the samples was read against a standard curve of chondroitin sulfate C.
Quantitative PCR
Cells were harvested at the days indicated from biological triplicates for RNA isolation with the RNeasy Mini Kit (Qiagen, Valencia, CA). Quantitative PCR was performed on an iCycler iQ system (BioRad) using a proprietary SYBR Green PCR Mix and primer sequences as described [21]. Melting curves were generated at the end of each run to ensure the presence of one single amplicon. Gene expression levels were analyzed with the 2(−ΔΔC(t)) method [23] from target gene C(t) values and respective C(t) values obtained for the reference gene GAPDH.
Burr-hole fracture model and ESC transplantation
All animal protocols were carried out as approved by the University of Calgary Animal Care Committee. For the fracture model, we selected immunocompetent mice that were strain-matched to the ESCs (ie, Sv129; Jackson Laboratory, Bar Harbor, ME). For surgery, mice were anesthetized using isofluorane. Buprenophine (0.05 mg/kg) and Derapen™ (1 mL/15 kg) were administered subcutaneously. A small incision was made on the medial side of the left knee. Using a high-speed microdrill (Fine Science Tools) and a 0.7-mm burr, a hole was drilled though the medial cortex and though the medullary cavity of the metaphysis. For ESC transplantations, two intact bioreactor aggregates (day 30 osteogenic or chondrogenic) were inserted into the fracture immediately after the injury was induced. The skin wound was then closed using Vetbond tissue adhesive (3 M). The mice were returned to their cages and allowed to bear weight immediately after surgery. As this was a pilot transplantation study, only one mouse was prepared for each cell type and each day of sacrifice (n = 1). Control fractures received no cell transplants and were allowed to heal without therapeutic intervention. Mice were sacrificed using cervical dislocation. The left tibia was excised and the soft tissue surrounding it was not removed. The tibiae were fixed in 10% neutral-buffered formalin (NBF) for a minimum of 5 days. Microcomputed tomography (MicroCT) scanning was conducted on the tibiae after fixation. The tibiae were then decalcified, embedded in paraffin, sectioned, and stained with hematoxylin and eosin (H&E).
Microcomputed tomography (microCT)
The tibia was scanned after fixation in 10% NBF. Imaging was performed at a nominal isotropic resolution of 10 μm covering a range of 5.7 mm from the proximal end (Micro-CT 35, Scanco Medical, Brüttisellen, Switzerland). The X-ray source had an energy level of 55 kVp, a current of 145 mA, and an integration time of 100 ms. The reconstructed images were processed (Image Processing Language, v5.08b) by filtering (sigma of 0.8, support of 1.0) to reduce noise, and the mineralized tissue was segmented by global thresholding (11.2% of maximum gray-scale value). The resulting binary image was reconstructed into a 3-dimensional image for morphological analysis.
Immunofluorescence
Aliquots of bioreactor-generated aggregates were obtained on day 30 of differentiation, washed in PBS, and fixed overnight in 4% PFA in PBS at 4°C. Aggregates were permeabilized in 0.5% saponin in PBS at 4°C overnight, rinsed once in PBS, and then blocked in 3% BSA at 4°C overnight. Primary antibodies (see Table 1)were diluted 1:50 in 3% BSA, added to the cell samples, and incubated overnight at 4°C. The aggregates were then washed 3 times with PBS and blocked again overnight at 4°C. Following the block, the aggregates were incubated with an appropriate Alexa Fluor 488 secondary antibody (Molecular Probes, Eugene, OR) and Toto-3 (Molecular Probes) overnight at 4°C. After incubation, the aggregates were washed 3 time with PBS and mounted on slides with mountant (9:1 glycerol:PBS). Spacers (270 μm) were adhered to slides prior to mounting to avoid aggregate compression. Slides were analyzed using a Zeiss 510 confocal Microscope with 488, 568, and 633 nm filters. Images were prepared using Zeiss LSM image browsing software.
Table 1.
Primary Antibodies Used for Immunofluorescent Analyses
| Marker | Source | Indication |
|---|---|---|
| Oct-4 | Chemicon | (Octomer-4) pluripotency |
| Gata-6 | Santa Cruz | Early endoderm, smooth muscle |
| Brachyury | Santa Cruz | Early mesoderm |
| BSP | Hybridoma Bank | (Bone Sialoproteins I & II) Osteoblasts |
| OCN | Santa Cruz | Osteoblasts |
| Endothelial cell | Hybridoma Bank | Endothelial cell marker except CNS |
| Aggrecan | Santa Cruz | Chondrocytes |
| Collagen II | Hybridoma Bank | Chondrocytes |
| Collagen X | Hybridoma Bank | Chondrocytes |
Primary antibodies used in the current study are listed.
BSP, bone sialoprotein; OCN, osteocalcin; CNS, central nervous system.
Teratoma formation assay
Fox Chase CB-17 SCID mice were obtained from Charles River. Animal protocols were carried out as approved by the University of Calgary Animal Care Committee. Cells were collected from static and bioreactor culture systems on days 0, 10, 20, and 30 of differentiation, dissociated with trypsin/EDTA (Invitrogen), and 106 cells were injected into the skin fold of the inner thigh. After 18 days, animals were sacrificed and emerging tissue material was dissected and examined histologically. Tissue was fixed overnight in 4% PFA at 4°C and then embedded in paraffin. Sections were stained with H&E.
Statistical analysis
Data from the metachromatic tests and quantitative PCR assays were statistically analyzed using an ANOVA with Sheffe's post-hoc test and the STATA 9.0 software package. For each assay, the values from the osteogenic, chondrogenic, and control treatment groups were compared at each time point.
Results
Characterization of bioreactor-differentiated ESCs
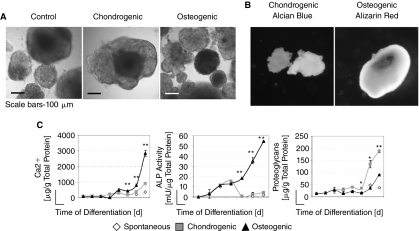
Over the first 24 h following inoculation of the cells into the bioreactor culture systems, ESCs aggregated into small clumps. By day 30 of differentiation, the aggregates had grown quite large with diameters of up to 500 μm (Fig. 1A). In the chondrogenic cultures, some aggregates stained completely for Alcian blue, while other aggregates had distinct regions that were positively stained (Fig. 1B). We did not observe any aggregates that did not stain at least partially for Alcian blue, suggesting that all aggregates contained glycosaminoglycans, albeit at varying degrees.
FIG. 1.
Extracellular matrix proteins on day 30 of differentiation. (A) Embryonic stem cell (ESC) aggregates from day 30 of bioreactor differentiation. (B) Aggregates expressed Alcian blue and Alizarin red. (C) Calcium content, alkaline phosphatase activity, and proteoglycan content in each bioreactor-differentiated culture are shown. An analysis of variance (ANOVA) was performed at each time point (*P < 0.01, **P < 0.001).
In the osteogenic cultures, mineralized extracellular matrix was detected in distinct pockets throughout the aggregates through Alizarin red staining (Fig. 1B). Furthermore, both calcium content and alkaline phosphatase activity were found to increase over the course of bioreactor differentiation in all cultures, but the increase was most substantial for both in the osteogenic cultures. Specifically, by day 30, osteogenic cultures possessed 3,000 μg of calcium per total grams of protein—a 3-fold increase over the chondrogenic cultures and a nearly 10-fold increase over the spontaneously differentiated control cultures (Fig. 1C). The alkaline phosphatase activity reached 54.5 mU per total protein content by day 30 in the osteogenic cultures, whereas the chondrogenic and control cultures reached only 4.4 mU and 1.9 mU per total protein, respectively (Fig. 1C). Proteoglycan content was found to increase to the greatest degree in the chondrogenic cultures by day 30 of differentiation. Specifically, the level of proteoglycans reached nearly 200 μg per total grams of protein by day 30, whereas proteoglycan content in the osteogenic and spontaneously differentiated control culture were detected at 91.5 μg and 37.1 μg per total protein content, respectively (Fig. 1C). Interestingly, there was a sharp increase in the calcium content (in the osteogenic cultures) and proteoglycan content (in the chondrogenic cultures) on day 25, suggesting that terminal differentiation of the cells was only occurring in the later stages of the bioprocess.
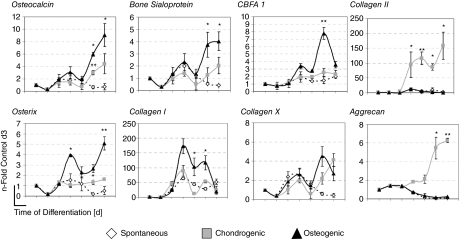
With respect to gene expression patterns, all lineage-specific markers were generally found to increase with differentiation (Fig. 2). Markers for the osteoblast lineage, osteocalcin, and bone sialoprotein, as well as the chondrocyte lineage, collagen type II, and aggrecan, were up-regulated over the course of each respective differentiation. In each case, peak expressions of these genes were observed by day 30 of differentiation in the bioreactors. Further, the pre-osteoblast marker collagen type I was detected at an earlier phase of osteogenic differentiation, with the greatest peak by day 10. Collagen type X was up-regulated toward the later stages of differentiation.
FIG. 2.
Time-course analysis for bioreactor-differentiated cultures. Expression of osteoblast- and chondrocyte-specific genes was assessed through quantitative PCR (normalized to GAPDH and standardized to undifferentiated embryonic stem cell [ESCs], n = 3). An ANOVA was performed at each time point (*P < 0.01, **P < 0.001).
Transplantation of bioreactor-differentiated ESC-derived cells
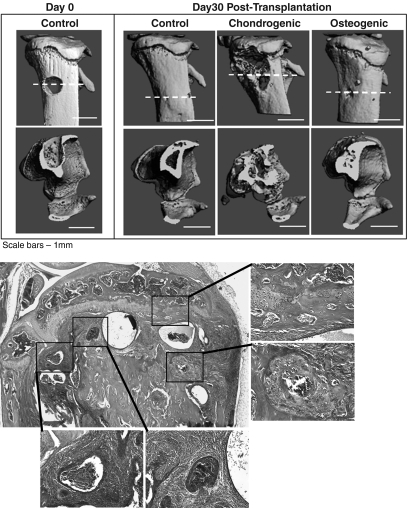
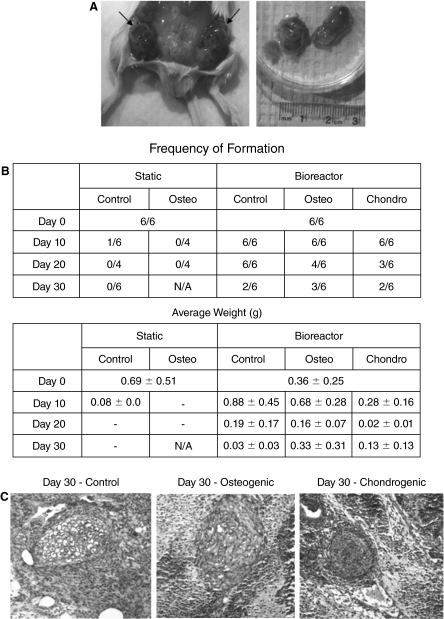
To test the in vivo functionality of the bioreactor-differentiated cells, we developed a burr-hole fracture model in the proximal murine tibia. MicroCT imaging showed that untreated (control) fractures healed effectively in ∼30 days. Specifically, new cortical bone had formed at the injury site and a cross-sectional view of the bone (lower panel of Fig. 3A) revealed that new mineralized tissue had reformed a morphology that was nearly normal. Transplantation of the ESC-derived chondrogenic and osteogenic bioreactor aggregates showed no obvious influence, either positive or negative, to the healing process by days 10 and 20 post-transplantation (data not shown). However, by day 30, severe bone mineral loss was observed at the proximal tibia in the mouse that had received ESC aggregates exposed to the chondrogenic differentiation protocol (Fig. 3A). The presence of disorganized, dense collections of cells of varying morphologies, characteristic of a teratoma, and a lack of bone mineral were observed in histological sections of this tibia (Fig. 3B). The transplantation of the ESC-derived osteogenic aggregates also appeared to alter the healing of the fracture, with excessive mineral present at the injury site by day 30 post-transplantation (Fig. 3A).
FIG. 3.
In vivo transplantation of bioreactor-differentiated cells. (A) Microcomputed tomography (MicroCT) images of the fracture site on day 0 and day 30 following transplantation (the dotted red line indicates the view plane of the images in the lower panel). Control fractures were untreated. (B) Histopathology (H&E staining) of the fracture at day 30 post-transplantation of day 30 bioreactor-differentiated chondrogenic cells. The presence of random cell types and lack of mineralized tissue below the growth plate are clear.
Immunofluorescent analysis of bioreactor-differentiated ESC-derived cells
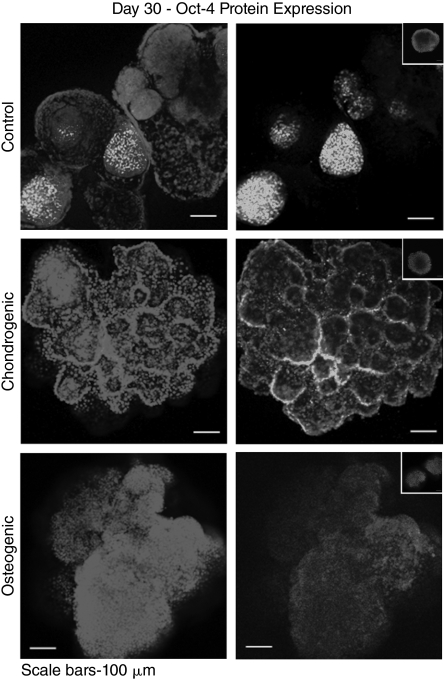
Whole-mount immunofluorescence and confocal microscopy revealed that cells differentiated in the bioreactor cultures showed nuclear expression of Oct-4 at all time points of induced differentiation including day 30 (Fig. 4). It is noteworthy that the localization of the cells expressing Oct-4 within the aggregates varied in each treatment. Specifically, in the untreated (spontaneously differentiated cultures without medium supplements), cells expressing Oct-4 tended to localize centrally in the aggregates; in the cultures treated with chondrogenic inducing agents, cells expressing Oct-4 tended to localize peripherally in the aggregates; in the cultures treated with osteogenic inducing agents, cells expressing Oct-4 were sparse and scattered randomly throughout the aggregates. Also of note, the nuclear staining of these aggregates depicted completely heterogeneous cellular arrangements, suggesting that a variety of cell/tissue types may be present within the aggregates (Fig. 4).
FIG. 4.
Pluripotency marker expression. Immunofluorescence of Oct-4 in aggregates from the bioreactor cultures on day 30 of bioreactor differentiation. The secondary antibody controls for each treatment group did not show nonspecific binding (insets).
Teratoma formation assays
Tissues generated in SCID mice from either static or bioreactor cultures that were treated with differentiation factors (osteogenic or chondrogenic) or spontaneously differentiated (control) for 0, 10, 20, or 30 days were excised and sampled for histopathology on day 18 after transplantation (Fig. 5A). Cells differentiated in static cultures showed a rapid and progressive decrease in the size and frequency of teratoma formation as a function of time of in vitro differentiation (Fig. 5B). Specifically, no teratomas were produced by 18 days in vivo when the cells were differentiated in static culture systems for 20 days prior to being injected (Fig. 5B). In contrast, cells differentiated in suspension bioreactors remained tumorogenic throughout the differentiation period, with teratomas arising in up to 50% of the cases even by day 30 of differentiation (Fig. 5B). Cells of varying morphologies were present in all teratomas (Fig. 5C).
FIG. 5.
Tumorogenicity of bioreactor-differentiated cells. (A) Undifferentiated embryonic stem cells (ESCs) from static or suspension bioreactors form teratomas 18 days post-injection into a SCID mouse. (B) The frequency of teratoma formation diminishes rapidly when ESCs are differentiated in static culture but not in bioreactor culture systems. Excessive mineralization resulted in limited ability to collect the cells for the assay and these samples could therefore not be analyzed. These samples are noted in the table as N/A. (C) Representative sections of teratomas generated from ESCs that were differentiated for 30 days in either untreated, osteogenic, or chondrogenic bioreactor cultures, stained with H&E.
Discrepancy between mRNA and protein expression of lineage-specific markers in differentiated cultures
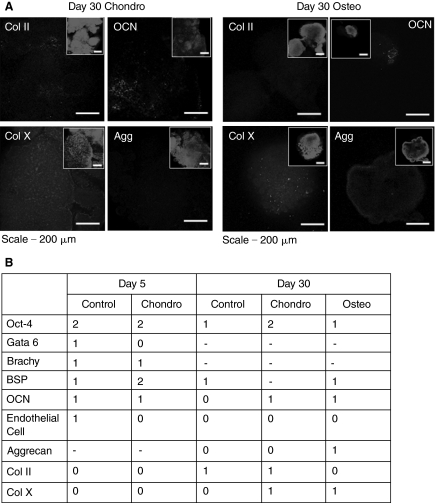
The gene expression patterns depicted in Figure 2 were not found to correlate well with the expression of the respective proteins. Specifically, whole-mount immunofluorescence and confocal microscopy revealed minimal expression of osteogenic and chondrogenic protein markers in bioreactor-differentiated cultures at all time points. Representative images showing the expression of four lineage-specific proteins—collagen II, osteocalcin, collagen X, and aggrecan—are depicted in Figure 6A for both the osteogenic and chondrogenic cultures. Minimal expression of each of these markers was detected over the course of the 30 day differentiation period (day 10 or day 20—data not shown). Brachyury, an early mesodermal marker, was found to be expressed at minimal levels on day 5 of differentiation. A marker specific for endothelial cells was also used in the characterization of the bioreactor aggregates; however, this marker was not detected in these cultures. Minimal expression of all other markers, except Oct-4, was observed (Fig. 6B).
FIG. 6.
Lineage-specific protein marker expression. (A) Representative images depict the expression of 4 markers (collagen II, osteocalcin, aggrecan, collagen X) on day 30 of bioreactor differentiation. (B) The degree of expression of each marker was graded from 0 to 2 (0, no positive cells; 1, few positive cells; 2, majority of cells in the aggregates were positive; dash (-), sample not assessed).
Discussion
For numerous degenerative diseases and injuries, replacement of the damaged tissues through stem cell therapies has recently been explored as a therapeutic alternative. Despite many innovations in this field, progress to meet clinical demands has been slow due to the technical limitations of large-scale expansion of stem cells and/or their progeny. Recently, effective bioreactor protocols for the large-scale expansion of murine ESCs have been developed [2,4,21]. These advances in stem cell expansion meet the need for large numbers of undifferentiated stem cells, but stop short of producing large numbers of tissue-specific progenitor and mature cells.
Following our prior ability to differentiate ESCs toward bone and cartilage in static culture [5,6], the results from the current study were surprising. By day 30 of bioreactor differentiation, we found positive expression of Alizarin red and Alcian blue in the osteogenic and chondrogenic cultures, respectively. Further, assays for calcium content, alkaline phosphatase activity, and proteoglycan content as well as the expression of lineage-specific genes—all commonly used indicators of differentiation—demonstrated that the cultures had undergone differentiation into the appropriate lineages. Although we realized the osteogenic and chondrogenic bioreactor cultures would not likely consist of pure populations of the appropriate cell types, these cells behaved quite differently than anticipated when transplanted into the burr-hole fracture. In the case of the chondrogenic aggregates, histopathology confirmed the presence of a tumor, with tissue arrangements similar to those reported by Wakitani et al. [15]. The formation of a tumor prompted us to further characterize the cell aggregates in the bioreactor cultures.
Surprisingly, all of the bioreactor differentiation cultures (spontaneous, osteogenic, and chondrogenic) revealed a high degree of nuclear Oct-4 expression in the aggregates, even after 30 days of induced differentiation in the absence of LIF, the factor normally responsible for maintaining pluripotency of the cells in vitro. The presence of undifferentiated cells in the chondrogenic and osteogenic bioreactor cultures was also confirmed by their teratogenicity in SCID mice. The persistence of any tumorigenic cells is the ultimate impediment to cell therapies using ESCs and while tumor formation was clearly observed in the chondrogenic bioreactor cultures, the presence of Oct-4 expression in the osteogenic bioreactor cultures is still worrisome given the small sample size and short time of the in vivo experiments.
Typically, Oct-4 expression is down-regulated in ESCs with differentiation in static culture systems [24], even when the ESCs are allowed to undergo spontaneous differentiation without the addition of specific medium supplements. In the current study, ESCs maintained positive nuclear expression of Oct-4, although the spatial distribution of these cells within the aggregates varied remarkably. In spontaneously differentiated bioreactor cultures, Oct-4 expression was localized to the center of the aggregates. This might be caused by concentration gradients due to limited diffusion of biochemical signals, oxygen, and nutrients into the center of the large aggregates [25]. However, in the chondrogenic-differentiated cultures, the Oct-4 expression was localized to the periphery of the aggregates—a region where the cells are exposed to fluid shear. The chondrogenic cultures were differentiated using TGF-β1 and BMP-2, two activators of the TGF-β-signaling pathway. This signaling pathway has recently been implicated in the inhibition of ESC differentiation [26–28]. Further, recent studies with endothelial cells have demonstrated that the TGF-β receptor can be activated through fluid shear and strain, causing downstream regulation of gene expression [29,30]. We did not observe Oct-4 expression in the ESC-derived osteoblasts or chondrocytes when the cells were differentiated in the static culture systems. In our ESC-derived chondrogenic bioreactor cultures, it is therefore conceivable that the concentrations gradients alone or in combination with co-activation of the TGF-β-signaling pathway, through fluid shear and ligand binding, up-regulated markers of ESC pluripotency and inhibited differentiation of the cells.
Overall, we have found that medium supplementation alone is insufficient to drive complete differentiation of ESCs in suspension bioreactors. This could explain why reports of successful bioreactor differentiation protocols for ESCs typically incorporate encapsulation [13,31], extracellular matrix molecules and/or microcarriers [14,32,33], or genetic engineering [31,34] in addition to the use of medium supplementation. By characterizing the environmental conditions in the bioreactors and the mechanism(s) by which these conditions influence ESC differentiation, we will be able to develop a more effective bioprocess for the large-scale production of ESC-derived specialized cells.
Acknowledgments
J.T.T. was awarded scholarships from the Natural Sciences and Engineering Research Council of Canada (NSERC), the Alberta Heritage Foundation for Medical Research (AHFMR), the Faculty of Graduate Studies at the University of Calgary, and the Skeletal Regenerative Medicine Team with the Canadian Institute of Health Research (CIHR). N.z.N. was awarded a fellowship from AHFMR. Y.E.W. was an AHFMR summer student. M.S.K. and D.E.R. received funding through an NSERC Collaborative Health Research Project. D.E.R. is an AHFMR Senior Scholar. J.R.M. is an Investigator of The Arthritis Society. The research was funded by operating grants from CIHR, NIH (5R21AR53738-2) and the Canadian Stem Cell Network Centre of Excellence.
Author Disclosure Statement
The authors have nothing to disclose.
References
- 1.Kraus KH. Kirker-Head C. Mesenchymal stem cells and bone regeneration. Vet Surg. 2006;35:232–242. doi: 10.1111/j.1532-950X.2006.00142.x. [DOI] [PubMed] [Google Scholar]
- 2.Cormier JT. zur Nieden NI. Rancourt DE. Kallos MS. Expansion of undifferentiated murine embryonic stem cells as aggregates in suspension culture bioreactors. Tissue Eng. 2006;12:3233–3245. doi: 10.1089/ten.2006.12.3233. [DOI] [PubMed] [Google Scholar]
- 3.zur Nieden NI. Price FD. Davis LA. Everitt RE. Rancourt DE. Gene profiling on mixed embryonic stem cell populations reveals a biphasic role for beta-catenin in osteogenic differentiation. Mol Endocrinol. 2007;21:674–685. doi: 10.1210/me.2005-0438. [DOI] [PubMed] [Google Scholar]
- 4.Fok EY. Zandstra PW. Shear-controlled single-step mouse embryonic stem cell expansion and embryoid body-based differentiation. Stem Cells. 2005;23:1333–1342. doi: 10.1634/stemcells.2005-0112. [DOI] [PubMed] [Google Scholar]
- 5.zur Nieden NI. Kempka G. Ahr HJ. In vitro differentiation of embryonic stem cells into mineralized osteoblasts. Differentiation. 2003;71:18–27. doi: 10.1046/j.1432-0436.2003.700602.x. [DOI] [PubMed] [Google Scholar]
- 6.zur Nieden NI. Kempka G. Rancourt DE. Ahr HJ. Induction of chondro-, osteo- and adipogenesis in embryonic stem cells by bone morphogenetic protein-2: effect of cofactors on differentiating lineages. BMC Dev Biol. 2005;5:1. doi: 10.1186/1471-213X-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kramer J. Hegert C. Guan K. Wobus AM. Muller PK. Rohwedel J. Embryonic stem cell-derived chondrogenic differentiation in vitro: activation by BMP-2 and BMP-4. Mech Dev. 2000;92:193–205. doi: 10.1016/s0925-4773(99)00339-1. [DOI] [PubMed] [Google Scholar]
- 8.Hegert C. Kramer J. Hargus G. Muller J. Guan K. Wobus AM. Muller PK. Rohwedel J. Differentiation plasticity of chondrocytes derived from mouse embryonic stem cells. J Cell Sci. 2002;115:4617–4628. doi: 10.1242/jcs.00171. [DOI] [PubMed] [Google Scholar]
- 9.Buttery LD. Bourne S. Xynos JD. Wood H. Hughes FJ. Hughes SP. Episkopou V. Polak JM. Differentiation of osteoblasts and in vitro bone formation from murine embryonic stem cells. Tissue Eng. 2001;7:89–99. doi: 10.1089/107632700300003323. [DOI] [PubMed] [Google Scholar]
- 10.Duplomb L. Dagouassat M. Jourdon P. Heymann D. Differentiation of osteoblasts from mouse embryonic stem cells without generation of embryoid body. In Vitro Cell Dev Biol Anim. 2007;43:21–24. doi: 10.1007/s11626-006-9010-4. [DOI] [PubMed] [Google Scholar]
- 11.Yamashita A. Krawetz R. Rancourt DE. Loss of discordant cells during micro-mass differentiation of embryonic stem cells into the chondrocyte lineage. Cell Death Differ. 2009;16:278–286. doi: 10.1038/cdd.2008.149. [DOI] [PubMed] [Google Scholar]
- 12.Stolberg S. McCloskey KE. Can shear stress direct stem cell fate? Biotechnol Prog. 2009;25:10–19. doi: 10.1002/btpr.124. [DOI] [PubMed] [Google Scholar]
- 13.Randle WL. Cha JM. Hwang YS. Chan KL. Kazarian SG. Polak JM. Mantalaris A. Integrated 3-dimensional expansion and osteogenic differentiation of murine embryonic stem cells. Tissue Eng. 2007;13:2957–2970. doi: 10.1089/ten.2007.0072. [DOI] [PubMed] [Google Scholar]
- 14.Tielens S. Declercq H. Gorski T. Lippens E. Schacht E. Cornelissen M. Gelatin-based microcarriers as embryonic stem cell delivery system in bone tissue engineering: an in-vitro study. Biomacromolecules. 2007;8:825–832. doi: 10.1021/bm060870u. [DOI] [PubMed] [Google Scholar]
- 15.Wakitani S. Takaoka K. Hattori T. Miyazawa N. Iwanaga T. Takeda S. Watanabe TK. Tanigami A. Embryonic stem cells injected into the mouse knee joint form teratomas and subsequently destroy the joint. Rheumatology (Oxford) 2003;42:162–165. doi: 10.1093/rheumatology/keg024. [DOI] [PubMed] [Google Scholar]
- 16.Uusitalo H. Rantakokko J. Ahonen M. Jamsa T. Tuukkanen J. KaHari V. Vuorio E. Aro HT. A metaphyseal defect model of the femur for studies of murine bone healing. Bone. 2001;28:423–429. doi: 10.1016/s8756-3282(01)00406-9. [DOI] [PubMed] [Google Scholar]
- 17.Einhorn TA. Lane JM. Burstein AH. Kopman CR. Vigorita VJ. The healing of segmental bone defects induced by demineralized bone matrix. A radiographic and biomechanical study. J Bone Joint Surg Am. 1984;66:274–279. [PubMed] [Google Scholar]
- 18.Bostrom M. Lane JM. Tomin E. Browne M. Berberian W. Turek T. Smith J. Wozney J. Schildhauer T. Use of bone morphogenetic protein-2 in the rabbit ulnar nonunion model. Clin Orthop Relat Res. 1996:272–282. doi: 10.1097/00003086-199606000-00034. [DOI] [PubMed] [Google Scholar]
- 19.Bruder SP. Kraus KH. Goldberg VM. Kadiyala S. The effect of implants loaded with autologous mesenchymal stem cells on the healing of canine segmental bone defects. J Bone Joint Surg Am. 1998;80:985–996. doi: 10.2106/00004623-199807000-00007. [DOI] [PubMed] [Google Scholar]
- 20.Einhorn TA. Clinically applied models of bone regeneration in tissue engineering research. Clin Orthop Relat Res. 1999;3675:S59–S67. doi: 10.1097/00003086-199910001-00007. [DOI] [PubMed] [Google Scholar]
- 21.zur Nieden NI. Cormier JT. Rancourt DE. Kallos MS. Embryonic stem cells remain highly pluripotent following long term expansion as aggregates in suspension bioreactors. J Biotechnol. 2007;129:421–432. doi: 10.1016/j.jbiotec.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 22.Farndale RW. Sayers CA. Barrett AJ. A direct spectrophotometric microassay for sulfated glycosaminoglycans in cartilage cultures. Connect Tissue Res. 1982;9:247–248. doi: 10.3109/03008208209160269. [DOI] [PubMed] [Google Scholar]
- 23.Livak KJ. Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 24.Palmqvist L. Glover CH. Hsu L. Lu M. Bossen B. Piret JM. Humphries RK. Helgason CD. Correlation of murine embryonic stem cell gene expression profiles with functional measures of pluripotency. Stem Cells. 2005;23:663–680. doi: 10.1634/stemcells.2004-0157. [DOI] [PubMed] [Google Scholar]
- 25.Sen A. Kallos MS. Behie LA. Expansion of mammalian neural stem cells in bioreactors: effect of power input and medium viscosity. Brain Res Dev Brain Res. 2002;134:103–113. doi: 10.1016/s0165-3806(01)00328-5. [DOI] [PubMed] [Google Scholar]
- 26.Dreesen O. Brivanlou AH. Signaling pathways in cancer and embryonic stem cells. Stem Cell Rev. 2007;3:7–17. doi: 10.1007/s12015-007-0004-8. [DOI] [PubMed] [Google Scholar]
- 27.Saha S. Ji L. de Pablo JJ. Palecek SP. TGFbeta/Activin/Nodal pathway in inhibition of human embryonic stem cell differentiation by mechanical strain. Biophys J. 2008;94:4123–4133. doi: 10.1529/biophysj.107.119891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu RH. Sampsell-Barron TL. Gu F. Root S. Peck RM. Pan G. Yu J. Antosiewicz-Bourget J. Tian S. Stewart R. Thomson JA. NANOG is a direct target of TGFbeta/activin-mediated SMAD signaling in human ESCs. Cell Stem Cell. 2008;3:196–206. doi: 10.1016/j.stem.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ohno M. Cooke JP. Dzau VJ. Gibbons GH. Fluid shear stress induces endothelial transforming growth factor beta-1 transcription and production. Modulation by potassium channel blockade. J Clin Invest. 1995;95:1363–1369. doi: 10.1172/JCI117787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cucina A. Sterpetti AV. Borrelli V. Pagliei S. Cavallaro A. D'Angelo LS. Shear stress induces transforming growth factor-beta 1 release by arterial endothelial cells. Surgery. 1998;123:212–217. [PubMed] [Google Scholar]
- 31.Bauwens C. Yin T. Dang S. Peerani R. Zandstra PW. Development of a perfusion fed bioreactor for embryonic stem cell-derived cardiomyocyte generation: oxygen-mediated enhancement of cardiomyocyte output. Biotechnol Bioeng. 2005;90:452–461. doi: 10.1002/bit.20445. [DOI] [PubMed] [Google Scholar]
- 32.Bielby RC. Boccaccini AR. Polak JM. Buttery LD. In vitro differentiation and in vivo mineralization of osteogenic cells derived from human embryonic stem cells. Tissue Eng. 2004;10:1518–1525. doi: 10.1089/ten.2004.10.1518. [DOI] [PubMed] [Google Scholar]
- 33.Kim S. Kim SS. Lee SH. Eun Ahn S. Gwak SJ. Song JH. Kim BS. Chung HM. In vivo bone formation from human embryonic stem cell-derived osteogenic cells in poly(d,l-lactic-coglycolic acid)/hydroxyapatite composite scaffolds. Biomaterials. 2008;29:1043–1053. doi: 10.1016/j.biomaterials.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 34.Zandstra PW. Bauwens C. Yin T. Liu Q. Schiller H. Zweigerdt R. Pasumarthi KB. Field LJ. Scalable production of embryonic stem cell-derived cardiomyocytes. Tissue Eng. 2003;9:767–778. doi: 10.1089/107632703768247449. [DOI] [PubMed] [Google Scholar]