Abstract
Objective
To explore the influence of reproductive aging, body mass index (BMI) and the menstrual cycle on adiponectin (AD) and leptin concentrations.
Design
Cross-sectional comparison in age- and BMI-matched non-obese volunteers with regular cycles (CO, n = 19) or in early menopause (EPM, n = 19), aged 40–52 years, and a young cycling group (CY, n = 21), aged 20–30 years. Measures: sex steroids, fasting AD, leptin, insulin, glucose, adiponectin/leptin (A/L) ratio and HOMA-IR. In ovulatory women, AD, estradiol and progesterone were assessed weekly across the same menstrual cycle.
Results
Insulin, glucose, HOMA-IR, A/L ratio and leptin values were similar across the three study groups. AD differed, with the highest concentrations in the EPM group (CY: 13.0 ± 0.9 µg/ml vs. CO: 14.0 ± 1.1 µg/ml vs. EPM: 17.7 ± 1.5 µg/ml; p = .05). Values among cycling women were similar. When the cycling groups were combined into a premenopausal (PRE) group and compared to EPM women by BMI (> or ≤ 25 kg/m2), leptin concentrations were higher and A/L ratios lower in PRE and EPM overweight subgroups vs. normal-weight subgroups. AD was lower in overweight, cycling women and higher in the normal-weight EPM subgroup compared to normal-weight PRE women. In cycling women, AD did not vary across the menstrual cycle.
Conclusion
Non-obese, midlife women experience minimal adverse effects from reproductive aging on insulin sensitivity and adipokine secretion. The menstrual cycle is not a key mediator of AD. Early menopause has differential, BMI-dependent effects on adipokine secretion.
Keywords: menopause, menstrual cycle, adipokines
The risk for insulin resistance (IR) increases after menopause and may be related to the decline in estrogen (Carr, 2003). In cycling women, estrogen differentially inhibits lipolysis in adipose tissue and promotes the deposition of fat in the gluteo-femoral region (Pedersen, Kristensen, Hermann, Katzenellenbogen, & Richelsen, 2004). With estrogen suppression at menopause, central fat mass increases, producing a more android pattern of fat distribution (Poehlman, Toth, & Gardner, 1995). The postmenopausal accumulation of intra-abdominal fat is presumed to be disease promoting, as this fat depot is a major predictor of insulin resistance (Cnop et al., 2002) and, in turn, the metabolic syndrome, cardiovascular disease (CVD) and diabetes (Carr et al., 2004; Kannel et al., 1991; Reusch, 2002). However, aging (Guo, Zeller, Chumlea, & Siervogel, 1999) is also believed to accelerate the risk for these conditions. It thus remains uncertain to what extent the decline of estrogen contributes to this risk.
The discovery that adipose tissue is an active endocrine organ (Miner, 2004) has provided the opportunity to qualify its role in the pathogenesis of the metabolic syndrome, CVD and diabetes. Adiponectin (AD), the most abundant adipocytokine, improves insulin sensitivity and inhibits inflammatory mediators that adversely affect glucose and lipid metabolism (Ahima, 2006). Inversely related to total and central adiposity (Cnop et al., 2003), low levels of AD are associated with insulin resistance and have been documented in persons with type 2 diabetes, coronary artery disease and the metabolic syndrome (Frystyk et al., 2007; Trujillo & Scherer, 2005; Weyer et al., 2001).
AD concentrations are sexually dimorphic, with higher levels in females, implying a role for the sex steroids in its regulation (Nishizawa et al., 2002). However, evidence for a causal relationship between estradiol (E2) and AD has been inconsistent. Increased (Gavrila et al., 2003; Lee et al., 2009), decreased (Chu, Cosper, Orio, Carmina, & Lobo, 2006) and unchanged (Jurimae, Rembel, Jurimae, & Rehand, 2005; Milewicz et al., 2005) AD concentrations have all been observed with menopause. However, nearly half of the existing studies failed to measure serum E2 as a direct marker of menopause status, and only one (Benetti-Pinto et al., in press) tightly controlled for age, a variable directly associated with AD concentrations (Cnop et al., 2003). Moreover, most subjects in existing studies were overweight or obese, a clinical feature closely associated with diminished AD production (Arita et al., 1999). These potential confounders have precluded a direct assessment of the relationship between estrogen status and adiponectin.
Leptin, the first adipocytokine to be characterized, influences several metabolic functions (Meier & Gressner, 2004). Leptin levels are directly proportional to body mass index (BMI) and fat mass, with greater expression demonstrated in the subcutaneous fat depots (Van Harmelen et al., 1998). High leptin concentrations are associated with insulin resistance (Margetic, Cazzola, Pegg, & Hill, 2002). As with AD, leptin secretion is sexually dimorphic, with higher levels in women (Zoico et al., 2004). Evidence for a causal relationship between leptin and E2 has also been inconsistent, with increased (Chu et al., 2006), decreased (Hong et al., 2007), and unchanged (Gavrila et al., 2003) leptin concentrations all associated with menopause.
Taken together, these discordant findings suggest that multiple factors are likely to mediate changes in adipokine secretion in aging women. Recently, the AD-to-leptin ratio (A/L) has been shown to serve as a novel marker of insulin sensitivity (Inoue, Yano, Yamakado, Maehata, & Suzuki, 2006); however, it has seldom been assessed for changes with menopause.
In the United States unparalleled numbers of women are expected to transition to menopause over the next decade (North American Menopause Society, 2007), with an accompanying increased risk for obesity and metabolic disease (Park et al., 2003). As health care professionals distinguished by their attention to health promotion and disease prevention, nurses play a role in the early detection and management of preventable conditions in women. Scientific evidence is necessary to characterize the clinical factors, including menopause, related to metabolic disease risk, such that targeted nursing assessment and management strategies can be developed to preserve women’s health during normal aging.
The purpose of this study was to define the influence of reproductive aging, body mass index and the menstrual cycle on adipokine secretion. Using a cross-sectional comparative group design, we examined adiponectin and leptin concentrations and measures of insulin sensitivity in three groups of healthy non-obese women, differing by age (younger cycling vs. older cycling) and reproductive stage (age-matched older cycling vs. early postmenopausal). In a subset of cycling women, we prospectively assessed adiponectin concentrations across the menstrual cycle to determine the effects of ovulation. Archived blood samples from a recently completed parent study of menopause effects on bone health (Lukacs et al., 2006) were used to assess the adipokines and insulin sensitivity parameters. Data on general clinical features (i.e., age, body mass index, sex steroids) of the study sample previously reported as part of the earlier study are presented here as background context for the new study question.
Methods
Study Sample
Approval was obtained from the University of Michigan Hospital's Institutional Review Board for a protocol involving analysis of new data using archived human blood samples. Fifty-nine healthy volunteers from the parent study (Lukacs et al., 2006) were categorized into three study groups by age and menstrual status: 21 cycling young women (CY; 20–30 years), 19 cycling older women (CO; 40–52 years) and 19 women in the early postmenopause period (EPM; 40–52 years; mean 2.8 ± 0.5 years postmenopause; Soules et al., 2001).
BMI for the participants ranged from 20 to 28 kg/m2. The women reported no history of smoking, chronic medical conditions (i.e., heart disease, diabetes, polycystic ovarian syndrome), or current use of oral contraceptives, hormone therapy, anabolic or corticosteroids; had not been pregnant or breast feeding in the past 6 months nor engaged in dieting or exercise of more than 5 hr/week; and reported infrequent (less than 1 drink/week) alcohol consumption. Plasma values for liver function, hemoglobin, hematocrit, glucose, testosterone, free testosterone, dehydroepiandosterone sulfate (DHEAS), and thyroid stimulating hormone were within normal female ranges. In EPM participants, screening values for follicular stimulating hormone (FSH) were ≥ 30 IU/L, and all women reported a final spontaneous menstrual period at least 12 months prior to study. Menstruating women had a history of menses every 21–45 days.
Protocol and Procedures
Participants in the parent study (Lukacs et al., 2006) completed serial blood sampling at the General Clinical Research Center (GCRC) at the University of Michigan Hospital Systems. Fasting samples included glucose, triglycerides, and cholesterol. Mean values of four hourly samples for FSH and two samples (9:00 am and 12 noon) for estradiol (E2) and progesterone were used for analysis. Height and weight measurements were obtained via measurement to calculate BMI: weight (kilograms)/height (meters squared).
CY and CO women were studied in the early follicular phase (cycle Day 4 ± 1; Day 1 = the first day of menses) of a menstrual cycle and returned to the GCRC for three additional blood samples at weekly intervals for hormone measures (E2, progesterone). Menstrual cycle length was determined from the first day of menstrual bleeding to the day before the next bleeding episode.
Assays
Plasma samples were assayed for E2, progesterone, and FSH as previously described (Lukacs et al., 2006). Androgens (total testosterone, free testosterone and DHEAS) were measured by the hospital laboratory as part of the endocrine screening test panel. Fasting plasma glucose (FPG) was determined by glucose oxidase method in the laboratories of the University of Michigan Hospital System (Ann Arbor, MI). The lower limit of detection (LOD) was 20mg/dl; inter-assay coefficient of variation (CV) was 2.6%.
This study analyzed new data on plasma adiponectin, leptin and insulin concentrations from archived fasting baseline blood samples; AD concentrations were also measured from the archived weekly blood samples of menstruating women. AD and leptin values were used to calculate the ratio of AD to leptin (A/L ratio). Fasting plasma glucose (FPG) and fasting insulin (FI) concentrations were used to estimate insulin resistance by homeostasis model assessment (HOMA-IR): FI (µU/ml) × FPG (mmol/L) / 22.5 (Matthews et al., 1985) with the Oxford HOMA calculator (Levy, Matthews, & Hermans, 1998) program v2.2 (http://www.dtu.ox.ac.uk/index.php?maindoc=/homa/index.php). For the prospective assessment of the menstrual cycle, stored plasma was sufficient only to determine AD values.
Plasma samples for AD, L and FI were measured in duplicate by double antibody radioimmunoassay (RIA) (Linco Diagnostics, Inc, St. Charles, MO). The LOD for insulin was 2 µU/ml, for leptin 0.5ng/ml and for AD 1 µg/ml. Intra- and inter-assay CVs were 3.2% and 4.3%, respectively, for insulin; 3.3% and 6.0%, respectively, for leptin; and 1.6% and 7.0%, respectively, for AD. For the purposes of statistical analysis, values below the assay LOD were set at the value of assay sensitivity.
Statistical Analysis
Descriptive data are presented as mean ± SEM. As hormone measures and physiologic variables were positively skewed, we used natural log-transformed values in the analysis and treated measures as continuous variables. Differences in AD concentrations and parameters of insulin sensitivity (FI, FPG, HOMA-IR, A/L ratio, lipids) between the three study groups were evaluated by one-way ANOVA with post hoc Tukey. Correlations among AD concentrations, age, BMI, sex steroids, and insulin sensitivity parameters were performed using Pearson r. Group differences in categorical variables were compared using Chi square or Fisher's exact test. Two-tailed p values ≤ 0.05 were regarded as statistically significant.
To examine the influence of ovulation on AD concentrations, we included data from subjects (n = 21) who completed all four serial blood draws during the study cycle and had documented evidence of ovulation (progesterone ≥ 3 ng/ml) on at least one of the weekly follow-up blood tests. Repeated measures ANOVA was used to examine changes in AD at the four time points: the early follicular (EF) baseline evaluation and three weekly (EF+1, EF+2, EF+3) follow-up time points in the same menstrual cycle. As menstrual cycle lengths varied and the weekly samples reflected different times within individuals, the weekly sample with the highest progesterone value and a rising E2 level from baseline was used to anchor the midluteal (ML) phase value. AD concentrations from the EF and ML phases were compared using the t-test. All analyses were performed with SPSS software (version 14.0; SPSS, Chicago, IL).
Results
Clinical Characteristics
Subjects identified themselves as Caucasian (80%), Hispanic (8%), Asian (7%) and African American (5%). Study groups did not differ by race (χ2 = 3.07, p = 0.2). Table 1 presents clinical characteristics of the three study groups. CO and EPM subjects were similar in age (matched within 1–2 years of each other by design) and older than the CY group. Testosterone values differed as expected, with higher concentrations demonstrated in CY women. E2 was similar in the CO and CY women, but mean FSH was higher (p = .0001) in the CO group compared to the CY group. Using the criteria developed by the Stages of Reproductive Aging Workshop (Soules et al., 2001), CO women were in the late reproductive stage while CY women were in the peak reproductive stage.
Table 1.
Clinical Characteristics by Study Group.
| Characteristic | Cycling Young (n = 21) |
Cycling Old (n = 19) |
Early Postmenopause (n = 19) |
|---|---|---|---|
| Age (years) | 24.5 ± 0.8 | 48.9 ± 0.5a | 49.0 ± 0.7a |
| BMI (kg/m2) | 23.4 ± 0.4 | 24.0 ± 0.7 | 24.1 ± 0.8 |
| FSH (IU/L) | 4.8 ± 0.3 | 16.6 ± 3.1a | 87.0 ± 7.5a,b |
| E2 (pg/ml) | 23.8 ± 2.5 | 33.7 ± 7.8 | 6.7 ± 1.3a,b |
| Testosterone (ng/ml) | 0.4 ± 0.03 | 0.2 ± 0.02a | 0.2 ± 0.02a |
| Free Testosterone (pg/ml) | 0.9 ± 0.07 | 0.5 ± 0.06a | 0.5 ± 0.08a |
| TSH (mU/L) | 1.9 ± 0.2 | 2.8 ± 0.3 | 2.6 ± 0.2 |
| Cholesterol (mg/dl) | 171.0 ± 6.0 | 171.6 ± 9.4 | 215.5 ± 10.6a,b |
| Triglycerides (mg/dl) | 81.0 ± 8.1 | 87.7 ± 14.7 | 86.7 ± 13.1 |
Note. Values are mean ± SEM, determined on menstrual cycle Day 5. Post hoc Tukey analyses were performed if analysis of variance revealed significant group effect. Normality was ensured by logarithmic transformation.
BMI = body mass index; E2 = estradiol; FSH = follicle stimulating hormone; TSH = thyroid stimulating hormone.
p ≤ .05 vs. cycling young women.
p ≤ .02 vs. cycling older women.
The majority of the sample (69%) was of normal weight (BMI ≤ 25 kg/m2). There were no differences in BMI among the study groups. Triglyceride concentrations were similar among the subjects but cholesterol was higher in the early postmenopause women compared to both CO and CY (p = .001) women.
Insulin Sensitivity Parameters
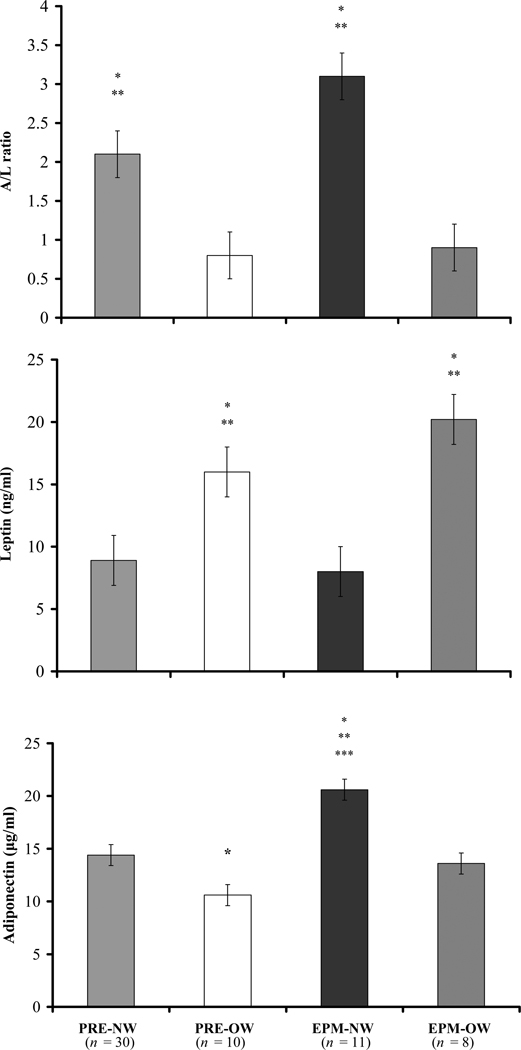
There were no differences in insulin sensitivity values (FI, FPG, HOMA-IR, A/L ratio) among the three study groups (data not shown). BMI was negatively correlated with the A/L ratio (r = −0.70; p < .0001), positively associated with FI (r = 0.28; p = .03) and HOMA-IR (r = 0.28; p = .03), and showed a trend for a positive relationship with FPG (r = 0.24; p = .06). To evaluate the interaction of BMI (normal weight [NW]: BMI ≤ 25 kg/m2; overweight [OW]: BMI > 25kg/m2) and menopause status on insulin sensitivity parameters, subjects were stratified into four groups: premenopausal normal-weight (PRE-NW; n = 30), premenopausal overweight (PRE-OW; n = 10), early postmenopausal normal-weight (EPM-NW; n = 11) and early postmenopausal overweight (EPM-OW; n = 8). As group sizes were small, we used the Kruskall Wallis ANOVA with post hoc Mann Whitney U tests to examine differences among the four groups. By ANOVA, no differences in measures of FI, FPG or HOMA-IR were observed among the groups, although trends in the expected directions were apparent as indicated by p values of < .1 (Table 2). A/L ratio values were lower (less insulin sensitivity) in the OW groups compared to the NW groups regardless of menopause status (Table 2 and Figure 1, top panel).
Table 2.
Insulin Sensitivity Measures of Study Participants Classified by Menopause Status and Body Mass Index.
| Insulin Sensitivity Measure |
Premenopause Normal Weight (PRE-NW) (n = 30) |
Premenopause Overweight (PRE-OW) (n = 10) |
Early Postmenopause Normal Weight (EPM-NW) (n = 11) |
Early Postmenopause Overweight (EPM-OW) (n = 8) |
|---|---|---|---|---|
| FI (µU/ml)* | 10.0 ± 1.8 | 12.3 ± 4.0 | 6.3 ± 0.6 | 10.5 ± 1.8 |
| FPG (mg/dl) | 85.7 ± 1.4 | 86.3 ± 2.0 | 87.8 ± 1.9 | 89.6 ± 2.8 |
| HOMA-IR | 1.3 ± 0.2 | 1.5 ± 0.5 | 0.8 ± 0.08 | 1.3 ± 0.2 |
| A/L ratio | 2.1 ± 0.2a,b | 0.8 ± 0.2 | 3.1 ± 0.5a,b | 0.9 ± 0.4 |
Note. Values are mean ± SEM, determined on menstrual cycle Day 5. Mann Whitney U post hoc pairwise comparisons were used if Kruskal Wallis ANOVA demonstrated significant group effect.
A/L ratio = adiponectin-to-leptin ratio; FI = fasting insulin; FPG = fasting plasma glucose; HOMA-IR = homeostasis model assessment for insulin resistance: HOMA-IR was estimated using the formula FI (µU/ml) × FPG (mmol/L)/22.5 (Matthews et al., 1985) with the Oxford HOMA calculator program v2.2 (http://www.dtu.ox.ac.uk/index.php?maindoc=/homa/index.php).
Insulin values below the assay detection limit (2 µU/ml) are set at the assay detection limit.
p < .01 vs. PRE-OW.
p < .01 EPM-OW.
Figure 1.
Adiponectin-to-leptin (A/L) ratio and leptin and adiponectin concentrations of study participants by menopausal status and weight groups. Premenopausal = PRE; early postmenopausal = EPM; normal-weight = NW (BMI ≤ 25 kg/m2); overweight = OW (BMI > 25 kg/m2). Data are mean ± SEM. Significant group differences determined by Kruskall Wallace ANOVA and post hoc Mann Whitney U test.
A/L ratio: *p < .01 vs. PRE-OW; **p < .01 EPM-OW.
Leptin: *p < .002 vs. PRE-NW; **p < .004 vs. EPM-NW.
Adiponectin: *p = .01 vs. PRE-NW; **p < .0002 vs. PRE-OW; ***p = .03 vs. EPM-OW
Adipokines
By ANOVA, AD differed by study group, with the highest concentrations found in the EPM group (CY: 13.0 ± 0.9 µg/ml vs. CO: 14.0 ± 1.1 µg/ml vs. EPM: 17.7 ± 1.5 µg/ml; p = .05), while leptin values were similar across the groups (CY: 10.9 ± 1.1 µg/ml vs. CO: 10.5 ± 1.4 µg/ml vs. EPM: 13.1 ± 2.0 µg/ml; p = NS). When subjects were reclassified by menopause status and BMI, EPM-NW women demonstrated higher levels of AD than their overweight counterparts and both groups of premenopausal women (Figure 1, bottom panel). Lower AD levels were also observed in PRE-OW compared to PRE-NW women. Leptin concentrations were higher in both overweight groups regardless of stage of reproductive aging (Figure 1 middle panel).
Factors Associated with Adipokines
AD levels were negatively correlated with BMI (r = −0.34; p = .009), leptin (r = −0.31; p = .02), and triglycerides (r = −0.38; p = 0.003) and positively associated with the A/L ratio (r = 0.71; p < .0001). A trend for an inverse relationship between AD and insulin (r = −0.24; p = .06) and HOMA-IR (r = −0.25; p = .06) was observed. Age was not directly correlated with AD (r = 0.23; p = 0.8) but became significant when controlling for BMI (r = 0.29; p < .03). Leptin was positively correlated with BMI (r = 0.72; p < .0001), cholesterol (r = 0.28; p = .03), triglycerides (r = 0.43; p = .001), FPG (r = 0.40; p = .002), insulin (r = 0.38; p = 0.003), and HOMA-IR values (r = 0.40; p = .002) but negatively associated with the A/L ratio (r = −0.89; p < .0001). Leptin did not demonstrate an association with age (r = 0.035; p = NS). Neither AD nor leptin concentrations were correlated with E2, testosterone or free testosterone levels.
Menstrual Cycle Effects on Adiponectin
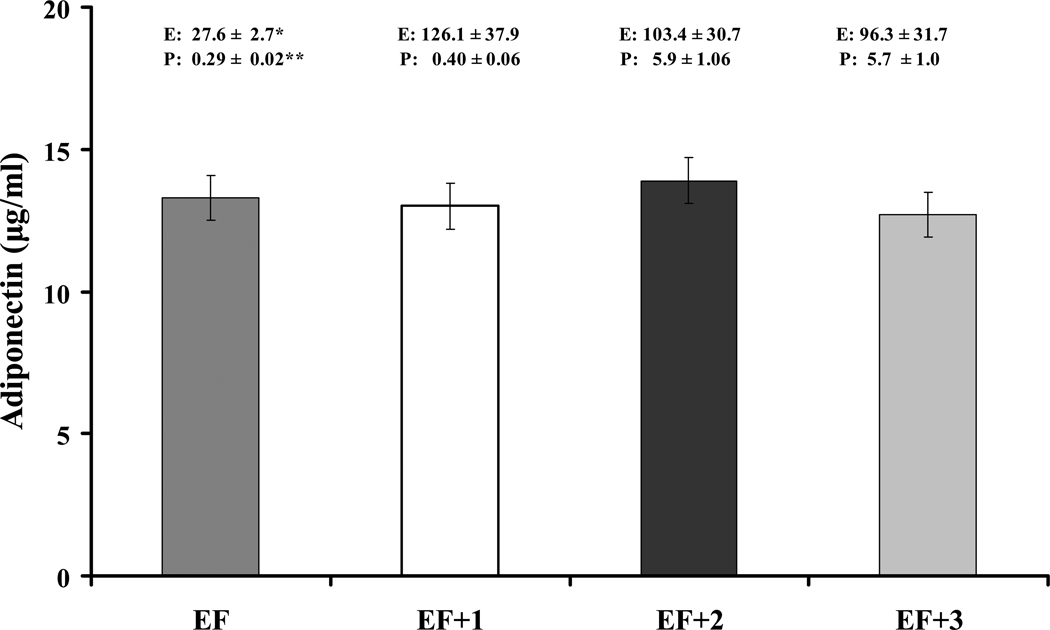
The subset of cycling women were 37.2 ± 2.7 years of age on average, of normal weight (mean BMI = 23.3 ± 0.6 kg/m2) with a mean cycle length of 30.3 ± 0.9 days. By repeated measures ANOVA, mean E2 and progesterone concentrations changed significantly as expected across the menstrual cycle, but no differences in AD secretion were detected (Figure 2). When the mean EF and ML values were compared, E2 and progesterone values again differed, but AD concentrations were similar (EF: 13.3 ± 0.9 µg/ml vs. ML: 13.1 ± 1.0 µg/ml; t = 0.33; p = .75). Neither weight (NW, OW) nor age (young, old) status demonstrated an interaction with AD across the cycle. AD concentrations were not correlated with E2 or progesterone values across the cycle.
Figure 2.
Comparison of adiponectin concentrations in the early follicular (EF) phase and weekly intervals (EF + 1 week, EF + 2 weeks, EF + 3 weeks) during the menstrual cycle (n = 21; F = 1.6; p = .21). Estradiol (E2; pg/ml) and Progesterone (P; ng/ml). Repeated measures ANOVA. Mean ± SEM. *F = 18.0; p < .0001. **F = 44.5; p < .0001.
Discussion
Using a cross-sectional, case-controlled approach, we did not observe an aging effect on AD secretion in non-obese midlife women. In older cycling women with adequate E2, AD values were similar to those of the younger group despite a mean age difference of more than 20 years. In contrast, early postmenopausal women of similar age as the CO group but with negligible estrogen levels demonstrated significantly higher AD concentrations than the young cycling women. When the confounder of high BMI was removed, this menopause effect on AD was heightened. Taken together, these findings support an inhibitory role for estrogen on AD secretion during the premenopausal years that is removed in early menopause.
Our data suggest that an elevated BMI prevents the early postmenopausal AD rise. When the CY and CO women were combined into one premenopausal group and compared to early postmenopause women, with both groups further stratified by BMI as normal weight (NW) or overweight (OW), AD levels were higher in EPM-NW women compared to all other groups, demonstrating the dual effects of normal body weight and estrogen loss. In contrast, AD was similar between PRE and EPM overweight women, suggesting that high BMI inhibited the potential rise in AD early in the menopause transition. Our results extend those of earlier work in mostly obese women with the metabolic syndrome (Chu et al., 2006; Hong et al., 2007) demonstrating that the adverse (suppressive) effect of body weight on AD secretion is present even in healthy, overweight women who do not meet the clinical definition of obesity.
Most prior investigations have been cross sectional, and researchers have observed either decreased (Chu et al., 2006; Sieminska et al., 2006) or unchanged (Hong et al., 2007; Milewicz et al., 2005; Nishizawa et al., 2002) AD concentrations after menopause. Differences in sample populations such as study groups with known metabolic conditions (Chu et al., 2006; Hong et al., 2007; Sieminska et al., 2006) or unaddressed confounders such as advanced age (Hong et al., 2007; Sieminska et al., 2006) may explain the discrepancies. The failure to confirm the reproductive aging stage of participants with both E2 and FSH measures (Jurimae et al., 2005; Nishizawa et al., 2002) or control for the timing of adipokine sampling in premenopausal women (Chu et al., 2006) may also account for differences in study results. The higher AD concentrations observed in our early postmenopausal group, however, are congruent with findings from a recent longitudinal study that demonstrated a significant but small increase in AD across the menopause transition in healthy women evaluated for changes in cardiovascular risk factors and fat mass measures (Lee et al., 2009).
In contrast, the few studies of AD secretion across the menstrual cycle demonstrate very consistent findings. An analysis of data in patients (mean age 33 years) with endometriosis and other gynecological complaints suggested that AD levels do not differ between the proliferative and secretory phase of a menstrual cycle, times when estrogen and progesterone are known to change dramatically (Takemura et al., 2005). In two pilot studies of lean women (Dafopoulos, Sourlas, Kallitsaris, Pournaras, & Messinis, in press; Kleiblova, Springer, & Haluzik, 2006), no differences in AD values were observed across the cycle phases. Our findings in a larger sample of both young and older cycling women corroborate this earlier work and confirm the stability of AD secretion despite changes in the sex steroid milieu with ovulation.
The paradoxical findings between the menopause and menstrual-cycle arms in our study suggest there is an inhibitory effect of estrogen on AD during the reproductive years that remains stable and unaffected by dramatic elevations in the sex steroid milieu with ovulation. In early menopause, as estrogen declines below early follicular phase levels, this inhibition is removed. In addition, the lack of a menstrual cycle effect fails to implicate progesterone in this ovarian inhibition and suggests that follicular-phase levels of estradiol are sufficient to maximally suppress AD secretion.
Measures of insulin sensitivity were similar among the three study groups and did not deteriorate as expected after menopause. The preservation of insulin sensitivity in early menopause in conjunction with the rise in AD is counterintuitive, given the reported increases in central adiposity associated with estrogen loss (Poehlman, Toth, & Gardner, 1995) and the inverse association of AD and insulin resistance with these fat depots (Cnop et al., 2003). Although we did not assess body fat characteristics, it is possible that our postmenopausal subjects, who were early in the postmenopausal period (mean 2.8 years), had not yet developed significant enough increases in central adiposity to suppress AD secretion. A later (> 5 years) postmenopausal study group would provide the opportunity to examine the duration of menopause as a contributing factor to this interaction between estradiol, central adiposity, AD secretion and insulin resistance risk. In addition to incorporating years postmenopause as a study variable, future work should include measures of central and peripheral adiposity to clarify this relationship. Finally, our observation of a significantly blunted A/L ratio in both pre- and early-postmenopausal overweight groups, in the face of unaltered values of other measures of insulin sensitivity, supports emerging data for use of this ratio as a more sensitive marker of insulin sensitivity (Inoue, Yano, Yamakado, Maehata, & Suzuki, 2006).
Leptin concentrations were positively associated with BMI, insulin and HOMA-IR values in the present study, which corroborates previous reports (Chu et al., 2006; Hong et al., 2007; Kontogianni, Dafni, Routsias, & Skopouli, 2004). The higher levels of leptin demonstrated in overweight women compared to their normal-weight counterparts, regardless of menopause status, confirm the findings of prior studies, well controlled for BMI and menopause status, (Hadji et al., 2000; Haffner, Mykkanen, & Stern, 1997) that demonstrated that body size and not estrogen status is the major mediator of L secretion.
The tight control for the confounders of age and BMI and the accurate characterization of E2 status with serial samples used to calculate a mean value are major strengths of this study. The cross-sectional design precludes a determination of causality and the small number of minority women in the sample limits generalizability, especially to populations most at risk for the adverse effects of obesity. Without measures of adiposity, we could not account for the effects of body fat distribution on adipokine concentrations in conjunction with E2 status.
Our findings are limited to women in the early postmenopausal period and cannot be extended to those in the late (> 5years) postmenopausal period (Soules et al., 2001). In some analyses, CY and CO women were categorized as premenopausal but were likely in different reproductive stages. While the cycling young women in this study were in the peak reproductive stage, the cycling older women who had elevations in FSH and regular menstrual cycles, as determined by menstrual diaries at screening, were most likely in either the late reproductive stage or on the cusp of the early menopausal transition where some menstrual-cycle length variability is occurring (Soules et al., 2001).
In summary, our findings support the following hypotheses: 1) in the absence of obesity, midlife women demonstrate minimal adverse aging effects on insulin sensitivity and adipokine secretion; 2) menopause is not a major mediator of leptin secretion; 3) even in non-obese women, an elevated BMI (25–30 kg/m2) plays a key role in adverse effects on both leptin and AD secretion before and after menopause; 4) the elevation in AD in the early postmenopausal period, coupled with the lack of change in AD across the menstrual cycle, suggests that follicular phase concentrations of E2 may be sufficient to maximally suppress AD secretion in normal-weight women.
These findings also support the protective effects of a normal BMI in early menopause and substantiate the importance of addressing body-weight management in the clinical care of women across the lifespan. Future studies using larger, more diverse study populations are needed to confirm these findings, further define the interactive effects of menopause on women's health, and ultimately guide the development of targeted nursing interventions to promote health and reduce metabolic disease risk.
Acknowledgments
This research was conducted while Dr. Rouen was completing her doctoral work under the mentorship of Dr. Reame, Rhetaugh Graves Dumas Professor of Nursing, and Dr. Lukacs, Assistant Research Scientist, School of Nursing at the University of Michigan, Ann Arbor, MI. We wish to thank Pamela Olton, Alice Rolfes-Curl and Vasantha Padmanabhan (director) of the pediatric endocrinology laboratories of the University of Michigan Hospitals for performing the reproductive hormone assays and for their assistance with the preparation of plasma samples for the adipokine assays. We are especially grateful to the women who served as research participants.
Research Support
Supported by grants from Pfizer, Inc. (Lukacs, PI) and Novo Nordisk, Inc. (Reame, PI). Also by NIH grants 5-M01-00042 (General Clinical Research Center, University of Michigan) and R01-AG15083-04 (Reame, PI) to the University of Michigan and P30NR010677 (Bakken, PI) to Columbia University. Additional support from the Janet Gatherer Boyles and Carl Pursell Award from the School of Nursing, University of Michigan (Lukacs, PI).
Contributor Information
Patricia A. Rouen, McAuley School of Nursing, University of Detroit Mercy, 4001 W. McNichols Road., Detroit, MI 48221, rouenpa@udmercy.edu, Phone: (313) 993-1739 Fax: (313) 993-1271.
Jane L. Lukacs, School of Nursing, University of Michigan, Ann Arbor, MI lukacsj@umich.edu.
Nancy E. Reame, School of Nursing, Columbia University, New York, NY nr2188@columbia.edu.
References
- Ahima RS. Metabolic actions of adipocyte hormones: Focus on adiponectin. Obesity. 2006;14 Suppl 1:9S–15S. doi: 10.1038/oby.2006.276. [DOI] [PubMed] [Google Scholar]
- Arita Y, Kihara S, Ouchi N, Takahashi M, Madea K, Miyagawa J, et al. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochemical and Biophysical Research Communications. 1999;257:79–83. doi: 10.1006/bbrc.1999.0255. [DOI] [PubMed] [Google Scholar]
- Benetti-Pinto CL, Castro N, Grassiotto O, Garmes HM. Leptin and adiponectin blood levels in women with premature ovarian failure and age- and weight-matched women with normal menstrual cycles. Menopause. doi: 10.1097/gme.0b013e3181b00dad. In press Retrieved from preprint archive at http://www.journals.lww.com/menopausejournal. [DOI] [PubMed] [Google Scholar]
- Carr DB, Utzschneider KM, Hull RL, Kodama K, Retzlaff BM, Brunzell JD, et al. Intra-abdominal fat is a major determinant of the national cholesterol education program adult treatment panel III criteria for the metabolic syndrome. Diabetes. 2004;53:2087–2094. doi: 10.2337/diabetes.53.8.2087. [DOI] [PubMed] [Google Scholar]
- Carr MC. The emergence of the metabolic syndrome with menopause. Journal of Clinical Endocrinology and Metabolism. 2003;88:2404–2411. doi: 10.1210/jc.2003-030242. [DOI] [PubMed] [Google Scholar]
- Chu MC, Cosper P, Orio F, Carmina E, Lobo RA. Insulin resistance in postmenopausal women with metabolic syndrome and the measurements of adiponectin, leptin, resistin, and ghrelin. American Journal of Obstetrics & Gynecology. 2006;194:100–104. doi: 10.1016/j.ajog.2005.06.073. [DOI] [PubMed] [Google Scholar]
- Cnop ME, Landchild MJ, Vidal J, Havel P, Knowles NG, Carr DR, et al. The concurrent accumulation of intra-abdominal and subcutaneous fat explains the association between insulin resistance and plasma leptin concentrations. Diabetes. 2002;51:1005–1015. doi: 10.2337/diabetes.51.4.1005. [DOI] [PubMed] [Google Scholar]
- Cnop M, Havel PJ, Utzschneider KM, Carr DB, Sinha MK, Boyko, et al. Relationship of adiponectin to body fat distribution, insulin sensitivity and plasma lipoproteins: Evidence for independent roles of age and sex. Diabetologica. 2003;46:459–469. doi: 10.1007/s00125-003-1074-z. [DOI] [PubMed] [Google Scholar]
- Dafopoulus K, Sourlas D, Kallitsaris A, Pournaras S, Messinis I. Blood ghrelin, resistin, and adiponectin concentrations during the menstrual cycle. Fertility and Sterility. doi: 10.1016/j.fertnstert.2008.07.1773. In press Retrieved from preprint archive at http://www.sciencedirect.com. [DOI] [PubMed] [Google Scholar]
- Frystyk J, Berne C, Berglund L, Jensevik K, Flyvbjerg A, Zethelius B. Serum adiponectin is a predictor of coronary heart disease: A population-based 10-year follow-up study in elderly men. Journal of Clinical Endocrinology and Metabolism. 2007;92:571–576. doi: 10.1210/jc.2006-1067. [DOI] [PubMed] [Google Scholar]
- Gavrila A, Chan JL, Yiannakouris N, Kontogianni M, Miller LC, Orlova C, et al. Serum adiponectin levels are inversely associated with overall and central fat distribution but are not directly regulated by acute fasting or leptin administration in humans: Cross-sectional and interventional studies. Journal of Clinical Endocrinology and Metabolism. 2003;88:4823–4831. doi: 10.1210/jc.2003-030214. [DOI] [PubMed] [Google Scholar]
- Guo SS, Zeller C, Chumlea WC, Siervogel RM. Aging, body composition, and lifestyle: The Fels longitudinal study. American Journal of Clinical Nutrition. 1999;70:405–411. doi: 10.1093/ajcn/70.3.405. [DOI] [PubMed] [Google Scholar]
- Hadji P, Hars O, Bock K, Sturm G, Bauer T, Emons G, et al. The influence of menopause and body mass index on serum leptin concentrations. European Journal of Endocrinology. 2000;143:55–60. doi: 10.1530/eje.0.1430055. [DOI] [PubMed] [Google Scholar]
- Haffner SM, Mykkanen L, Stern MP. Leptin concentrations in women in the San Antonio Heart Study: Effect of menopausal status and postmenopausal hormone replacement therapy. American Journal of Epidemiology. 1997;146:581–585. doi: 10.1093/oxfordjournals.aje.a009317. [DOI] [PubMed] [Google Scholar]
- Hong SC, Yoo SW, Cho GJ, Kim T, Hur JY, Park YK, et al. Correlation between estrogens and serum adipocytokines in premenopausal and postmenopausal women. Menopause. 2007;14:835–840. doi: 10.1097/GME.0b013e31802cddca. [DOI] [PubMed] [Google Scholar]
- Inoue M, Yano M, Yamakado M, Maehata E, Suzuki S. Relationship between the adiponectin-leptin ratio and parameters of insulin resistance in subjects without hyperglycemia. Metabolism: Clinical and Experimental. 2006;55:1248–1254. doi: 10.1016/j.metabol.2006.05.010. [DOI] [PubMed] [Google Scholar]
- Jurimae J, Rembel K, Jurimae T, Rehand M. Adiponectin is associated with bone mineral density in perimenopausal women. Hormone and Metabolism Research. 2005;37:297–302. doi: 10.1055/s-2005-861483. [DOI] [PubMed] [Google Scholar]
- Kannel WB, Cupples LA, Ramaswami R, Stokes J, III, Kreger BE, Higgins M. Regional obesity and risk of cardiovascular disease, the Framingham study. Journal of Clinical Epidemiology. 1991;44:183–190. doi: 10.1016/0895-4356(91)90265-b. [DOI] [PubMed] [Google Scholar]
- Kleiblova P, Springer D, Haluzik M. The influence of hormonal changes during the menstrual cycle on serum adiponectin concentrations in healthy women. Physiological Research. 2006;55:661–666. doi: 10.33549/physiolres.930977. [DOI] [PubMed] [Google Scholar]
- Kontogianni MD, Dafni UG, Routsias JG, Skopouli FN. Blood leptin and adiponectin as possible mediators of the relation between fat mass and BMD in perimenopausal women. Journal of Bone and Mineral Research. 2004;19:546–551. doi: 10.1359/JBMR.040107. [DOI] [PubMed] [Google Scholar]
- Lee CG, Carr MC, Murdoch SJ, Mitchell E, Woods NF, Wener MH, et al. Adipokines, inflammation, and visceral adiposity across the menopausal transition: A prospective study. Journal of Clinical Endocrinology and Metabolism. 2009;94:1104–1109. doi: 10.1210/jc.2008-0701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy JC, Matthews DR, Hermans MP. Correct homeostasis model assessment (HOMA) evaluation uses the computer program. Diabetes Care. 1998;21:2191–2192. doi: 10.2337/diacare.21.12.2191. [DOI] [PubMed] [Google Scholar]
- Lukacs JL, Booth S, Kleerekoper M, Ansbacher R, Rock CL, Reame NE. Differential associations for menopause and age in measures of vitamin K, osteocalcin, and bone density: A cross-sectional exploratory study in healthy volunteers. Menopause. 2006;13:799–808. doi: 10.1097/01.gme.0000227023.89062.43. [DOI] [PubMed] [Google Scholar]
- Margetic S, Cazzola C, Pegg GG, Hill RA. Leptin: A review of its peripheral actions and interactions. International Journal of Obesity. 2002;26:1407–1433. doi: 10.1038/sj.ijo.0802142. [DOI] [PubMed] [Google Scholar]
- Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: Insulin resistance and beta cell function from fasting glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- Meier U, Gressner A. Endocrine regulation of energy metabolism: Review of pathobiochemical and clinical chemical aspects of leptin, ghrelin, adiponectin and resistin. Clinical Chemistry. 2004;50:1511–1525. doi: 10.1373/clinchem.2004.032482. [DOI] [PubMed] [Google Scholar]
- Milewicz A, Zatonska K, Demissie M, Jedrzejuk D, Dunajska K, Ilow R, et al. Serum adiponectin concentration and cardiovascular risk factors in climacteric women. Gynecological Endocrinology. 2005;20:68–73. doi: 10.1080/09513590400020989. [DOI] [PubMed] [Google Scholar]
- Miner JL. The adipocyte as an endocrine cell. Journal of Animal Science. 2004;82:935–942. doi: 10.2527/2004.823935x. [DOI] [PubMed] [Google Scholar]
- Nishizawa H, Shimomura I, Kishida K, Maeda N, Kuriyama H, Nagaretani H, et al. Androgens decrease plasma adiponectin, an insulin-sensitizing adipocyte-derived protein. Diabetes. 2002;51:2734–2741. doi: 10.2337/diabetes.51.9.2734. [DOI] [PubMed] [Google Scholar]
- North American Menopause Society. Menopause practice: A clinician’s guide. 3rd Ed. Cleveland, OH: Author; 2007. [Google Scholar]
- Park YW, Zhuu S, Palaniappan L, Heshka S, Carnethon MR, Heymsfield SB. The metabolic syndrome: Prevalence and associated risk factor findings in the US population from the third National Health and Nutrition Examination Survey, 1988–1994. Archives of Internal Medicine. 2003;163:427–436. doi: 10.1001/archinte.163.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen SB, Kristensen K, Hermann P, Katzenellenbogen JA, Richelsen B. Estrogen controls lipolysis by up-regulating α2A-adrenergic receptors directly in human adipose tissue through the estrogen receptor α. Implications for the female fat distribution. Journal of Clinical Endocrinology and Metabolism. 2004;89:1869–1878. doi: 10.1210/jc.2003-031327. [DOI] [PubMed] [Google Scholar]
- Poehlman ET, Toth MJ, Gardner AW. Changes in energy balance and body composition at menopause: A controlled longitudinal study. Annals of Internal Medicine. 1995;123:673–675. doi: 10.7326/0003-4819-123-9-199511010-00005. [DOI] [PubMed] [Google Scholar]
- Reusch JE. Current concepts in insulin resistance, type 2 diabetes and the metabolic syndrome. American Journal of Cardiology. 2002;90 Supplement:19G–26G. doi: 10.1016/s0002-9149(02)02555-9. [DOI] [PubMed] [Google Scholar]
- Sieminska L, Wojciechowska C, Foltyn W, Kajdaniuk D, Kos-Kudla B, Marek B, et al. The relation of serum adiponectin and leptin levels to metabolic syndrome in women before and after menopause. Polish Journal of Endocrinology. 2006;57:16–22. [PubMed] [Google Scholar]
- Soules MR, Sherman S, Parrott E, Rebar R, Santoro N, Utian W, et al. Executive summary: Stages of reproductive aging workshop (STRAW) Fertility & Sterility. 2001;76:874–878. doi: 10.1016/s0015-0282(01)02909-0. [DOI] [PubMed] [Google Scholar]
- Takemura Y, Osuga Y, Harada M, Hirata T, Koga K, Morimoto C, et al. Serum adiponectin concentrations are decreased in women with endometriosis. Human Reproduction. 2005;20:3510–3513. doi: 10.1093/humrep/dei233. [DOI] [PubMed] [Google Scholar]
- Trujillo ME, Scherer PE. Adiponectin—journey from an adipocyte secretory protein to a biomarker of the metabolic syndrome. Journal of Internal Medicine. 2005;257:167–175. doi: 10.1111/j.1365-2796.2004.01426.x. [DOI] [PubMed] [Google Scholar]
- van Harmelen V, Reynisdottir S, Eriksson P, Thorne A, Hoffstedt J, Lonnqvist F, et al. Leptin secretion from subcutaneous and visceral adipose tissue in women. Diabetes. 1998;47:913–917. doi: 10.2337/diabetes.47.6.913. [DOI] [PubMed] [Google Scholar]
- Weyer C, Funahashi T, Tanaka S, Hotta K, Matsuzawa Y, Pratley RE, et al. Hypoadiponectinemia in obesity and type 2 diabetes: Close association with insulin resistance and hyperinsulinemia. Journal of Clinical Endocrinology and Metabolism. 2001;86:1930–1935. doi: 10.1210/jcem.86.5.7463. [DOI] [PubMed] [Google Scholar]
- Zoico E, DiFrancesco V, Mazzali G, Vettor R, Fantin F, Bissoli L, et al. Adipocytokines, fat distribution and insulin resistance in elderly men and women. Journals of Gerontology. 2004;59A:935–938. doi: 10.1093/gerona/59.9.m935. [DOI] [PubMed] [Google Scholar]