Abstract
Diffuse cutaneous mastocytosis (DCM) is a rare, severe, variant of cutaneous mastocytosis. The authors report the case of a male infant who developed maculae and maculopapulae on his legs and abdomen when aged 3.5 months, which spread to all body surfaces within weeks. Diagnosis of DCM was made at the age of 6 months when he had developed extensive bullous eruptions, generalised pruritus, flushing and abdominal pain. Treatment was started with oral dimethindine maleate. At the age of 18 months, oral sodium cromoglicate (SCG) was introduced. At the age of 23 months, additional treatment was started with a cutaneous emulsion containing 4% SCG. Continued treatment with oral dimethindine maleate, oral SCG with the dose maintained at 25 mg/kg/day, and SCG 4% cutaneous emulsion applied two to four times daily has resulted in a steady improvement of symptoms and skin appearance.
Background
Mastocytosis describes a group of disorders characterised by the abnormal growth, accumulation, or both, of mast cells, in one or more organ systems.1 Mastocytosis is divided into two main subgroups, systemic mastocytosis (SM) and cutaneous mastocytosis (CM). Mastocytosis affects approximately 0.9 in 10 000 people in the European Union.
Diffuse cutaneous mastocytosis (DCM) is a rare, severe variant of CM. It occurs mainly in infants, with the first signs often appearing soon after birth. The skin is infiltrated by increased numbers of mast cells. Degranulation of the mast cells produces oedema and a leather grain skin appearance. Blistering is common, and there are also nodules and plaques. In many cases, there is a generalised erythroderma, sometimes soon after birth and DCM is more likely than other forms of CM to be associated with systemic symptoms including shock, diarrhoea and abdominal pain.
There is no curative treatment for mastocytosis and treatment consists primarily of symptomatic drugs and other treatments that deal with the symptoms experienced by individual patients.2 The main groups of drugs used are histamine receptor blockers, both H1 and H2 antagonists, leukotriene antagonists, acetylsalicylic acid and other non-steroidal anti-inflammatory drugs, oral sodium cromoglicate (SCG), oral corticosteroids and ultraviolet (UV) light irradiation.
The disease described in this case report, DCM, is rare, but very distressing. There are no established treatments for infants. The primary treatment we used, oral SCG, is licensed in USA for the treatment of SM, including patients with cutaneous symptoms, but its use in children below the age of 2 years is not recommended. Other cases using this treatment below this age have been published but the dose used has been variable. We found that adjusting the dose on an mg/kg/day basis did result in symptomatic improvement, and we believe that this is an important information to be followed and expanded. Although the symptoms affect mainly the skin, no topical treatments are recommended. In addition to oral SCG, we used a cutaneous emulsion containing 4% SCG. This was helpful. This is the first report of this use of this emulsion in this disease.
Case presentation
A male infant aged 3.5 months, developed irregular, dark brown spots on his legs, which rapidly spread to his abdomen, neck and temples. After about 3 weeks, he developed blisters in his groin. When aged 5 months, he was seen by one of us (SC). At that time, he had generalised maculae and maculopapulae covering most of his skin including his face and head. A provisional diagnosis of urticaria pigmentosa (UP)/DCM was made. Darier’s sign was positive: stroking the abdomen resulted in a red and later white urticaria reaction associated with a large red weal and after 5-min marked flushing of the face. The reaction lasted 30 min. One month later, when he had developed extensive bullous eruptions, generalised pruritus, flushing and abdominal pain, the diagnosis was confirmed as DCM. Treatment was started with oral dimethindine maleate. Four months later, treatment was changed to a combination of cetirizine and ketotifen, but within a short time he reacted with flushing of the face and extremities followed by oedema of the same areas followed by severe blistering and diarrhoea. To deal with this reaction, he was admitted to hospital and treated with a corticosteroid infusion. He reacted in a similar way but not so severely to desloratidine and levocetirizine. Treatment has therefore continued with dimethindine maleate which has been well tolerated.
Over the course of the next 9 months in addition to the symptoms described, he had attacks of more severe symptoms. These started with flushing, red eyes, abdominal pain, vomiting and reddening of the skin. The following day, he developed fluid containing blisters all over his body apart from his face, palms and soles. The blisters cracked, emptied and then filled with fluid again before turning to red crusts, which lasted about a week and then fell off. The whole attack lasted about 10–14 days. It was found that the only treatment that helped was a 100-mg prednisone suppository. During the first 12 months, he required one corticosteroid suppository every month. figure 1 shows the child, 1 day after the onset of an attack.
Figure 1.
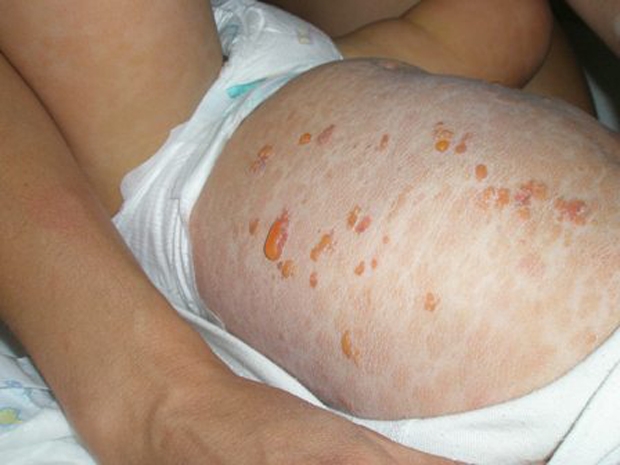
Patient aged 17 months showing lesions of diffuse cutaneous mastocytosis during flare-up.
The parents declined a skin biopsy, and 12 months after the diagnosis had been made this was confirmed with a raised serum tryptase. Additional treatment was started with oral SCG at a dose of a 50 mg five times daily. This resulted in an improvement in the abdominal symptoms of abdominal pain and diarrhoea.
The child’s mother was in contact with the mothers of other children with DCM and learnt of the availability of a cutaneous emulsion containing 4% SCG (Altoderm), which they had obtained and were using it as a topical treatment of DCM. She contacted one of us (AME), who is involved in the clinical development of this emulsion for the treatment of atopic dermatitis. Supplies are available on a named-patient basis for the treatment of rare diseases.
The severity of skin symptoms (itching, flushing and wealing) using a 0–3 scale, where 0=no symptoms, 1=mild symptoms, 2=moderate symptoms and 3=severe symptoms were recorded by the mother for 7 days before the commencement of treatment with the 4% SCG cutaneous emulsion and daily thereafter. Treatment with the 4% SCG cutaneous emulsion began at the age of 23 months.
The daily mean scores for all skin symptoms for 7 days before treatment with the 4% SCG skin lotion was started and those for the first 100 days of treatment are shown in figure 2. This shows the skin symptoms initially worsened, then improved and then became more severe again. His weight at this time was 15 kg, making the daily dose of oral SCG 16 mg/kg/day. By day 100 of treatment, when symptoms had worsened, his weight had increased to 16 kg, and it was decided to increase the dose of oral SCG to 25 mg/kg/day. This was done in two stages, initially to 350 mg/day for 5 days and then to 400 mg/day. During the second 100 days of treatment with this regimen, there was a slow but steady improvement in skin symptoms as seen in figure 3. During flare-ups of skin symptoms, the use of the 4% emulsion was increased to three to fou times a day, reducing to once or twice a day when symptoms improved.
Figure 2.
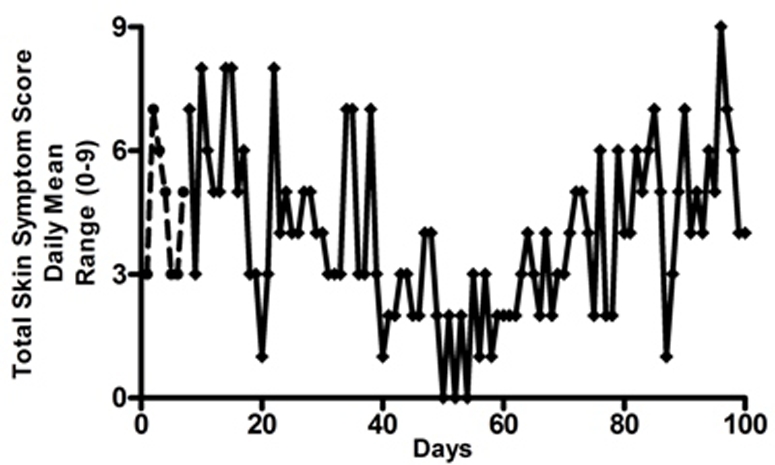
Total daily symptom score recorded by mother (range 0–9). Symptoms recorded, itching, flushing, whealing (hives), using 0–3 scale. 0=no symptoms, 1=mild, 2=moderate, 3=severe. Interrupted line=before introduction of sodium cromoglicate (SCG) 4% cutaneous emulsion. Day 0=aged 23 months. Solid line=first 100 days of using SCG 4% cutaneous emulsion.
Figure 3.
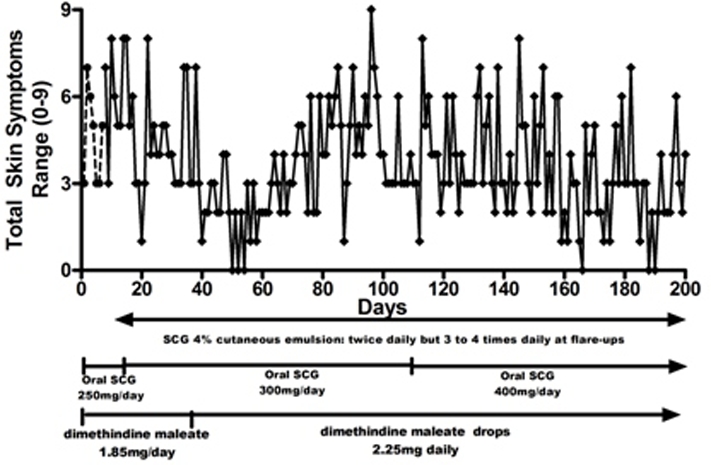
Total daily symptom score recorded by mother (range 0–9). Similar symptoms to figure 2. Interrupted line=before introduction of sodium cromoglicate (SCG) 4% cutaneous emulsion. Day 0=aged 23 months. Solid line=first 200 days of using SCG 4% cutaneous emulsion. Dose of oral SCG increased from day 110.
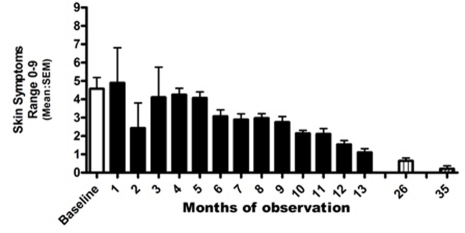
Daily diary cards of skin symptoms were kept from the age of 23 months to 36 months and again for 1 month aged 45 months (after 24 months) and for 2 weeks aged 54 months (after 35 months). The change in skin symptoms is seen in figure 4, which shows mean monthly scores for total skin symptoms (apart from the final record which is for 2 weeks only). No prednisone suppositories have been required during this time. The current dose of oral SCG had been increased to 500 mg/day to maintain the daily dose at 25 mg/kg/day. The dose of dimethindine maleate had been increased to 3 mg/day. Measures of serum tryptase made throughout this period are shown in figure 5.
Figure 4.
Skin symptoms recorded by mother during period of observation. Columns=monthly mean scores of three symptoms. White column before introduction of sodium cromoglicate (SCG) 4% cutaneous emulsion. Black columns=months of treatment with dimethidine maleate, oral SCG and SCG 4% cutaneous emulsion. Boxed column=1-months records at after 24 months of treatment aged 3 years 11 months.
Figure 5.
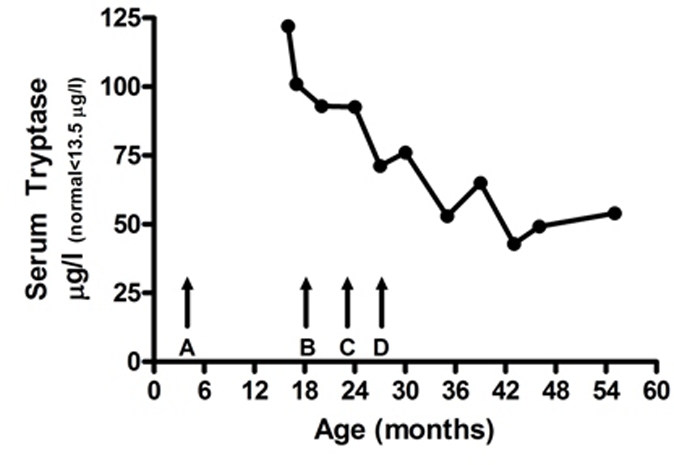
Serum tryptase. (Normal<13.5 µg/l.) Arrows=time points. (A) Onset of symptoms. (B) Start of oral sodium cromoglicate (SCG) treatment. (C) Start of treatment with 4% cutaneous emulsion. (D) Adjustment of oral SCG to 20–25 mg/kg/day and increased frequency of daily use of cutaneous emulsion.
Investigations
Serum tryptase (figure 4).
Differential diagnosis
The diagnosis of CM is based on typical skin lesions: four subtypes are recognised, urticaria pigmentosa (UP), DCM, mastocytoma and telangiectasia macularis eruptive perstans (TMEP). Darier’s sign, local wealing of the lesions in UP and DCM when the skin lesions are rubbed or scratched is usually positive. DCM is differentiated by the age of onset, the nature of the skin lesions and the association of other symptoms.
Treatment
Described in case presentation.
Outcome and follow-up
Improvement in symptoms has continued and is seen 3 monthly at paediatric dermatology clinic. Dosage of antihistamine is kept according to manufacturers’ guidelines. Dosage of oral SCG is maintained at 25 mg/kg/day. Use of SCG cutaneous emulsion varies from once to twice daily according to skin symptoms.
Discussion
This is the first report of this combined use of oral and topical SCG together with antihistamine for the treatment of DCM.
DCM is the rarest form of CM. It presents earlier than the other variants, usually before the age of 3 years, differs in appearance in that the classical pigmented lesions of UP are absent, blistering is common and the entire cutaneous area is affected. Also systemic symptoms, diarrhoea, abdominal pain, hypotension and shock are common. There is a general consensus about the avoidance of mast cell triggers, and the use of antimediator drugs, specifically, H1 and H2 antihistamines and oral SCG.3 This protocol for management refers to these but provides little guidance on how these should be used, and at what dosage, in infants with DCM, in whom the disease can present soon after birth.
SCG is a bischromone, the disodium salt of a strong acid (pKa 2.0) with molecular weight 512. It is freely soluble in water up to about 5% and is insoluble in fats. It was initially developed as inhaled drug for the treatment of asthma4 and subsequently as a nasal spray for allergic rhinitis, as eye drops for allergic conjunctivitis and as an oral powder or solution for food allergy. In the USA, it is known as cromolyn sodium and is licensed, in the USA only, as an orphan drug for the treatment of mastocytosis. For this indication, it is presented as an oral, aqueous solution (Gastrocrom).
Two pharmacological activities of SCG relevant to its use in SM and CM have been identified.
-
(1)
It is a ‘mast cell stabiliser’. It interferes in the release of inflammatory and other chemical mediators from mast cells and either blocks or reduces the amount released.4 The effect is dependent on the source of the mast cells, the challenge used and the outcome measured.5 The effect on mast cells in the skin is uncertain but is probably dependent on dose, the challenge used and the outcome measured.6 7 The effect as a mast cell stabiliser is dose dependent. The purported receptor on mast cells has recently been recognised.8
-
(2)
It has a direct effect on sensory nerves probably by an inhibitory effect on sensory nerve activation. This was first demonstrated on the activity of ‘C’ fibre sensory nerve endings in the dog lung.9 This effect has been shown to be at a much lower dose level than that required to stabilise mast cells.10 Itch sensation is thought to be mediated by a subgroup of ‘C’ fibres in the skin, and SCG has been shown to reduce the severity of itch when administered either topically11 or orally.12
The first report of the use of oral SCG for the treatment of SM was in 1974.13 This was in an 18-year-old boy. Good control of gastrointestinal symptoms was achieved with doses of 160 mg/day to 220 mg/day.
There have been four placebo-controlled trials of oral SCG in SM.14–17 Three were conducted in adults. In the first,14 oral SCG resulted in marked amelioration of pruritus, wealing, flushing, diarrhoea, abdominal pain and disorders of cognitive function. The other trials also reported symptomatic improvement. One trial included three children, age not specified, using a dose of 200 mg/day or 400 mg/day.15 The recommended dose of the licensed oral product in USA is 800 mg/day in adults and 400 mg/day in children aged 2–12 years. It is not recommended in children below the age of 2 years. The dose should not exceed 40 mg/kg/day.
There have been no formal clinical trials of oral SCG in DCM. There have been 10 case reports (13 children) of the use of oral SCG in children with CM.18–27 In one of these,18 SCG was also applied topically, initially as a 10% concentration in propylene glycol and later as a 10% concentration in petroleum jelly. With both applications, an initial slight improvement was not long lasting. In the children in whom oral SCG was used, in two cases, the dose used was not recorded; in the others, the dose used varied from an initial dose of 10 mg 12 hourly, with a maintenance dose of 160 mg to 200 mg/day to 300 mg/day. In two reports, there was no improvement in skin symptoms: in eight children, an improvement was reported with a lessening of both bullae and pruritus. In two cases, symptoms worsened if the oral SCG was stopped and improved again when re-started. No adverse effects have been reported.
The 4% cutaneous emulsion we used in this case is being developed for the treatment of atopic dermatitis in children.28 It is a unique formulation designed to improve the absorption of SCG into and through the dermis.29 It has to be gently rubbed onto the skin. For children with a positive Darier’s sign, as in this case, the first few applications may result in worsening of symptoms, but this soon stops and skin symptoms improve. Otherwise, there have been no adverse effects during 3 years of treatment.
In our case, it cannot be absolutely claimed that the treatment used did provide therapeutic benefit. Reviews of DCM report that the disease usually resolves spontaneously. A review of CM states that 50% of pediatric CM patients have improvement of symptoms over time with 50% having complete resolution by adolescence. Of the other 50%, most have reduction of symptoms, but 10–15% have persistent symptoms into adulthood.30 In the proposed protocol for management,3 it states that partial remission of DCM occurs in the third to fifth year of life.
In our case, the records kept by the mother demonstrated symptomatic improvement once the dose of oral SCG was raised to and maintained at 25 mg/kg/day. She also thought that the application of the cutaneous emulsion of SCG was beneficial. Improvement began at 2 years of age. It is also uncertain whether the reduction in serum tryptase is spontaneous or results from treatment. As serum tryptase levels remained raised, but the child being almost symptom free, it suggested that the disease is still active but that the treatment is effective in controlling symptoms.
From our case and a review of other cases of DCM in infants below the age of 2 years, a combination of oral SCG and topical SCG emulsion together with an antihistamine is a safe and useful symptomatic treatment to use. As the child grows, the dose of the antihistamine should be increased as recommended and that of oral SCG, maintained at 25 mg/kg/day in two or three divided doses. Initially, SCG cutaneous emulsion can be applied up to three or four times daily according to response with a maintenance dose of once or twice daily.
Learning points.
-
▶
CM should be considered in any infant presenting with generalised skin symptoms with blistering and bullae.
-
▶
Darier’s sign (urtication after stroking the skin lesions) should be tested for in all cases of infants with skin symptoms.
-
▶
If using treatment with oral SCG, dosage should be on an mg/kg/day basis with gradual increase in dose.
Footnotes
Competing interests AME was employed by the originators of sodium cromoglicate, Fisons Pharmaceuticals from 1974 to 1995. He is a consultant to the manufacturers of Altoderm, a 4% cutaneous emulsion of sodium cromoglicate, Thornton & Ross. ŠČ has no competing interest.
Patient consent Obtained.
References
- 1.Valent P, Horny HP, Escribano L, et al. Diagnostic criteria and classification of mastocytosis: a consensus proposal. Leuk Res 2001;25:603–25 [DOI] [PubMed] [Google Scholar]
- 2.Escribano L, Akin C, Castells M, et al. Mastocytosis: current concepts in diagnosis and treatment. Ann Hematol 2002;81:677–90 [DOI] [PubMed] [Google Scholar]
- 3.Heide R, Beishuizen A, De Groot H, et al. Mastocytosis in children: a protocol for management. Pediatr Dermatol 2008;25:493–500 [DOI] [PubMed] [Google Scholar]
- 4.Cox JS, Beach JE, Blair AM, et al. Disodium cromoglycate (Intal). Adv Drug Res 1970;5:115–96 [PubMed] [Google Scholar]
- 5.Pearce FL, Al-Laith M, Bosman L, et al. Effects of sodium cromoglycate and nedocromil sodium on histamine secretion from mast cells from various locations. Drugs 1989;37(Suppl 1):37–43 [DOI] [PubMed] [Google Scholar]
- 6.Okayama Y, Benyon RC, Rees PH, et al. Inhibition profiles of sodium cromoglycate and nedocromil sodium on mediator release from mast cells of human skin, lung, tonsil, adenoid and intestine. Clin Exp Allergy 1992;22:401–9 [DOI] [PubMed] [Google Scholar]
- 7.Walsh LJ. Ultraviolet B irradiation of skin induces mast cell degranulation and release of tumour necrosis factor-alpha. Immunol Cell Biol 1995;73:226–33 [DOI] [PubMed] [Google Scholar]
- 8.Yang Y, Lu JY, Wu X, et al. G-protein-coupled receptor 35 is a target of the asthma drugs cromolyn disodium and nedocromil sodium. Pharmacology 2010;86:1–5 [DOI] [PubMed] [Google Scholar]
- 9.Dixon M, Jackson DM, Richards IM. The action of sodium cromoglycate on ‘C’ fibre endings in the dog lung. Br J Pharmacol 1980;70:11–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Allistone A, Collier JG, Davidson RN, et al. The effect of intravenous sodium cromoglycate on the bronchoconstriction induced by sulphur dioxide inhalation in man. Clin Sci 1985;68:227–32 [DOI] [PubMed] [Google Scholar]
- 11.Vieira Dos Santos R, Magerl M, Martus P, et al. Topical sodium cromoglicate relieves allergen- and histamine-induced dermal pruritus. Br J Dermatol 2010;162:674–6 [DOI] [PubMed] [Google Scholar]
- 12.Vessal G, Sagheb MM, Shilian S, et al. Effect of oral cromolyn sodium on CKD-associated pruritus and serum tryptase level: a double-blind placebo-controlled study. Nephrol Dial Transplant 2010;25:1541–7 [DOI] [PubMed] [Google Scholar]
- 13.Dolovich J, Punthakee ND, MacMillan AB, et al. Systemic mastocytosis: control of lifelong diarrhea by ingested disodium cromoglycate. Can Med Assoc J 1974;111:684–5 [PMC free article] [PubMed] [Google Scholar]
- 14.Soter NA, Austen KF, Wasserman SI. Oral disodium cromoglycate in the treatment of systemic mastocytosis. N Engl J Med 1979;301:465–9 [DOI] [PubMed] [Google Scholar]
- 15.Czarnetzki BM, Behrendt H. Urticaria pigmentosa: clinical picture and response to oral disodium cromoglycate. Br J Dermatol 1981;105:563–7 [DOI] [PubMed] [Google Scholar]
- 16.Frieri M, Alling DW, Metcalfe DD. Comparison of the therapeutic efficacy of cromolyn sodium with that of combined chlorpheniramine and cimetidine in systemic mastocytosis. Results of a double-blind clinical trial. Am J Med 1985;78:9–14 [DOI] [PubMed] [Google Scholar]
- 17.Horan RF, Sheffer AL, Austen KF. Cromolyn sodium in the management of systemic mastocytosis. J Allergy Clin Immunol 1990;85:852–5 [DOI] [PubMed] [Google Scholar]
- 18.Sauder DN, Bergfield WF, Krakauer RS. Disodium cromoglycate therapy in mastocytosis. The mast cell – its role in health and disease. Pepys J, Edwards AM, eds. England: Pitman Medical, 1979 [Google Scholar]
- 19.Evans S, Vickers CF. Bullous urticaria pigmentosa (cutaneous mastocytosis) and sodium cromoglycate therapy. Acta Derm Venereol 1981;61:572–5 [PubMed] [Google Scholar]
- 20.Welch EA, Alper JC, Bogaars H, et al. Treatment of bullous mastocytosis with disodium cromoglycate. J Am Acad Dermatol 1983;9:349–53 [DOI] [PubMed] [Google Scholar]
- 21.Golitz LE, Weston WL, Lane AT. Bullous mastocytosis: diffuse cutaneous mastocytosis with extensive blisters mimicking scalded skin syndrome or erythema multiforme. Pediatr Dermatol 1984;1:288–94 [DOI] [PubMed] [Google Scholar]
- 22.Oranje AP, Soekanto W, Sukardi A, et al. Diffuse cutaneous mastocytosis mimicking staphylococcal scalded-skin syndrome: report of three cases. Pediatr Dermatol 1991;8:147–51 [DOI] [PubMed] [Google Scholar]
- 23.Burger M, Hartmann AA, Wallis S, et al. Bullous cutaneous mastocytosis. Z Hautkr 1991;66:889–91 [Google Scholar]
- 24.Cook J, Stith M, Sahn EE. Bullous mastocytosis in an infant associated with the use of a nonprescription cough suppressant. Pediatr Dermatol 1996;13:410–14 [DOI] [PubMed] [Google Scholar]
- 25.Haustein UF, Bedri M. [Bullous mastocytosis in a child]. Hautarzt 1997;48:127–9 [DOI] [PubMed] [Google Scholar]
- 26.Ping-shen F, Meng FU, Wen-ju L, et al. Bullous mastocytosis. J Clin Dermatol 2007;36:26–7 [Google Scholar]
- 27.Silva I, Carvalho S, Pinto PL, et al. Mastocytosis: a rare case of anaphylaxis in paediatric age and literature review. Allergol Immunopathol (Madr) 2008;36:154–63 [PubMed] [Google Scholar]
- 28.Stainer R, Matthews S, Arshad SH, et al. Efficacy and acceptability of a new topical skin lotion of sodium cromoglicate (Altoderm) in atopic dermatitis in children aged 2–12 years: a double-blind, randomized, placebo-controlled trial. Br J Dermatol 2005;152:334–41 [DOI] [PubMed] [Google Scholar]
- 29.Edwards AM, Matthews S, Arshad SH. Systemic absorption of sodium cromoglicate from a new cutaneous emulsion (Altoderm) in children with atopic dermatitis. Eur J Dermatol 2010;20:864–5 [DOI] [PubMed] [Google Scholar]
- 30.Briley LD, Phillips CM. Cutaneous mastocytosis: a review focusing on the pediatric population. Clin Pediatr (Phila) 2008;47:757–61 [DOI] [PubMed] [Google Scholar]