Abstract
Xylitol is widely used as a sweetener in foods and medications. Xylitol ingestion causes a small blood glucose rise, and it is commonly used as an alternative to high-energy supplements in diabetics. In previous studies, a xylitol metabolite, xylulose-5-phosphate, was shown to activate carbohydrate response element binding protein, and to promote lipogenic enzyme gene transcription in vitro; however, the effects of xylitol in vivo are not understood. Here we investigated the effects of dietary xylitol on lipid metabolism and visceral fat accumulation in rats fed a high-fat diet. Sprague-Dawley rats were fed a high-fat diet containing 0 g (control), 1.0 g/100 kcal (X1) or 2.0 g/100 kcal (X2) of xylitol. After the 8-week feeding period, visceral fat mass and plasma insulin and lipid concentrations were significantly lower in xylitol-fed rats than those in high-fat diet rats. Gene expression levels of ChREBP and lipogenic enzymes were higher, whereas the expression of sterol regulatory-element binding protein 1c was lower and fatty acid oxidation-related genes were significantly higher in the liver of xylitol-fed rats as compared with high-fat diet rats. In conclusion, intake of xylitol may be beneficial in preventing the development of obesity and metabolic abnormalities in rats with diet-induced obesity.
Keywords: xylitol, visceral fat, lipid metabolism, lower insulin level, high fat diet
Introduction
Visceral fat obesity has become a severe problem in the world and is a major risk factor for many diseases, such as metabolic syndrome, atherosclerosis, cardiovascular disease, stroke, and even cancer.(1–3) Recently, it has been established that obesity is caused by various environmental factors, including dietary energy content and nutrient composition.(4,5) A high-fat diet induces obesity, hyperglycemia, dyslipidemia and hyperinsulinemia, leading to insulin resistance and type 2 diabetes.(6,7) In rodents, such overnutrition as high-fat feeding activates sterol regulatory-element binding protein 1c (SREBP-1c) in the liver, and the increased accumulation of fat promotes metabolic syndrome.(8,9) In addition, SREBP-1c plays a key role in the mechanism of high-fat-induced obesity and insulin resistance in the liver.(10) A high-fat diet leads to the increased expression of fatty acid oxidation-related genes including peroxisome proliferators-activated receptor (PPAR) α in the liver to allow adaptation to the high intake of fat.(8) However, an impaired ability to increase fat oxidation in response to a high-fat diet leads to the development of obesity and insulin resistance.(11)
Xylitol is a five-carbon sugar alcohol with an energy value of 3 kcal/g. Significant quantities of xylitol have been detected in a wide variety of plants, including fruits and vegetables such as plums, strawberries, raspberries, and cauliflower.(12) It is used widely as a low-calorie sweetener in medications, dental care products, chewing gums, and candies. Xylitol is absorbed from the small intestine by passive diffusion and is mostly metabolized in the liver.(13) The ingestion of xylitol causes a smaller rise in plasma glucose and insulin concentrations than does the ingestion of glucose in healthy men and diabetics.(14,15) Hence, it has been used in patients with diabetes mellitus as an energy source in place of other carbohydrates. In the liver, xylitol is phosphorylated and metabolized to xylulose 5-phosphate (Xu5P), an intermediate of the nonoxidative branch of the pentose phosphate pathway.(16–18) The xylitol metabolite Xu5P specifically activates both nuclear transport and the DNA-binding activities of carbohydrate response element binding protein (ChREBP) through the activation of protein phosphatase 2A (PP2A) in vitro.(19,20) ChREBP is a transcription factor that activates lipogenic enzyme genes, such as acetyl coenzyme A carboxylase (ACC) and fatty acid synthase (FAS), and that stimulates lipogenesis in the liver.(21,22) Enhanced hepatic lipogenesis result in steatosis and obesity.(23,24) If xylitol stimulates lipogenesis, it might induce steatosis and obesity, leading to metabolic syndrome. In addition, the long-term effects of xylitol intake on lipid metabolism are not fully understood.
In the present study, we investigated the effects of dietary xylitol on visceral fat accumulation and lipid metabolism in rats fed a high-fat diet.
Materials and Methods
Animals
Sprague-Dawley (SD) rats were purchased from the Japan SLC (Hamamatsu, Japan) and used in all experiments. The rats were individually caged in the facility under a 12-h light/dark cycle and constant temperature (23 ± 2°C). Prior to the initiation of our study, the rats were fed a standard rodent diet (MF; Oriental Yeast, Tokyo, Japan) and water ad libitum. This study was approved by the Tokushima University Animal Use Committee, and the rats were maintained according to guidelines of Tokushima University for care of laboratory animals.
Diet and experimental design of long-term xylitol feeding test
Eighteen male, 9-week-old SD rats (weight 290–310 g) were used in a long-term xylitol feeding test. After acclimation for 2 weeks, the rats were divided into 3 groups (6 rats per group) and subjected to the following studies. The rats were fed one of three high-fat-based diets containing different amounts of xylitol: 0 g (HFD, control), 1.0 g/100 kcal (X1) or 2.0 g/100 kcal (X2). The exact composition of each diet is shown in Table 1. Xylitol was substituted with starch, because sucrose contents have greater effects on serum lipid levels, postprandial glucose level, and body fat mass than starch.(26–28) To eliminate impact of differences in energy states on body fat mass, isocaloric pair-feeding was performed through the feeding period. Food intake was monitored daily, and body weight was recorded weekly throughout the feeding period. After 8 week, blood samples were collected from the tail vein for the determination of plasma glucose and insulin levels after 12 h of food deprivation. The rats were then anesthetized with diethylether and blood was withdrawn from the jugular vein for the determination of all other measurements. After the rats were killed by exsanguination, organs including the liver, visceral fat and soleus muscle were collected, weighted and stored at −80°C until analysis.
Table 1.
Composition of experimental diets
| Composition | Diet |
||
|---|---|---|---|
| HFD | X1 | X2 | |
| g/kg diet | |||
| Milk casein | 222.5 | 222.5 | 222.5 |
| L-Cystine | 2.5 | 2.5 | 2.5 |
| Lard | 150.0 | 150.0 | 150.0 |
| Soybean oil | 50.0 | 50.0 | 50.0 |
| Cornstarch | 312.3 | 267.3 | 222.3 |
| α-Cornstarch | 50.0 | 50.0 | 50.0 |
| Sucrose | 100.0 | 100.0 | 100.0 |
| Xylitol | 0.0 | 45.0 | 90.0 |
| Vitamin mixture† | 12.5 | 12.5 | 12.5 |
| Mineral mixture† | 50.0 | 50.0 | 50.0 |
| Fiber (cellulose) | 45.0 | 45.0 | 45.0 |
| Choline bitartrate | 3.2 | 3.2 | 3.2 |
| tert-butylhydroquinone | 0.01 | 0.01 | 0.01 |
| Vitamin E acetate | 2.0 | 2.0 | 2.0 |
HFD, high-fat diet; X1, high-fat diet containing xylitol at 1.0 g/100 kcal; X2, high-fat diet containing xylitol at 2.0 g/100 kcal. † AIN-93M(25).
Plasma glucose, insulin and lipids measurements
Plasma glucose levels were measured by the glucose dehydrogenase method using an Accu-Chek blood glucose meter (Roche Diagnostics, Mainz-Hechtsheim, Germany). Plasma insulin levels were measured by ELISA (Morinaga, Yokohama, Japan). Plasma triglyceride, total cholesterol and non-esterified fatty acids (NEFA) concentrations were measured by enzymatic methods using Triglyceride E, Cholesterol E and NEFA C-tests (Wako, Osaka, Japan), respectively.
Hepatic lipids concentration
Hepatic lipids were extracted from 1.0 g of liver with chloroform/methanol (2:1 v/v), according to the method of Folch et al.(29) Triglyceride and cholesterol concentrations were determined in the extracted samples by commercial kits (Triglyceride E and Cholesterol E-tests).
RNA preparation and quantitative RT-PCR
Total RNA was isolated from frozen liver and mesenteric adipose tissue samples with TRIzol Regent (Invitrogen, Carlsbad, CA) and RNeasy kit (QIAGEN, Tokyo, Japan), respectively. First-standard cDNAs were synthesized with M-MLV reverse transcriptase (Invitrogen) and oligo-dT primer. We performed real-time PCR by using the primers described in Table 2, and SYBR green dye (SYBR Premix Ex Taq; TAKARA BIO, Shiga, Japan) in a LightCycler real-time PCR system (Roche Diagnostics), according to the manufacturer’s instructions. The relative amounts of mRNA were calculated with β-actin mRNA as the invariant control. The ratio for the data from the HFD group was set arbitrarily at 1.
Table 2.
Sequence of oligonucleotide primers for quantitative RT-PCR analysis
| Gene name | Size (bp) | Accession No. | Primer sequence |
|---|---|---|---|
| PPARγ | 147 | AF156665 | F: 5'-GGAAACTTGTGCAAGGTTGGA-3' |
| R: 5'-CAGGCTCTACTTTGATCGCA-3' | |||
| adiponectin | 140 | NM_144744 | F: 5'-GGAAACTTGTGCAAGGTTGGA-3' |
| R: 5'-GGTCACCCTTAGGACCAAGA-3' | |||
| HSL | 229 | X51415 | F: 5'-AGAGCCATCAGACAGCCCCGAGAT-3' |
| R: 5'-TGACGAGTAGAGGGGCATGTGGAG-3' | |||
| ATGL | 148 | NM_001108509 | F: 5'-GAGATGTGCAAACAGGGCTA-3' |
| R: 5'-CAGTCCTCTCCTCAGTCACG-3' | |||
| SREBP-1c | 190 | AF286470 | F: 5'-GGAGCCATGGATTGCACATTT-3' |
| R: 5'-TCCTTCCGAAGGTCTCTCCTC-3' | |||
| ChREBP | 113 | AB074517 | F: 5'-CAGCTTCTCGACTTGGACTG-3' |
| R: 5'-TTGCCAACATAAGCGTCTTC-3' | |||
| ACC | 233 | J03803 | F: 5'-CCAGTCTACATCCGCTTGGCTGAG-3' |
| R: 5'-AGTCGCCAGTAGAAGAAGGTGCGG-3' | |||
| FAS | 104 | M76767 | F: 5'-TGGGCCCATCTTCTTAGCC-3' |
| R: 5'-GGAACAGCGCAGTACCGTAGA-3' | |||
| PPARα | 112 | M88529 | F: 5'-TGTATGAAGCCATCTTCACG-3' |
| R: 5'-GGCATTGAACTTCATAGCGA-3' | |||
| ACO | 114 | J02752 | F: 5'-ATGGCAGTCCGGAGAATACCC-3' |
| R: 5'-CCTCATAACGCTGGCTTCGAGT-3' | |||
| UCP2 | 107 | NM_019354 | F: 5'-TCTCCCAATGTTGCCCGAAA-3' |
| R: 5'-GGGAGGTCGTCTGTCATGAG-3' | |||
| PGC-1α | 109 | AY237127 | F: 5'-TGTTCGATGTGTCGCCTTGT-3' |
| R: 5'-GAACGAGAGCGCATCCTTTG-3' | |||
| CYP7A1 | 127 | NM_012942 | F: 5'-CACCTTTGACGACATGGAGAAG-3' |
| R: 5'-TGCTTTCATTGCTTCAGGACTC-3' | |||
| ABCG5 | 111 | NM_053754 | F: 5'-TGTGTTACTGGACTCTGGGC-3' |
| R: 5'-CAAGCAGCACAAGTGTCAGA-3' | |||
| β-actin | 171 | NM_031144 | F: 5'-GTCCCAGTATGCCTCTGGTCGTAC-3' |
| R: 5'-CCACGCTCGGTCAGGATCTTCATG-3' |
F, forward; R, reverse; PPAR, peroxisome proliferator-activated receptor; HSL, hormone sensitive lipase; ATGL, adipose trigyceride lipase; SREBP-1c, sterol regulatory-element binding protein 1c; ChREBP, carbohydrate response-element binding protein; ACC, acetyl coenzyme A carboxylase; FAS, fatty acid synthase; ACO, acyl coenzyme A oxidase; UCP2, uncoupling protein 2; PGC-1α, peroxisome proliferator-activated receptor-gamma coactivator 1α; CYP7A1, cholesterol 7α hydroxylase; ABCG5, ATP-binding cassette subfamily G member 5.
Primary culture of hepatocytes
Hepatocytes were isolated from male SD rats aged 6–10 weeks using the collagenase perfusion method.(30) The cells were cultured in glucose-free Dulbecco’s modified Eagle’s medium (Invitrogen) supplemented with 1 nmol/l of insulin (Sigma, St. Louis, MO), 1 nmol/l of dexamethasone (Sigma), 10% (v/v) fetal bovine serum (Invitrogen), and 1% (v/v) penicillin-streptomycin (Sigma). After 6 h of attachment, the medium was removed and changed to fresh medium. After an overnight culture, cells were stimulated with 5 mmol/l xylitol for 16 h, after which total RNA was extracted (n = 3 in each experiment) and cDNA synthesis and real-time PCR analysis was performed.
Effects of xylitol on postprandial glucose and insulin levels in rats
Male SD rats aged 10–13 weeks were used in an oral sucrose tolerance test. Before 4 days of the oral study, rats underwent a catheterization to insert catheter into the left femoral vein as described previously.(31) After an overnight fast (18–20 h), sucrose (1 g/kg body weight), either alone or with xylitol (0.25 g/kg body weight), was orally administered to rats. Mannitol, a type of sugar alcohol, was used as a reference against xylitol. Blood samples were taken from the femoral vein of unanesthetized rats at 0, 30, 60 and 120 min after administration.
Statistical analysis
Values are expressed as means ± SEM. Significance of the differences was determined by analysis of variance (ANOVA) or the Kruskal-Wallis test, followed by the post-hoc test. Differences with a value of p<0.05 were considered significant. Statistical tests were performed with Excel-Toukei 2006 (SSRI, Tokyo, Japan).
Results
Xylitol supplimentation suppresses visceral fat accumulation induced by HFD
During the 8-week feeding period, the energy intake was similar in all groups (Table 3). Diarrhea was not found in any rats throughout the study. After the experimental period, body weight did not differ among the groups; however, the accumulation of visceral fat was significantly smaller in the xylitol-fed (X1 and X2) groups than in the control (HFD) group (p<0.05; Table 3). In particular, the relative weight of mesenteric fat was significantly lower in the xylitol-fed (X2) group than in the HFD group by 23.2% (p<0.05; Table 3). In addition, the relative weight of epididymal fat was significantly lower in the xylitol-fed (X1 and X2) groups than in the HFD group by 15.5% and 17.0%, respectively (p<0.05; Table 3). The relative weights of soleus muscle and liver, and the hepatic triglyceride and cholesterol concentrations were not affected by dietary interventions (Table 3).
Table 3.
Energy intake, body and organ weights, and hepatic lipid concentrations in rats fed three different diets for 8 week
| HFD | X1 | X2 | |
|---|---|---|---|
| Energy intake, kcal/d | 95.5 ± 1.1 | 94.1 ± 1.2 | 93.5 ± 1.2 |
| Body weight, g | 543.2 ± 10.2 | 525.1 ± 9.6 | 540.4 ± 9.9 |
| Visceral fat, g/kg body weight | 96.7 ± 3.8 | 84.2 ± 5.3* | 81.7 ± 4.2* |
| Mesenteric fat | 27.5 ± 1.3 | 25.4 ± 1.3 | 21.1 ± 1.0* |
| Epididymal fat | 30.2 ± 2.1 | 25.6 ± 1.5* | 25.1 ± 1.9* |
| Retroperitoneal fat | 39.0 ± 1.7 | 33.2 ± 3.9 | 35.5 ± 2.1 |
| Soleus muscle, g/kg body weight | 0.64 ± 0.02 | 0.68 ± 0.02 | 0.66 ± 0.04 |
| Liver, g/kg body weight | 29.7 ± 1.1 | 29.6 ± 1.1 | 28.4 ± 1.2 |
| Liver triglyceride, µmol/γ tissue | 21.1 ± 4.3 | 27.3 ± 5.4 | 22.6 ± 5.6 |
| Liver cholesterol, µmol/γ tissue | 3.7 ± 0.6 | 4.5 ± 0.6 | 3.9 ± 0.7 |
Values are mean ± SEM, n = 6. *p<0.05 vs HFD group.
Xylitol supplementation suppresses the increrased insulinemia and lipidemia induced by HFD
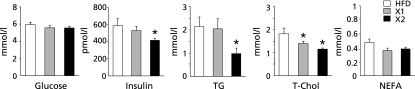
The plasma glucose and non-esterified fatty acid (NEFA) concentrations did not differ among the three groups (Fig. 1). The plasma insulin and triglyceride concentrations were significantly lower in the xylitol-fed (X2) group than in the HFD group by 29.3% and 54.5%, respectively (p<0.05; Fig. 1). The plasma total cholesterol concentrations in the xylitol-fed (X1 and X2) groups were significantly reduced as compared with the HFD group (p<0.05; Fig. 1).
Fig. 1.
Plasma glucose, insulin, and lipids levels in rats fed three different diets for 8 week. Values are mean ± SEM (n = 6 for each group). *p<0.05 vs HFD group. TG, triglyceride; T-Chol, total cholesterol; NEFA, non-esterified fatty acid.
Adipose gene expression in xylitol long-term feeding test
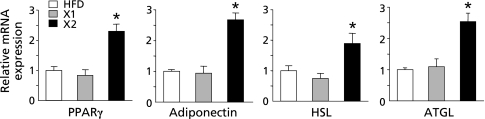
As shown in Table 3, the visceral fat mass, especially mesenteric and epididymal fat mass, was significantly lower in the xylitol-fed (X1 and X2) groups than in the HFD group. Therefore, we examined the expression of lipid metabolism-related genes in the mesenteric adipose tissue by real-time RT-PCR. The mRNA levels of PPARγ, a key regulator of adipocyte differentiation, and the insulin-sensitizing hormone adiponectin were significantly up-regulated in the xylitol-fed (X2) group as compared with the HFD group (p<0.05; Fig. 2). Moreover, the mRNA levels of hormone sensitive lipase (HSL) and adipose triglyceride lipase (ATGL), the lipolytic enzymes in adipocytes, were significantly elevated in the xylitol-fed (X2) group as compared with the HFD group (p<0.05; Fig. 2).
Fig. 2.
Adipose gene expression in rats fed three different diets for 8 week. mRNA levels of genes in the mesenteric fat tissues were determined by quantitative RT-PCR analysis. Values are mean ± SEM (n = 5 for each group). *p<0.05 vs HFD group. The ratio for the data from the HFD group was set arbitrarily at 1. PPARγ, peroxisome proliferator-activated receptor γ; HSL, hormone sensitive lipase; ATGL, adipose triglyceride lipase.
Hepatic gene expression in xylitol long-term feeding test
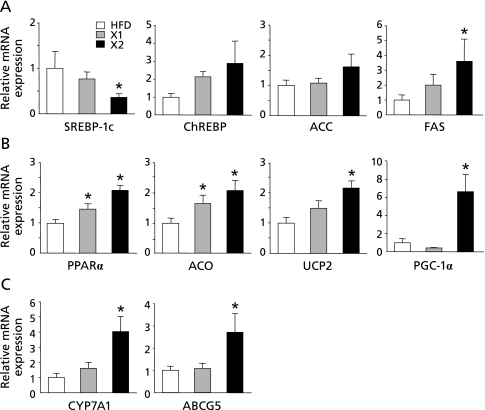
To investigate the molecular mechanisms underlying the effects of xylitol on hepatic lipid metabolism in vivo, we examined the expression levels of lipid metabolism-related genes in the liver by real-time RT-PCR. The mRNA levels of ChREBP, and the lipogenic enzymes ACC and FAS were higher in the xylitol-fed (X1 and X2) groups than in the HFD group (p<0.05; Fig. 3A). On the other hand, the mRNA level of another lipogenic transcription factor SREBP-1c, which regulated principally by insulin,(32) was significantly lower in the xylitol-fed (X2) group than in the HFD group (p<0.05; Fig. 3A). The mRNA levels of the transcription factors PPARα and PPARγ coactivator 1α (PGC-1α) which regulate fatty acid oxidation were significantly higher in the xylitol-fed (X2) group than in the HFD group (p<0.05; Fig. 3B). The mRNA levels of acyl-coenzyme A oxidase (ACO) and uncoupling protein 2 (UCP2), downstream target genes of PPARα were significantly higher in the xylitol-fed (X1 and X2) groups than in the HFD group (p<0.05; Fig. 3B). Additionally, the expression levels of genes involved in bile acid synthesis and cholesterol metabolism such as cholesterol 7α hydroxylase (CYP7A1) and ATP-binding cassette, subfamily G, member 5 (ABCG5) increased in the xylitol-fed (X2) group compared with HFD group (p<0.05; Fig. 3C).
Fig. 3.
Hepatic gene expression in rats fed three different diets for 8 week. mRNA levels of genes related to (A) lipogenesis, (B) fatty acid oxidation, and (C) cholesterol metabolism in the liver were determined by quantitative RT-PCR analysis. Values are mean ± SEM (n = 6 for each group). *p<0.05 vs HFD group. The ratio for the data from the HFD group was set arbitrarily at 1. SREBP-1c, sterol regulatory-element binding protein 1c; ChREBP, carbohydrate response-element binding protein; ACC, acetyl coenzyme A carboxylase; FAS, fatty acid synthase; PPARα, peroxisome proliferator-activated receptor α; ACO, acyl coenzyme A oxidase; UCP2, uncoupling protein 2; PGC-1α, peroxisome proliferator-activated receptor-gamma coactivator 1α; CYP7A1, cholesterol 7α hydroxylase; ABCG5, ATP-binding cassette subfamily G member 5.
Effect of xylitol on gene expression in rat primary hepatocytes
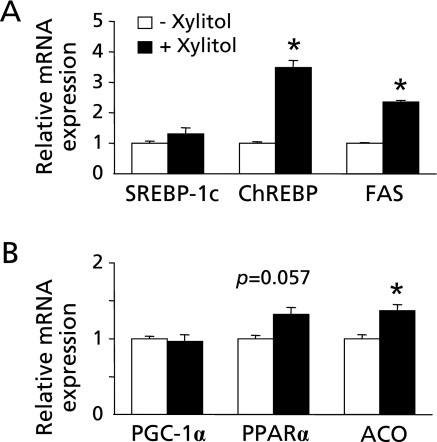
Xylitol is metabolized predominantly in the liver,(13) so we next investigated the direct effect of xylitol on lipid metabolism-related gene expression using rat primary hepatocytes. Xylitol induced lipogenic gene expressions including ChREBP and FAS (p<0.05; Fig. 4A), as well as previously reported.(19,33) Moreover, xylitol also induced fatty acid oxidation-related gene expressions including PPARα and ACO as observed in long-term feeding test (p = 0.057 and p<0.05; Fig. 4B). However, the direct effect of xylitol on PPARα and ACO seems to be not so potent. In addition, xylitol stimulation did not change the expression level of SREBP-1c differently from the result in vivo (Fig. 4A). These results indicated that xylitol could also have the indirect effect on hepatic gene expression.
Fig. 4.
Effects of xylitol on gene expression in rat primary hepatocytes. Xylitol stimulation was performed in rat primary hepatocytes, and mRNA levels of genes related to (A) lipogenesis and (B) fatty acid oxidation were determined by quantitative RT-PCR analysis. Values are mean ± SEM, n = 3. *p<0.05 vs control (- xylitol) group.
Xylitol suppresses postprandial rises in blood glucose and insulin in rats
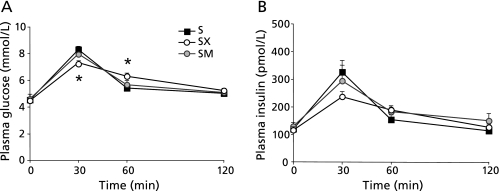
In the long-term xylitol feeding test, fasting plasma insulin level was reduced in the xylitol-fed groups than in the HFD group (Fig. 1). One potential factor that regulates lipogenic gene expression is insulin. Thus, we investigated whether xylitol suppress the postprandial rises in blood glucose and insulin by performing the oral co-administration with sucrose to rats as described previously.(34,35) The plasma glucose levels were significantly reduced by the coadministration of xylitol at 30 min after the sucrose administration (p<0.05; Fig. 5A). In addition, the coadministration of xylitol tended to decrease the plasma insulin levels (Fig. 5B). There were no significant differences in the plasma glucose and insulin levels between the sucrose alone and the coadministration of mannitol (Fig. 5 A and B). These data showed that xylitol could suppress postprandial rise in glucose and insulin levels.
Fig. 5.
Effect of oral sucrose administration with or without xylitol on plasma glucose and insulin levels. After fasting for 18–20 h, the rats were orally administrated sucrose (1 g/kg body weight) either alone or with xylitol or mannitol (0.25 g/kg body weight). Blood samples were taken at 0, 30, 60 and 120 min after administration. (A, B) Time-dependent curve for plasma levels of (A) glucose and (B) insulin. Values are mean ± SEM, n = 4–7. *p<0.05 vs S group. S, sucrose (1 g/kg body weight); SX, sucrose (1 g/kg body weight) with xylitol (0.25 g/kg body weight); SM, sucrose (1 g/kg body weight) with mannitol (0.25 g/kg body weight).
Discussion
In the present study, we demonstrated that long-term intake of xylitol suppressed the accumulation of visceral fat and the increase in plasma insulin and lipids concentrations in rats fed a high-fat diet. Intake of xylitol stimulated the expression of fatty acid oxidation genes in the liver, and lipid degradation and adiponectin genes in the adipose tissue. Furthermore, in oral sucrose tolerance test, we found for the first time that xylitol ingestion lowered postprandial hyperglycemia. The dose of xylitol in this study is the non-effective dose in causing diarrhea, and within the limits of orally administered physiological amounts (1–4 g/kg body weight daily) of xylitol on human and rats identified previously.(36,37)
The adipose tissue of xylitol-fed rats showed significantly higher levels of mRNAs encoding PPARγ, adiponectin, HSL and ATGL. These data indicates a miniaturization of adipocytes and lipolysis were caused in the adipose tissue, that they could contribute to lowering fat mass in xylitol-fed rats. PPARγ is mainly expressed in adipose tissue, which triggers adipocyte differentiation,(38) and it up-regulates the expression level of adiponectin in small adipocytes.(39) Adiponectin is an important modulator of insulin sensitivity and activates PPARα, thereby stimulating fatty acid oxidation in the liver.(40) In addition, PPARγ agonists can stimulate lipolysis by increasing the expression levels of lipolysis-related enzymes, including HSL and ATGL in adipose tissue.(41) Therefore, these observations suggest that the elevation in PPARγ expression in the adipose tissue of rats fed a high-fat diet with xylitol might contribute to the suppression of visceral fat accumulation and increased level of adiponectin mRNA that occurs concomitantly with the provision of fatty acid as an energy source to the liver. At present, however, it is uncertain how dietary xylitol promotes the expression of PPARγ in adipose tissue. Because xylitol is mainly metabolized in the liver(16–18) and little is taken up into adipose tissue,(42,43) the effects of xylitol on adipose tissue may be not a direct effect. Additional studies are needed to investigate the underlying mechanism.
In the liver, two main transcription factors regulate the gene expression of lipogenic enzymes: one is SREBP-1c, whose expression can be up-regulated by insulin;(32) the other is ChREBP, which can be activated by the xylitol metabolite Xu5P through the activation of PP2A, independently of insulin.(21,22) Although it has been reported that SREBP-1c and ChREBP synergistically regulate lipogenesis,(44) the liver of xylitol-fed rats showed the increase in the expression of ChREBP and the reduction of SREBP-1c in the long-term xylitol feeding test, and caused the suppression of visceral fat accumulation, resulting from not trending toward an increase in lipid synthesis. When we tried to confirm the direct effect of xylitol on above-mentioned genes expression related to hepatic lipid metabolism by using rat primary hepatocytes, xylitol induced the gene expression of ChREBP, but did not affect SREBP-1c. In oral sucrose tolerance test, xylitol supplementation exhibited a suppression of postprandial hyperglycemia. Therefore, the suppression of plasma insulin levels in xylitol-fed rats of the long-term test could be attributed to the suppression of postprandial hyperglycemia. The smaller demand for insulin secretion in xylitol-fed rats can contribute to lower the level of plasma insulin and the hepatic lipid metabolism-related gene expression. Because SREBP-1c is regulated mainly by insulin, a low level of SREBP-1c mRNA expression in vivo study might be caused by the suppression of insulin secretion in xylitol-fed rats.
In the current in vivo study, the liver of xylitol-fed rats showed a significant increase in mRNAs levels of the genes encoding PPARα, PGC-1α, ACO and UCP2, which are related to fatty acid oxidation.(45,46) Activation of PPARα not only suppresses adipocyte hypertrophy in adipose tissue but also stimulates fatty acid oxidation in the liver.(47) In addition, the rate of fatty acid oxidation in the liver is a major determinant of plasma triglyceride levels.(48) The increased fatty acid oxidation-related gene expression may also contribute to the suppression of visceral fat accumulation in xylitol-fed rats. However, it is likely that the direct effect of xylitol on the induction of PPARα and oxidation-related enzyme is not much powerful as shown Fig. 4B. The other candidate for the regulator of fatty acid oxidation can be suspected insulin. The attenuation of hyperinsulinemia enhances fat oxidation rates, and assist in preventing obesity and insulin resistance.(49,50) And, insulin negative-regulator of fatty acid oxidation-related gene expression including PPARα.(51,52) Therefore, it is possible that hepatic oxidation-related gene expression in xylitol-fed rats could be affected by insulin. Taken together, hepatic lipid metabolism in xylitol-fed rats might be affected predominantly by the suppression of insulin, and contribute to lower visceral fat accumulation. Because xylitol intake attenuated the increase in plasma insulin induced by HFD and postprandial hyperglycemia, it may be possible that intake of xylitol prevents the onset or progression of type 2 diabetes. However, further studies using model animals of diabetes are needed.
Conclusion
To date, xylitol is used widely in foods and medications, but its metabolite has been reported to activate ChREBP, up-regulating the gene transcription of lipogenic enzymes in vitro. This discrepancy suggests caution in the use of xylitol for the patients with obesity, type 2 diabetes, and other metabolic disorders. Our study has demonstrated that the intake of xylitol in vivo did not cause problems with lipogenesis, because of the suppression of high-fat induced-visceral fat accumulation. In addition, xylitol may have some beneficial effects such as lower postprandial hyperglycemia. These preferable effects suggest that xylitol intake may be useful to control or prevent humans from obesity, diabetes, and other metabolic disorders.
Acknowledgments
This work was supported by the Grants-in-Aid for Scientific Research (B) (to E.T.), for Young Scientists (to H.A. and Y.T.) from the Ministry of Education, Culture, Sports, Science and Technology in Japan.
We thank Assistant Professor Nagakatsu Harada (Department of Nutrition and Metabolism) for excellent technical assistance, and Kazusa Sato, Asami Toya and Ayumi Kuwada for essential experimental assistance.
Abbreviations
- ABCG5
ATP-binding cassette subfamily G member 5
- ACC
acetyl coenzyme A carboxylase
- ACO
acyl coenzyme A oxidase
- ATGL
adipose trigyceride lipase
- ChREBP
carbohydrate response-element binding protein
- CYP7A1
cholesterol 7α hydroxylase
- FAS
fatty acid synthase
- HFD
high-fat diet
- HSL
hormone sensitive lipase
- PGC-1α
peroxisome proliferator-activated receptor-gamma coactivator 1α
- PPAR
peroxisome proliferator-activated receptor
- SREBP-1c
sterol regulatory-element binding protein 1c
- UCP2
uncoupling protein 2
- X1
high-fat diet containing xylitol at 1.0 g/100 kcal
- X2
high-fat diet containing xylitol at 2.0 g/100 kcal
Conflict of interest
No potential conflicts of interest were disclosed.
References
- 1.Després JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2009;444:881–887. doi: 10.1038/nature05488. [DOI] [PubMed] [Google Scholar]
- 2.Nammi S, Koka S, Chinnala KM, Boini KM. Obesity: an overview on its current perspectives and treatment options. Nutr J. 2003;3:3. doi: 10.1186/1475-2891-3-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akagiri S, Naito Y, Ichikawa H, et al. A mouse model of metabolic syndrome; Increase in visceral adipose tissue precedes the development of fatty liver and insulin resistance in high-fat-diet fed-male KK/Ta mice. J Clin Biochem Nutr. 2008;42:150–157. doi: 10.3164/jcbn.2008022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bray GA, Lovejoy JC, Smith SR, et al. The influence of different fats and fatty acids on obesity, insulin resistance and inflammation. J Nutr. 2002;132:2488–2491. doi: 10.1093/jn/132.9.2488. [DOI] [PubMed] [Google Scholar]
- 5.Watanabe K, Arozal W, Tanaka H, et al. Beneficial effect of food substitute containing L-arginine, omega-3 poly unsaturated fatty acid, and ribonucleic acid in preventing or improving metabolic syndrome: a study in 15 overweight patients and a study of fatty acid metabolism in animals. J Clin Biochem Nutr. 2009;44:266–174. doi: 10.3164/jcbn.08-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martins IJ, Redgrave TG. Obesity and post-prandial lipid metabolism. Feast or famine? J Nutr Biochem. 2004;15:130–141. doi: 10.1016/j.jnutbio.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 7.Surwit RS, Kuhn CM, Cochrane C, McCubbin JA, Feinglos MN. Diet-induced type II diabetes in C57BL/6J mice. Diabetes. 1988;37:1163–1167. doi: 10.2337/diab.37.9.1163. [DOI] [PubMed] [Google Scholar]
- 8.Kennedy AR, Pissios P, Out H, et al. A high-fat, ketogenic diet induces a unique metabolic state in mice. Am J Physiol Endocrinol Metab. 2007;292:E1724–E1739. doi: 10.1152/ajpendo.00717.2006. [DOI] [PubMed] [Google Scholar]
- 9.Murase T, Mizuno T, Omachi T, et al. Dietary diacylglycerol suppresses high fat and high sucrose diet-induced body fat accumulation in C57BL/6J mice. J Lipid Res. 2001;42:372–378. [PubMed] [Google Scholar]
- 10.Ide T, Shimano H, Yahagi N, et al. SREBPs suppress IRS-2-mediated insulin signalling in the liver. Nat Cell Biol. 2004;6:351–357. doi: 10.1038/ncb1111. [DOI] [PubMed] [Google Scholar]
- 11.Heilbronn LK, Gregersen S, Shirkhedkar D, Hu D, Campbell LV. Impaired fat oxidation after a single high-fat meal in insulin-sensitive nondiabetic individuals with a family history of type 2 diabetes. Diabetes. 2007;56:2046–2053. doi: 10.2337/db06-1687. [DOI] [PubMed] [Google Scholar]
- 12.Georgieff M, Moldawer LL, Bistrian BR, Blackburn GL. Xylitol, an energy source for intravenous nutrition after trauma. JPEN J Parenter Enteral Nutr. 1985;9:199–209. doi: 10.1177/0148607185009002199. [DOI] [PubMed] [Google Scholar]
- 13.Dills WL., Jr Sugar alcohols as bulk sweeteners. Annu Rev Nutr. 1989;9:161–186. doi: 10.1146/annurev.nu.09.070189.001113. [DOI] [PubMed] [Google Scholar]
- 14.Goto Y, Anzai M, Chiba M, Ohneda A, Kawashima S. Clinical effects of xylitol on carbohydrate and lipid metabolism in diabetes. Lancet. 1965;2:918–921. doi: 10.1016/s0140-6736(65)92900-4. [DOI] [PubMed] [Google Scholar]
- 15.Natah SS, Hussien KR, Tuominen JA, Koivisto VA. Metabolic response to lactitol and xylitol in healthy men. Am J Clin Nutr. 1977;65:947–950. doi: 10.1093/ajcn/65.4.947. [DOI] [PubMed] [Google Scholar]
- 16.Mäkinen KK. Biochemical principles of the use of xylitol in medicine and nutrition with special consideration of dental aspects. Experientia Suppl. 1978;30:1–160. doi: 10.1007/978-3-0348-5757-4. [DOI] [PubMed] [Google Scholar]
- 17.Mäkinen KK. Can the pentitol-hexitol theory explain the clinical observations made with xylitol? Med Hypotheses. 2000;54:603–613. doi: 10.1054/mehy.1999.0904. [DOI] [PubMed] [Google Scholar]
- 18.Woods HF, Krebs HA. Xylitol metabolism in the isolated perfused rat liver. Biochem J. 1973;134:437–443. doi: 10.1042/bj1340437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kabashima T, Kawaguchi T, Wadzinski BE, Uyeda K. Xylulose 5-phosphate mediates glucose-induced lipogenesis by xylulose 5-phosphate-activated protein phosphatase in rat liver. Proc Natl Acad Sci USA. 2003;100:5107–5112. doi: 10.1073/pnas.0730817100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kawaguchi T, Takenoshita M, Kabashima T, Uyeda K. Glucose and cAMP regulate the L-type pyruvate kinase gene by phosphorylation/dephosphorylation of the carbohydrate response element binding protein. Pro Natl Acad Sci USA. 2001;98:13710–13715. doi: 10.1073/pnas.231370798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iizuka K, Bruick RK, Liang G, Horton JD, Uyeda K. Deficiency of carbohydrate response element-binding protein (ChREBP) reduces lipogenesis as well as glycolysis. Proc Natl Acad Sci USA. 2004;101:7281–7286. doi: 10.1073/pnas.0401516101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ishii S, Iizuka K, Miller BC, Uyeda K. Carbohydrate response element binding protein directly promotes lipogenic enzyme gene transcription. Proc Natl Acad Sci USA. 2004;101:15597–15602. doi: 10.1073/pnas.0405238101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kusunoki J, Kanatani A, Moller DE. Modulation of fatty acid metabolism as a potential approach to the treatment of obesity and the metabolic syndrome. Endocrine. 2006;29:91–100. doi: 10.1385/ENDO:29:1:91. [DOI] [PubMed] [Google Scholar]
- 24.Postic C, Girard J. The role of the lipogenic pathway in the development of hepatic steatosis. Diabetes Metab. 2008;34:643–648. doi: 10.1016/S1262-3636(08)74599-3. [DOI] [PubMed] [Google Scholar]
- 25.Reeves PG, Nielsen FH, Fahey GC., Jr AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr. 1993;123:1939–1951. doi: 10.1093/jn/123.11.1939. [DOI] [PubMed] [Google Scholar]
- 26.Daly M. Sugars, insulin sensitivity, and the postprandial state. Am J Clin Nutr. 2003;78:865S–872S. doi: 10.1093/ajcn/78.4.865S. [DOI] [PubMed] [Google Scholar]
- 27.Fried SK, Rao SP. Sugars, hypertriglyceridemia, and cardiovascular disease. Am J Clin Nutr. 2003;78:873S–880S. doi: 10.1093/ajcn/78.4.873S. [DOI] [PubMed] [Google Scholar]
- 28.Vermunt SH, Pasman WJ, Schaafsma G, Kardinaal AF. Effects of sugar intake on body weight: a review. Obes Rev. 2003;4:91–99. doi: 10.1046/j.1467-789x.2003.00102.x. [DOI] [PubMed] [Google Scholar]
- 29.Folch J, Less M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 30.Uebanso T, Taketani Y, Fukaya M, et al. Hypocaloric high-protein diet improves fatty liver and hypertriglyceridemia in sucrose-fed obese rats via two pathways. Am J Physiol Endocrinol Metab. 2009;297:E67–E84. doi: 10.1152/ajpendo.00014.2009. [DOI] [PubMed] [Google Scholar]
- 31.Fukaya M, Mizuno A, Arai H, et al. Mechanism of rapid-phase insulin response to elevation of portal glucose concentration. Am J Physiol Endocrinol Metab. 2007;293:E515–E522. doi: 10.1152/ajpendo.00536.2006. [DOI] [PubMed] [Google Scholar]
- 32.Foretz M, Guichard C, Ferré P, Foufelle F. Sterol regulatory element binding protein-1c is a major mediator of insulin action on the hepatic expression of glucokinase and lipogenesis-related genes. Proc Natl Acad Sci USA. 1999;96:12737–12742. doi: 10.1073/pnas.96.22.12737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mourrieras F, Foufelle F, Foretz M, Morin J, Bouche S, Ferre P. Induction of fatty acid synthase and S14 gene expression by glucose, xylitol and dihydroxyacetone in cultured rat hepatocytes is closely correlated with glucose 6-phosphate concentrations. Biochem J. 1997;326:345–349. doi: 10.1042/bj3260345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Uebanso T, Arai H, Taketani Y, et al. Extracts of Momordica charantia suppress postprandial hyperglycemia in rats. J Nutr Sci Vitaminol (Tokyo) 2007;53:482–488. doi: 10.3177/jnsv.53.482. [DOI] [PubMed] [Google Scholar]
- 35.Matsuo T, Izumori T. d-Psicose inhibits intestinal alpha-glucosidase and suppresses the glycemic response after ingestion of carbohydrates in rats. J Clin Biochem Nutr. 2009;45:202–206. doi: 10.3164/jcbn.09-36. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 36.Makinen KK, Ylikahri R, Makinen PL, Soderling E, Hamalainen M. Turku sugar studies XXIII. Comparison of metabolic tolerance in human volunteers to high oral doses of xylitol and sucrose after long-term regular consumption of xylitol. Int J Vitam Nutr Res Suppl. 1982;22:29–49. [PubMed] [Google Scholar]
- 37.Hamalainen M, Makinen KK. Effect of peroral xylitol on the concentration levels of lipids and electrolytes in rat tessues. Nutr Res. 1983;3:497–510. [Google Scholar]
- 38.Desvergne B, Wahli W. Peroxisome proliferator-activated receptors: nuclear control of metabolism. Endocr Rev. 1999;20:649–688. doi: 10.1210/edrv.20.5.0380. [DOI] [PubMed] [Google Scholar]
- 39.Hammarstedt A, Sopasakis VR, Gogg S, Jansson PA, Smith U. Improved insulin sensitivity and adipose tissue dysregulation after short-term treatment with pioglitazone in non-diabetic, insulin-resistant subjects. Diabetologia. 2005;48:96–104. doi: 10.1007/s00125-004-1612-3. [DOI] [PubMed] [Google Scholar]
- 40.Yamauchi T, Kamon J, Waki H, et al. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med. 2001;7:941–946. doi: 10.1038/90984. [DOI] [PubMed] [Google Scholar]
- 41.Festuccia WT, Laplante M, Berthiaume M, Gélinas Y, Deshaies Y. PPARgamma agonism increases rat adipose tissue lipolysis, expression of glyceride lipases, and the response of lipolysis to hormonal control. Diabetologia. 2006;49:2427–2436. doi: 10.1007/s00125-006-0336-y. [DOI] [PubMed] [Google Scholar]
- 42.Quadflieg KH, Brand K. Comparison of xylitol and glucose metabolism in nonhepatic rat tissues. Z Ernahrungswiss. 1976;15:345–354. doi: 10.1007/BF02020503. [DOI] [PubMed] [Google Scholar]
- 43.Wang MC, Meng HC. Xylitol metabolism in extrahepatic tissues. Z Ernahrungswiss Suppl. 1971;11:8–16. [PubMed] [Google Scholar]
- 44.Dentin R, Girard J, Postic C. Carbohydrate responsive element binding protein (ChREBP) and sterol regulatory element binding protein-1c (SREBP-1c): two key regulators of glucose metabolism and lipid synthesis in liver. Biochimie. 2005;87:81–86. doi: 10.1016/j.biochi.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 45.Mandard S, Müller M, Kersten S. Peroxisome proliferator-activated receptor alpha target genes. Cell Mol Life Sci. 2004;61:393–416. doi: 10.1007/s00018-003-3216-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vega RB, Huss JM, Kelly DP. The coactivator PGC-1 cooperates with peroxisome proliferator-activated receptor alpha in transcriptional control of nuclear genes encoding mitochondrial fatty acid oxidation enzymes. Mol Cell Biol. 2000;20:1868–1876. doi: 10.1128/mcb.20.5.1868-1876.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tsuchida A, Yamauchi T, Takekawa S, et al. Peroxisome proliferator-activated receptor (PPAR) alpha activation increases adiponectin receptors and reduces obesity-related inflammation in adipose tissue: comparison of activation of PPARalpha, PPARgamma, and their combination. Diabetes. 2004;54:3358–3370. doi: 10.2337/diabetes.54.12.3358. [DOI] [PubMed] [Google Scholar]
- 48.Ide T, Ontko JA. Increased secretion of very low density lipoprotein triglyceride following inhibition of long chain fatty acid oxidation in isolated rat liver. J Biol Chem. 1981;256:10247–10255. [PubMed] [Google Scholar]
- 49.Brand-Miller JC, Holt SH, Pawlak DB, McMillan J. Glycemic index and obesity. Am J Clin Nutr. 2002;76:281S–285S. doi: 10.1093/ajcn/76/1.281S. [DOI] [PubMed] [Google Scholar]
- 50.van Can JG, Ijzerman TH, van Loon LJ, Brouns F, Blaak EE. Reduced glycaemic and insulinaemic responses following isomaltulose ingestion: implications for postprandial substrate use. Br J Nutr. 2009;102:1408–1143. doi: 10.1017/S0007114509990687. [DOI] [PubMed] [Google Scholar]
- 51.Daitoku H, Yamagata K, Matsuzaki H, Hatta M, Fukamizu A. Regulation of PGC-1 promoter activity by protein kinase B and the forkhead transcription factor FKHR. Diabetes. 2003;52:642–649. doi: 10.2337/diabetes.52.3.642. [DOI] [PubMed] [Google Scholar]
- 52.Steineger HH, Sørensen HN, Tugwood JD, Skrede S, Spydevold O, Grautvik KM. Dexamethasone and insulin demonstrate marked and opposite regulation of the steady-state mRNA level of the peroxisomal proliferator-activated receptor (PPAR) in hepatic cells. Hormonal modulation of fatty-acid-induced transcription. Eur J Biochem. 1994;225:967–974. doi: 10.1111/j.1432-1033.1994.0967b.x. [DOI] [PubMed] [Google Scholar]