Abstract
Chromatin remodeling is an important step in promoter activation during cellular lineage commitment and differentiation. We show that the ability of the C/EBPα transcription factor to direct adipocyte differentiation of uncommitted fibroblast precursors and to activate SWI/SNF-dependent myeloid-specific genes depends on a domain, C/EBPα transactivation element III (TE-III), that binds the SWI/SNF chromatin remodeling complex. TE-III collaborates with C/EBPα TBP/TFIIB interaction motifs during induction of adipogenesis and adipocyte-specific gene expression. These results indicate that C/EBPα acts as a lineage-instructive transcription factor through SWI/SNF-dependent modification of the chromatin structure of lineage-specific genes, followed by direct promoter activation via recruitment of the basal transcription–initiation complex, and provide a mechanism by which C/EBPα can mediate differentiation along multiple cellular lineages.
Keywords: Adipocyte, C/EBP, chromatin, differentiation, SWI/SNF, transcription
The activation of cellular genes is a multistep process requiring modulation of promoter and enhancer chromatin structure as a prerequisite for the subsequent binding of specific and basal transcription factors that direct gene transcription. Modification of chromatin involves direct modification of the histones by kinases, methylases, and acetylases, as well as reorganization of nuclesome structure by chromatin remodeling machines (for review, see Muller and Leutz 2001). The role of chromatin remodeling in lineage-specific gene expression has been investigated in the globin locus, where it has been found that the erythroid Kruppel-like factor (EKLF) transcription factor recruits the SWI/SNF complex to the β-globin promoter, resulting in displacement of nucleosomes from and hypersensitivity of the core promoter region (Armstrong et al. 1998; Lee et al. 1999). EKLF binding to the proximal β-globin promoter region is required for β-globin transcription in vivo, and EKLF-deficient mice die from severe β-thalassemia (Perkins et al. 1995). Recently, it was reported that dominant-negative variants of the SWI/SNF catalytic core subunits, human Brahma (hBrm) and Brahma-related gene 1 (Brg1), which lack the ATPase activity necessary for chromatin remodeling, inhibit MyoD-dependent induction of muscle-specific gene transcription in fibroblasts (de La Serna et al. 2001). These observations indicate that SWI/SNF-mediated chromatin remodeling is an integral part of developmental decisions mediated by transcriptional activators.
C/EBPα, the founding member of the C/EBP family, was originally isolated as an activator of liver-specific transcription. Subsequently, C/EBP proteins have been shown to regulate the differentiation of several cell types. C/EBPα is required in vivo for the differentiation of adipocytes, neutrophil granulocytes, and eosinophils (Wang et al. 1995; Zhang et al. 1997). Moreover, C/EBPα is capable of inducing uncommitted progenitors to differentiate along the granulocyte, eosinophil, and adipose lineages (Freytag et al. 1994; Nerlov et al. 1998; Radomska et al. 1998), classifying it as a lineage instructive transcription factor. The induction of eosinophil lineage commitment by C/EBPs appears to be caused by their ability to down-regulate the expression of the Friend of GATA (FOG) protein, allowing cooperative activation by C/EBP proteins and GATA-1 (Querfurth et al. 2000). The activation of chromatin-embedded myeloid genes in heterologous cell types, such as fibroblasts, has been accomplished by a combination of Myb and C/EBP transcription factors (Burk et al. 1993; Ness et al. 1993), and this has been shown to involve the recruitment of the SWI/SNF chromatin-remodeling complex by the N-terminal CR1 region of C/EBPβ (Kowenz-Leutz and Leutz 1999). In the case of the adipose lineage, the molecular mechanisms are less clear. The inductive role of C/EBPα in adipogenesis has been correlated to its ability to up-regulate the expression of PPARγ (Wu et al. 1999), an essential in vivo regulator of adipogenesis (Barak et al. 1999; Lowell 1999; Rosen et al. 1999), as well as the ability of C/EBPα to directly activate adipocyte-specific promoters (Christy et al. 1989; Cheneval et al. 1991).
To address the molecular mechanisms underlying adipocyte differentiation, we have determined the structural requirements for C/EBPα-mediated conversion of uncommitted fibroblast precursors to adipocytes. We find that the C/EBPα transactivation element III (TE-III; Nerlov and Ziff 1994) is required for adipose conversion, as well as activation of chromatin-embedded myeloid- and adipocyte-specific genes, including PPARγ. This region of C/EBPα interacts with the SWI/SNF chromatin remodeling complex, and collaborates with TBP/TFIIB-interacting motifs in C/EBPα during adipogenesis, indicating that integration of chromatin remodeling and recruitment of basal transcription factors forms the basis for the lineage instructive capacity of C/EBPα.
Results
The C/EBPα TE-III is required for adipose conversion of NIH3T3 fibroblasts
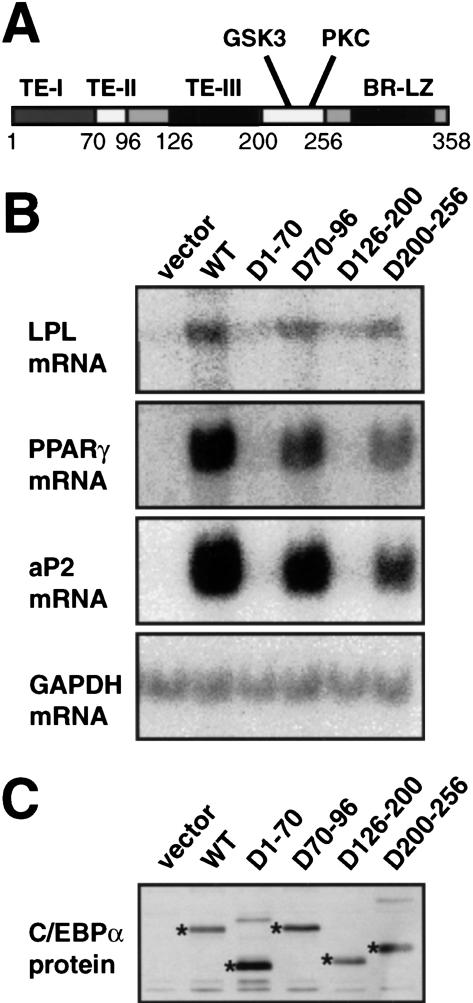
C/EBPα is able to mediate the transition of uncommitted mesenchymal precursor cells to mature adipocytes (Freytag et al. 1994). In the case of NIH3T3 fibroblasts, induced adipogenesis occurs without up-regulation of the endogenous C/EBPα gene (Wu et al. 1995; Yeh et al. 1995), making this system well-suited for structure-function analysis of C/EBPα. We infected subconfluent NIH3T3 cultures with pBabePuro-based virus expressing wild-type C/EBPα, as well as C/EBPα lacking each of the three identified C/EBPα transactivation elements (TE-I through III; Nerlov and Ziff 1994) or amino acids 200–256, containing the putative GSK3 (T222,T226,S230; Ross et al. 1999) and PKC (S248) phosphorylation sites in C/EBPα (Fig. 1A). These experiments showed that TE-I and TE-III were required for the induction of expression of the lipoprotein lipase (LPL), PPARγ, and aP2 mRNAs (Fig. 1B), all coding for proteins characteristically expressed in adipocytes. Deletion of TE-II or the GSK3 and PKC phosphorylation sites (aa 200–256) did not significantly affect C/EBPα-mediated induction of adipocyte gene expression (Fig. 1B), which occurred with kinetics comparable to cells transduced with wild-type C/EBPα (data not shown). For all mutants, gene expression correlated with morphological adipocyte differentiation and triglyceride accumulation induced by the C/EBPα alleles (Porse et al. 2001). Stability of the various mutant C/EBPα proteins did not appear to play a role in their differential ability to induce adipocyte differentiation, as judged by their similar expression levels determined by Western blotting (Fig. 1C).
Figure 1.
C/EBPα domains required for adipogenesis in vitro. (A) Domain structure of the C/EBPα protein. The transactivation elements (TE) I through III, the phosphorylation sites for glycogen synthase kinase 3 (GSK3), and protein kinase C (PKC), and the basic region-leucine zipper (BR-LZ) DNA binding domain are indicated. (B) NIH3T3 fibroblasts were transduced with pBabePuro (vector), pBabePuro-based retrovirus encoding wild-type C/EBPα, or C/EBPα derivatives lacking TE-I (D1–70), TE-II (D70–96), TE-III (D126–200), or the GSK3 and PKC phosphorylation sites (D200–256). After 2 wk of selection for expression of the retroviral construct total cellular RNA was analyzed for the presence of the following mRNAs by Northern blotting: lipoprotein lipase (LPL), peroxisome-proliferator activating receptor γ (PPARγ), aP2–422 (aP2), and glyceraldehyde phosphate dehydrogenase (GAPDH). (C) Parallel cultures were lysed in SDS sample buffer and equivalent amounts of total cellular protein analyzed for C/EBPα expression by Western blotting using the C103 anti-C/EBPα antibody. Bands corresponding to C/EBPα derivatives are indicated with asterisks.
The C/EBPα TE-III is required for interaction with the SWI/SNF chromatin remodeling complex
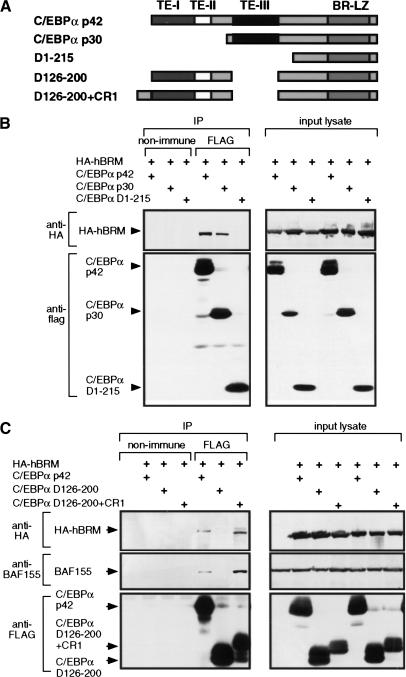
The functions embedded in the essential TE-I and TE-III regions were further examined. We have found TE-I to be necessary for the ability of C/EBPα to repress E2F mediated transcription, and that this is required for adipocyte differentiation (Porse et al. 2001). The function of TE-III was suggested by the observation that this region has little homology in other C/EBPs, including C/EBPβ. The distinct properties of C/EBPα and C/EBPβ were addressed recently by in vivo gene replacement by Chen et al. (2000), who substituted the mouse C/EBPα coding sequence with that of C/EBPβ (generating mice with the C/EBPαβ/β genotype), and observed that differentiation of white adipose tissue was severely impaired. The C/EBPβ coding sequence used to replace C/EBPα lacked the murine equivalent of the C/EBPβ CR1 SWI/SNF interaction domain (Kowenz-Leutz and Leutz 1999). This suggested that the lack of proper adipocyte differentiation in the C/EBPαβ/β mice was caused by a defect in SWI/SNF recruitment. We therefore tested the ability of C/EBPα to interact with SWI/SNF components, and whether TE-III was necessary and sufficient for this interaction. For this purpose, full-length C/EBPα (p42), C/EBPα p30 (a C/EBPα form initiated from the in-frame methionine at position 118), and a deletion mutant lacking the entire transactivation domain, including TE-III, (D1–215; Fig. 2A) were C-terminally FLAG-tagged and cotransfected with HA-tagged hBrm into the Brahma-negative C33A cell line. The ability of these C/EBPα derivatives to interact with hBrm was then analyzed by immunoprecipitation with the M2 anti-FLAG monoclonal antibody, followed by Western blot analysis of the immunoprecipitate with the PBR–205C monoclonal anti-HA antibody (Fig. 2B). In this assay the C/EBPα p42 and p30 proteins, but not C/EBPα D1-215, were found to associate with hBrm, consistent with TE-III being a C/EBPα SWI/SNF interaction domain. Indeed, deletion of TE-III was sufficient to eliminate the C/EBPα–hBrm coprecipitation (Fig. 2C). Furthermore, after deletion of TE-III, hBrm coprecipitation could be restored by fusing the heterologous CR1 SWI/SNF recruiting domain from C/EBPβ to the C/EBPα N terminus (Fig. 2A and C, lane 7). The BAF155 core SWI/SNF subunit also coprecipitated with C/EBPα (Fig. 2C). These results show that TE-III is both necessary and sufficient for C/EBPα to interact with the core SWI/SNF complex in a cellular setting, and that the structurally unrelated CR1 domain from C/EBPβ can functionally replace TE-III in this respect. Furthermore, these results show that the N-terminal part (amino acids 1–117) of the C/EBPα transactivation domain unique to C/EBPα p42 is not required for SWI/SNF interaction by C/EBPα.
Figure 2.
Interaction between TE-III and the SWI/SNF complex. (A) Structure of the C/EBPα derivatives used for coimmunoprecipitation assays. The transactivation elements (TE−) I through III, and the basic region-leucine zipper DNA binding domain (BR-LZ) are indicated. CR1 is the conserved region 1 from C/EBPβ (Kowenz-Leutz et al. 1994). All C/EBPα derivatives were C-terminally FLAG tagged for coimmunoprecipitation analysis. (B,C) 5 × 106 C33A cells were calcium phosphate-transfected with 5μg of the indicated CMV-driven C/EBPα-FLAG and HA-hBrm expression vectors. Lysates were prepared and immunoprecipitated (IP) with M2 anti-FLAG antibody (FLAG) or with nonimmune serum (non-immune). Precipitated proteins were detected by Western blotting with PRB-205C antiHA monoclonal antibody (HA–hBrm), M2 anti-FLAG monoclonal antibody (C/EBPα-FLAG) and anti-BAF155 polyclonal antibody (BAF155). Western blot analysis of the lysates used for immunoprecipitation is shown (lysate) to verify the expression of the various input proteins.
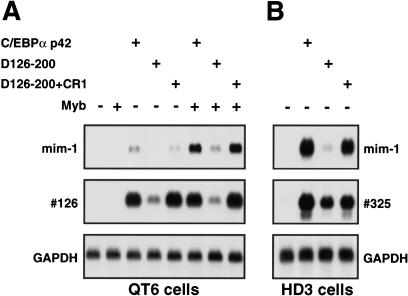
The ability of C/EBPα TE-III to function as a SWI/SNF-recruiting domain on chromosomal loci was tested by analyzing the C/EBPα-mediated induction of the mim-1 and #126 myeloid-specific transcripts (Ness et al. 1993). Both of these genomic loci are activated by C/EBPβ in a SWI/SNF-dependent manner in nonmyeloid cell types, and in the case of mim-1 in collaboration with Myb (Kowenz-Leutz and Leutz 1999). QT6 cells were transfected with wild-type C/EBPα and selected derivatives, both in the presence and the absence of c-Myb expression, and the expression of mim-1 and #126 was analyzed by Northern blotting (Fig. 3A). C/EBPα efficiently activated both #126 and mim-1 transcription, the latter in collaboration with c-Myb. This activation was strongly diminished by deletion of TE-III. However, TE-III deletion could be functionally compensated by N-terminal addition of the C/EBPβ CR1 domain. Similar results were obtained in HD3 erythroblasts; here Myb is already present, and strong TE-III-dependent activation of mim-1 transcription was observed (Fig. 3B). Again TE-III could be functionally replaced by CR1. The activation of the #325 locus, which is activated by C/EBPβ in a SWI/SNF independent manner (Kowenz-Leutz and Leutz 1999), is shown as a control for the presence of C/EBPα transactivation in all cases. Together these data show that C/EBPα TE-III mediates activation of SWI/SNF-dependent chromosomal loci, indicating that it indeed has SWI/SNF recruiting activity, and that this is a required function during C/EBPα-mediated activation of myeloid-specific transcription.
Figure 3.
TE-III dependent activation of chromosomal myeloid-specific loci. (A) Poly(A) RNA from 8 × 106 QT6 fibroblasts transfected with CMV–C/EBPα expression vectors and pCRNCM–Myb expression vector (+) or the corresponding empty expression vectors, was analyzed by Northern blotting for expression of the myeloid-specific mim-1 and #126 transcripts, and for expression of GAPDH mRNA as an internal control. (B) Poly(A) RNA from 2 × 107 HD3 cells transfected with the indicated CMV–C/EBPα expression vectors was analyzed by Northern blotting for expression of the myeloid-specific mim-1, #325, and GAPDH transcripts.
SWI/SNF recruitment is essential for C/EBPα-mediated adipogenesis
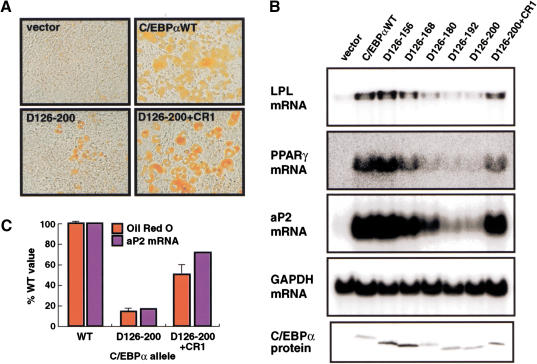
To assess whether SWI/SNF recruitment was the essential function of TE-III during adipocyte differentiation, we transduced NIH3T3 cells with retrovirus encoding C/EBPα, the TE-III deletion mutant, or the CR1 add-back protein. As can be seen from Figure 4A, this analysis showed that although deletion of TE-III strongly reduced morphological adipocyte differentiation, the CR1 domain could efficiently rescue the ability of C/EBPα to induce morphological adipocyte differentiation (Fig. 4A). Analysis of adipocyte-specific gene expression showed that the ability of C/EBPα to activate endogenous adipocyte-specific genes (with kinetics comparable to wild-type C/EBPα; data not shown), including that of PPARγ, was abolished by progressive deletion of TE-III, and restored by the CR1 add-back (Fig. 4B). The level of rescue was estimated by quantification of triglyceride accumulation and aP2 mRNA (Fig. 4C), and found to be 50% and 70%, respectively. The <100% rescue efficiency may be caused by the presence of both SWI/SNF recruiting and direct transactivation functions within TE-III, only the former being supplied by CR1 (see Discussion).
Figure 4.
Rescue of adipogenesis by the C/EBPβ CR1 domain. NIH3T3 cells were infected with pBabePuro control virus (vector) or virus encoding the indicated C/EBPα derivatives and allowed to differentiate for 2 wk. Adipocyte morphology was analyzed by Oil Red O staining (A), and expression of the LPL, PPARγ, and aP2 adipocyte mRNAs and C/EBPα protein determined (B) as in Figure 1B. (C) Level of adipocyte differentiation determined by quantitative Oil Red O staining (red columns; error bars indicate standard deviations; n = 3) and measurement of aP2 mRNA accumulation (after normalization to GAPDH) for the indicated C/EBPα alleles. The background value obtained from cells transduced with empty vector have been subtracted.
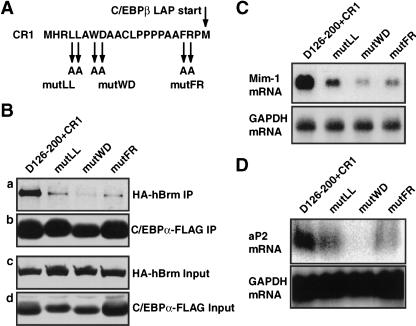
To further investigate the correlation between SWI/SNF recruitment and activation of adipocyte gene expression by C/EBPα, we introduced point mutations into residues of CR1 conserved in vertebrate C/EBPβ molecules (Fig. 5A). CR1 was chosen for this analysis rather than TE-III, because the latter, in addition to its SWI/SNF recruiting activity, harbors activating functions and negative regulatory elements (Friedman and McKnight 1990; Nerlov and Ziff 1994) that could potentially confound the analysis. These CR1 mutations were introduced into the C/EBPα D126–200+CR1 background and analyzed for their effect on SWI/SNF interaction in coimmunoprecipitation assays (as in Fig. 2). The mutations were found to inhibit (mutLL, mutFR) or abolish (mutWD) the interaction of the C/EBPα D126-200+CR1 molecule and the SWI/SNF complex, as determined by coimmunoprecipitation of HA–hBrm (Fig. 5B, panel a), even though the mutant proteins were expressed and immunoprecipitated at the same level as the wild-type CR1 fusion (Fig. 5B, panels b and d). The deficiencies of these mutant molecules in direct SWI/SNF interaction were paralleled by their decreased ability to activate the SWI/SNF-dependent mim-1 locus after cotransfection into HD3 erythroblasts (Fig. 5C), as well as aP2 gene expression (Fig. 5D) and morphological differentiation (data not shown) in NIH3T3 cells on retroviral transduction. A strong correlation therefore exists between SWI/SNF recruitment, the ability to activate of SWI/SNF-dependent loci, and the adipogenic potential of CR1.
Figure 5.
Mutation analysis of CR1. (A) Amino acid sequence of mouse CR1, indicating the positions of residues mutated to alanines in the mutLL, mutWD, and mutFR CR1 variants, respectively. (B) Coimmunoprecipitation analysis of C/EBPα D126–200+CR1 and its mutant derivatives was performed as in Figure 2B. The HA–hBrm coprecipitated with the C/EBPα–FLAG-derivatives is shown in panel a, the precipitated C/EBPα–FLAG in panel b. The levels of the same proteins in the input lysate for the coimmunoprecipitation are shown in panels c and d, respectively. (C) The ability of C/EBPα D126–200+CR1 and its mutant derivatives to induce mim-1 expression in HD3 erythroblasts was analyzed as in Figure 3B. (D) The induction of aP2 mRNA in retrovirally transduced NIH3T3 cells determined as in Figure 1B.
SWI/SNF and TBP/TFIIB binding motifs of C/EBPα cooperate during adipocyte differentiation
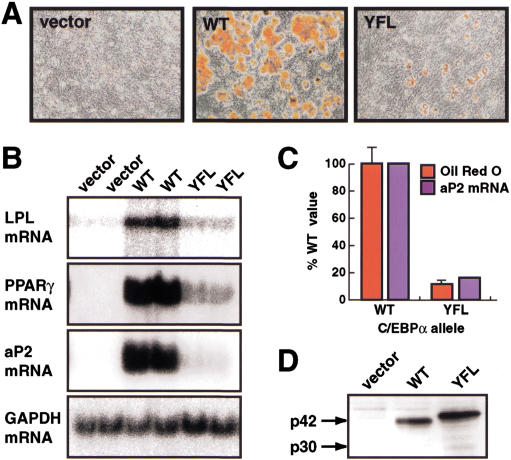
The C/EBPα transactivation domain contains amino acid motifs, conserved between the activating C/EBP isoforms, which interact with the basal transcriptional machinery, both in vitro and in a cellular context (Nerlov and Ziff 1995). These motifs reside in the N-terminal part of the C/EBPα transactivation domain, which is not required for SWI/SNF interaction. Therefore, we introduced into NIH3T3 cells a version of C/EBPα in which residues critical for its interaction with the basal transcription factors TBP and TFIIB have been mutated (C/EBPα Y67A, FL77, 78AA, or YFL, mutant; Nerlov and Ziff 1995), and analyzed the effect on adipogenesis and adipocyte-specific gene expression. NIH3T3 cells transduced with C/EBPα YFL differentiated poorly, both by morphological criteria (Fig. 6A) and as measured by their expression of LPL, PPARγ, and aP2 mRNAs (Fig. 6B). Quantification of Oil Red O staining and aP2 mRNA expression indicated a 80%–90% loss of adipogenic potential in the YFL mutant (Fig. 6C), despite expression of the mutant protein to a similar extent as wild-type C/EBPα (Fig. 6D). These results show that the C/EBPα TBP/TFIIB interaction motifs are required for C/EBPα-mediated activation of the chromosomal loci encoding the adipocyte-specific markers LPL, PPARγ, and aP2.
Figure 6.
Requirement of C/EBPα TBP/TFIIB interaction motifs for adipogenesis. NIH3T3 cells were infected with pBabePuro control virus (vector), virus encoding wild-type (WT) C/EBPα, or the Y67A, FL77,78AA C/EBPα mutant (YFL) and allowed to differentiate for 2 wk. Parallel cultures were analyzed for morphological adipocyte differentiation (A) and expression of the LPL, PPARγ, and aP2 adipocyte mRNAs (B) as in Figures 4A and 1B, respectively. Quantification of accumulated triglyceride (by Oil Red O staining) and aP2 mRNA (C) was done as in Figure 4C, and C/EBPα protein (D) as in Figure 1C.
Discussion
SWI/SNF action in differentiation and lineage-specific gene expression
The results presented here show that C/EBPα interacts with the SWI/SNF complex via its TE-III, and that TE-III is required for C/EBPα-mediated activation of SWI/SNF-dependent myeloid genes, as well as for C/EBPα-mediated adipogenesis. The structurally unrelated C/EBPβ CR1 SWI/SNF recruiting domain could replace TE-III, and mutation of residues within CR1 that compromised its SWI/SNF recruiting capacity reduced or abolished its ability to compensate for deletion of TE-III. Together, these results strongly support that SWI/SNF recruitment is the critical function of TE-III, which is complemented by CR1. In addition to the SWI/SNF recruiting function identified here, TE-III has been shown previously to participate in direct promoter activation (Friedman and McKnight 1990; Nerlov and Ziff 1994), which is not the case for CR1 (Kowenz-Leutz and Leutz 1999). This is likely to account for <100% rescue of adipocyte gene expression that we observe. Previous reports have shown that SWI/SNF is involved in the transcription of the erythroid lineage-specific β-globin locus and in activation of chromosomal myeloid-specific loci (Armstrong et al. 1998; Kowenz-Leutz and Leutz 1999; Lee et al. 1999). More recently, de la Serna et al. (2001) have shown that dominant-negative hBrm and Brg1 molecules block the induction of myogenesis and muscle-specific gene expression by MyoD in NIH3T3 cells. The present results show that SWI/SNF recruitment by a lineage-specific transcription factor directly mediates lineage commitment and differentiation in a mammalian species, and in addition provide a molecular mechanism by which C/EBPα can mediate the differentiation of distinct cell types.
Generating specificity of C/EBPα function
The C/EBPα transcription factor has the capability to execute various differentiation programs. In hematopoiesis, both eosinophil and neutrophil lineage commitment can be induced by C/EBPα (Nerlov et al. 1998; Radomska et al. 1998), and adipogenesis can be initiated by C/EBPα in uncommitted mesenchymal precursor cells (Freytag et al. 1994) or, in collaboration with PPARγ, in myocytes (Hu et al. 1995). These observations suggest that C/EBPα provides a fundamental function generally required for the activation of specific differentiation programs, and that the collaborating factors (PPARγ, GATA-1, Myb, PU.1) serve to direct this function to appropriate gene loci. The requirement for the SWI/SNF interacting TE-III domain of C/EBPα in both adipose conversion of NIH3T3 cells and in activation of SWI/SNF-dependent myeloid-specific gene expression (in the case of mim-1, in collaboration with c-Myb) provides evidence that the capacity to recruit SWI/SNF chromatin remodeling complexes is such a function. This is further supported by the previous demonstration that providing a “selector” molecule such as c-Myb (which by itself does not recruit SWI/SNF) with an SWI/SNF recruiting domain rendered it functionally independent of C/EBP activity for myeloid-specific gene activation (Kowenz-Leutz and Leutz 1999). Together, these observations lead us to propose that SWI/SNF recruitment is an integral part of C/EBPα-dependent (and C/EBPβ-dependent) differentiation processes. As mentioned above, the inability of a C/EBPβ molecule lacking the CR1 SWI/SNF binding domain to replace C/EBPα in adipogenesis, without affecting liver gene expression (Chen et al. 2000), provides in vivo evidence that SWI/SNF recruitment is indeed relevant for adipogenesis.
Collaboration between chromatin remodeling and direct promoter activation
Whereas the p42-specific part of the C/EBPα transactivation domain is dispensable for the interaction between C/EBPα and the SWI/SNF complex, it still harbors functions required for adipocyte (this paper) and eosinophil differentiation (Nerlov et al. 1998). Mutation of two TBP and TFIIB interaction motifs (Nerlov and Ziff 1995) that reside in this part of C/EBPα almost completely blocks induction of adipocyte-specific genes, demonstrating that abolishing the capacity of C/EBPα to interact with the basal transcription apparatus blocks C/EBPα-mediated adipogenesis. In vitro analysis of β-globin promoter activation by EKLF has shown that SWI/SNF mediated promoter chromatin remodeling was necessary, but not sufficient, for promoter activity (Armstrong et al. 1998), and Imbalzano et al. (1994) have shown that SWI/SNF-mediated chromatin remodeling facilitates binding of TBP to a nucleosomal template. All these results are consistent with a model in which C/EBPα induces SWI/SNF-mediated chomatin remodeling, facilitating subsequent recruitment of the basal transcriptional machinery, and indicate that such mechanisms operate generally during cellular lineage commitment and differentiation.
Materials and methods
DNA constructs
Rat C/EBPα deletion mutants (Nerlov and Ziff 1994) and transactivation domain point mutant (C/EBPα Y,FL; Nerlov and Ziff 1995) have been described previously. All C/EBPα derivatives were cloned into pBabePuro (Morgenstern and Land 1990) for retroviral infection as BamHI–EcoRI fragments. C/EBPαD126-200+CR1 and mutant derivatives were amplified by PCR from pBabePuro rαD126-200 using a 5′ primer encoding the first 21 amino acids of full-length mouse C/EBPβ (CR1; Kowenz-Leutz et al. 1994) or primers encoding mutant versions of CR1. The PCR fragment was cloned into pBabePuro using BamHI and EcoRI. All C/EBPα derivatives produced by PCR were confirmed by DNA sequencing. The pCRNCM and pCRNCM–Myb expression vector has been described previously (Lim et al. 1992). C-terminal FLAG tagging of C/EBPα was performed using an oligonucleotide adapter, and FLAG fusion proteins were expressed from pcDNA3 (Invitrogen).
Retroviral infection and adipocyte differentiation
NIH3T3 cells (provided by Dr. Karsten Kristiansen, University of Southern Denmark, Denmark) and Phoenix-E ecotropic retroviral packaging cells (from Dr. G. Nolan, Stanford University, San Francisco, California) were grown in Dulbecco's modified Eagles medium (DMEM) containing 10% FBS. Retroviral stocks were obtained by transiently transfecting Phoenix-E cells with pBabePuro-based proviral constructs using calcium phosphate coprecipitation. Forty-eight hours after transfection culture supernatants were harvested and filtered through 0.45 μm sterile filters (Millipore). The resulting viral stocks were used to infect subconfluent layers (30%–50% confluence) of NIH3T3 cells overnight in the presence of 100 μg/mL polybrene (Sigma). After infection, cells were cultured in DMEM plus 10% FBS (without any addition of adipogenic effectors) for 2 wk in puromycin-containing medium (1 μg/mL, Sigma) to allow differentiation to occur. Parallel cultures were used for preparation of total cellular protein or RNA preparation, or fixed and stained with Oil Red O (Ramirez-Zacarias et al. 1992).
Endogenous gene activation assay
HD3 erythroblasts and quail QT6 fibroblasts (Beug et al. 1979) were grown in DMEM supplemented with 8% fetal calf serum, 2% heat-inactivated chicken serum, and antibiotics. To monitor endogenous gene activation in HD3 or QT6 cells, 2 × 107 or 8 × 106 cells, respectively, were transfected with C/EBPα and Myb expression vectors using DEAE-dextran (Kowenz-Leutz et al. 1994), and RNA harvested 16–24 h posttransfection.
RNA isolation and Northern blotting
Total cellular RNA was prepared according to Chromzynski and Sacchi (1987). Poly(A) RNA was isolated using magnetic oligo(dT) beads according to the manufacturer's instructions (Dynal). RNA (25 μg of total RNA from NIH3T3 cells; poly(A) RNA from 2 × 107 HD3 cells or 8 × 106 QT6 cells) was run on a 1.2% agarose gel (in 20 mM MOPS at pH 7.0, 50 mM Na-acetate, 1 mM EDTA, and 1.5% formaldehyde) for 500 Vh. RNA was capillary blotted overnight onto BiodyneB membranes (Life Technologies) or Hybond N+ membranes (Amersham). Blots were prehybridized (30 min at 65°C) and hybridized (overnight at 65°C) in Quick-Hyb hybridization solution (Stratagene). Stringent washes were at 65°C in 0.2× SSC 0.1% SDS (60°C for QT6 and HD3 blots). The Northern blots were analyzed using a Fuji BAS2500 phosphorimager and ImageGauge software, or exposed to Kodak XAR X-ray film. cDNA probes were human LPL cDNA (Wion et al. 1987), mouse PPARγ, and aP2 cDNAs (obtained from Dr. Karsten Kristiansen, University of Southern Denmark, Denmark), mouse GAPDH (Hanauer and Mandel 1984) and chicken mim-1, #126, and GAPDH (Nakano and Graf 1992). Probes were labeled with [α-32P]dCTP (Amersham-Pharmacia) using random priming (RadPrime; GIBCO BRL) according to the instructions supplied by the manufacturer.
Western blotting
Virally infected NIH3T3 cells were lysed in SDS sample buffer, incubated at 95°C for 5 min, centrifuged to remove insoluble components, and the lysates separated on 12.5% SDS-PAGE gels (200 Vh). Proteins were transferred to PVDF membranes (Millipore) using semi-dry transfer. Membranes were blocked in TBS-T (100 mM Tris at pH 7.5, 150 mM NaCl, 0.1% Tween 20) containing 10% nonfat dry milk overnight at 4°C, incubated with primary antibody (C103 rabbit anti-C/EBPα antiserum; Nerlov and Ziff 1994), PRB-205C anti-HA antibody (BAbCO, Freiburg, Germany), M2 anti-FLAG antibody (Sigma) or anti-BAF155 antiserum (Kowenz-Leutz and Leutz 1999) in TBS-T for 1 h at room temperature, followed by appropriate horseradish peroxidase-conjugated secondary antibody (Amersham-Pharmacia; 1:5000 in TBS-T; 1 h at room temperature) with three 5′ washes in TBS-T after each incubation. Blots were developed using enhanced chemiluminescence (ECL; Amersham-Pharmacia).
Coimmunoprecipitation
A total of 5 × 106 C33A cells were transfected with 5 μg of the expression vectors indicated, using calcium phosphate coprecipitation. Cell lysates were prepared and immunoprecipitated as described previously (Kowenz-Leutz and Leutz 1999) using anti-FLAG M2 antibodies (Kodak). Immunoprecipitates were boiled in SDS sample buffer and subjected to Western blotting.
Acknowledgments
We thank Drs. Kristian Helin, Karsten Kristiansen and Gary Nolan for kind gifts of plasmids and cell lines. This work was supported by the Danish Medical Research Council, the Danish Cancer Society, the Novo Nordisk Foundation (C.N.), and the Deutsche Forschungsgemeinschaft (DFG LE770/1-2) (A.L.).
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Footnotes
E-MAIL aleutz@mdc-berlin.de; FAX 49-30-9406-3298.
E-MAIL nerlov@embl-monterotundo.it; FAX 39-06-9009-1272.
Article and publication are at http://www.genesdev.org/cgi/doi/10.1101/gad.209901.
References
- Armstrong JA, Bieker JJ, Emerson BM. A SWI/SNF-related chromatin remodeling complex, E-RC1, is required for tissue-specific transcriptional regulation by EKLF in vitro. Cell. 1998;95:93–104. doi: 10.1016/s0092-8674(00)81785-7. [DOI] [PubMed] [Google Scholar]
- Barak Y, Nelson MC, Ong ES, Jones YZ, Ruiz-Lozano P, Chien KR, Koder A, Evans RM. PPARγ is required for placental, cardiac, and adipose tissue development. Mol Cell. 1999;4:585–595. doi: 10.1016/s1097-2765(00)80209-9. [DOI] [PubMed] [Google Scholar]
- Beug H, von Kirchbach A, Doderlein G, Conscience JF, Graf T. Chicken hematopoietic cells transformed by seven strains of defective avian leukemia viruses display three distinct phenotypes of differentiation. Cell. 1979;18:375–390. doi: 10.1016/0092-8674(79)90057-6. [DOI] [PubMed] [Google Scholar]
- Burk O, Mink S, Ringwald M, Klempnauer KH. Synergistic activation of the chicken mim-1 gene by v-myb and C/EBP transcription factors. EMBO J. 1993;12:2027–2038. doi: 10.1002/j.1460-2075.1993.tb05852.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SS, Chen JF, Johnson PF, Muppala V, Lee YH. C/EBPβ, when expressed from the C/ebpα gene locus, can functionally replace C/EBPα in liver but not in adipose tissue. Mol Cell Biol. 2000;20:7292–7299. doi: 10.1128/mcb.20.19.7292-7299.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheneval D, Christy RJ, Geiman D, Cornelius P, Lane MD. Cell-free transcription directed by the 422 adipose P2 gene promoter: Activation by the CCAAT/enhancer binding protein. Proc Natl Acad Sci. 1991;88:8465–8469. doi: 10.1073/pnas.88.19.8465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Christy RJ, Yang VW, Ntambi JM, Geiman DE, Landschulz WH, Friedman AD, Nakabeppu Y, Kelly TJ, Lane MD. Differentiation-induced gene expression in 3T3-L1 preadipocytes: CCAAT/enhancer binding protein interacts with and activates the promoters of two adipocyte-specific genes. Genes & Dev. 1989;3:1323–1335. doi: 10.1101/gad.3.9.1323. [DOI] [PubMed] [Google Scholar]
- de La Serna IL, Carlson KA, Imbalzano AN. Mammalian SWI/SNF complexes promote MyoD-mediated muscle differentiation. Nat Genet. 2001;27:187–190. doi: 10.1038/84826. [DOI] [PubMed] [Google Scholar]
- Freytag SO, Paielli DL, Gilbert JD. Ectopic expression of the CCAAT/enhancer-binding protein α promotes the adipogenic program in a variety of mouse fibroblastic cells. Genes & Dev. 1994;8:1654–1663. doi: 10.1101/gad.8.14.1654. [DOI] [PubMed] [Google Scholar]
- Friedman AD, McKnight SL. Identification of two polypeptide segments of CCAAT/enhancer-binding protein required for transcriptional activation of the serum albumin gene. Genes & Dev. 1990;4:1416–1426. doi: 10.1101/gad.4.8.1416. [DOI] [PubMed] [Google Scholar]
- Hanauer A, Mandel JL. The glyceraldehyde 3 phosphate dehydrogenase gene family: Structure of a human cDNA and of an X chromosome linked pseudogene; amazing complexity of the gene family in mouse. EMBO J. 1984;3:2627–2633. doi: 10.1002/j.1460-2075.1984.tb02185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu E, Tontonoz P, Spiegelman BM. Transdifferentiation of myoblasts by the adipogenic transcription factors PPARγ and C/EBPα. Proc Natl Acad Sci. 1995;92:9856–9860. doi: 10.1073/pnas.92.21.9856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imbalzano AN, Kwon H, Green MR, Kingston RE. Facilitated binding of TATA-binding protein to nucleosomal DNA. Nature. 1994;370:481–485. doi: 10.1038/370481a0. [DOI] [PubMed] [Google Scholar]
- Kowenz-Leutz E, Leutz A. A C/EBPβ isoform recruits the SWI/SNF complex to activate myeloid genes. Mol Cell. 1999;4:735–743. doi: 10.1016/s1097-2765(00)80384-6. [DOI] [PubMed] [Google Scholar]
- Kowenz-Leutz E, Twamley G, Ansieau S, Leutz A. Novel mechanism of C/EBPβ (NF-M) transcriptional control: Activation through derepression. Genes & Dev. 1994;8:2781–2791. doi: 10.1101/gad.8.22.2781. [DOI] [PubMed] [Google Scholar]
- Lee CH, Murphy MR, Lee JS, Chung JH. Targeting a SWI/SNF-related chromatin remodeling complex to the β-globin promoter in erythroid cells. Proc Natl Acad Sci. 1999;96:12311–12315. doi: 10.1073/pnas.96.22.12311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim F, Kraut N, Framptom J, Graf T. DNA binding by c-Ets-1, but not v-Ets, is repressed by an intramolecular mechanism. EMBO J. 1992;11:643–652. doi: 10.1002/j.1460-2075.1992.tb05096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowell BB. PPARγ: An essential regulator of adipogenesis and modulator of fat cell function. Cell. 1999;99:239–242. doi: 10.1016/s0092-8674(00)81654-2. [DOI] [PubMed] [Google Scholar]
- Morgenstern JP, Land H. Advanced mammalian gene transfer: High titre retroviral vectors with multiple drug selection markers and a complementary helper-free packaging cell line. Nucleic Acids Res. 1990;18:3587–3596. doi: 10.1093/nar/18.12.3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller C, Leutz A. Chromatin remodeling in development and differentiation. Curr Opin Genet Dev. 2001;11:167–174. doi: 10.1016/s0959-437x(00)00175-1. [DOI] [PubMed] [Google Scholar]
- Nakano T, Graf T. Identification of genes differentially expressed in two types of v-myb-transformed avian myelomonocytic cells. Oncogene. 1992;7:527–534. [PubMed] [Google Scholar]
- Nerlov C, Ziff EB. Three levels of functional interaction determine the activity of CCAAT/enhancer binding protein-α on the serum albumin promoter. Genes & Dev. 1994;8:350–362. doi: 10.1101/gad.8.3.350. [DOI] [PubMed] [Google Scholar]
- ————— CCAAT/enhancer binding protein-α amino acid motifs with dual TBP and TFIIB binding ability co-operate to activate transcription in both yeast and mammalian cells. EMBO J. 1995;14:4318–4328. doi: 10.1002/j.1460-2075.1995.tb00106.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nerlov C, McNagny KM, Doderlein G, Kowenz-Leutz E, Graf T. Distinct C/EBP functions are required for eosinophil lineage commitment and maturation. Genes & Dev. 1998;12:2413–2423. doi: 10.1101/gad.12.15.2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ness SA, Kowenz-Leutz E, Casini T, Graf T, Leutz A. Myb and NF-M: Combinatorial activators of myeloid genes in heterologous cell types. Genes & Dev. 1993;7:749–759. doi: 10.1101/gad.7.5.749. [DOI] [PubMed] [Google Scholar]
- Perkins AC, Sharpe AH, Orkin SH. Lethal β-thalassaemia in mice lacking the erythroid CACCC-transcription factor EKLF. Nature. 1995;375:318–322. doi: 10.1038/375318a0. [DOI] [PubMed] [Google Scholar]
- Porse B, Åskov T, Xu X, Lindberg B, Wewer U, Friis-Hansen L, Nerlov C. E2F repression by C/EBPα is required for adipogenesis and granulopoiesis in vivo. Cell. 2001;107:247–258. doi: 10.1016/s0092-8674(01)00516-5. [DOI] [PubMed] [Google Scholar]
- Querfurth E, Schuster M, Kulessa H, Crispino JD, Doderlein G, Orkin SH, Graf T, Nerlov C. Antagonism between C/EBPβ and FOG in eosinophil lineage commitment of multipotent hematopoietic progenitors. Genes & Dev. 2000;14:2515–2525. doi: 10.1101/gad.177200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radomska HS, Huettner CS, Zhang P, Cheng T, Scadden DT, Tenen DG. CCAAT/enhancer binding protein α is a regulatory switch sufficient for induction of granulocytic development from bipotential myeloid progenitors. Mol Cell Biol. 1998;18:4301–4314. doi: 10.1128/mcb.18.7.4301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez-Zacarias JL, Castro-Munozledo F, Kuri-Harcuch W. Quantitation of adipose conversion and triglycerides by staining intracytoplasmic lipids with Oil red O. Histochemistry. 1992;97:493–497. doi: 10.1007/BF00316069. [DOI] [PubMed] [Google Scholar]
- Rosen ED, Sarraf P, Troy AE, Bradwin G, Moore K, Milstone DS, Spiegelman BM, Mortensen RM. PPARγ is required for the differentiation of adipose tissue in vivo and in vitro. Mol Cell. 1999;4:611–617. doi: 10.1016/s1097-2765(00)80211-7. [DOI] [PubMed] [Google Scholar]
- Ross SE, Erickson RL, Hemati N, MacDougald OA. Glycogen synthase kinase 3 is an insulin-regulated C/EBPα kinase. Mol Cell Biol. 1999;19:8433–8441. doi: 10.1128/mcb.19.12.8433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ND, Finegold MJ, Bradley A, Ou CN, Abdelsayed SV, Wilde MD, Taylor LR, Wilson DR, Darlington GJ. Impaired energy homeostasis in C/EBPα knockout mice. Science. 1995;269:1108–1112. doi: 10.1126/science.7652557. [DOI] [PubMed] [Google Scholar]
- Wion KL, Kirchgessner TG, Lusis AJ, Schotz MC, Lawn RM. Human lipoprotein lipase complementary DNA sequence. Science. 1987;235:1638–1641. doi: 10.1126/science.3823907. [DOI] [PubMed] [Google Scholar]
- Wu Z, Xie Y, Bucher NL, Farmer SR. Conditional ectopic expression of C/EBPβ in NIH-3T3 cells induces PPARγ and stimulates adipogenesis. Genes & Dev. 1995;9:2350–2363. doi: 10.1101/gad.9.19.2350. [DOI] [PubMed] [Google Scholar]
- Wu Z, Rosen ED, Brun R, Hauser S, Adelmant G, Troy AE, McKeon C, Darlington GJ, Spiegelman BM. Cross-regulation of C/EBPα and PPARγ controls the transcriptional pathway of adipogenesis and insulin sensitivity. Mol Cell. 1999;3:151–158. doi: 10.1016/s1097-2765(00)80306-8. [DOI] [PubMed] [Google Scholar]
- Yeh WC, Cao Z, Classon M, McKnight SL. Cascade regulation of terminal adipocyte differentiation by three members of the C/EBP family of leucine zipper proteins. Genes & Dev. 1995;9:168–181. doi: 10.1101/gad.9.2.168. [DOI] [PubMed] [Google Scholar]
- Zhang DE, Zhang P, Wang ND, Hetherington CJ, Darlington GJ, Tenen DG. Absence of granulocyte colony-stimulating factor signaling and neutrophil development in CCAAT enhancer binding protein α-deficient mice. Proc Natl Acad Sci. 1997;94:569–574. doi: 10.1073/pnas.94.2.569. [DOI] [PMC free article] [PubMed] [Google Scholar]