Abstract
Kurozu moromimatsu is the sediment of Kurozu, a jar-fermented Japanese black vinegar produced from unpolished rice. Here, we examined the protective effects of Kurozu moromimatsu in a diethylnitrosamine-induced model of hepatocellular carcinoma. Thirty-two F344 rats were divided into two groups; the control group received basal CE-2 diet, and the Kurozu moromimatsu group received CE-2 diet containing Kurozu moromimatsu. At 16 weeks after initial intraperitoneal administration of diethylnitrosamine (150 mg/kg/week), serum was collected from half the rats. These rats were sacrificed and the liver was resected for histological examination of hematoxylin-eosin-stained sections and assay of matrix metalloproteinase-2 and matrix metalloproteinase-9 levels in tumor tissues. Glutathione S-transferase placental form-positive foci were evaluated by immunostaining for glutathione S-transferase placental form. The remaining rats were maintained for evaluation of survival. There were no significant differences of serum transaminases, tumor necrosis factor-alpha, and also no marked hepatic histological differences, between the two groups. However, the size of hepatocellular carcinomas was greatly decreased and the levels of activated matrix metalloproteinase-2 and -9 were significantly reduced in the Kurozu moromimatsu group. Further, survival was significantly prolonged in the Kurozu moromimatsu group compared with the control. These results indicate that Kurozu moromimatsu inhibited the growth of hepatocellular carcinoma.
Keywords: Kurozu moromimatsu, hepatocellular carcinoma
Introduction
Kurozu is a traditional black vinegar produced from unpolished rice and has been widely used in Japan as seasoning for food or as a health supplement. Kurozu is produced mainly in Kagoshima, Japan, by fermentation of vinegar with several bacilli for more than one year in earthenware jars. The supernatant is Kurozu, and the sediment at the bottom of the jars is called Kurozu moromimatsu (Kurozu-M), and contains high levels of amino acids, minerals, organic materials and so on.
Anti-cancer effects of ethyl acetate extract of Kurozu have been reported in vitro(1) and in an animal model of colon cancer.(2) We previously reported that Kurozu-M inhibited development of human colon cancer cells, LoVo cells, in a transplanted rodent model.(3) On the other hand, the therapeutic effect of Kurozu-M on hepatocellular carcinoma (HCC) has not yet been reported in animals or humans. Recently, an anti-cancer effect of coffee drink on HCC has been reported in humans,(4,5) and the presence of dietary factors that inhibit HCC has been detected in an animal model.(6) However, effective dietary factors to inhibit the development of HCC remain to be identified.
The most common cause of HCC is thought to be hepatitis C virus (HCV), followed by hepatitis B virus (HBV) infection in Japan, and the number of deaths from HCC is more than 30,000/year; it is the third most important cause of cancer death in males in Japan. Moreover, HCC is the fifth most common cancer worldwide.(7) Recently, drugs directed against HCC-related molecular targets have entered clinical trial.(8) However, at present, the impact on the survival rate in HCC patients is limited owing to high frequencies of recurrence and metastasis and the background of often-severe liver dysfunction.
Several matrix metalloproteinase (MMP)s, which are zinc-dependent endopeptidases degrading the extracellular matrix, are related to development and metastasis of various solid cancers, including HCC.(8) Moreover, MMPs can promote the activation of insulin-like growth factor, which promotes tumor development and growth.(9,10) In particular, MMP-2 and MMP-9 (gelatinases) play important roles in tumor angiogenesis and cancer growth. HCC is typically a hypervascular cancer, and angiogenesis is important in its early stages.(11) Therefore, inhibition of angiogenesis, for example, by inhibition of activation of MMPs, might improve the prognosis of HCC patients.
In this work, we examined the protective effects of Kurozu-M in a dietheylnitrosamine (DEN)-induced HCC experimental model, focusing on suppression of activated MMPs.
Materials and Methods
Experimental animal model
The experimental procedures were approved by the Animal Experimentation Committee, School of Medicine, Tokai University, Japan. Thirty-two F344 male rats (8 weeks of age) were obtained from CLEA Japan Inc. (Tokyo, Japan) and bred under specific pathogen-free conditions at a room temperature of 24–25°C, under constant humidity, with a 12-h light/dark cycle. The rats were randomized into two groups; the control group received basal CE-2 diet (CLEA Japan Inc., Tokyo, Japan) (n = 16) and the Kurozu-M group received CE-2 diet including 2% Kurozu-M (Sakamoto Kurozu Inc., Kagoshima, Japan) (n = 16). The volume of Kurozu-M added to the diet of rats was chosen based on the typical volume of ingestion by humans, adjusted for body weight. Basal CE-2 diet and special diet including Kurozu-M were started from a week before the initial administration of DEN (Sigma, St. Louis). Administrations of DEN (150 mg/kg/week) were performed by intraperitoneal injection 3 times during 3 weeks. The concentration of iron in basal CE-2 diet was 32.2 mg/100 g (data from manufacturer) and that of CE-2 diet containing 2% Kurozu-M, used as the experimental diet in this study, was calculated to be 31.7 mg/100 g (based on iron concentration of 4.8 mg/100 g in Kurozu-M).
Preliminary examinations showed that liver tumors more than 10 mm in size at the major axis were present in all rats (n = 5) of the control group at 12 weeks after initial administration of DEN.
Serum examinations
At 16 weeks after initial administration of DEN, serum levels of albumin, transaminases (AST and ALT), hyaluronic acid, tumor necrosis factor (TNF)-α (ELISA), interleukin (IL)-2 (ELISA) were measured (SRL Inc., Tokyo, Japan) in half of the rats (each group; n = 8) of both groups.
Histology and immunostaining
Immediately after collection of serum, the same rats (each group; n = 8) were sacrificed under anesthesia induced with isoflurane (Wako Pure Chemicals, Osaka, Japan) and the livers were resected. The presence of liver tumors was confirmed macroscopically. Moreover, microscopic examination of liver tissues by hematoxylin-eosin (HE) staining was performed as usual. Further, glutathione S-transferase placental form (GST-P) was examined as a marker of HCC and precancerous lesions. Procedures of GST-P staining were as follows. Livers were fixed, embedded in paraffin and sectioned. Sections of 3 µm thickness were treated with rabbit anti-rat GST-P antibody (MBL International Co., Nagoya, Japan) and then with secondary antibody and avidin-biotin complex (Ultratech HRP Kit; Beckman Coulter Inc., Ontario, Canada). The sites of peroxidase binding were visualized with diaminobenzidine. Sections were counterstained with hematoxylin. Evaluation of GST-P-positive foci was performed based on size (tranverse diameter) and number per slice (transverse axis) of the right lobe of liver in all 16 rats. Semi-quantitative analysis of GST-P-positive foci was done using Adobe Photoshop CS3 Extended. All evaluations were performed by a pathologist who was blinded as to the treatment the animals had received.
MMP-2 assay
Levels of activity of MMP-2 were assayed with a commercial kit (Amersham Pharmacia Biotech, Buckinghamshire, UK). Tissues of HCCs were homogenized by centrifugation, and the supernatant was used as the sample. Samples were added to wells coated with MMP-2 antibody and incubated. Each sample was activated with 0.5 mM aminophenylmercuric acetate (APMA). The absorbance was read at 405 nm on a microplate reader. The method used was as described in our previous report.(3)
MMP-9 assay
Standards and samples were run in the same manner as described for MMP-2 on a microplate coated with MMP-9 antibody, and 1 mM APMA was used for activation. The absorbance was read at 405 nm on a microplate reader. The method used was as described in our previous report.(3)
Survival rates
The remaining rats (each group; n = 8) in both groups were continued on the same diets and under same conditions. Survival was examined during 48 weeks after the initial administration of DEN. Differences in the survival rates of the non-sacrificed rats (each group; n = 8) among the two groups after initial DEN administration were analyzed by the Kaplan-Meier method.
Statistical analysis
Statistical analysis of serum parameters, MMP-2 and MMP-9 levels, and numbers and sizes of GST-P-positive foci in the liver among rats of the two groups (each group; n = 8) was done with the unpaired t test. The criterion of significance was p<0.05. Levels of serum albumin, transaminases, hyaluronic acid, TNF-α, IL-2 and MMP-2 and MMP-9 levels were given as mean and standard deviation.
Results
Serum examinations
Levels of serum albumin (g/dl) were 4.3 ± 0.2 in the control group and 4.2 ± 0.2 in the Kurozu-M group. Levels of serum AST/ALT (IU/l) were 236 ± 67/206 ± 51 in the control group and 209 ± 64/183 ± 49 in the Kurozu-M group. There was no significant difference in the levels of albumin or AST/ALT between the two groups. Moreover, the levels of serum hyaluronic acid (ng/ml) were 37.8 ± 3.8 in the control group, and 35.1 ± 2.8 in the Kurozu-M group. There was no significant difference in the level of hyaluronic acid between the two groups.
As regards the levels of cytokines, the levels of TNF-α (pg/ml) were 3.4 ± 1.7 in the control group and 3.1 ± 1.8 in the Kurozu-M group. Levels of IL-2 (U/ml) were 1.6 ± 0.6 in the control group and 1.4 ± 0.4 in the Kurozu-M group. There were no significant differences in the levels of either of the cytokines between the two groups.
Histology and GST-P staining
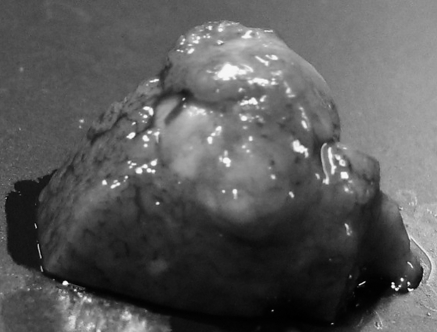
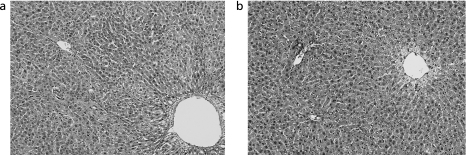
In macroscopic examination of the resected liver, white tumors with a size of more than 10 mm at the major axis were apparent in all rats in the control group (Fig. 1). Such large tumors were not found in the Kurozu-M group. In microscopic examination of HE-stained liver sections, focal necrosis of liver cells and invasion of inflammatory cells in portal areas (corresponding to mild chronic hepatitis in humans) were found in both groups. However, fibrosis or piecemeal necrosis was not obvious. Moreover, there were no marked background changes of liver tissues in the two groups (Fig. 2 a and b).
Fig. 1.
Photograph of a representative resected liver tumor. Multiple white tumors were apparent in the control group. The diameter of the tumor shown, at the major axis, was 14 mm.
Fig. 2.
Findings of liver tissues. Focal necrosis of liver cells and invasion of inflammatory cells in portal areas were found in both groups. However, fibrosis or piecemeal necrosis was not obvious. (a: control group, b: Kurozu-M group; HE ×200).
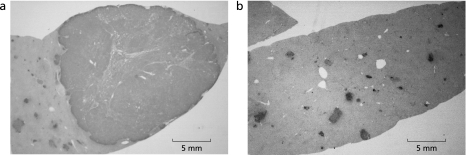
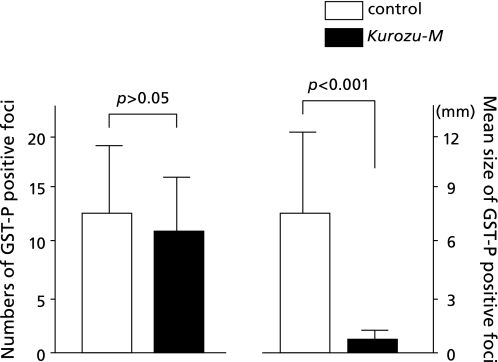
As for GST-P staining, the numbers of GST-P-positive foci were 12.2 ± 6.8 in the control group, and 10.7 ± 5.3 in the Kurozu-M group. There was no significant difference between the two groups. However, large HCC with a diameter of more than 10 mm was noted in liver in all animals of the control group. On the other hand, in the Kurozu-M group, no lesion of more than 3 mm in diameter was found in any liver. The size (mm) of GST-P-positive foci in the Kurozu-M group (0.89 ± 0.46) was significantly (p<0.001) reduced compared with the control (7.35 ± 5.31) (Fig. 3 and 4).
Fig. 3.
Findings of GST-P staining. Large GST-P-positive foci with a diameter of 20 mm was noted in liver in the control group (a). On the other hand, in the Kurozu-M group, no lesion of more than 3 mm in diameter was found in liver (b).
Fig. 4.
Numbers and size of GST-P-positive foci. The numbers of GST-P-positive foci were 12.2 ± 6.8 in the control group, and 10.7 ± 5.3 in the Kurozu-M group. There was no significant difference between the two groups. The size (mm) of GST-P-positive foci in the Kurozu-M group (0.89 ± 0.46) was significantly (p<0.001) reduced compared with the control (7.35 ± 5.31).
MMP-2 and MMP-9 levels in tumor tissues
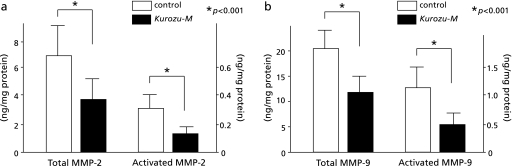
Total MMP-2 amounted to 6.7 ± 2.6 ng/mg protein in the control group and 3.6 ± 1.7 ng/mg protein in Kurozu-M group. The corresponding values for activated MMP-2 were 0.28 ± 0.12 ng/mg protein and 0.12 ± 0.06 ng/mg protein, respectively. Total MMP-9 amounted to 20.6 ± 4.6 ng/mg protein in the control group and 12.0 ± 2.7 ng/mg protein in Kurozu-M group. The corresponding values for activated MMP-9 were 1.17 ± 0.38 ng/mg protein and 0.51 ± 0.19 ng/mg protein, respectively. Total and activated MMP-2 and MMP-9 levels were all significantly (p<0.001) reduced in the Kurozu-M group compared with the control group (Fig. 5).
Fig. 5.
MMP-2 and MMP-9 levels (ng/mg protein). Total MMP-2 amounted to 6.7 ± 2.6 in the control group and 3.6 ± 1.7 in Kurozu-M group. The corresponding values for activated MMP-2 were 0.28 ± 0.12 and 0.12 ± 0.06, respectively (a). Total MMP-9 amounted to 20.6 ± 4.6 in the control group and 12.0 ± 2.7 in Kurozu-M group. The corresponding values for activated MMP-9 were 1.17 ± 0.38 and 0.51 ± 0.19, respectively (b). Total and activated MMP-2 and MMP-9 levels were all significantly (p<0.001) reduced in the Kurozu-M group compared with the control group.
Survival
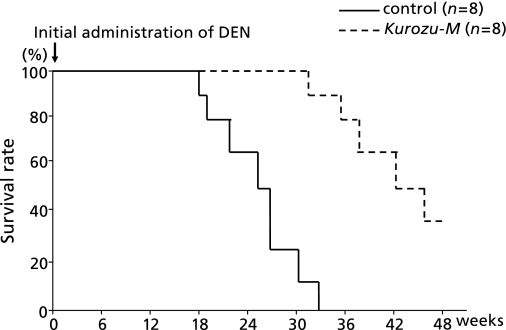
In the control group, all 8 rats died within 36 weeks after initial DEN administration. On the other hand, the survival rate in the Kurozu-M group was 37.5% (3/8) at 48 weeks. The survival rate in the Kurozu-M group was significantly higher than that in the control group (p<0.001) (Fig. 6).
Fig. 6.
Survival rate by the Kaplan-Meier method. In the control group, all 8 rats died within 36 weeks after initial administration of DEN. On the other hand, the survival rate in the Kurozu-M group was 37.5% (3/8) at 48 weeks. The survival rate in the Kurozu-M group was significantly higher than that in the control group (p<0.001).
Discussion
Kurozu-M treatment markedly inhibited growth of HCC and improved survival in the DEN-induced HCC animal model. One of the mechanisms of its anti-cancer effects was considered to be suppression of activated MMP-2 and MMP-9, which degrade the extracellular matrix, thereby promoting the growth and metastasis of various solid cancers.
Occurrence of HCC is generally associated with liver fibrosis or inflammatory changes. Clinically, chronic hepatitis or liver cirrhosis (LC) with HCV infection often leads to HCC. For example, the occurrence rate of HCC in LC patients with HCV infection is 6–8%/year in Japan.(12) In patients with hepatitis, inflammation and regeneration in the liver are associated with development of HCC,(13) though exceptionally, HCC rarely develops in patients with autoimmune hepatitis. However, our study revealed that the extents of histological liver fibrosis or inflammatory change, as well as the serum levels of transaminases or hyaluronic acid as a marker of liver fibrosis, were not significantly different between the Kurozu-M group and the control. Therefore, inhibition of HCC growth in the Kurozu-M group in this study was not associated with differences in background factors. It has been reported that iron overload, which induces oxidative stress, aggravates liver diseases(14) and increases the risk of malignancy.(15) However, the levels of iron in the diets of the control and Kurozu-M groups were essentially the same in this study.
Increased levels of the gelatinases; MMP-2 and MMP-9 are considered prognostic for solid cancers, including HCC.(16) In fact, it has been suggested that inhibition of gelatinases can suppress development of HCC in an animal model.(17) Since activated MMP-2 promotes angiogenesis,(18) suppression of MMP-2 and MMP-9 by Kurozu-M may have led to inhibition of HCC growth in our model. We found that the number of GST-P-positive foci (which were estimated foci corresponding to low-grade dysplastic nodules) in the Kurozu-M group showed no difference from the control, but the size of developed GST-P-positive foci was greatly decreased. Thus, a possible explanation is that Kurozu-M inhibited angiogenesis, which may not greatly influence the initial progression from premalignant tumor, but may be an important promoting factor at the stage of high-grade dysplastic nodules or early HCC. This would be consistent with our findings.
Activation of MMPs is important for carcinogenesis. MMP-2 is activated by membrane type (MT)-1-MMP.(19) It is also activated by peroxylnitrite(20) which exhibits cytotoxicity via direct oxidation of sulfhydryl groups, leading to severe tissue damage. We have already reported that Kurozu-M inhibits nitrotyrosine generation in colon cancer cells.(3) Nitrotyrosine is produced in vivo via two pathways, i.e., from the reaction of tyrosine with peroxylnitrite, which is generated from O2•− and nitric oxide (NO),(21) and from the reaction of tyrosine with nitrite, catalyzed by myeloperoxidase (MPO).(22) Therefore, Kurozu-M might reduce MMP-2 activation by inhibiting the generation of peroxylnitrite. In addition, MMP-9 is activated by MMP-2,(23) as well as by several cytokines.(24) However, in our study, serum levels of TNF-α and IL-2 were not increased.
The active component of Kurozu-M remains to be identified. Acetic acid, which is the main component of Kurozu, is not included in Kurozu-M. However, Kurozu-M contains amino acids, oligopeptide, organic materials including metabolites of lactic acid or Koji molds, minerals, saccharides, lipids, etc. Candidate active components include branched chain amino acid (BCAA), which are widely used for the treatment of protein malnutrition in LC patients. It was reported that BCAA suppressed insulin-resistance-based carcinogenesis in diabetic rats(7) and suppressed angiogenesis in a chemical carcinogenesis model.(7) Furthermore, various other amino acids have anti-oxidative activity, acting as radical scavengers.(25) Therefore, various amino acids may suppress the generation of O2•− or peroxylnitrite, thereby suppressing activation of MMP-2. On the other hand, oligosaccharides are also candidate active components, because oligosaccharides from agar suppressed NO generation in vitro.(26) Oligosaccharides also reduced the activity of inducible NO synthase (iNOS),(27) which might lead to a decrease of peroxylnitrite generation. Organic materials, including metabolites of lactic acid or Koji formed in the earthenware jars are also potential anti-oxidants,(28) and anticancer agents such as bleomycin or mitomycin C or their derivatives are known to be produced as bacterial metabolites.
In conclusion, Kurozu-M inhibited the growth of HCC in a DEN-induced HCC animal model and prolonged survival. The active component(s) has not yet been identified, but our results suggest that Kurozu-M may be beneficial as a dietary supplement in humans.
Acknowledgments
This work was supported by grants in 2009 Tokai University School of Medicine Research Aid, 2009 and 2010 Grant-in-Aid for Scientific Research in Japan Society for the Promotion of Science (No. 21659295 and No. 22659106) and 2009 Grant-in-Aid for Japanese Society for Parenteral and Enteral Nutrition.
Abbreviations
- Kurozu-M
Kurozu moromimatsu
- HCC
hepatocellular carcinoma
- HCV
hepatitis C virus
- HBV
hepatitis B virus
- MMP
matrix metalloproteinase
- DEN
diethylnitrosamine
- TNF
tumor necrosis factor
- IL
interleukin
- HE
hematoxylin-eosin
- GST-P
glutathione S-transferase placental form
- APMA
aminophenylmercuric acetate
- LC
liver cirrhosis
- MT
membrane type
- NO
nitric oxide
- MPO
myeloperoxidase
- BCAA
branched chain amino acid
- iNOS
inducible nitric oxide synthase
References
- 1.Nanda K, Miyoshi N, Nakamura Y, et al. Extract of vinegar ”Kurosu” from unpolished rice inhibits the proliferation of human cancer cells. J Exp Clin Cancer Res. 2004;23:69–75. [PubMed] [Google Scholar]
- 2.Shimoji Y, Kohno H, Nanda K, et al. Extract of Kurosu, a vinegar from unpolished rice, inhibits azoxymethane-induced colon carcinogenesis in male F344 rats. Nutr Cancer. 2004;49:170–173. doi: 10.1207/s15327914nc4902_8. [DOI] [PubMed] [Google Scholar]
- 3.Fukuyama N, Jujo S, Ito I, et al. Kurozu moromimatsu inhibits tumor growth of Lovo cells in a mouse model in vivo. Nutrition. 2007;23:81–86. doi: 10.1016/j.nut.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 4.Masterton GS, Hayes PC. Coffee and the liver: a potential treatment for liver disease? Eur J Gastroenterol Hepatol. 2010;22:1277–1283. doi: 10.1097/MEG.0b013e32833cca96. [DOI] [PubMed] [Google Scholar]
- 5.Bravi F, Bosetti C, Tavani A, La Vecchia C. Coffee drinking and hepatocellular carcinoma: an update. Hepatology. 2009;50:1317–1318. doi: 10.1002/hep.23272. [DOI] [PubMed] [Google Scholar]
- 6.Weng CJ, Chau CF, Yen GC, Liao JW, Chen DH, Chen KD. Inhibitory effects of ganoderma lucidum on tumorigenesis and metastasis of human hepatoma cells and animal models. J Agric Food Chem. 2009;57:5049–5057. doi: 10.1021/jf900828k. [DOI] [PubMed] [Google Scholar]
- 7.Yoshiji H, Noguchi R, Kitade M, et al. Branched-chain amino acids suppress insulin-resistance-based hepatocarcinogenesis in obese diabetic rats. J Gastroenterol. 2009;44:483–491. doi: 10.1007/s00535-009-0031-0. [DOI] [PubMed] [Google Scholar]
- 8.Thomas M. Molecular targeted therapy for hepatocellular carcinoma. J Gastroenterol. 2009;44(Suppl. 19):136–141. doi: 10.1007/s00535-008-2252-z. [DOI] [PubMed] [Google Scholar]
- 9.Shimizu M, Deguchi A, Hara Y, Moriwaki H, Weinstein IB. EGCG inhibits activation of the insulin-like growth factor-1 receptor in human colon cancer cells. Biochem Biophys Res Commun. 2005;334:947–953. doi: 10.1016/j.bbrc.2005.06.182. [DOI] [PubMed] [Google Scholar]
- 10.Hemers E, Duval C, McCaig C, Handley M, Dockray GJ, Varro A. Insulin-like growth factor binding protein-5 is a target of matrix metalloproteinase-7: implications for epithelial-mesenchymal signaling. Cancer Res. 2005;65:7363–7369. doi: 10.1158/0008-5472.CAN-05-0157. [DOI] [PubMed] [Google Scholar]
- 11.Sakamoto M, Hirohashi S, Shimosato Y. Early stages of multistep hepatocarcinogenesis: adenomatous hyperplasia and early hepatocellular carcinoma. Hum Pathol. 1991;22:172–178. doi: 10.1016/0046-8177(91)90039-r. [DOI] [PubMed] [Google Scholar]
- 12.Toshikuni N, Izumi A, Nishino K, et al. Comparison of outcomes between patients with alcoholic cirrhosis and those with hepatitis C virus-related cirrhosis. J Gastroenterol Hepatol. 2009;24:1276–1283. doi: 10.1111/j.1440-1746.2009.05851.x. [DOI] [PubMed] [Google Scholar]
- 13.Miyakawa K, Tarao K, Ohshige K, et al. High serum alanine aminotransferase levels for the first three successive years can predict very high incidence of hepatocellular carcinoma in patients with Child Stage A HCV-associated liver cirrhosis. Scand J Gastroenterol. 2009;44:1340–1348. doi: 10.3109/00365520903222681. [DOI] [PubMed] [Google Scholar]
- 14.Asare GA, Kew MC, Mossanda KS, Paterson AC, Siziba K, Kahler-Venter CP. Effects of exogenous antioxidants on dietary iron ovrtload. J Clin Biochem Nutr. 2009;44:85–94. doi: 10.3164/jcbn.08-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bae YJ, Yeon JY, Sung CJ, Kim HS, Sung MK. Dietary intake and serum levels of iron in relation to oxidative stress in breast cancer patients. J Clin Biochem Nutr. 2009;45:355–360. doi: 10.3164/jcbn.09-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Turpeenniemi-Hujanen T. Gelatinases (MMP-2 and -9) and their natural inhibitors as prognostic indicators in solid cancers. Biochimie. 2005;87:287–297. doi: 10.1016/j.biochi.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 17.Murugan RS, Vinothini G, Hara Y, Nagai S. Black tea polyphenols target matrix metalloproteinases, RECK, proangiogenic molecules and histone deacetylase in a rat hepatocarcinogenesis model. Anticancer Res. 2009;29:2301–2305. [PubMed] [Google Scholar]
- 18.Ohta M, Konno H, Tanaka T, et al. Effect of combination therapy with matrix metalloproteinase inhibitor MMI-166 and mitomycin C on the growth and liver metastasis of human colon cancer. Jpn J Cancer Res. 2001;92:688–695. doi: 10.1111/j.1349-7006.2001.tb01149.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muñoz-Nájar UM, Neurath KM, Vumbaca F, Claffey KP. Hypoxia stimulates breast carcinoma cell invasion through MT1-MMP and MMP-2 activation. Oncogene. 2006;25:2379–2392. doi: 10.1038/sj.onc.1209273. [DOI] [PubMed] [Google Scholar]
- 20.Migita K, Maeda Y, Abiru S, et al. Peroxynitrite-mediated matrix metalloproteinase-2 activation in human hepatic stellate cells. FEBS Lett. 2005;579:3119–3125. doi: 10.1016/j.febslet.2005.04.071. [DOI] [PubMed] [Google Scholar]
- 21.Ahmad R, Rasheed Z, Ahsan H. Biochemical and cellular toxicology of peroxynitrite: implications in cell death and autoimmune phenomenon. Immunopharmacol Immunotoxicol. 2009;31:388–396. doi: 10.1080/08923970802709197. [DOI] [PubMed] [Google Scholar]
- 22.Eiserich JP, Hristova M, Cross CE, et al. Formation of nitric oxide-derived inflammatory oxidants by myeloperoxidase in neutrophils. Nature. 1998;391:393–397. doi: 10.1038/34923. [DOI] [PubMed] [Google Scholar]
- 23.Wittrant Y, Theoleyre S, Couillaud S, Dunstan C, Heymann D, Rédini F. Relevance of an in vitro osteoclastogenesis system to study receptor activator of NF-κB ligand and osteoprotegerin biological activities. Exp Cell Res. 2004;293:292–301. doi: 10.1016/j.yexcr.2003.10.016. [DOI] [PubMed] [Google Scholar]
- 24.Steinbrenner H, Ramos MC, Stuhlmann D, Mitic D, Sies H, Brenneisen P. Tumor promoter TPA stimulates MMP-9 secretion from human keratinocytes by activation of superoxide-producing NADPH oxidase. Free Radic Res. 2005;39:245–253. doi: 10.1080/10715760500053487. [DOI] [PubMed] [Google Scholar]
- 25.Wu G. Amino acids: metabolism, functions, and nutrition. Amino Acids. 2009;37:1–17. doi: 10.1007/s00726-009-0269-0. [DOI] [PubMed] [Google Scholar]
- 26.Enoki T, Okuda S, Kudo Y, Takashima F, Sagawa H, Kato I. Oligosaccharides from agar inhibit pro-inflammatory mediator release by inducing heme oxygenase 1. Biosci Biotechnol Biochem. 2010;74:766–770. doi: 10.1271/bbb.90803. [DOI] [PubMed] [Google Scholar]
- 27.Daddaoua A, Puerta V, Requena P, et al. Goat milk oligosaccharides are anti-inflammatory in rats with hapten-induced colitis. J Nutr. 2006;136:672–676. doi: 10.1093/jn/136.3.672. [DOI] [PubMed] [Google Scholar]
- 28.Fukuda Y, Tao Y, Tomita T, et al. A traditional Japanese medicine mitigates TNBS-induced colitis in rats. Scand J Gastroenterol. 2006;41:1183–1189. doi: 10.1080/00365520600575704. [DOI] [PubMed] [Google Scholar]