Abstract
The inhibitory activity on intestinal α-glucosidase by cyanidin-3-rutinoside was examined in vitro and in vivo. The IC50 values of cyanidin-3-rutinoside against intestinal maltase, and sucrase were 2,323 ± 14.8 and 250.2 ± 8.1 µM, respectively. The kinetic analysis revealed that intestinal sucrase was inhibited by cyanidin-3-rutinoside in a mixed-type manner. The synergistic inhibition also found in combination of cyanidin-3-rutinoside with acarbose against intestinal maltase and sucrase. The oral administration of cyanidin-3-rutinoside (100 and 300 mg/kg) plus maltose or sucrose to normal rats, postprandial plasma glucose was markedly suppressed at 30–90 min after loading. Furthermore, the normal rats treated with acarbose and cyanidin-3-rutinoside (30 mg/kg) showed greater reduction of postprandial plasma glucose than the group treated with acarbose alone. These results suggest that cyanidin-3-rutinoside retards absorption of carbohydrates by inhibition of α-glucosidase which may be useful as a potential inhibitor for prevention and treatment of diabetes mellitus.
Keywords: cyandin-3-rutinoside, α-glucosidase, synergism, acarbose
Introduction
Diabetes mellitus is one of the chronic diseases characterized by hyperglycemia, dyslipidemia, and protein metabolism which result from defects in both regulations of insulin secretion and/or insulin action. The prevalence of diabetic patients worldwide has dramatically increased due to modern lifestyle changes and an increase of consumption of high-carbohydrate diets.(1) Recently, it has been reported that postprandial hyperglycemia is an important contributing factor for the development of diabetic complications.(2) Current scientific evidence has demonstrated the possibility of successfully preventing the onset of diabetes by controlling postprandial hyperglycemia through the inhibition of α-glucosidase and α-amylase activities, resulting in aggressive delay of carbohydrate digestion to absorbable monosaccharide.(3) The suppression of postprandial hyperglycemia subsequently delays the progression of micro- and macro-vascular complications such as microangiopathy, cardiovascular, and cerebrovascular diseases.(4)
Much effort has been extended in search of effective dietary plants and fruits for the development of functional foods in order to prevent and treat diabetes mellitus. They provide the bioactive compounds with beneficial health effects, and their consumption has been associated with reduced risk of developing diabetes through inhibition of α-glucosidase and pancreatic α-amylase activities.(5–7) Anthocyanins are versatile and plentiful flavonoid pigments which are widely distributed in various human diets through crops, vegetables, fruits, and red wine, suggesting the benefit of daily intake of certain amounts of these compounds from plant-based diets.(8) Interestingly, anthocyanins have been intensively investigated for their mechanisms related to anti-diabetic effect.(9) Numerous studies have documented that anthocyanins potentially inhibit intestinal α-glucosidases which have renewed interest in the studies on delaying postprandial hyperglycemia.(10,11) Cyanidin-3-rutinoside, a natural anthocyanin, has been found in litchi, black current, capulin and sweet cherry.(12) Our previous study reveals that cyanidin-3-rutinoside exhibits an inhibitory effect on yeast α-glucosidase in a non-competitive manner.(12) However, no information is available about the inhibitory effect of cyanidin-3-rutinoside related to intestinal α-glucosidase, and studies regarding the combined effect of cyanidin-3-rutinoside and acarbose have not been undertaken in vitro and in vivo.
The current study was therefore carried out to investigate the inhibitory effect of cyanidin-3-rutinoside against intestinal α-glucosidase. In addition, the study was conducted to evaluate the types of kinetic inhibition on intestinal α-glucosidase. Furthermore, the study was designed to investigate the combined effects of cyanidin-3-rurinoside and acarbose on inhibition of intestinal α-glucosidase. Finally, anti-hyperglycemic effect of cyanidin-3-rutinoside was performed in normal rats by oral maltose and sucrose tolerance test.
Materials and Methods
Materials
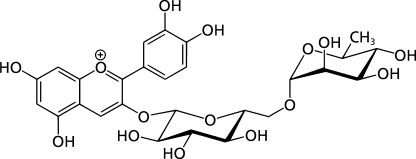
Rat intestinal acetone powder, 3,5-dinitrosalicylic acid, glucose oxidase kits, quercetin-3-rutinoside were purchased from Sigma Chemical Co. Ltd. (St. Louis, MO). Acarbose was obtained from Bayer, Germany. All others chemicals used were of analytical grade. Cyanidin-3-rutinoside chloride (C3R) was synthesized from quercetin-3-rutinoside according to the previous method.(13) After purification, the chemical structure of C3R (Fig. 1) was confirmed by using 1H-nuclear magnetic resonance (NMR), 13C-NMR, Mass spectrometry data.
Fig. 1.
The chemical structure of cyanidin-3-rutinoside (C3R).
In vitro assay for the intestinal α-glucosidase inhibitory activity
α-Glucosidase inhibitory activity was followed according to our previous report.(14) Briefly, rat intestinal acetone powder (100 mg) was homogenized in 3 ml of 0.9% NaCl solution. After centrifugation (12,000 g × 30 min), the crude enzyme (maltase = 0.68 units/mg protein, sucrase = 0.10 units/mg protein) was incubated with 37 mM maltose (70 µL) or 56 mM sucrose (40 µL) in 0.1 M phosphate buffer pH 6.9, and 20 µL of various concentrations of C3R at 37°C for 30 min (maltase assay) and 60 min (sucrase assay). The mixtures were suspended in boiling water for 10 min to stop the reaction. The concentration of glucose released from the reaction mixtures was determined by using glucose oxidase kits.
Enzyme kinetics
In order to investigate the type of inhibition, the enzyme kinetic analysis was performed according to the above reaction. Maintaining the quantity of sucrase constant at 0.10 units/mg protein and C3R (from 0.1 to 1.0 mM) was measured in various concentrations of sucrose. The type of inhibition was calculated on the basis of Lineweaver–Burk by reciprocally plotted data (substrate concentration on horizontal axis and velocity on vertical axis).
Combined effect of cyanidin-3-rutinoside and acarbose on intestinal α-glucosidase inhibitory activity
Acarbose was combined with or without low concentration of C3R. The reaction was performed according to the abovementioned assay. Results are expressed as the percentage inhibition of the corresponding control values.
Experimental animals
Male Wistar rats (180–200 g) were obtained from the National Laboratory Animal Center, Mahidol University, Salaya, Thailand. All animal experiments were conducted according to the ethical guidelines outlined in the Guide for Care and Use of Laboratory Animals. Animal facilities and protocol were approved by the Laboratory Animal Care and Use Committee at Faculty of Veterinary Science, Chulalongkorn University, Thailand. Wistar rats were housed in individual stainless steel cages in a room maintained at 25 ± 1°C on a 12:12-h light-dark cycle. They were fed standard laboratory chow with water ad libitum and fasted overnight before the experiments.
Effect of cyanidin-3-rutinoside and its combination with acarbose on plasma glucose concentration by the oral maltose or sucrose tolerance test
Rats were randomly assigned to 5 groups of 6 animals. Briefly, after overnight fasting, group 1 was orally administered a vehicle (distilled water). Group 2, 3 and 4 received C3R (30, 100, 300 mg/kg). Group 5 received acarbose (3 mg/kg). Thereafter, either maltose (3 g/kg) or sucrose (3 g/kg) solution was administered as the second administration at 5 min after the first administration. Blood samples were collected from the tail vein at 0 (before administration), 30, 60, 90, 120 and 180 min after substrate administration.
The combined effect of C3R and acarbose on plasma glucose concentration was performed according to above-mentioned method. Group 1 was orally administered a vehicle. Group 2 received C3R (30 mg/kg). Group 3 received acarbose (3 mg/kg). The last group received C3R (30 mg/kg) plus acarbose (3 mg/kg). Heparin-containing blood samples were immediately centrifuged (2,000 × g), and the plasma was separated and frozen at −20°C until analyzed for glucose concentration. Plasma glucose concentrations were determined by using the glucose oxidase kits. The areas under the curve (AUC) of plasma glucose concentration were calculated using a modification of the trapezoidal rule.
Statistical analysis
Data were expressed as means ± SEM The IC50 values were calculated from plots of log concentration of inhibitor concentration versus percentage inhibition curves by using Sigma Plot 10.0. Statistical analysis was performed by Student t test and one-way ANOVA. The least significant difference (LSD) test was used for mean comparisons and p<0.05 was considered to be statistically significant.
Results
The IC50 values for the intestinal maltase and sucrase activities
C3R inhibited the intestinal sucrase activity in a concentration-dependent manner, whereas it slightly inhibited the intestinal maltase activity. The IC50 values of C3R are summarized in Table 1. The results showed that there was quite selective inhibition on intestinal sucrase because the IC50 value for intestinal maltase activity was much higher than that of sucrase activity. However, C3R was much less potent than that of acarbose on the intestinal maltase and sucrase inhibition.
Table 1.
The IC50 values for intestinal α-glucosidase (maltase and sucrase) by cyanidin-3-rutinoside and acarbose.
| Compounds | IC50 values (µM) |
|
|---|---|---|
| Maltase | Sucrase | |
| Cyanidin-3-rutinoside | 2,323 ± 14.8 | 250.2 ± 8.1 |
| Acarbose | 2.7 ± 0.1 | 29.6 ± 3.5 |
Results are expressed as means ± SEM, n = 3.
The kinetic Inhibition of cyanidin-3-rutinoside on intestinal sucrase
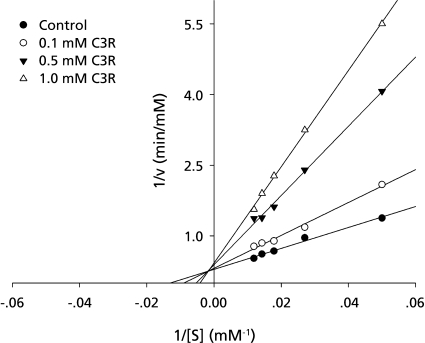
To further explore the inhibitory characteristics of C3R, the kinetic assay was performed using Lineweaver-Burk double reciprocal plots. The inhibitory mechanisms on the intestinal sucrase by C3R are shown in Fig. 2. A Lineweaver-Burk plot of C3R generated straight lines which had different intersections on the X-axis in the second quadrant, indicating that type of inhibition was of the mixed competitive and noncompetitive type.
Fig. 2.
Lineweaver-Burk plot for inhibitory activity of C3R on the intestinal sucrase.
The combined effect of cyanidin-3-rutinoside with acarbose on the inhibition of α-glucosidase in vitro
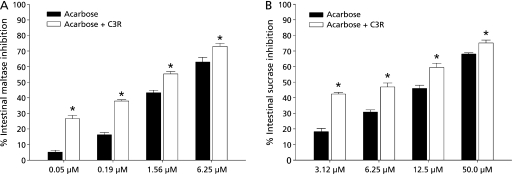
It was of interest to establish whether C3R and acarbose might interact synergistically or additively on the inhibition of intestinal α-glucosidase activity. Accordingly, an assay was then performed on the solutions containing acarbose alone or in mixture with a low concentration of C3R (1 µM) which did not show the inhibitory effect against intestinal maltase and sucrase activities. The combined effect of acarbose with C3R on intestinal maltase and sucrase inhibition are shown in Fig. 3. After the addition of C3R to the assay system with various concentrations of acarbose, the percentage intestinal maltase and sucrase inhibition increased when compared with acarbose alone (Fig. 3). The results showed that the percentage inhibition of mixtures was greater than the sum of acarbose and C3R. These findings suggest that combination of C3R and acarbose produced the synergistic inhibition against the intestinal maltase and sucrase.
Fig. 3.
The percentage enzyme inhibition of acarbose and its combination with C3R (1 µM) on (A) intestinal maltase, and (B) intestinal sucrase. Results are expressed as mean ± SEM, n = 3. *p<0.001 compared to acarbose alone.
Anti-hyperglycemic effect of cyanidin-3-rutinoside on plasma glucose concentration in vivo
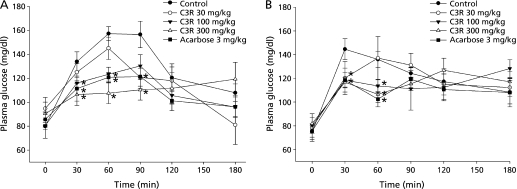
A single oral administration study of C3R with intestinal maltase and sucrase inhibitory activities was performed to clarify its anti-hyperglycemic effect in normal rats by oral maltose, and sucrose tolerance test. Fig. 4 shows the change in plasma glucose concentration during the 180 min of experiment in maltose- and sucrose-loaded normal rats. The results of the present study clearly show that a single oral administration of C3R (100 and 300 mg/kg) significantly suppressed a rise of plasma glucose concentration at 30, 60 and 90 min after maltose and sucrose administration (Fig. 4 A and B). Thereafter, plasma glucose concentration returned to baseline level at 120 min. The AUCs of rats treated with C3R (100 and 300 mg/kg) were significantly lower than those of untreated rats (Fig. 5). However, C3R (30 mg/kg) did not exert any significant effect on reduction of plasma glucose concentrations after oral maltose, and sucrose loading.
Fig. 4.
The effects of C3R on plasma glucose concentration by oral (A) maltose and (B) sucrose tolerance test. Results are expressed as means ± SEM, n = 6. *p<0.05 compared with the control group.
Fig. 5.
The effects of C3R on the total areas under the curve (AUC) of plasma glucose concentration by oral (A) maltose, and (B) sucrose tolerance test. Results are expressed as means ± SEM, n = 6. *p<0.05 compared with the control group.
To confirm the synergistic effect from in vitro, the normal rats were fed C3R (30 mg/kg) combined with acarbose at a dose of 3 mg/kg (maltose and sucrose loading). There were no significant differences in reduction of plasma glucose concentration between C3R (30 mg/kg) and the control group. Given the combined administration of acarbose and C3R (30 mg/kg) to the rats, it was found that plasma glucose concentration was significantly lower than that of acarbose alone at 30 min after administration of maltose and sucrose (Table 2). In addition, the AUCs of rats treated with acarbose plus C3R was significantly lower than that of rats treated with acarbose alone.
Table 2.
The combined effect of C3R and acarbose on plasma glucose concentration in oral maltose and sucrose tolerance test
| Groups | Plasma glucose (mg/dl) |
AUC (mg/dl.min) | |||||
|---|---|---|---|---|---|---|---|
| 0 min | 30 min | 60 min | 90 min | 120 min | 180 min | ||
| Maltose loading | |||||||
| Control | 75.8 ± 6.7 | 163.7 ± 8.8 | 148.7 ± 5.4 | 132.4 ± 4.6 | 117.0 ± 6.5 | 96.1 ± 10.0 | 23,400.1 ± 660.6 |
| C3R (30 mg/kg) | 86.6 ± 9.7 | 145.6 ± 9.0 | 152.3 ± 12.5 | 138.0 ± 10.3 | 114.2 ± 12.8 | 100.9 ± 7.1 | 22,899.1 ± 1934.6 |
| Acarbose (3 mg/kg) | 80.1 ± 5.1 | 139.4 ± 6.5* | 120.8 ± 4.1* | 124.4 ± 7.3 | 109.2 ± 4.6 | 96.1 ± 12.2 | 20,920.7 ± 500.5* |
| C3R(30 mg/kg) + Acabose (3 mg/kg) | 84.0 ± 7.3 | 118.6 ± 5.6*,# | 119.1 ± 2.3* | 108.6 ± 4.7*,# | 107.2 ± 8.5 | 93.5 ± 7.6 | 17,859.1 ± 432.1*,# |
| Sucrose loading | |||||||
| Control | 85.1 ± 2.9 | 134.4 ± 7.8 | 159.3 ± 6.0 | 156.5 ± 11.0 | 120.4 ± 11.6 | 107.8 ± 7.3 | 23,413.6 ± 915.2 |
| C3R (30 mg/kg) | 90.5 ± 3.2 | 125.1 ± 7.0 | 148.3 ± 7.3 | 125.6 ± 7.1* | 116.0 ± 4.5 | 81.2 ± 10.3 | 21,459.2 ± 1,312.7 |
| Acarbose (3 mg/kg) | 85.6 ± 6.0 | 114.3 ± 3.5* | 125.1 ± 6.5* | 121.4 ± 9.0* | 101.2 ± 8.3 | 99.2 ± 5.3 | 19,325.4 ± 373.6* |
| C3R (30 mg/kg) + Acabose (3 mg/kg) | 94.0 ± 5.7 | 99.8 ± 4.1*,# | 109.7 ± 5.4*,# | 105.3 ± 5.6*,# | 95.3 ± 2.7 | 94.8 ± 6.8 | 17,786.3 ± 200.3*,# |
Data are expressed as mean ± SEM, n = 6 rats per group. *p<0.05 compared to control, #p<0.05 compared to acarbose.
Discussion
Cyanidin and its glycosides represent one of the major groups of naturally occurring anthocyanins. Their mechanisms related to anti-diabetic effect have been comprehensively identified including the stimulatory insulin secretion,(15) and prevention of insulin resistance.(16) C3R, one of anthocyanins, displays a wide range of biological activities including antioxidant,(17) anti-inflammatory,(18) and anti-cancer.(19) Previous studies reported that C3R dose-dependently inhibited yeast α-glucosidase and it was a more potent inhibitor than quercetin-3-rutinoside.(12) The current study demonstrates that C3R has anti-hyperglycemic activity through inhibition of intestinal α-glucosidase (maltase and sucrase) which plays a major role in carbohydrate digestion. Inhibition of intestinal sucrase by C3R was conspicuous, whereas intestinal maltase was not so strongly inhibited. According to a previous study, the presence of monosaccharide moiety at the 3-O-position of cyanidin dramatically increased the potency of intestinal sucrase inhibition.(20) When comparing the IC50 values of C3R and cyanidin glycosides from the previous study, it seems that C3R shows significant increase in potency of intestinal sucrase inhibition over cyanidin and its glycosides, indicating that the introduction of a dissacharide (rutinose) in the 3-O-position of cyanidin may play a more important factor for increasing the intestinal sucrase inhibitory activity than the presence of monosaccharide.
The effect of C3R on postprandial hyperglycemia was investigated in normal rats by oral loading of maltose and sucrose. The postprandial hyperglycemia was suppressed after co-administration of C3R and substrates. The evidence confirms the findings from the in vitro study, indicating that the effect of C3R on the delay of dietary carbohydrate as well as disaccharides digestion is due to its inhibition of α-glucosidase activity in the small intestine, leading to suppression of postprandial hyperglycemia. Considering the data obtained from this investigation, it suggests that inhibition of α-glucosidase may be one of the possible mechanisms of C3R on the reduction of plasma glucose. Moreover, we hypothesize that C3R may also mediate its antihyperglycemic action via other mechanisms such as stimulating insulin secretion and activating glucose uptake in muscle and adipose tissues. However, it was reported when black currant anthocyanins containing C3R (2.08 µmol/kg of body weight) was orally administered to human, C3R could be detected in the plasma and the Cmax value was 46.3 ± 22.5 nmol/L, indicating that C3R was poorly absorbed to blood circulation in humans.(21) Due to its low bioavailability, it is possible that C3R does not appear to alter mechanisms for antihyperglycemic effect through peripheral tissues such as stimulating insulin secretion or activating glucose uptake. Hassimotto et al.(22) proposed that one mechanism of uptake of C3R in the small intestine probably involves sodium-dependent glucose transporter 1 (SGLT1). Glucose and galactose are also transported across the brush border membrane of the enterocytes by SGLT1. Form this evidence, C3R may compete with glucose for binding site of SGLT1 which may delay in glucose absorption, thus, eliciting a rise in postprandial hyperglycemia. Another possible mechanism of C3R may be involved in this pathway. To prove this hypothesis, a further study is needed to pinpoint the inhibitory effect of C3R on glucose transporter in small intestine.
Acarbose is an anti-diabetic drug used to treat type 2 diabetes mellitus and, in some countries, pre-diabetes. A recent report has shown that treatment of acarbose was associated with a 25% reduction in the incidence of diabetes in subjects with impaired glucose tolerance.(23) The administration of acarbose is associated with a 20% reduction of the peak of postprandial hyperglycemia. This effect may last for as much as 5 h, with an increase in the time of glucose absorption that prevents glucotoxicity and the consequent hyperinsulinaemia.(24) In general, interactions between anthocyanins and pharmaceutical drugs have received minimal study. It is possible that dietary intake of fruits and plants enriched with C3R may interact with acarbose in diabetic patients who use this drug to control plasma glucose level, such that it can have a detrimental impact on treatment outcome. Therefore, it is interesting and important to determine whether C3R produces a synergistic or additive inhibition with acarbose against intestinal α-glucosidases. Our present study reveals that combination of acarbose with C3R significantly produces a synergistic effect, suggesting that it would have significant clinical benefit of combination therapy on controlling postprandial hyperglycemia in diabetic patients. In addition, combined therapy with C3R may reduce the dose of acarbose, the progressive increase in optimal drug dosage, and costs associated with pharmaceutical disease management.
Postprandial hyperglycemia is one of the earliest observable abnormalities in diabetes mellitus. It has been established that an increase in postprandial hyperglycemia could contribute to the increase of hemoglobin glycosylation (HbA1c) by up to 25% in inadequately controlled patients with type 2 diabetes.(25) The decrease in HbA1c level could reduce the incidence of chronic vascular complications in diabetic patients.(23) HbA1c is the product of non-enzymatic reaction between glucose and amine moiety of hemoglobin. This reaction, called glycosylation, involves lots of other proteins, and it is the principal mechanism causing glucotoxicity which generates free radicals which directly damage proteins, lipids, and nucleic acids and contribute toward tissue damage,(26) induces oxidizing vascular wall lipids and accelerates atherogenesis in diabetic patients.(27) It has been reported that C3R has antioxidant activity by being free radical scavengers.(28) This suggests that intake of C3R would have another beneficial effect in diabetic patients, since it prevents oxidative damage to cells by its antioxidant activity and thus helps to slow down the development of diabetic complications.
Considerable amounts of anthocyanins are ingested as constituents of the human diet, 180–215 mg daily.(21) C3R is a natural colorant found in red sweet cherry, blackcurrant and other fruits. For example, red sweet cherry (Kordia) contains C3R, as a major anthocyanin, yielding at 184 mg/100 g fresh weight.(18) Moreover, it has been found that the abundant anthocyanins in mulberry pigment are cyanidin 3-O-rutinoside (60%) and cyanidin 3-O-glucoside (38%).(29) It is possible that consumption of C3R-enriched fruits may suppress postprandial hyperglycaemia, helping to the prevention of diabetic complications. Unfortunately, clinical data on consumption of C3R-enriched fruits in diabetic patients are limited. Further comprehensive pharmacological investigations of C3R-enriched fruits are required to evaluate its toxicity and clinical efficacy for potential application in pre-diabetic or diabetic patients.
The present investigation suggests that C3R is effective in the lowering of postprandial hyperglycemia by mediating inhibition of intestinal α-glucosidase. It also produces the synergistic inhibition when combined with acarbose. Further, C3R delays digestion and absorption of dietary carbohydrates in the small intestine, leading to suppression of an increased blood glucose level after a meal. Consumption of C3R-enriched fruit and plants may help to improve postprandial hyperglycemia which would offer a greater benefit for the treatment and prevention of diabetic complications. Furthermore, C3R can be developed as a nutraceutical food for prevention and treatment of diabetes mellitus.
Acknowledgments
This research work was financially supported by the Government Research Budget. The authors would like to thank The Medical Food Research and Development Center, which has been financially and institutionally supported by Chulalongkorn University.
Abbreviations
- AUC
areas under the curve
- C3R
Cyanidin-3-rutinoside chloride
- HbA1c
hemoglobin glycosylation
- LSD
least significant difference
- NMR
nuclear magnetic resonance
- SGLT1
sodium-dependent glucose transporter 1
References
- 1.Misra A, Khurana L. Obesity and the metabolic syndrome in developing countries. J Clin Endocrinol Metab. 2008;93(11 Suppl 1):S9–S30. doi: 10.1210/jc.2008-1595. [DOI] [PubMed] [Google Scholar]
- 2.Ceriello A. Postprandial hyperglycemia and diabetes complications is it time to treat. Diabetes. 2005;54:1–7. doi: 10.2337/diabetes.54.1.1. [DOI] [PubMed] [Google Scholar]
- 3.Raptis SA, Dimitriadis GD. Oral hypoglycemic agents: insulin secretagogues, alpha-glucosidase inhibitors and insulin sensitizers. Exp Clin Endocrinol Diabetes. 2001;109(Suppl 2):S265–S287. doi: 10.1055/s-2001-18588. [DOI] [PubMed] [Google Scholar]
- 4.Chiasson JL. Acarbose for the prevention of diabetes, hypertension, and cardiovascular disease in subjects with impaired glucose tolerance: the Study to Prevent Non-Insulin-Dependent Diabetes Mellitus (STOP-NIDDM) Trial. Endocr Pract. 2006;12:25–30. doi: 10.4158/EP.12.S1.25. [DOI] [PubMed] [Google Scholar]
- 5.Adisakwattana S, Pudhom K, Yibchok-Anun S. Influence of the methanolic extract from Abutilon indicum leaves in normal and streptozotocin-induced diabetic rats. Afr J Biotechnol. 2009;8:2011–2015. [Google Scholar]
- 6.Yibchok-Anun S, Jittaprasatsin W, Somtir D, Bunlunara W, Adisakwattana S. Insulin secreting and α-glucosidase inhibitory activity of Coscinium fenestratum and postprandial hyperglycemia in normal and diabetic rats. J Med Plants Res. 2009;3:646–651. [Google Scholar]
- 7.Adisakwattana S, Jiphimai P, Prutanopajai P, Chanathong B, Sapwarobol S, Ariyapitipan T. Evaluation of alpha-glucosidase, alpha-amylase and protein glycation inhibitory activities of edible plants. Int J Food Sci Nutr. 2010;61:295–305. doi: 10.3109/09637480903455963. [DOI] [PubMed] [Google Scholar]
- 8.Galvano F, La Fauci L, Vitaglione P, Fogliano V, Vanella L, Felgines C. Bioavailability, antioxidant and biological properties of the natural free-radical scavengers cyanidin and related glycosides. Ann Ist Super Sanita. 2007;43:382–393. [PubMed] [Google Scholar]
- 9.Ghosh D, Konishi T. Anthocyanins and anthocyanin-rich extracts: role in diabetes and eye function. Asia Pac J Clin Nutr. 2007;16:200–208. [PubMed] [Google Scholar]
- 10.Adisakwattana S, Charoenlertkul P, Yibchok-Anun S. alpha-Glucosidase inhibitory activity of cyanidin-3-galactoside and synergistic effect with acarbose. J Enzyme Inhib Med Chem. 2009;24:65–69. doi: 10.1080/14756360801906947. [DOI] [PubMed] [Google Scholar]
- 11.Matsui T, Ebuchi S, Kobayashi M, et al. Anti-hyperglycemic effect of diacylated anthocyanin derived from Ipomoea batatas cultivar Ayamurasaki can be achieved through the alpha-glucosidase inhibitory action. J Agric Food Chem. 2002;50:7244–7248. doi: 10.1021/jf025913m. [DOI] [PubMed] [Google Scholar]
- 12.Adisakwattana S, Ngamrojanavanich N, Kalampakorn K, Tiravanit W, Roengsumran S, Yibchok-Anun S. Inhibitory activity of cyanidin-3-rutinoside on alpha-glucosidase. J Enzyme Inhib Med Chem. 2004;19:313–316. doi: 10.1080/14756360409162443. [DOI] [PubMed] [Google Scholar]
- 13.Elhabiri M, Figueiredo P, Fougerousse A, Brouillard R. A convenient method for conversion of flavonoids into anthocyanins. Tetrahedron Lett. 1995;36:4611–4614. [Google Scholar]
- 14.Adisakwattana S, Chantarasinlapin P, Thammarat H, Yibchok-Anun S. A series of cinnamic acid derivatives and their inhibitory activity on intestinal alpha-glucosidase. J Enzyme Inhib Med Chem. 2009;24:1194–1200. doi: 10.1080/14756360902779326. [DOI] [PubMed] [Google Scholar]
- 15.Jayaprakasam B, Vareed SK, Olson LK, Nair MG. Insulin secretion by bioactive anthocyanins and anthocyanidins present in fruits. J Agric Food Chem. 2005;53:28–31. doi: 10.1021/jf049018+. [DOI] [PubMed] [Google Scholar]
- 16.Sasaki R, Nishimura N, Hoshino H, et al. Cyanidin 3-glucoside ameliorates hyperglycemia and insulin sensitivity due to downregulation of retinol binding protein 4 expression in diabetic mice. Biochem Pharmacol. 2007;74:1619–1627. doi: 10.1016/j.bcp.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 17.Tulio AZ, Jr., Reese RN, Wyzgoski FJ, et al. Cyanidin 3-rutinoside and cyanidin 3-xylosylrutinoside as primary phenolic antioxidants in black raspberry. J Agric Food Chem. 2008;56:1880–1888. doi: 10.1021/jf072313k. [DOI] [PubMed] [Google Scholar]
- 18.Mulabagal V, Lang GA, De Witt DL, Dalavoy SS, Nair MG. Anthocyanin content, lipid peroxidation and cyclooxygenase enzyme inhibitory activities of sweet and sour cherries. J Agric Food Chem. 2009;57:1239–1246. doi: 10.1021/jf8032039. [DOI] [PubMed] [Google Scholar]
- 19.Chen PN, Chu SC, Chiou HL, Kuo WH, Chiang CL, Hsieh YS. Mulberry anthocyanins, cyanidin 3-rutinoside and cyanidin 3-glucoside, exhibited an inhibitory effect on the migration and invasion of a human lung cancer cell line. Cancer Lett. 2006;235:248–259. doi: 10.1016/j.canlet.2005.04.033. [DOI] [PubMed] [Google Scholar]
- 20.Akkarachiyasit S, Charoenlertkul P, Yibchok-Anun S, Adisakwattana S. Inhibitory activities of cyanidin and its glycosides and synergistic effect with acarbose against Intestinal α-Glucosidase and Pancreatic α-Amylase. Int J Mol Sci. 2010;11:3387–3396. doi: 10.3390/ijms11093387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matsumoto H, Inaba H, Kishi M, Tominaga S, Hirayama M, Tsuda T. Orally administered delphinidin 3-rutinoside and cyanidin 3-rutinoside are directly absorbed in rats and humans and appear in the blood as the intact forms. J Agric Food Chem. 2001;49:1546–1551. doi: 10.1021/jf001246q. [DOI] [PubMed] [Google Scholar]
- 22.Hassimotto NM, Genovese MI, Lajolo FM. Absorption and metabolism of cyanidin-3-glucoside and cyanidin-3-rutinoside extracted from wild mulberry (Morus nigra L.) in rats. Nutr Res. 2008;28:198–207. doi: 10.1016/j.nutres.2007.12.012. [DOI] [PubMed] [Google Scholar]
- 23.Chiasson JL, Josse RG, Gomis R, Hanefeld M, Karasik A, Laakso M, STOP-NIDDM trial research group Acarbose for the prevention of Type 2 diabetes mellitus: the STOP-NIDDM Trial. Lancet. 2002;359:2072–2077. doi: 10.1016/S0140-6736(02)08905-5. [DOI] [PubMed] [Google Scholar]
- 24.Hanefeld M, Fischer S, Schulze J, et al. Therapeutic potentials of acarbose as first-line drug in NIDDM insufficiently treated with diet alone. Diabetes Care. 1991;14:732–737. doi: 10.2337/diacare.14.8.732. [DOI] [PubMed] [Google Scholar]
- 25.Lebovitz HE. Postprandial hyperglycaemic state: importance and consequences. Diabetes Res Clin Pract. 1998;40(Suppl):S27–S28. [PubMed] [Google Scholar]
- 26.Takagi Y, Kashiwagi A, Tanaka Y, Asahina T, Kikkawa R, Shigeta Y. Significance of fructose-induced protein oxidation and formation of advanced glycation end product. J Diabetes Complications. 1995;9:87–91. doi: 10.1016/1056-8727(94)00022-g. [DOI] [PubMed] [Google Scholar]
- 27.Cho SJ, Roman G, Yeboah F, Konishi Y. The road to advanced glycation end products: a mechanistic perspective. Curr Med Chem. 2007;14:1653–1671. doi: 10.2174/092986707780830989. [DOI] [PubMed] [Google Scholar]
- 28.Muselík J, García-Alonso M, Martín-López MP, Žemlička M, Rivas-Gonzalo JC. Measurement of antioxidant activity of wine catechins, procyanidins, anthocyanins and pyranoanthocyanins. Int J Mol Sci. 2007;8:797–809. [Google Scholar]
- 29.Qin C, Li Y, Niu W, Ding Y, Zhang R, Shang X. Analysis and characterisation of anthocyanins in mulberry fruit. Czech J Food Sci. 2010;28:117–126. [Google Scholar]