Abstract
While individuals with psychiatric illnesses are widely considered a special class of research subjects regarding decisional capacity and coercion vulnerability, those with physical illnesses often are not. IRB members (N = 127) read vignettes that described clinical research targeting one of two levels of disease severity (high/low) for psychiatric or medical diagnoses. They then rated decisional capacity, coercion, and risks for hypothetical research subjects. IRB members viewed psychiatric subjects as having greater vulnerability to coercion and less decisional capacity than medical subjects, even when medical illness was of a severity likely to engender psychiatric comorbidities. These results suggest that IRB members may inflate the vulnerability and decisional incapacity of psychiatric subjects, while discounting vulnerability and incapacity in medical subjects.
Keywords: coercion, decisional capacity, institutional review boards, research ethics, risk, vulnerability
Introduction
An enduring bioethical concern associated with the conduct of clinical research involves the protection of vulnerable subjects. The Belmont Report (National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research, 1979) focused particularly on children, prisoners, and those institutionalized with psychiatric disorders. Since then, concerns have broadened to the general population of persons with “mental disorders,” as reflected in the report of the National Bioethics Advisory Commission (NBAC, 1998) for persons with mental disorders who were being recruited into research studies involving their particular disorder. While the NBAC report proposed a variety of safeguards for such subjects (e.g., regarding the roles for legally authorized representatives in providing proxy consent for research participation, the use of independent professionals to monitor consent processes), a number of objections have been raised to the report. Many reflect a concern that actually preceded the NBAC report: Increased restrictions on research involving persons with psychiatric disorders, no matter how well intentioned, may reflect and reinforce stigmatization of those persons (Hirschfeld, Winslade, & Krause, 1997). Because stigmatization of mental illness already is widespread in the general public (Corrigan, 2004; Corrigan & Penn, 1999) and among medical professionals (Keane, 1990; Lawrie et al., 1998), some also have argued that further restriction on consent processes for psychiatric research ignores the existing research literature that supports the decision-making capabilities of people with certain types of mental illness (Chen, Miller, & Rosenstein, 2002; Roberts, 2002a). Instead, restrictions may foster paternalism and potentially obstruct needed psychiatric research (Oldham, Haimowitz, & Delano, 1999).
Research on decisional capacity in people with schizophrenia and major depression illustrates the complexity of these issues. Such research has shown that approximately 90% of schizophrenic subjects exhibit adequate decisional capacity if they are exposed to additional educational materials beyond the standard consent form (Carpenter et al., 2000; Moser et al., 2002) and also that they remember relevant information about those materials (Wirshing, Wirshing, Marder, Liberman, & Mintz, 1998). Similarly, although there is some evidence of decisional impairment in depressed patients involving their clinical care (Roth et al., 1982), little impairment in decisional capacity has been documented among outpatient research participants with major depression (Appelbaum, Grisso, Frank, O’Donnell, & Kupfer, 1999). Hence, if the consent process is well managed, the data suggest that people with certain severe mental illnesses do not appear to require extensive restrictions on the consent process in order to ethically manage their participation in biomedical research. Of course, there is considerable disagreement over what constitutes a well-managed consent process (de Champlain & Patenaude, 2006).
Similar concerns regarding decisional capacity have been raised regarding research on medical disorders (Oldham et al., 1999). Various medical disorders have been identified in which decisional capacity may be compromised (Michaud, Murray, & Bloom, 2001), including cancer (Miller, 2001; Pereira, Hanson, & Bruera, 1997; Schaeffer et al., 1996), acute trauma (Cohen, McCue, & Green, 1993; Prentice, Antonson, Leibrock, Kelso, & Sears, 1993), cerebrovascular disease (Slyter, 1998), myocardial infarction (Smithline, Mader, & Crenshaw, 1999), diabetes (Strachan, Deary, Ewing, & Frier, 1997), and severe pain (Pearlman et al., 1993; Sullivan, Rapp, Fitzgibbon, & Chapman, 1997). In fact, recent estimates indicate that up to 40% of hospitalized medical patients may demonstrate diminished decisional capacity (Raymont et al., 2004). Despite this evidence attesting to the vulnerability of prospective research participants with medical disorders, institutional review boards (IRBs) may not afford levels of protection for medically vulnerable individuals that are comparable to those afforded to psychiatrically vulnerable individuals (Miller, 2001; Roberts, 2002a).
There has been little empirical attention to factors that may influence the judgments of IRB members. The purpose of the present study, therefore, was to examine IRB member judgments of clinical research studies as a function of condition type (psychiatric vs. medical) and illness severity (low vs. high). To do this, we asked IRB members to read vignettes that described hypothetical clinical research studies and to (a) judge the decisional capacity of the research subjects, (b) judge research subjects’ susceptibility to coercion, and (c) evaluate study risks, including both the risk/benefit ratio for subjects and the legal risk to the institution. Two diagnoses were nested within each condition type: schizophrenia and major depressive disorder for the psychiatric condition, and cancer and neuropathic pain for the medical condition. Relative to those with medical conditions, potential research subjects with psychiatric conditions were expected to be viewed as more likely to demonstrate consent-related decisional incapacity and more vulnerable to coercion. Psychiatric studies also were expected to yield lower estimates of benefit/risk and higher estimates of legal risk to the institution. These effects were expected to be moderated by illness severity, such that higher levels of illness severity would augment judgments of decisional incapacity, coercion, and risk for studies of medical disorders only.
Methods
Participants
A convenience sample of 127 IRB members was recruited from two Midwestern university IRBs, including both full and alternate members who had served on their respective IRBs within the previous three years. The sample represented 33% of the 384 people who were asked to participate. There were 66 (52.0%) men and 61 (48.0%) women with a mean age of 47.3 years (SD = 11.2), including 58 (45.7%) university faculty, 44 (34.6%) university non-faculty, and 25 (19.7%) non-university community members. Most participants (n = 104, 81.9%) were full IRB members, and 23 (18.1%) were alternate members. Mean years of IRB service was 4.0 (SD = 4.0).
Design
Written vignettes describing hypothetical clinical research studies were developed that varied according to condition type (psychiatric vs. medical) and illness severity (low vs. high). A third independent variable, diagnosis, was nested within each of the two condition types (depression vs. schizophrenia for psychiatric studies; diabetic neuropathic pain vs. cancer for medical studies). Thus, the study was a 2 × 2 × [2] between subjects design with one nested factor, resulting in eight vignettes.
Materials and Measures
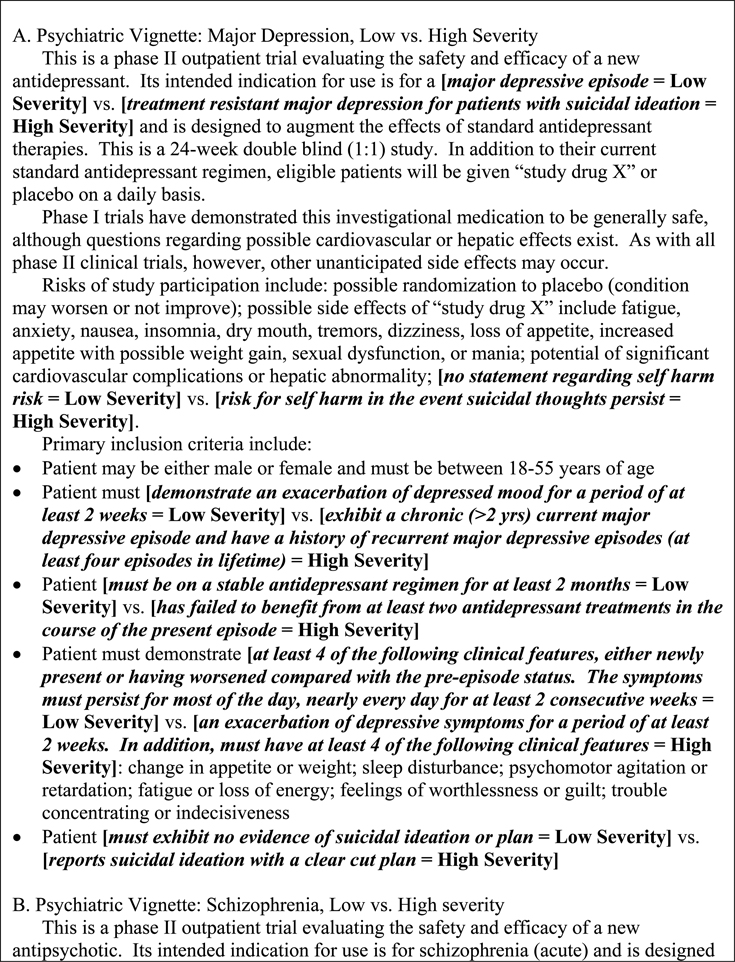
Vignettes presented details of a clinical research trial of an adjunctive medication (adjunctive to standard-of-care treatment). The content of each vignette reflected either a psychiatric or medical condition of either low or high severity. Within the psychiatric condition, the four vignettes presented either a study of low-severity depression, low-severity schizophrenia, high-severity depression, or high-severity schizophrenia. Within the medical condition, the four vignettes presented either a study of low-severity diabetic neuropathic pain, low-severity cancer, high-severity diabetic neuropathic pain, or high-severity cancer. Vignettes are presented in Figure 1.
FIG. 1.
Vignettes for the four diagnostic conditions. Bracketed terminology in bold italics differed between the low and high severity conditions.
The primary dependent variables for the study included judgments of decisional capacity, coercion, and risk, as displayed in Table 1. Two secondary dependent variables—ratings of the physical and mental illness severity of the type of person being recruited for the clinical trial—were also included as manipulation checks. Ratings for the latter items were made on 0–10 Likert-type scales (0 = very low severity; 5 = moderate severity; 10 = very high severity). Responses to these items were used to gauge the fidelity of the experimental inductions for condition type and illness severity. Participants were expected to perceive people with psychiatric disorders as more mentally ill than people with medical disorders, and vice versa. Further, participants were expected to perceive people with low severity conditions as less ill than people with high severity conditions.
Table 1.
Primary Dependent Measures
| Decisional Capacity |
|
| Coercion |
Coercion is defined as the process of compelling a person to act, or refrain from acting, contrary to free choice. In clinical research, this may take the form of verbal persuasion or inducement to participate in a study.
|
| Risk |
|
Procedure
IRB members were initially contacted by email to alert them that they would be receiving study materials by regular mail within two weeks. Study materials containing one of the eight experimental vignettes were mailed at random to potential participants. Recipients first read an agreement statement regarding voluntary participation. Those who agreed to participate then completed the materials and returned them in a postage-paid envelope. A second set of study materials was mailed to all potential participants after two weeks (because the study was anonymous, we could not track respondents), at which time non-responders again were encouraged to complete and return experimental materials. There were no further attempts to recruit participants following the second mailing.
Analyses
All analyses were performed using SPSS for Windows (version 13; SPSS Inc., Chicago, IL). Mixed-model analysis of variance (ANOVA) for nested designs was used to analyze the data for continuous measures (0–10 scales). All independent variables were tested simultaneously for each dependent measure. Simple effects analyses were used to decompose significant interaction effects and nested effects. Ratings of decisional capacity were made on a dichotomous scale (yes vs. no). Chi-square tests of association were used to analyze this variable as a function of each independent variable separately.
Results
The 127 participants were distributed across the eight study vignettes as follows: medical condition/low severity/neuropathic pain, n = 12; medical condition/high severity/neuropathic pain, n = 17; medical condition/low severity/cancer, n = 16; medical condition/high severity/cancer, n = 15; psychiatric condition/low severity/depression, n = 15; psychiatric condition/high severity/depression, n = 19; psychiatric condition/low severity/schizophrenia, n = 18; psychiatric condition/high severity/schizophrenia, n = 15.
Manipulation Checks
Participants rated the physical and mental illness severity of the patients described in the vignettes in order to evaluate whether the experimental manipulations of severity and condition type produced the expected differences in participants’ perceptions of patient illness severity. As shown in Table 2, participants rated physical and mental illness severity as higher in the high severity conditions, relative to the low severity conditions. Participants rated physical illness severity as higher in the medical conditions relative to the psychiatric conditions. Conversely, participants rated mental illness severity as higher in the psychiatric conditions relative to the medical conditions. These results support the fidelity of the severity and condition inductions.
Table 2.
Manipulation Check Results for Inductions of Illness Severity and Condition Type
| Dependent Measures | |||
|---|---|---|---|
| Induction | Levels | Physical Illness Severity Mean (SD) |
Mental Illness Severity M (SD) |
| Illness Severity | Low | 4.0 (2.3) | 5.7 (3.1) |
| High | 5.4 (2.8) | 7.1 (3.0) | |
| F(1,119)=15.2* | F(1,119)=21.8* | ||
| Condition | Medical | 6.3 (2.1) | 4.2 (3.0) |
| Psychiatric | 3.3 (2.3) | 8.4 (1.3) | |
| F(1,119)=71.5* | F(1,119)=148.4* | ||
p < .001.
Decisional Capacity
Nearly all participants (95.0%) believed that patients with medical conditions were capable of providing independent informed consent to participate in the research, while only 38.8% of participants believed this about patients with psychiatric conditions, χ2(1) = 44.1, p < .001. Illness severity had no significant association with ratings of consent capability. Under circumstances of low severity, 68.9% of participants indicated that patients were consent capable, while 62.1% indicated that they were capable of consent under circumstances of high severity, χ2(1) = 0.6, p = .43. Within the medical conditions, diagnosis had no significant association with ratings of consent capability: 90.3% of participants indicated cancer patients were capable of consent, and 100% indicated neuropathic pain patients were capable, χ2(1) = 3.0, p = .09. Within the psychiatric conditions, depressed patients were perceived as capable of giving consent by 64.7% of participants, while schizophrenic patients were rated as capable by only 12.1%, χ2(1) = 19.5, p < .001.
Coercion and Risk
Participants rated the risk of coercion and vulnerability to coercion for patients described in the vignettes. They also rated the legal risk to the institution associated with the study and the degree to which presumed study benefits outweighed presumed study risks. As shown in Table 3, participants rated coercion risk, coercion vulnerability, and legal risk as higher for psychiatric than for medical conditions. In addition, participants were less confident that study benefits outweighed study risks for psychiatric conditions relative to medical conditions. Participants rated legal risk as higher for high severity conditions relative to low severity conditions.
Table 3.
Effects of Illness Severity and Condition Type on Ratings of Coercion and Risk
| Dependent Measures | ||||
|---|---|---|---|---|
| Induction/Levels | Coercion Risk M (SD) |
Coercion Vulnerability M (SD) |
Legal Risk M (SD) |
Benefits Outweigh Risks M (SD) |
| Illness Severity | ||||
| Low | – | – | 4.5 (2.5) | – |
| High | – | – | 5.3 (2.8) | – |
| F(1,119)=4.5* | ||||
| Condition | ||||
| Medical | 3.0 (2.5) | 5.0 (2.5) | 3.7 (1.9) | 6.3 (2.0) |
| Psychiatric | 4.7 (2.8) | 6.7 (2.3) | 6.0 (2.8) | 5.5 (2.5) |
| F(1,119)=14.9** | F(1,119)=19.1** | F(1,119) = 29.6** | F(1,119) = 4.1* | |
p < .05.
p < .001.
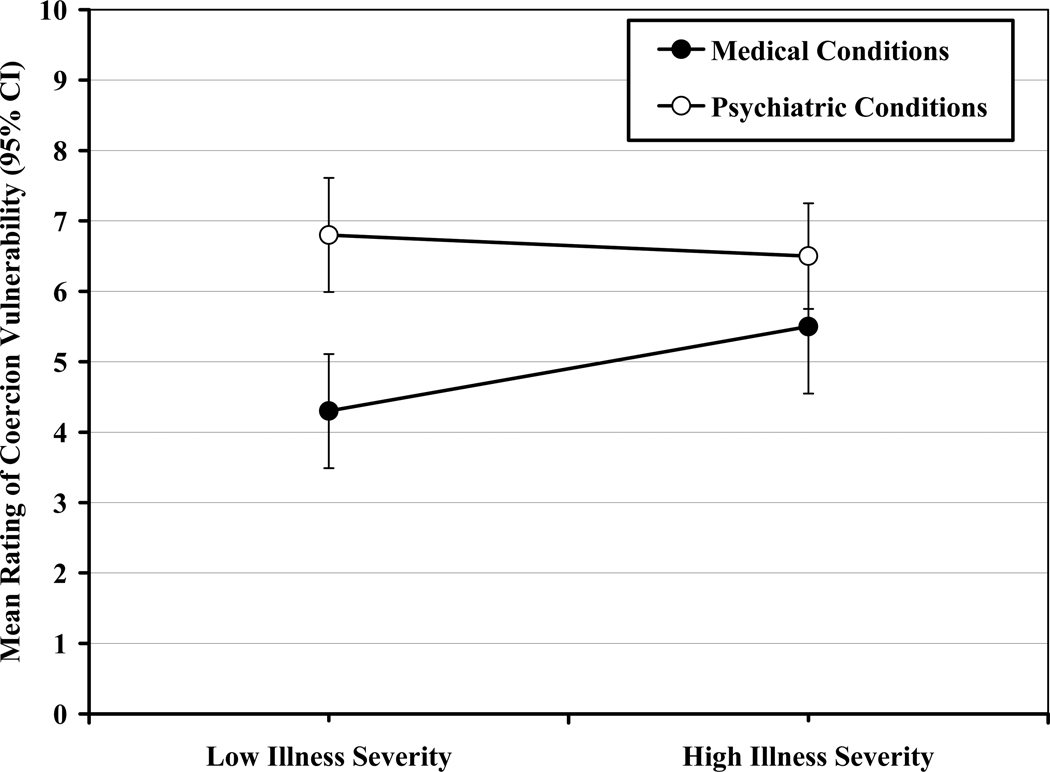
Only one significant interaction effect emerged, between illness severity and condition type for ratings of vulnerability to coercion, F(1,119) = 4.2, p < .05. As shown in Figure 2, simple effects analyses indicated that, under circumstances of low severity, participants rated coercion risk higher for psychiatric conditions relative to medical conditions, F(1,124) = 15.9, p < .001. Under circumstances of high severity, ratings of coercion risk were not significantly different between psychiatric and medical conditions, F(1,124) = 3.1, p = .08.
FIG. 2.
Interaction of illness severity and condition type for ratings of coercion vulnerability.
The nested effect of diagnosis was significant for ratings of coercion risk and coercion vulnerability. Simple effects analyses indicated that ratings of coercion risk were significantly higher for cancer patients (M = 4.2, SD = 2.7) relative to neuropathic pain patients (M = 1.7, SD = 1.6), F(1,124) = 12.0, p = .001. Coercion risk was also perceived as significantly higher for schizophrenic patients (M = 5.4, SD = 2.4) relative to depressed patients (M = 3.9, SD = 2.8), F(1,124) = 5.1, p < .05. For coercion vulnerability, ratings were higher for cancer patients (M = 6.0, SD = 2.4) relative to neuropathic pain patients (M = 3.8, SD = 2.3), F(1,124) = 11.4, p = .001. There was no significant difference in ratings of coercion vulnerability between schizophrenic patients (M = 7.1, SD = 2.4) and depressed patients (M = 6.3, SD = 2.2), F(1,124) = 1.4, p = .24.
Discussion
Consistent with hypotheses, IRB members viewed studies involving research subjects with psychiatric conditions as more problematic with regard to decisional capacity, coercion, and risk than those involving medical conditions. Moreover, participants made adjustments for illness severity with regard to coercion vulnerability for patients with low severity medical conditions, but not for patients with low severity psychiatric conditions. Psychiatric patients were perceived as vulnerable to coercion regardless of illness severity. The findings with regard to decisional capacity were perhaps the most striking. Whereas nearly all participants (95%) saw medical patients as having adequate decisional capacity, less than two in five participants (39%) indicated that patients with psychiatric disorders demonstrated such capacity.
These results appear to reflect sensitivity among IRB members to the protection of subjects with psychiatric diagnoses who might participate in clinical research. Viewed most positively, this sensitivity may reflect attentiveness to human subjects protection issues, consistent with the NBAC report. IRB member judgments regarding decisional capacity, however, were consistently lower than empirical findings reported in the literature, including studies of people with major depression (Appelbaum et al., 1999) and schizophrenia (Carpenter et al., 2000; Moser et al., 2002). Our results indicate that about 35% of participants thought that depressed patients did not have the capacity to provide informed consent; 88% of participants thought schizophrenics lacked decisional capacity.
While IRB member sensitivities to the protection of psychiatric research subjects may exceed the empirical need, the study design precludes an empirical explanation of mediating factors that might explain the pattern of results. Nonetheless, several speculative explanations may apply. For example, it is possible that some IRB members, aware that most people with major depression are capable of informed consent, still were inclined to take steps to insure the protection of those with impaired capability. For those IRB members, the prospect of inappropriately enrolling (failing to appropriately consent) as few as one of 20 prospective subjects may have constituted excessive exposure to risk. Similarly, it is possible that IRB members were less familiar with psychiatric illnesses, relative to medical illnesses, leading them to apply more conservative standards to inferences that they make about psychiatric disorders (Fiske & Taylor, 1991). Finally, it is possible that IRB members, like much of society, attach stigma to psychiatric diagnoses, causing them to discount the abilities and over-estimate the disabilities that people with mental illness possess. If the latter, stigmatization may amplify human subject protections concerns in ways that, paradoxically, could hinder research progress in the field (Hirschfeld et al., 1997).
Aside from the heightened sensitivity to the decisional capabilities of subjects considered for psychiatric studies, the results seem to reflect insensitivity to psychiatric comorbidities in people with medical conditions, including those with severe disorders. Depression is a common, well-recognized comorbidity in patients with cancer (Matsushita, Matsushima, & Maruyama, 2005; Norton et al., 2005; O’Mahony et al., 2005), as it is in patients with severe, debilitating pain (Banks & Kerns, 1996). While psychiatric comorbidities are common and often clinically significant elements of severe medical illness, IRB members apparently did not weigh the potential comorbidities heavily when evaluating risks to decisional capacity or coercion in the medical trials (Roberts, 2002b). Indeed, these comorbidities seemingly were not weighed at all by IRB members when considering severe neuropathic pain.
It is not evident why psychiatric comorbidity considerations figured minimally or not at all in IRB members’ judgments of medical trials. Since psychiatric distress was not explicitly mentioned in the medical vignettes, IRB members simply may have failed to consider such comorbidities secondary to their lack of salience. Hence, specific information, for example, regarding the prevalence of depression in cancer patients, may have engendered higher levels of concern regarding risk, coercion, and decisional capacity (Redelmeier, Koehler, Liberman, & Tversky, 1995). Alternatively, IRB members may have considered the psychiatric factors carefully, but decided that they were of relatively less importance relative to the possible medical benefits of participation, especially for cancer patients.
There are a number of limitations that should be considered when interpreting the results. First, we should note the preliminary nature of the data reported here. The data were derived from a convenience sample that represented approximately 33% of all potential participants. Both the non-random nature of the sample and the response rate raise questions about the generalizability of the results. Second, study participants were asked to make judgments based on a limited amount of information. While this methodology has been shown to effectively reflect attitudes, judgments, and response tendencies (Fiske & Taylor, 1991), the amount of information provided in the study falls far short of the information provided to IRB members when reviewing an actual protocol for human subject protections considerations. Moreover, IRB members were asked to make these judgments independently, in contrast to many IRB decisions that are made in a group context after deliberation. Hence, extrapolation of these results to actual IRB decision-making needs further investigation. Finally, the vignettes represented only two psychiatric and two medical conditions. There are numerous other medical and psychiatric conditions that could have been selected and that might have yielded different results. The degree to which the results apply broadly to other medical and psychiatric conditions is open to question.
These limitations notwithstanding, the results suggest that IRB member judgments may apply different standards of human subjects protections as a function of the type of condition being studied. In regard to psychiatric research, IRB member judgments are characterized by heightened sensitivity to issues of risk, coercion, and decisional capacity for informed consent. In regard to medical research, IRB member judgments seem to reflect a relative insensitivity to the potential for psychiatric comorbidities to affect medical patients, such that IRB members fail to identify threats to decisional capacity and coercion to a degree comparable to that of psychiatric studies. In light of the level of responsibility that IRBs assume in their oversight of medical and psychiatric research, further empirical examination of factors that influence member judgments and IRB decision-making processes appears warranted.
Best Practices
As noted above, the generalizability of these results to actual decision-making that occurs in IRBs must be considered carefully. That said, the results point to differences in judgments as a function of condition type under study (psychiatric vs. medical) that may operate among IRB members in their reviews of clinical research protocols. While the sensitivity of IRB members to issues of vulnerability and risk may hinder the conduct of psychiatric research, that sensitivity likely serves to provide adequate (or more than adequate) protections for psychiatric research participants. The apparently enhanced sensitivity to human subjects protection in psychiatric research, while potentially problematic, is perhaps less of a concern than the relative insensitivity to the vulnerabilities of medical participants. Because the vulnerability of medical research participants may be underestimated, they may be more likely to be exposed to risk. Hence, this research suggests the importance of particular attention among IRB members to vulnerable medical participants.
Research Agenda
These preliminary findings suggest a lack of consistency in IRB member judgments of decisional capacity, coercion, and risk depending on the condition (psychiatric vs. medical) being studied. Because the data derive from a convenience sample of IRB members at several local institutions, it would be useful to replicate the study in a larger, more representative sample of IRB members. Should the results prove generalizable, further research should examine the degree to which the stigmatization of psychiatric patients affects IRB judgments. If stigmatization accounts for relatively little of the demonstrated difference, research should examine other features of the research protocols that may contribute to such judgments. For example, medical protocols may omit specific information relative to the psychiatric vulnerabilities of medical participants. Previous research has shown that observers may discount information that lacks specificity or salience (Redelmeier, Koehler, Liberman, & Tversky, 1995). Research that targets this phenomenon could clarify whether specificity of information is a factor leading to the under-estimation of risks and/or vulnerabilities in the IRB setting.
Educational Implications
Because the data reported in this article are preliminary in nature, some caution is justified when considering their educational implications. Nonetheless, the results suggest the potential importance of two educational directives. First, there is an apparent need to educate IRB members regarding the capacities of psychiatric patients for providing autonomous informed consent. The data suggest that the capacity of such patients is widely questioned. While it is crucial that IRBs offer this patient population oversight that insures protections, IRB members may stigmatize and/or be overly sensitive to the vulnerabilities of this population to a degree that hinders research.
A second point of potential educational value involves the psychiatric vulnerabilities of participants in medical studies. At present, these vulnerabilities appear to be given little weight by IRB members as they consider issues related to decisional capacity, coercion, and risk, despite abundant evidence that many medical populations may be psychiatrically vulnerable, secondary to comorbidities, at the time that they are considered for research participation. Further education regarding the vulnerabilities of these groups could be beneficial for both IRB members and for investigators that work with these groups, especially in terms of sensitizing them to the factors that may impact on a research subject’s capacity to provide autonomous informed consent.
Acknowledgments
This work was supported in part by National Institute of Mental Health grant # R01 MH075958.
Contributor Information
Rebecca Luebbert, Southern Illinois University-Edwardsville (USA).
Raymond C. Tait, Saint Louis University (USA)
John T. Chibnall, Saint Louis University (USA)
Teresa L. Deshields, Siteman Cancer Center, Barnes-Jewish Hospital (USA)
References
- Appelbaum PS, Grisso T, Frank E, O’Donnell S, Kupfer DJ. Competence of depressed patients for consent to research. American Journal of Psychiatry. 1999;156:1380–1384. doi: 10.1176/ajp.156.9.1380. [DOI] [PubMed] [Google Scholar]
- Banks SM, Kerns RD. Explaining high rates of depression in chronic pain: A diathesis-stress framework. Psychological Bulletin. 1996;119:95–110. [Google Scholar]
- Carpenter WT, Jr, Gold JM, Lahti AC, Queern CA, Conley RR, Bartko JJ, Kovnick J, Appelbaum PS. Decisional capacity for informed consent in schizophrenia research. Archives of General Psychiatry. 2000;57:533–538. doi: 10.1001/archpsyc.57.6.533. [DOI] [PubMed] [Google Scholar]
- Chen DT, Miller FG, Rosenstein DL. Enrolling decisionally impaired adults in clinical research. Medical Care. 2002;40 Suppl. 9:V20–V29. doi: 10.1097/01.MLR.0000023952.15394.88. [DOI] [PubMed] [Google Scholar]
- Cohen LM, Mccue JD, Green GM. Do clinical and formal assessments of the capacity of patients in the intensive care unit to make decisions agree? Archives of Internal Medicine. 1993;153:2481–2485. [PubMed] [Google Scholar]
- Corrigan P. How stigma interferes with mental health care. American Psychologist. 2004;59:614–625. doi: 10.1037/0003-066X.59.7.614. [DOI] [PubMed] [Google Scholar]
- Corrigan PW, Penn DL. Lessons from social psychology on discrediting psychiatric stigma. American Psychologist. 1999;54:765–776. doi: 10.1037//0003-066x.54.9.765. [DOI] [PubMed] [Google Scholar]
- de Champlain J, Patenaude J. Review of a mock research protocol in functional neuroimaging by Canadian research ethics boards. Journal of Medical Ethics. 2006;32:530–534. doi: 10.1136/jme.2005.012807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiske ST, Taylor SE. Social cognition. 2nd ed. New York: McGraw-Hill; 1991. [Google Scholar]
- Hirschfeld RMA, Winslade W, Krause TL. Protecting subjects and fostering research: Striking the proper balance. Archives of General Psychiatry. 1997;54:121–123. doi: 10.1001/archpsyc.1997.01830140031005. [DOI] [PubMed] [Google Scholar]
- Keane M. Contemporary beliefs about mental illness among medical students: Implications for education and practice. Academic Psychiatry. 1990;14:172–177. doi: 10.1007/BF03341291. [DOI] [PubMed] [Google Scholar]
- Lawrie SM, Martin K, Mcneill G, Drife J, Chrystie P, Reid A, Wu P, Nammary S, Ball J. General practitioners’ attitudes to psychiatric and medical illness. Psychology and Medicine. 1998;28:1463–1467. doi: 10.1017/s0033291798007004. [DOI] [PubMed] [Google Scholar]
- Matsushita T, Matsushima E, Maruyama M. Psychological state, quality of life, and coping style in patients with digestive cancer. General Hospital Psychiatry. 2005;27:125–132. doi: 10.1016/j.genhosppsych.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Michaud CM, Murray CJ, Bloom BR. Burden of disease – implications for future research. Journal of the American Medical Association. 2001;285:535–539. doi: 10.1001/jama.285.5.535. [DOI] [PubMed] [Google Scholar]
- Miller M. Phase I cancer trials: A crucible of competing priorities. International Anesthesiology Clinics. 2001;39:13–33. doi: 10.1097/00004311-200107000-00004. [DOI] [PubMed] [Google Scholar]
- Moser DJ, Schultz SK, Arndt S, Benjamin ML, Fleming FW, Brems CS, Paulsen JS, Appelbaum PS, Andreasen NC. Capacity to provide informed consent for participation in schizophrenia and HIV research. American Journal of Psychiatry. 2002;159:1201–1207. doi: 10.1176/appi.ajp.159.7.1201. [DOI] [PubMed] [Google Scholar]
- National Bioethics Advisory Commission (NBAC) Research involving persons with mental disorders that may affect decisionmaking capacity. Rockville, MD: Author; 1998. [Google Scholar]
- National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research. Belmont report: Ethical principles and guidelines for the protection of human subjects of research. Federal Register Document 79-12065. 1979
- Norton TR, Manne SL, Rubin S, Hernandez E, Carlson J, Bergman C, Rosenblum N. Ovarian cancer patients’ psychological distress: The role of physical impairment, perceived unsupportive family and friend behaviors, perceived control, and self-esteem. Health Psychology. 2005;24:143–152. doi: 10.1037/0278-6133.24.2.143. [DOI] [PubMed] [Google Scholar]
- O’Mahony S, Goulet J, Kornblith A, Abbatiello G, Clarke B, Kless-Siegel S, Breitbart W, Payne R. Desire for hastened death, cancer pain and depression: Report of a longitudinal observational study. Journal of Pain and Symptom Management. 2005;29:446–457. doi: 10.1016/j.jpainsymman.2004.08.010. [DOI] [PubMed] [Google Scholar]
- Oldham JM, Haimowitz S, Delano SJ. Protection of persons with mental disorders from research risk: A response to the report of the National Bioethics Advisory Commission. Archives of General Psychiatry. 1999;56:688–693. doi: 10.1001/archpsyc.56.8.688. [DOI] [PubMed] [Google Scholar]
- Pearlman RA, Cain KC, Patrick DL, Appelbaum-Maizel M, Starks HE, Jecker NS, Uhlmann RF. Insights pertaining to patient assessments of states worse than death. Journal of Clinical Ethics. 1993;4:33–41. [PubMed] [Google Scholar]
- Pereira J, Hanson J, Bruera E. The frequency and clinical course of cognitive impairment in patients with terminal cancer. Cancer. 1997;79:835–842. [PubMed] [Google Scholar]
- Prentice ED, Antonson DL, Leibrock LG, Kelso TK, Sears TD. IRB review of a phase II randomized clinical trial involving incompetent patients suffering from severe closed head injury. IRB Review of Human Subjects Research. 1993;15:1–7. [PubMed] [Google Scholar]
- Raymont V, Bingley W, Buchanan A, David AS, Hayward P, Wessely S, Hotopf M. Prevalence of mental incapacity in medical inpatients and associated risk factors: Cross-sectional study. Lancet. 2004;364:1421–1427. doi: 10.1016/S0140-6736(04)17224-3. [DOI] [PubMed] [Google Scholar]
- Redelmeier DA, Koehler DJ, Liberman V, Tversky A. Probability judgment in medicine: Discounting unspecified possibilities. Medical Decision Making. 1995;15:227–230. doi: 10.1177/0272989X9501500305. [DOI] [PubMed] [Google Scholar]
- Roberts LW. Ethics and mental illness research. Psychiatric Clinics of North America. 2002a;25:525–545. doi: 10.1016/s0193-953x(01)00014-4. [DOI] [PubMed] [Google Scholar]
- Roberts LW. Informed consent and the capacity for voluntarism. American Journal of Psychiatry. 2002b;159:705–712. doi: 10.1176/appi.ajp.159.5.705. [DOI] [PubMed] [Google Scholar]
- Roth LH, Lidz CW, Meisel A, Soloff PH, Kaufman K, Spiker DG, Foster FG. Competency to decide about treatment or research: An overview of some empirical data. International Journal of Law and Psychiatry. 1982;5:29–50. [PubMed] [Google Scholar]
- Schaeffer MH, Krantz DS, Wichman A, Masur H, Reed E, Vinicky JK. The impact of disease severity on the informed consent process in clinical research. American Journal of Medicine. 1996;100:261–268. doi: 10.1016/S0002-9343(97)89483-1. [DOI] [PubMed] [Google Scholar]
- Slyter H. Ethical challenges in stroke research. Stroke. 1998;29:1725–1729. doi: 10.1161/01.str.29.8.1725. [DOI] [PubMed] [Google Scholar]
- Smithline HA, Mader TJ, Crenshaw BJ. Do patients with acute medical conditions have the capacity to give informed consent for emergency medicine research? Academic Emergency Medicine. 1999;6:776–780. doi: 10.1111/j.1553-2712.1999.tb01205.x. [DOI] [PubMed] [Google Scholar]
- Strachan MW, Deary IJ, Ewing FM, Frier BM. Is type II diabetes associated with an increased risk of cognitive dysfunction? A critical review of published studies. Diabetes Care. 1997;20:438–445. doi: 10.2337/diacare.20.3.438. [DOI] [PubMed] [Google Scholar]
- Sullivan M, Rapp S, Fitzgibbon D, Chapman CR. Pain and the choice to hasten death in patients with painful metastatic cancer. Journal of Palliative Care. 1997;13:18–28. [PubMed] [Google Scholar]
- Wirshing DA, Wirshing WC, Marder SR, Liberman RP, Mintz J. Informed consent: Assessment of comprehension. American Journal of Psychiatry. 1998;155:1508–1511. doi: 10.1176/ajp.155.11.1508. [DOI] [PubMed] [Google Scholar]