Abstract
The functions of sleep remain elusive, but a strong link exists between sleep need and neuronal plasticity. We tested the hypothesis that plastic processes during wake lead to a net increase in synaptic strength, and sleep is necessary for synaptic renormalization. We found that, in 3 Drosophila neuronal circuits, synapse size or number increases after a few hours of wake and decreases only if flies are allowed to sleep. A richer wake experience resulted in both larger synaptic growth and greater sleep need. Finally, we demonstrate that the gene Fmr1 (fragile X mental retardation 1) plays an important role in sleep-dependent synaptic renormalization.
Sleep is present in every species that has been carefully studied (1), including Drosophila melanogaster (2, 3), but its functions remain elusive. Increasing evidence points to a link between sleep need and neuronal plasticity (1, 4, 5). A recent hypothesis (6) suggests that a consequence of staying awake is a progressive increase in synaptic strength, as the awake brain learns and adapts to an ever-changing environment mostly through synaptic potentiation (7). However, such increase would soon become unsustainable, because stronger synapses consume more energy, occupy more space, require more supplies, and cannot be further potentiated, saturating the ability to learn. Thus, according to the synaptic homeostasis hypothesis, sleep may serve an essential function by promoting a homeostatic reduction in synaptic strength down to sustainable levels. Also, the hypothesis predicts that the more one learns and adapts (the more intense is the wake experience), the more one needs to sleep. Findings in rodents are consistent with this hypothesis. For instance, molecular and electrophysiological markers of synaptic strength are higher after wake and lower after sleep (8, 9). Moreover, presynaptic terminals of hypocretin neurons in zebrafish larvae undergo both circadian and sleep-wake dependent structural changes, the latter consistent with sleep-dependent downregulation (10). Finally, in the fly brain, overall levels of synaptic proteins increase after wake and decrease after sleep (11), and synaptic structural changes have been described after very long sleep deprivation (12). These results suggest that a role for sleep in synaptic homeostasis may hold in phylogenetically distant species, and may thus be of general importance.
The evidence in support of the synaptic homeostasis hypothesis is mainly correlative, and thus it is important to seek direct proof that sleep is necessary for synaptic renormalization, and do so at the level of individual synapses. Moreover, the synaptic homeostasis hypothesis predicts that behavioral paradigms that enhance wake-related plasticity in specific neural circuits should increase synaptic strength in those circuits as well as sleep need, but this prediction has never been tested. Finally, the cellular mechanisms that underlie synaptic and sleep changes remain unexplored. Here we exploited the power of Drosophila genetics, combined with confocal microscopy and behavioral analysis, to address these questions.
Changes in synaptic strength are often associated with changes in synaptic structure, including synapse number/size, although the link between structural and functional plasticity is complex (13-15). In mammals, the diameter and length of synaptic spines correlate with the size of the postsynaptic density and with the magnitude of electric signals transmitted to the dendritic shaft (16, 17). Moreover, the induction of synaptic potentiation leads to synapse/spine growth, while synaptic depression causes synapses/spines to retract or shrink (13-15). Similarly, in Drosophila, synaptic morphology at the neuromuscular junction changes depending on experience, and these changes correlate with synaptic strength (18). Previous in vivo experiments in mammals and flies measured overall changes in electrophysiological and molecular markers of synaptic strength, without cellular resolution, and without direct evidence for morphological changes in synaptic terminals. Here we selected 3 specific cell populations in the fly brain and asked whether sleep/wake affect synaptic density and size.
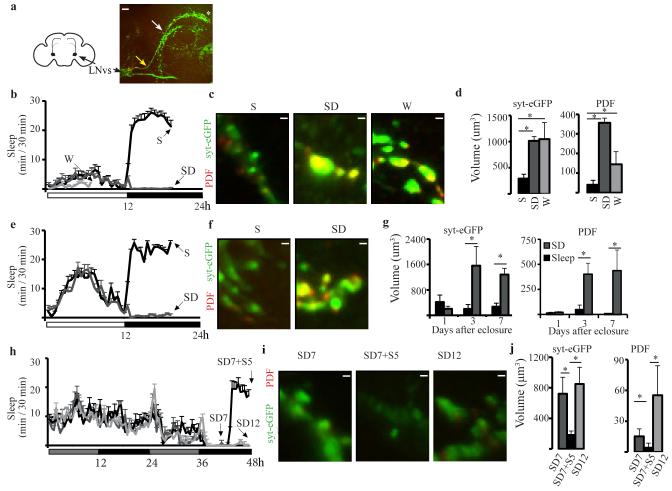
The first cell group we studied included the small ventral Lateral Neurons (LNvs), a subset of circadian oscillator neurons that are part of the wake promoting system (19) and express the neuropeptide pigment dispersing factor (PDF) (20)(Fig. 1a). To visualize changes in presynaptic morphology we used a PDF-GAL4 driver and expressed a UAS-syt-eGFP construct whose protein product colocalizes with native synaptic vesicles (21). We also measured PDF expression, because the latter is another marker of presynaptic boutons in small LNvs (22). First we tested adult females (7 day-old) collected either during the light period after 7h of mainly (>75%) spontaneous wake, or during the dark period after 7h of mostly sleep (>80%) or sleep deprivation (>90%)(Fig. 1b). Syt-eGFP and PDF staining were both higher in the presynaptic region of sleep deprived and spontaneously awake flies relative to sleeping flies (Fig. 1c,d), while no differences were found in the axonal processes extending from the cell bodies to the presynaptic region (both syt-eGFP and PDF p = 0.3, Kruskal-Wallis test), suggesting that the changes are independent of circadian time and specific to the presynaptic terminal. We then tested males, and because they have less consolidated wake during the day than females, we only collected flies at night, after sleep or sleep deprivation (Fig. 1e). Sleep deprived 3 and 7 day-old males consistently showed higher presynaptic syt-eGFP and PDF staining than sleeping flies (Fig. 1f,g). In contrast, 1 day-old flies showed low syt-eGFP and PDF staining after both sleep and sleep deprivation (Fig. 1f,g). The lack of PDF staining in very young flies suggests that these neurons are still inactive soon after eclosure. Moreover, because PDF promotes arousal, low PDF staining is consistent with flies being predominantly asleep after eclosure (3), even if mechanical stimulation was used to try to keep them awake, consistent with high sleep need and elevated arousal threshold in newborn mammals. Syt-eGFP staining did not change in newly eclosed flies, whose PDF levels were very low. Syt-eGFP and PDF expression were also measured in Per01 flies carrying a null mutation of the clock gene Period. Because Per01 mutants have no spontaneous consolidated sleep, flies were collected immediately after 7h of sleep deprivation, or after 5 additional h of either recovery sleep or sleep deprivation (Fig. 1h). Overall syt-eGFP and PDF staining in presynaptic terminals was reduced in Per01 mutants relative to wildtype flies, but still high after both 7 and 12h of sleep deprivation and low after recovery sleep (Fig. i,j).
Fig. 1. Sleep/wake presynaptic changes in small LNvs.
a. Left, schematic frontal section of fly brain with LNvs neurons projecting to the dorsal brain. Right, example of small LNvs axonal terminals stained for syt-eGFP (green). Yellow and white arrows point to where LNvs axons leave the posterior optic tract and to the first axonal bifurcation, respectively. Asterisk marks the tip of the terminal region whose volume was measured, as shown in c, f, i. b. Mean sleep duration in 7 day-old females used for imaging after spontaneous wake (W), sleep deprivation (SD), or sleep (S). Horizontal white and black bars indicate light and dark period, respectively. c. Examples of small LNvs axonal terminals stained for syt-eGFP (green), PDF (red, overlap yellow) and volume measurements (d) in females (S = 9, W = 9, SD = 5). e. Mean sleep duration in 7 day-old males used for imaging. f. Examples of axonal terminals in males. g. Mean volume measurements in males harvested 1, 3 and 7 days after eclosure (n = 5 / time point). h. Mean sleep duration in per01 males kept in constant darkness. At the onset of the second subjective night, flies underwent SD for 7 or 12h, or 7h SD followed by 5h of sleep. i. Examples of axonal terminals. j. Mean volume measurements (n = 7 /group). All bars = 1μm except in A (10μm). All panels show ± SEM.
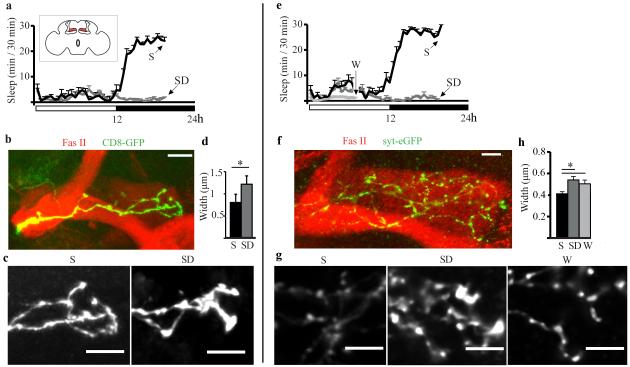
The second cell group we analyzed included γ neurons of the mushroom bodies (Fig. 2a, inset), because they can be targeted by mosaic analysis with a repressible cell marker (MARCM) to visualize single cells (23), show a relatively simple morphology, and undergo activity-dependent pruning (24). Moreover, the mushroom bodies are involved in sleep regulation (25, 26), and mutations altering cAMP/PKA signaling or Fmr1 expression in these brain regions affect both sleep need and experience-dependent structural plasticity (12, 27-29). Flies were collected at night after 7h of sleep or sleep deprivation (Fig. 2a), and dissected brains were immunostained for GFP-tagged CD8 to visualize neuronal membranes (Fig. 2b). We found that the axonal tips were larger after sleep deprivation than after sleep (Fig. 2c,d), consistent with an increase in volume of presynaptic terminals. To confirm this result, we generated fly stocks with γ MARCM clones expressing syt-eGFP, and flies were collected after 7h of mostly spontaneous wake, or during the dark period after 7h of mostly sleep or sleep deprivation (Fig. 2e). As expected, syt-eGFP tended to accumulate in puncta along lightly stained processes (Fig. 2f), in contrast to the diffuse CD8-GFP staining (Fig. 2b). Syt-eGFP puncta were larger in sleep deprived and spontaneously awake flies relative to sleeping flies (Fig. 2g,h).
Fig. 2. Sleep/wake presynaptic changes in the gamma lobe of the mushroom bodies.
a. Mean sleep duration in female flies used for imaging after 7h of S or SD at night. Inset, schematic frontal section of the fly brain showing gamma lobes in red. b. Example of MARCM clones tagged with CD8-GFP (green), which outlines gamma lobe neurons. Fasciclin II (Fas II, red) staining outlines the mushroom bodies. c. Representative images of CD8-GFP clones from S and SD flies. d. Mean width of axonal tips (females, S = 26, SD = 15). e. Mean sleep duration in flies used for imaging after W, SD or S. f. Representative gamma lobe with two MARCM-generated clones expressing syt-eGFP (green). g. Representative syt-eGFP puncta from S, SD, and W flies. h. Mean puncta width (males and females did not differ and were pooled; S = 34, SD = 26, W = 20). Mean number of tested puncta per lobe per fly was S = 28 ± 2, SD = 29 ± 2, W = 30 ± 2. All bars = 10μm. All panels show ± SEM.
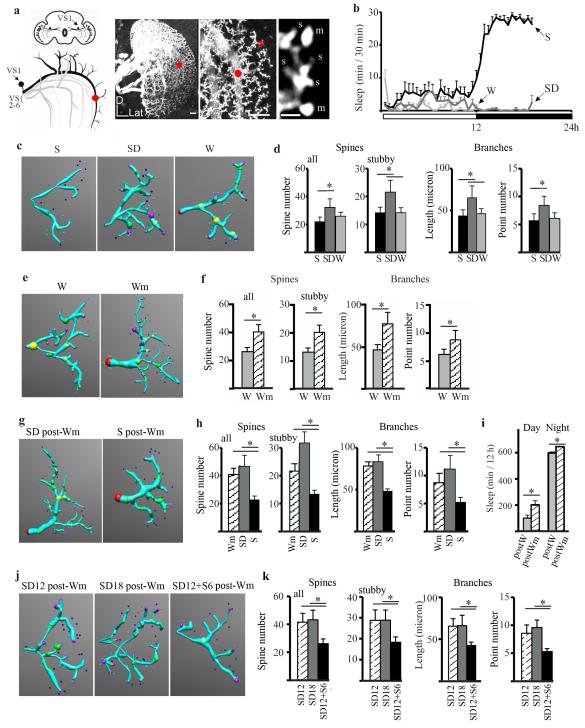
Next, we studied whether postsynaptic morphological changes also occur as a function of sleep and wake. To do so we focused on the first giant tangential neuron of the lobula plate vertical system. This cell (VS1, Fig. 3a) is unambiguously recognizable and its stereotyped dendritic tree shows small actin-enriched protrusions morphologically and functionally similar to mammalian dendritic spines (30). We compared flies that were spontaneously awake during the day, or that slept or were sleep deprived during the first 7h of the night (Fig. 3b). Single VS1 spines were visualized using an antibody against actin-GFP and counted in one easily identifiable branch (Fig. 3a,c, see Supporting Online Material). The total number of spines was similar in spontaneously awake and sleeping flies, but increased after sleep deprivation relative to both conditions, mainly because of an increase in stubby spines (which were the majority of scored spines, Fig. 3d, left). The number of mushroom spines did not change (p = 0.29, Kruskal-Wallis test). The increase in spine number after sleep loss was associated with increased branching and lengthening of the dendritic tree (Fig. 3d), while spine density (N of spine/branch length) was similar in all conditions (p = 0.20, Kruskal-Wallis test). Because sleep deprived female flies had been mostly awake during the previous light period, this suggests that these postsynaptic changes may need sustained periods of wake. Another possibility, not mutually exclusive, is that changes in VS1 spines require a wake condition richer than that experienced by flies spontaneously awake alone inside small glass tubes. Indeed, sleep deprived flies were kept awake using vibratory stimuli, resulting in the flies often falling from the top to the bottom of the tubes. Because visually-driven responses in VS neurons are stronger during flight than during non flight (31), it is possible that these cells were activated by the fall.
Fig. 3. Sleep/wake postsynaptic changes in VS1.
a. Left, frontal section of fly brain with VS neurons (branches and cell body are shown only for VS1, trunks are shown for all other VS neurons). Right, representative two-dimensional maximum intensity projections of three-dimensional image stacks of VS neurons (actin-GFP driven by DB331GAL4) at low and medium resolution (left and middle, bars = 10 μm) and high resolution (right, bar = 1μm). Red dot indicates the beginning of the scored branch. Red asterisk is above the region shown in the right panel. s, stubby, m, mushroom. b. Mean sleep duration in females used for imaging after W, SD, or S. c. Examples of model neurons (reconstructed using NeuroStudio (36) from S, SD, and W flies. Model shows dendritic processes as blue cylinders connecting user defined locations on the branch (large spheres) and spines (smaller spheres). d. Mean number of total and stubby spines, branch length and branch points (n = 10 flies /group). e, f. Examples of reconstructed neurons from flies awake for 12h in single tubes (W, n = 10) or in the fly mall (Wm, n = 12). g, h. Examples of reconstructed neurons from flies allowed to sleep (S postWm, n = 12) or sleep deprived (SD postWm, n = 11) after 12h in the fly mall. (Wm = 12, same flies as in f). i. Sleep time for the 24h following 12h in the fly mall (postWm, n = 76). Control flies (postW, n = 75) spent the same 12h awake in single tubes. j, k. Examples of reconstructed neurons from flies housed for 12h during the light period in the fly mall and then sleep deprived for 12h at night. Flies were then collected immediately (SD12, n = 9), sleep deprived for 6h (SD18, n = 7), or allowed to sleep for 6h (SD12 + S6, n = 10). All panels show ± SEM.
To test whether a rich wake experience that engages the VS circuit is sufficient to affect VS1 synaptic morphology, we housed up to 100 flies inside a large lighted chamber (“fly mall”) for an entire light period (12h). In the mall flies could fly at libitum, explore, and interact with each other. Flies were collected immediately after the mall experience and compared to flies that, as usual, had remained awake during the day in singles tubes. The enriched experience in the mall had profound morphological effects on the VS1 dendritic tree: total branch length increased due to the addition of more branches with spines (mainly stubby), resulting in an overall increase in spine number (Fig. 3e,f).
Once experience-dependent synaptic changes have occurred, are they stable and if not, is sleep necessary to bring synaptic morphology back to pre-enrichment levels? To answer these questions 2 other groups of flies were moved back to single tubes after 12h of mall experience; one group was allowed to sleep for 7h, while the other was kept awake as before using mechanical stimuli. In flies sleep deprived after enrichment branch length, branch points, and spine number were at levels similar to those seen in flies collected immediately after enrichment. In contrast, in flies that were allowed to sleep after the mall experience all morphological parameters reverted to the levels observed in awake flies kept in single tubes (Fig. 3g,h). Moreover, spine density was negatively correlated with the amount of sleep during the last 7h, as well as with the maximal duration of sleep bouts (Fig. S1). In another experiment flies were housed in the mall for 12h during the day, and then moved back to single tubes to record their sleep. During the 24h following the enrichment flies slept more, both during the day and at night (Fig. 3i). Finally, in the last experiment flies were housed in the mall for 12h during the day, moved back to single tubes and sleep deprived all night (12h), and then either collected immediately, allowed to sleep for 6h, or kept awake for 6 more hours. Consistent with the previous experiments, decreases in all morphological parameters were only seen in flies that could sleep (Fig. 3j,k), and spine density was negatively correlated with the amount of sleep during the last 6h, as well as with mean and maximal duration of sleep bouts (Fig. S2).
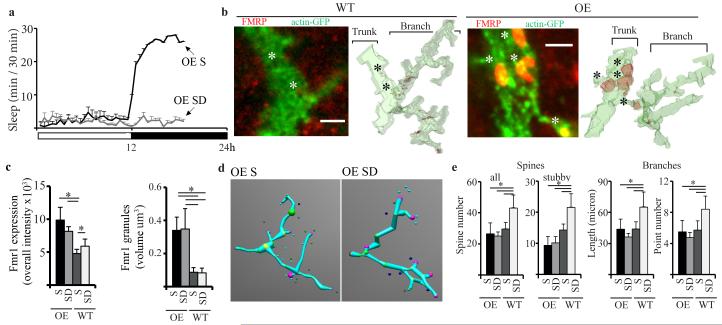
Previous experiments suggest that Fmr1 could mediate at least some of the effects of sleep/wake on synapses. Fmr1 protein product, FMRP, is present in dendritic spines and loss of FMRP in flies is associated with overgrown dendritic trees, larger synaptic boutons (32), and defects in developmental and activity-dependent pruning (22, 24). Importantly, Fmr1 overexpression results in the opposite phenotype, with dendritic and axonal underbranching and loss of synapse differentiation (32). Moreover, Fmr1 expression is reduced by sensory deprivation in flies (24), and increased by sensory stimulation and enrichment in mammals (33-35).
We recently showed that FMRP levels increase in the adult fly brain during wake relative to sleep, independent of time of day or light (29), suggesting that waking experience is sufficient to affect Fmr1 expression even after the end of development. We also showed that Fmr1 overexpression in either the whole brain or in the mushroom bodies is associated with a ~ 30% decrease in sleep duration (29), and we hypothesized that this reduced need for sleep occurs because chronically high Fmr1 levels may allow synaptic pruning to occur at all times, independent of sleep. If so, Fmr1 overexpressing (OE) flies should fail to show increased spine density after prolonged wake. We thus overexpressed Fmr1 specifically in the vertical and horizontal system of the lobula plate. OE flies were collected at night after 7h of either sleep or sleep deprivation (Fig. 4a), and compared to corresponding sleeping and sleep deprived wildtype controls. As expected, Fmr1 expression was concentrated in granules along the VS1 dendritic tree (Fig. 4b) and overall Fmr1 levels were higher in sleeping and sleep deprived OE flies than in their corresponding controls, due to larger Fmr1 granules in OE flies (Fig. 4c). Crucially, in contrast to wildtype controls, OE flies showed no increase in either spine number, branch length or branch points after sleep deprivation relative to sleep (Fig. 4d,e); all these parameters were similar between the 2 experimental groups, and their levels were close to those observed in wildtype flies after sleep (Fig.4 d,e). Finally, OE flies slept less than their wildtype controls during baseline (−9.6%, n of flies, WT = 110, OE = 62, p < 0.05, Mann-Whitney test) and showed a reduced sleep rebound after 12h of sleep deprivation at night (% of sleep recovered, OE 39%, WT = 49%; n of flies, OE = 293, WT = 420, p < 0.05, Mann-Whitney test; both groups lost > 90% sleep during SD). Thus, it seems that Fmr1 overexpression was sufficient to completely abolish the wake-dependent increase in VS1 spine number, while the effects on sleep were small. The latter result is not surprising, because sleep need presumably results from the overall amount of synaptic plasticity occurring during wake in many brain areas, while Fmr1 overexpression was restricted to a few VS neurons.
Fig. 4. Effects of Fmr1 overexpression on synaptic complexity and sleep need.
a. Mean sleep duration in OE female flies used for imaging after S and SD. b. Left, single confocal images of Fmr1 wildtype and OE VS1 neurons stained for FMRP (red) and actin-GFP (green, overlap yellow). Right, surface plots generated by segmenting the three-dimensional confocal stacks. FMRP localizes to granules clearly visible in OE but not in WT VS1 neurons. Bars = 2 μm. c. Mean overall (trunk+branch+spine regions) FMRP intensity (left), and mean granule volume (right) in WT and OE VS1 neurons. d. Examples of reconstructed neurons (as in Fig. 3c). e. Left, total and stubby spine number per branch in OE and WT harvested after S or SD. Right, mean branch length and number of branch points. All panels show ± SEM. (OE S = 9, OE SD = 8, WT S = 11, WT S = 8).
Sleep is perhaps the only major behavior still in search of a function. The results of this study support the hypothesis that plastic processes during wake lead to a net increase in synaptic strength in many brain circuits, and that sleep is required for synaptic renormalization. A wake-related increase in synapse number and strength, if unopposed, would lead to a progressive increase in energy expenditure and saturation of learning. A sleep-dependent synaptic homeostasis may explain why sleep is required to maintain cognitive performance (1). How sleep would bring about a net decrease in synaptic strength remains unknown, but in mammals potential mechanisms favoring synaptic depression during NREM sleep may require the repeated sequences of depolarization/synchronous firing and hyperpolarization/silence at ~1Hz observed in corticothalamic cells, as well as the low levels of neuromodulators such as noradrenaline and of plasticity-related molecules such as BDNF (6). To what extent such mechanisms may also apply to flies remains to be determined.
Supplementary Material
Acknowledgments
The study was supported by NIGMS (R01 GM075315 to CC), NIH Director’s Pioneer award (to GT), Army Research Office (DURIP Award W911NF-08-1-0169 to CC), and Canadian Institutes of Health Research (to DB). We thank Dr. Gaia Tavosanis for technical advice.
Footnotes
Supporting Online Material
Supplementary Figures S1 and S2
Materials and Methods
References and Notes
- 1.Cirelli C, Tononi G. PLoS Biol. 2008 Aug 26;6:e216. doi: 10.1371/journal.pbio.0060216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hendricks JC, et al. Neuron. 2000;25:129. doi: 10.1016/s0896-6273(00)80877-6. [DOI] [PubMed] [Google Scholar]
- 3.Shaw PJ, Cirelli C, Greenspan RJ, Tononi G. Science. 2000;287:1834. doi: 10.1126/science.287.5459.1834. [DOI] [PubMed] [Google Scholar]
- 4.Mignot E. PLoS Biol. 2008 Apr 29;6:e106. doi: 10.1371/journal.pbio.0060106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Diekelmann S, Born J. Nat Rev Neurosci. 2010 Feb;11:114. doi: 10.1038/nrn2762. [DOI] [PubMed] [Google Scholar]
- 6.Tononi G, Cirelli C. Sleep Med Rev. 2006 Feb;10:49. doi: 10.1016/j.smrv.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 7.Feldman DE. Annu Rev Neurosci. 2009;32:33. doi: 10.1146/annurev.neuro.051508.135516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vyazovskiy VV, Cirelli C, Pfister-Genskow M, Faraguna U, Tononi G. Nat Neurosci. 2008 Feb;11:200. doi: 10.1038/nn2035. [DOI] [PubMed] [Google Scholar]
- 9.Liu ZW, Faraguna U, Cirelli C, Tononi G, Gao XB. J Neurosci. 2010 Jun 23;30:8671. doi: 10.1523/JNEUROSCI.1409-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Appelbaum L, et al. Neuron. 2010 Oct 6;68:87. doi: 10.1016/j.neuron.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gilestro GF, Tononi G, Cirelli C. Science. 2009 Apr 3;324:109. doi: 10.1126/science.1166673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Donlea JM, Ramanan N, Shaw PJ. Science. 2009 Apr 3;324:105. doi: 10.1126/science.1166657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holtmaat A, Svoboda K. Nat Rev Neurosci. 2009 Sep;10:647. doi: 10.1038/nrn2699. [DOI] [PubMed] [Google Scholar]
- 14.Bhatt DH, Zhang S, Gan WB. Annu Rev Physiol. 2009;71:261. doi: 10.1146/annurev.physiol.010908.163140. [DOI] [PubMed] [Google Scholar]
- 15.Redondo RL, Morris RG. Nat Rev Neurosci. 2011 Jan;12:17. doi: 10.1038/nrn2963. [DOI] [PubMed] [Google Scholar]
- 16.Harris KM, Stevens JK. J Neurosci. 1989 Aug;9:2982. doi: 10.1523/JNEUROSCI.09-08-02982.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murthy VN, Schikorski T, Stevens CF, Zhu Y. Neuron. 2001 Nov 20;32:673. doi: 10.1016/s0896-6273(01)00500-1. [DOI] [PubMed] [Google Scholar]
- 18.Sigrist SJ, Reiff DF, Thiel PR, Steinert JR, Schuster CM. J Neurosci. 2003 Jul 23;23:6546. doi: 10.1523/JNEUROSCI.23-16-06546.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parisky KM, et al. Neuron. 2008 Nov 26;60:672. doi: 10.1016/j.neuron.2008.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Helfrich-Forster C. Genes Brain Behav. 2005 Mar;4:65. doi: 10.1111/j.1601-183X.2004.00092.x. [DOI] [PubMed] [Google Scholar]
- 21.Zhang YQ, Rodesch CK, Broadie K. Genesis. 2002 Sep-Oct;34:142. doi: 10.1002/gene.10144. [DOI] [PubMed] [Google Scholar]
- 22.Gatto CL, Broadie K. Front Neural Circuits. 2009;3:8. doi: 10.3389/neuro.04.008.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee T, Lee A, Luo L. Development. 1999 Sep;126:4065. doi: 10.1242/dev.126.18.4065. [DOI] [PubMed] [Google Scholar]
- 24.Tessier CR, Broadie K. Front Mol Neurosci. 2009;2:8. doi: 10.3389/neuro.02.008.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Joiner WJ, Crocker A, White BH, Sehgal A. Nature. 2006 Jun 8;441:757. doi: 10.1038/nature04811. [DOI] [PubMed] [Google Scholar]
- 26.Pitman JL, McGill JJ, Keegan KP, Allada R. Nature. 2006 Jun 8;441:753. doi: 10.1038/nature04739. [DOI] [PubMed] [Google Scholar]
- 27.Balling A, Technau GM, Heisenberg M. J Neurogenet. 1987 Apr;4:65. [PubMed] [Google Scholar]
- 28.Ganguly-Fitzgerald I, Donlea J, Shaw PJ. Science. 2006 Sep 22;313:1775. doi: 10.1126/science.1130408. [DOI] [PubMed] [Google Scholar]
- 29.Bushey D, Tononi G, Cirelli C. J Neurosci. 2009 Feb 18;29:1948. doi: 10.1523/JNEUROSCI.4830-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leiss F, et al. Dev Neurobiol. 2009 Mar;69:221. doi: 10.1002/dneu.20699. [DOI] [PubMed] [Google Scholar]
- 31.Maimon G, Straw AD, Dickinson MH. Nat Neurosci. 2010 Mar;13:393. doi: 10.1038/nn.2492. [DOI] [PubMed] [Google Scholar]
- 32.Pan L, Zhang YQ, Woodruff E, Broadie K. Curr Biol. 2004 Oct 26;14:1863. doi: 10.1016/j.cub.2004.09.085. [DOI] [PubMed] [Google Scholar]
- 33.Weiler IJ, et al. Proc Natl Acad Sci U S A. 1997 May 13;94:5395. [Google Scholar]
- 34.Todd PK, Mack KJ. Brain Res Mol Brain Res. 2000 Aug 14;80:17. doi: 10.1016/s0169-328x(00)00098-x. [DOI] [PubMed] [Google Scholar]
- 35.Irwin SA, et al. Neurobiol Learn Mem. 2005 May;83:180. doi: 10.1016/j.nlm.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 36.Rodriguez A, Ehlenberger DB, Dickstein DL, Hof PR, Wearne SL. PLoS ONE. 2008;3:e1997. doi: 10.1371/journal.pone.0001997. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.