Abstract
Most coinhibitory receptors regulate T cell responses through an immunoreceptor tyrosine-based inhibitory motif (ITIM) that recruits protein tyrosine phosphatases (PTP) to mediate inhibitory function. Because syndecan-4 (SD-4), the coinhibitor for DC-HIL, lacks such an ITIM, we posited that SD-4 links with a PTP in an ITIM-independent manner. We showed SD-4 to associate constitutively with the intracellular protein syntenin but not with the receptor-like PTP CD148 on human CD4+ T cells. Binding to DC-HIL allowed SD-4 to assemble with CD148 through the help of syntenin as a bridge, and this process upregulated the PTP activity of CD148, which is required for SD-4 to mediate DC-HIL’s inhibitory function. Using a mouse model, we found SD-4 to reside away from the immunological synapse formed between T cells and APC during activation of T cells. These findings indicate that SD-4 is unique among known T cell coinhibitors, in employing CD148 to inhibit T cell activation at a site distal from the synapse.
Keywords: CD148, Co-inhibitory receptors, DC-HIL, Syndecan-4, T cells
Introduction
T cells are activated through interaction of its TCR with Ag-bound MHC class molecules on APC, and this activation is regulated by coinhibitory signals delivered by binding of paired receptors and ligands on the two cells, respectively. Coinhibitory receptors include cytotoxic T-Lymphocyte antigen-4 (CTLA-4) [1], programmed cell death-1 (PD-1) [2] and B- and T-lymphocyte attenuator (BTLA) [3]. These receptors induce dominant negative signals via phosphorylation of a tyrosine residue in their ITIMs or IT switching motif (ITSM) that can bind various protein tyrosine phosphatases (PTP), including SHP-1 and SHP-2 [3;4], that are responsible ultimately for mediating inhibitory function.
DC-HIL is a transmebrane protein expressed constitutively by different APC [5]. It binds heparan sulfate-like structures on syndecan-4 (SD-4) expressed on activated (but not resting) T cells, and this binding attenuates strongly TCR-induced activation [5–7]. In the mouse model of contact hypersensitivity, SD-4 is expressed primarily by effector/memory (but not recently activated) T cells [8]. Blockade of the endogenous DC-HIL/SD-4 pathway via infusion of relevant soluble receptors or by gene disruption significantly worsens T cell-mediated inflammatory diseases (manuscript submitted). These outcomes support the concept that DC-HIL inhibits pre-primed immune responses via binding to SD-4. The intracellular mechanism responsible for SD-4-mediated T cell inhibition is undefined, and is our focus in this study.
SD-4 does not contain an ITIM and it is not known whether it can associate with PTP. Having shown that binding of SD-4 to DC-HIL induces tyrosine phosphorylation on SD-4 [7], we posited SD-4 to transduce a tyrosine based-signal that links with a PTP. Recently an intracellular PDZ protein syntenin was shown to bind to EFYA amino acids on the cytoplasmic tail-end of SD-4 [9], and an independent study suggested it also associates with GYIA on the tail-end of CD148 [10], which is a membrane-type PTP known to attenuate TCR signaling [11–13]. These reports suggested that ligation of SD-4 to DC-HIL may lead to assembly with CD148 that then allows the latter’s PTP activity to inhibit T cell activation. Indeed, we found SD-4 to associate with CD148 in T cells treated with DC-HIL and this association was required for SD-4 to mediate the inhibitory function of DC-HIL. Moreover, SD-4 resides away from the immunogical synapse (IS) formed in the interface between T cells and APC. These novel features distinguish SD-4 from other coinhibitors (like PD-1 and BTLA) that possess ITIM and associate directly with the IS.
Results and discussion
SD-4 assembles with CD148 on activated T cells following its binding to DC-HIL
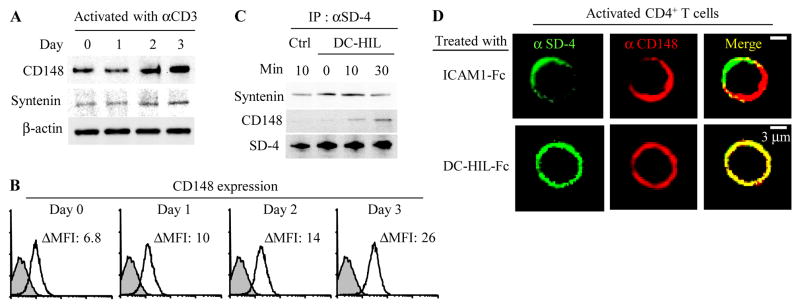
To determine whether SD-4 associates with CD148 through syntenin in activated T cells, we examined protein expression of syntenin and CD148 in human CD4+ T cells before and after stimulation with anti-CD3 Ab. Resting CD4+ T cells expressed syntenin and CD148 protein, respectively, that were upregulated up to 5-fold after anti-CD3 Ab treatment (Fig. 1A). Surface expression of CD148 was expressed and also upregulated in T cells (Fig. 1B). Similarly, PMA/ionomycin upregulated CD148 expression (data not shown). We then questioned whether SD-4 associates physically with the two molecules in CD4+ T cells activated with immobilized DC-HIL-Fc or control Ig. Whole cell extracts were prepared and then immunoprecipitated with anti-SD-4 Ab (Fig. 1C). Precipitates were then assayed for expression of syntenin, CD148, and SD-4. Regardless of the type of treatment, syntenin was co-precipitated constitutively. By contrast, CD148 was not co-precipitated from T cells treated with control Ig or immediately after treatment with DC-HIL-Fc (0 min), but it was precipitated from T cells 10 or 30 min such treatment. The amounts of precipitated SD-4 protein were similar among all samples. We next used confocal analysis to examine localization of SD-4 and CD148 on the surface of activated T cells following binding of DC-HIL (Fig. 1D). In T cells treated with ICAM1-Fc (soluble ICAM-1 receptor binds to T cells), SD-4 did not associate with CD148 (Pearson coefficient r = 0.301, p = 0.0003). By contrast, in T cells treated with DC-HIL-Fc, most SD-4 co-localized with CD148 (r = 0.915, p = 0.0001). These results indicate that SD-4 is linked constitutively to syntenin (but not to CD148), and that binding of DC-HIL to SD-4 in activated T cells allows the latter to associate with CD148.
Figure 1. Binding of SD-4 to DC-HIL leads it to associate with syntenin and CD148 on activated T cells.
(A) At varying times after stimulation of human CD4+ T cells with anti- CD3 Ab, protein expression of CD148, syntenin or β-actin was examined by immunoblotting. Stimulation index for CD148 protein expression is 1.7-fold increase on day 1; 2.3-fold on day 2; and 5.3 on day 3; and for syntenin, 1.2-fold increase on day 1; 1.6 on day 2; and 2.6 on day 3. (B) Kinetics of CD148 surface expression was also examined by flow cytometry. Expression level is expressed as mean fluorescent intensity (MFI) left after subtracting MFI of control staining from that of positive staining (ΔMFI). (C) At varying incubation times after treatment of PMA/ionomycin-activated CD4+ T cells with immobilized control Ig (Ctrl) or DC-HIL-Fc, SD-4 protein was immunoprecipitated from whole T cell extracts and examined by immunoblotting for precipitation of syntenin, CD148 or SD-4. (D) Activated CD4+ T cells were treated with DC-HIL-Fc or ICAM1-Fc, fluorescently labeled with anti-SD-4 (shown in green) or anti-CD148 Ab (in red) and co-localization analyzed using confocal microscopy. Among T cells treated with DC-HIL-Fc, 91% (60 of 66 cells examined) showed co-localization with CD148; and among T cells treated with ICAM-1-Fc, 87% (40 of 46 cells examined) did not co-localize. Data shown are representative of at least 2 experiments.
Engagement of DC-HIL with SD-4 augments PTP activity of CD148
Since CD148 is a membrane-type PTP known to attenuate TCR signaling [12], we thought that SD-4 might upregulate the PTP activity of CD148. We first asked whether DC-HIL-induced assembly of SD-4 and CD148 can trigger tyrosine phosphorylation of CD148 (Fig. 2A). Indeed, such phosphorylation was induced as early as 10 min after treatment with DC-HIL-Fc (but not with control Ig). Since this phosphorylation was known to activate CD148 [14], we determined whether the same assembly upregulates PTP activity (Fig. 2B). Thirty min after treating activated T cells with DC-HIL-Fc or control Ig, whole cell extracts were subjected to the CD148-associated PTP assay. Binding of DC-HIL led to a 4-fold increase in PTP activity of CD148 (vs. control Ig-treated T cells). Thus, ligation of DC-HIL to SD-4 leads to interaction with CD148, most likely through syntenin acting as a bridge, and these processes subsequently upregulated PTP activity.
Figure 2. Ligation of DC-HIL to SD-4 upregulates PTP activity of CD148, which is required for DC-HIL to inhibit T cell activation.
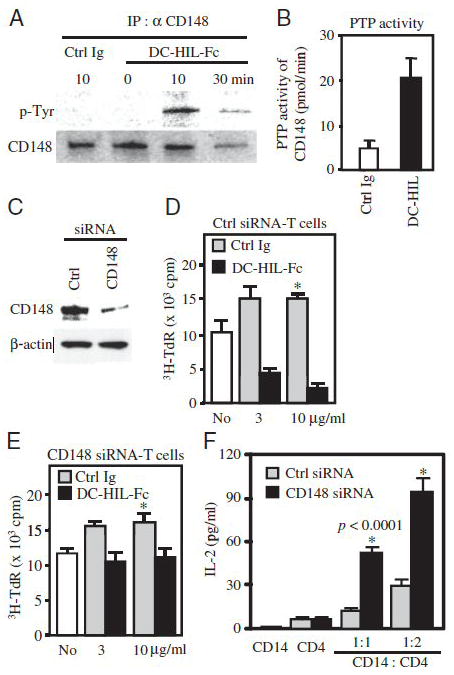
(A) After treatment of human activated CD4+ T cells with immobilized DC-HIL-Fc or control Ig, whole cell extracts were immunoprecipitated with anti-CD148 Ab and assayed by immunoblotting for expression of phosphorylated tyrosine (p-tyrosine) or CD148 using relevant Ab. (B) 30 min after culturing activated CD4+ T cells with immobilized DC-HIL or control Ig, CD148-associated PTP activity was measured (mean ± sd, n=3). (C) At 48 h after transfection of activated CD4+ T cells with control or CD148-specific siRNA, expression of CD148 and β-actin was examined by immunoblotting. (D and E) CD4+ T cells were transfected with control (D) or CD148-siRNA (E) and cultured with immobilized anti-CD3 Ab (1 μg/ml)/DC-HIL-Fc (black-filled bars) or anti-CD3/control Ig (gray bars, 3 or 10 μg/ml). T cells treated with anti-CD3 Ab alone (No) were also examined. T cell activation was measured by 3H-thymidine incorporation (mean ±sd, n=3). Basal levels of proliferation (without stimulation) of T cells transfected with control siRNA (D) and CD148-siRNA (E) were 538 ± 267 and 564 ± 158 cpm, respectively. *p<0.001 between T cells treated with DC-HIL-Fc and control. (F) Transfected CD4+ T cells were also co-cultured with allogenic CD14+ cells at two different cell ratios (1:1 and 1:2) for 6 days and IL-2 production measured. CD14+ cells (CD14) or CD4+ T cells (CD4) alone served as controls. Data shown are representative of at least 2 experiments.
Inhibitory function of DC-HIL requires CD148
We then used siRNA to knockdown CD148 in order to determine whether it is required for DC-HIL to exert inhibitory function. Human CD4+ T cells were transfected with CD148-targeted or control siRNA, cultured with immobilized anti-CD3 Ab for 2 days, and then assayed for protein expression of CD148 by immunoblotting (Fig. 2C). CD148-siRNA blocked CD148 expression in activated T cells by nearly 80% (vs. control siRNA-treated cells). There was no change in β-actin expression between siRNA-treated cells. siRNA-transfected T cells were also cultured with DC-HIL-Fc at different doses plus a constant dose of anti-CD3 Ab or control Ig/anti-CD3 Ab (Fig. 2D and E). Control siRNA-transfected T cells proliferated strongly to anti-CD3 Ab in the presence of control Ig, and this proliferation was blocked markedly by co-treatment with DC-HIL-Fc in a dose-dependent manner. By contrast, T cells knocked-down for CD148 also proliferated to anti-CD3 Ab as strongly as control siRNA-T cells, but it was not blocked (albeit minimally) by DC-HIL-Fc even at a higher dose. Moreover, we examined effect of CD148-knockdown on the allogeneic T cell response, in which siRNA-transfected CD4+ T cells were stimulated by allogeneic CD14+ cells that express DC-HIL (Fig. 2F). T cell activation was measured by IL-2 production. Neither CD14+ cells alone nor transfected CD4+ T cells alone produced significant amounts of IL-2. CD14+ cells stimulated CD148 siRNA-T cells to produce IL-2 at a level 3-fold greater than by control siRNA-T cells, consistent with data produced by blocking of SD-4 function on CD14+ cells with DC-HIL-Fc [7]. We conclude that the inhibitory function of DC-HIL is mediated through CD148.
Originally, the function of CD148 was characterized as a regulator of density-dependent cell growth inhibition and differentiation in non-lymphoid cells [14]. Subsequently, in T cells, CD148 was shown to be a negative regulator [11,12] since its overexpression in Jurkat T cells inhibited the anti-CD3 response, with marked reduction in levels of tyrosine phosphorylation of ZAP-70 and MAPK, both of which are critical signaling molecules for T cell activation. This report using an overexpression system raises the possibility that PTP activity of CD148 in T cells is controlled by expression level alone.
Actually, we showed CD4+ T cells knocked-down for CD148 expression to be no different from control cells (with high CD148 expression) in their ability to proliferate in response to anti-CD3 stimulation. We also showed that binding of DC-HIL induced tyrosine phosphorylation of CD148 and upregulated PTP activity. These data indicate that CD148 PTP activity is positively regulated by binding to ligands, consistent with a recent report that a mAb directed against the extracellular domain inhibited endothelial cell growth by increasing PTP activity [15]. Thus, we think that DC-HIL activates CD148’s PTP by binding to SD-4.
SD-4 resides outside the IS
Since CD148 was reported to reside outside the IS formed in the interface between T cells and DC [16], we examined whether SD-4 co-localize with CD3 following formation of the IS. To exclude DC expression of SD-4 (and PD-1), we produced OVA-specific DO.11.10 T cells transfected with SD-4 (or PD-1) gene tagged with the N-terminal V5 epitope. These cells were co-cultured with DC (pulsed with OVA peptide) for varying times, fixed, and stained fluorescently with anti-V5 and anti-CD3 Ab to locate DC-HIL (or PD-1) and the IS, respectively (Fig. 3). In the absence of DC, both SD-4 and PD-1 co-localized with CD3 on T cells (r = 0.611, p = 0.0001 for SD-4; and r = 0.597, p = 0.0002 for PD-1). By contrast, after contact with DC, they distributed separately from labeled CD3 marking the IS. PD-1 and CD3 migrated to the interface between T cells and DC as early as 10 min after making contact with DC (r = 0.894, p = 0.001 for co-localization between PD-1 and CD3), whereas SD-4 trafficked to an opposite direction (away from the interface) (r = 0.218, p = 0.0005). This opposing movement was not seen in the case of contact with unpulsed DC (Supplemental figure 1). These results indicate that SD-4 differs from PD-1 in location relative to the IS.
Figure 3. SD-4 does not associate directly with CD3-complex in the interface between DC and T cells.
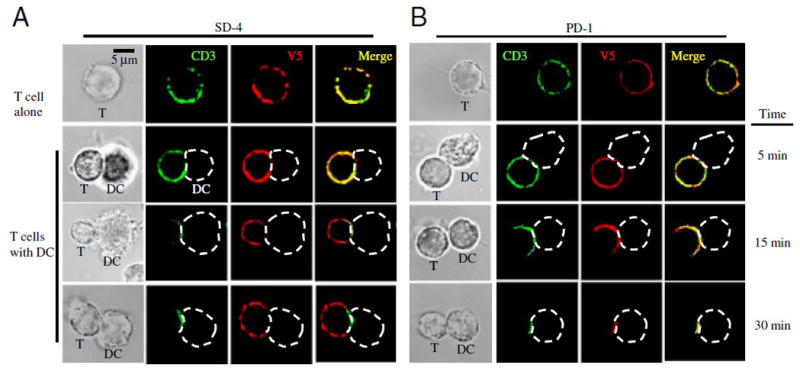
V5-SD-4- or V5-PD-1-transfected DO11.10 T cells were co-cultured without (T cell alone) or with OVA peptide-pulsed BM-DC for indicated times, fixed, and immunostained with anti-CD3 (labeled in green) or anti-V5 Ab (red). Images were taken and analyzed by phase contrast or confocal microscopy. Merged images are shown to document co-localization (scale bar, 5 μm). The location of DC in the DC/T cell conjugate is illustrated by white dashed lines. Among V5-PD-1-T cells (30 min), 93% (40 of 43 cells examined) showed a co-localized pattern with CD3; and among V5-SD-4-T cells, 93% (39 of 42 cells examined) did not co-localize. Second experiment showed similar results.
T cell activation requires contact between T cells and APC to bring TCR and peptide-loaded MHC together to the same complex (termed IS) that permits directed exchange of signals between the 2 cells. Coinhibitory receptors are also incorporated into the IS: In the absence of APC, CTLA-4 localizes mainly in lysosomes of T cells but are transported to the IS immediately after contact with APC [17]. Like CTLA-4, PD-1 and BTLA also accumulate in the IS in contact with their corresponding ligands on APC [18,19], suggestive of an early function by these inhibitory receptors during TCR-signaling. By contrast, SD-4 co-localizes with CD3 on T cells in the absence of APC, and then moves away from the IS after APC/T cell interaction, consistent with a previous report showing exclusion of CD148 from the IS [16]. These events should not imply exclusion of CD148 from regulation of T cell activation, but rather that CD148 may interact with the TCR/CD3 complexes during or after T cell-APC disengagement [16], when it may desphosphorylate activated signaling molecules left after coinhibitors within the IS have accomplished their respective tasks.
Concluding remarks
We conclude that binding of DC-HIL to SD-4 induces tyrosine phosphorylation of the latter’s intracellular domain, leading to assembly of CD148, likely with the help of syntenin acting as a bridge. These events upregulate PTP activity that in turn inhibits TCR-signals distal to the IS. SD-4 employs an ITIM-independent signal pathway to mediate T cell inhibition, which distinguishes it from other co-inhibitory receptors.
Materials and methods
Mice
Female BALB/c (6-wk-old) mice were used according to National Institutes of Health guidelines and approved by The University of Texas Southwestern Medical Center.
Isolation and culture of human T cells
Peripheral blood was obtained from healthy donors according to an Institutional Review Board-approved protocol and following participant consent. CD4+ T cells were isolated from the blood samples using CD4 T cell isolation kit (Miltenyi Biotec). Some cells were activated by 1 μg/ml anti-CD3 Ab (eBiosciences) or PMA (5 ng/ml) plus ionomycin (250 ng/ml) (Sigma-Aldrich).
Generation of transfectants
Mouse SD-4- or PD-1-coding sequence was attached with the N-terminal V5 epitope sequence and inserted into a lentiviral vector plasmid (pHR-SIN-CSGW-GFP) by replacing the GFP gene [20]. These lentiviruses were infected into mouse T cell hybridoma DO11.10 line (from J. Kappler and P. Marrack, National Jewish Medical and Research Center, Denver, CO). Post-infection, V5+ cells were enriched by flow cytometric sorting until they were >90% positive.
Immunoblotting and flow cytometry
At varying time points after activation of human CD4+ T cells with anti-CD3 Ab (1 μg/ml), whole cell extracts were prepared and applied to SDS-PAGE (10 μg/lane) and blotted with mouse anti-CD148, goat anti-syntenin, or rabbit anti-β-actin (each 1 μg/ml) and HRP-secondary Ab. Intensity of Ab-reactive bands was measured by ImageQuant (GE Healthcare) and expression level of CD148 or syntenin is expressed as a value relative to β-actin. These activated cells were also stained with anti-CD148 or control IgG and PE-anti-mouse IgG, followed by flow cytometry.
Immunofluorescent staining
To examine localization of SD-4 and CD148, activated human CD4+ T cells (2 × 105) were treated with immobilized DC-HIL-Fc (the extracellular domain fused to the IgG-Fc) [5] or ICAM1-Fc (20 μg/ml, R & D System) for 30 min, and labeled with anti-SD-4 Ab plus Alexa 488 anti-mouse IgG (2 μg/ml) or with anti-CD148 Ab plus Alexa 546 anti-rabbit IgG (2 μg/ml) (Invitrogen).
To examine localization of SD-4 relative to the IS, DC (1 × 106 cells) were harvested from BM cell (from BALB/c mice) culture of 6 d with 10 ng/ml GM-CSF (PeproTech), pulsed with OVA peptide (2 μg/ml), mixed 1:1 with transfected DO11.10 T cells, and incubated for 5- 30 min at 37°C. The cells were then allowed to settle onto poly-L-lysine-coated slides for 5 min. After fixing with 4% paraformaldehyde, slides were treated with anti-V5 Ab plus Alexa 546 anti-mouse IgG (2 μg/ml) and with anti-CD3 Ab (10 μg/ml) plus Alexa 488 anti-hamster IgG (2μg/ml). Stained cells were examined by confocal microscopy. Co-localization of SD-4 and CD3 (or CD148) was evaluated individually on 42–66 cells using Pearson correlation coefficient r.
Immunoprecipitation
Activated human CD4+ T cells (1 × 107) were cultured in a 100 mm petri dish precoated with DC-HIL-Fc or control Ig (20 μg/ml). At varying time points after incubation at 37°C, whole cell extracts (1 × 107 cells/ml) were prepared and incubated with anti-SD-4 Ab or control IgG (2μg/ml) for 3 h on ice, and then precipated with protein A-agarose (Pierce). After washing, immune complexes were eluted by boiling and subjected to SDS-PAGE/immunoblotting.
To assay phosphorylation of CD148 after treating activated CD4+ T cells with immobilized DC-HIL-Fc or control Ig, whole cell extracts were incubated with anti-CD148 Ab (2 μg/ml) and rabbit anti-mouse IgG (4 μg/ml) at 4°C for 3 h, and then precipitated with protein A-agarose. After washing, immune complexes were separated electrophoretically and then blotted using biotinylated anti-phospho-tyrosine (0.5 μg/ml) (Upstate) and HRP-streptavidin. Blotted membranes were reprobed with rabbit anti-CD148 Ab (1 μg/ml) and HRP-secondary Ab.
PTP assay
Human CD4+ T cells (1 × 106/ml) were cultured for 3 d with PMA/ionomycin, and then incubated with immobilized DC-HIL-Fc or control Ig (20 μg/ml) at 37°C for 30 min. An aliquot (5 × 106 cells equivalent) of whole cell extract was immunoprecipitated with anti-CD148 Ab or control IgG (2 μg) and protein A-agarose. PTP activity was measured using the Malachite Green Phosphatase Assay (Upstate Biotechnlogy Inc). Specific activity of CD148 was expressed as OD620 reading after subtracting OD620 reading of control IgG-precipitate from that of anti-CD148 Ab.
Knockdown of gene expression and T cell activation
CD148-siRNA (Cat#sc-35189) or control siRNA (Cat#sc-37007, Santa Cruz Biotechnology) was treated with 15 μl of Metafetene™ Pro (Biotex) for 30 min and then added to human CD4+ T cells (1 × 106). After incubating at 37°C for 4 h, cells were cultured in 10% FCS-RPMI for 2 d in the presence of PMA/ionomycin. Transfected cells were examined by immunoblotting for expression of CD148 or β-actin. For DC-HIL-mediated suppression, siRNA-transfected cells (2 × 105/well) were cultured for 2 d in ELISA wells precoated with a constant dose of anti-CD3 Ab (1 μg/ml) and varying doses of DC-HIL-Fc or control Ig. T cell activation was measured by 3H-thymidine incorporation (pulsing with 1 μCi/well in the last 20 h of the culture period). For the mixed lymphocyte reaction (MLR), transfected T cells (2 or 4 × 105/well) were also co-cultured with CD14+ cells (2 × 105/well) isolated from peripheral blood of an allogenic donor for 6 d. IL-2 production was measured by ELISA.
Supplementary Material
Acknowledgments
We thank Irene Dougherty and Megan Randolph for technical and secretary assistance. This research was supported by NIH grant (AI064927-05).
Abbreviations used
- BTLA
B- and T-lymphocyte attenuator
- IS
immunological synapse
- PD-1
programmed cell death-1
- PTP
protein tyrosine phosphatase
- SD-4
syndecan-4
Footnotes
Disclosure
The authors have no financial conflict of interest.
References
- 1.Walunas TL, Lenschow DJ, Bakker CY, Linsley PS, Freeman GJ, Green JM, Thompson CB, et al. CTLA-4 can function as a negative regulator of T cell activation. Immunity. 1994;1:405–413. doi: 10.1016/1074-7613(94)90071-x. [DOI] [PubMed] [Google Scholar]
- 2.Okazaki T, Iwai Y, Honjo T. New regulatory co-receptors: inducible co-stimulator PD-1. Curr Opin Immunol. 2002;14:779–782. doi: 10.1016/s0952-7915(02)00398-9. [DOI] [PubMed] [Google Scholar]
- 3.Watanabe N, Gavrieli M, Sedy JR, Yang J, Fallarino F, Loftin SK, Hurchla MA, et al. BTLA is a lymphocyte inhibitory receptor with similarities to CTLA-4 and PD-1. Nat Immunol. 2003;4:670–679. doi: 10.1038/ni944. [DOI] [PubMed] [Google Scholar]
- 4.Chemnitz JM, Parry RV, Nichols KE, June CH, Riley JL. SHP-1 and SHP-2 associate with immunoreceptor tyrosine-based switch motif of programmed death 1 upon primary human T cell stimulation, but only receptor ligation prevents T cell activation. J Immunol. 2004;173:945–954. doi: 10.4049/jimmunol.173.2.945. [DOI] [PubMed] [Google Scholar]
- 5.Chung JS, Sato K, Dougherty I, Cruz PD, Jr, Ariizumi K. DC-HIL is a negative regulator of T lymphocyte activation. Blood. 2007;109:4320–4327. doi: 10.1182/blood-2006-11-053769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chung JS, Dougherty I, Cruz PD, Jr, Ariizumi K. Syndecan-4 mediates the coinhibitory function of DC-HIL on T cell activation. J Immunol. 2007;179:5778–5784. doi: 10.4049/jimmunol.179.9.5778. [DOI] [PubMed] [Google Scholar]
- 7.Chung JS, Bonkobara M, Tomihari M, Cruz PD, Jr, Ariizumi K. The DC-HIL/syndecan-4 pathway inhibits human allogeneic T-cell responses. Eur J Immunol. 2009;39:965–974. doi: 10.1002/eji.200838990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Akiyoshi H, Chung JS, Tomihari M, Cruz PD, Jr, Ariizumi K. Depleting Syndecan-4+ T Lymphocytes Using Toxin-Bearing Dendritic Cell-Associated Heparan Sulfate Proteoglycan-Dependent Integrin Ligand: A New Opportunity for Treating Activated T Cell-Driven Disease. J Immunol. 2010;184:3554–3561. doi: 10.4049/jimmunol.0903250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grootjans JJ, Zimmermann P, Reekmans G, Smets A, Degeest G, Durr J, David G. Syntenin a PDZ protein that binds syndecan cytoplasmic domains. Proc Natl Acad Sci USA. 1997;94:13683–13688. doi: 10.1073/pnas.94.25.13683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iuliano R, Trapasso F, Sama I, Le PI, Martelli ML, Lembo F, Santoro M, et al. Rat protein tyrosine phosphatase eta physically interacts with the PDZ domains of syntenin. FEBS Lett. 2001;500:41–44. doi: 10.1016/s0014-5793(01)02580-7. [DOI] [PubMed] [Google Scholar]
- 11.Baker JE, Majeti R, Tangye SG, Weiss A. Protein tyrosine phosphatase CD148-mediated inhibition of T-cell receptor signal transduction is associated with reduced LAT and phospholipase Cgamma1 phosphorylation. Mol Cell Biol. 2001;21:2393–2403. doi: 10.1128/MCB.21.7.2393-2403.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tangye SG, Wu J, Aversa G, de Vries JE, Lanier LL, Phillips JH. Negative regulation of human T cell activation by the receptor-type protein tyrosine phosphatase CD148. J Immunol. 1998;161:3803–3807. [PubMed] [Google Scholar]
- 13.Tangye SG, Phillips JH, Lanier LL, de Vries JE, Aversa G. CD148: a receptor-type protein tyrosine phosphatase involved in the regulation of human T cell activation. J Immunol. 1998;161:3249–3255. [PubMed] [Google Scholar]
- 14.Holsinger LJ, Ward K, Duffield B, Zachwieja J, Jallal B. The transmembrane receptor protein tyrosine phosphatase DEP1 interacts with p120(ctn) Oncogene. 2002;21:7067–7076. doi: 10.1038/sj.onc.1205858. [DOI] [PubMed] [Google Scholar]
- 15.Takahashi T, Takahashi K, Mernaugh RL, Tsuboi N, Liu H, Daniel TO. A monoclonal antibody against CD148, a receptor-like tyrosine phosphatase, inhibits endothelial-cell growth and angiogenesis. Blood. 2006;108:1234–1242. doi: 10.1182/blood-2005-10-4296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin J, Weiss A. The tyrosine phosphatase CD148 is excluded from the immunologic synapse and down-regulates prolonged T cell signaling. J CellBiol. 2003;162:673–682. doi: 10.1083/jcb.200303040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Darlington PJ, Baroja ML, Chau TA, Siu E, Ling V, Carreno BM, Madrenas J. Surface cytotoxic T lymphocyte-associated antigen 4 partitions within lipid rafts and relocates to the immunological synapse under conditions of inhibition of T cell activation. J Exp Med. 2002;195:1337–1347. doi: 10.1084/jem.20011868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pentcheva-Hoang T, Chen L, Pardoll DM, Allison JP. Programmed death-1 concentration at the immunological synapse is determined by ligand affinity and availability. Proc Natl Acad Sci U S A. 2007;104:17765–17770. doi: 10.1073/pnas.0708767104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Owada T, Watanabe N, Oki M, Oya Y, Saito Y, Saito T, Iwamoto I, et al. Activation-induced accumulation of B and T lymphocyte attenuator at the immunological synapse in CD4+ T cells. J Leukoc Biol. 2009;87:425–432. doi: 10.1189/jlb.0309138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sato K, Yang XL, Yudate T, Chung JS, Wu J, Luby-Phelps K, Kimberly RP, et al. Dectin-2 is a pattern recognition receptor for fungi that couples with the Fc receptor γ chain to induce innate immune responses. J Biol Chem. 2006;281:38854–38866. doi: 10.1074/jbc.M606542200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.