Abstract
Objective
The Child Health Improvement through Computer Automation (CHICA) system is a decision-support and electronic-medical-record system for pediatric health maintenance and disease management. The purpose of this study was to explore CHICA's ability to screen patients for disorders that have validated screening criteria—specifically tuberculosis (TB) and iron-deficiency anemia.
Design
Children between 0 and 11 years were randomized by the CHICA system. In the intervention group, parents were asked about TB and iron-deficiency risk, and physicians received a tailored prompt. In the control group, no screens were performed, and the physician received a generic prompt about these disorders.
Results
1123 participants were randomized to the control group and 1116 participants to the intervention group. Significantly more people reported positive risk factors for iron-deficiency anemia in the intervention group (17.5% vs 3.1%, OR 6.6, 95% CI 4.5 to 9.5). In general, far fewer parents reported risk factors for TB than for iron-deficiency anemia. Again, there were significantly higher detection rates of positive risk factors in the intervention group (1.8% vs 0.8%, OR 2.3, 95% CI 1.0 to 5.0).
Limitations
It is possible that there may be more positive screens without improving outcomes. However, the guidelines are based on studies that have evaluated the questions the authors used as sensitive and specific, and there is no reason to believe that parents misunderstood them.
Conclusions
Many screening tests are risk-based, not universal, leaving physicians to determine who should have a further workup. This can be a time-consuming process. The authors demonstrated that the CHICA system performs well in assessing risk automatically for TB and iron-deficiency anemia.
Keywords: Tuberculosis, iron-deficiency anemia, CHICA, screening, computer decision-support system, visualization of data and knowledge, knowledge representations, communications, networking methods, mobility, uncertain reasoning and decision theory, languages, computational methods, advanced algorithms, methods for integration of information from disparate sources, distributed systems, agents, software engineering: architecture, data models, automated learning, developing/using clinical decision support (other than diagnostic) and guideline systems, discovery, text and data mining methods
Background
Well-child care is a service fundamental to pediatrics, representing nearly half of healthcare visits made by children in the USA.1 These visits are designed to encompass a variety of health-promoting and disease-preventing services, including growth and development assessments, screens for subtle or asymptomatic disease, and delivery of services such as immunizations.2 Because preventive service delivery to children is neither procedure-oriented nor hospital-based, the relative cost per visit is small compared to other medical problems encountered in the healthcare system. However, the 19.8 million children under the age of five in the USA should have each received 13 well-child visits by the time they reach school age.2 3 Therefore, the magnitude and potential impact of childhood preventive services delivery is large.
The importance of preventive care has been widely emphasized, and a growing body of preventive services is routinely proposed by various expert panels and professional organizations.2 4–6 They range all the way from newborn metabolic and hearing screening to counseling 5 year olds about head-injury prevention through bike-helmet use. As these recommendations proliferate, a growing body of research continues to document the benefits of these preventive services on such diverse topics as injury prevention,7 8 childhood tuberculosis,9 childhood literacy,10 11 healthy sleep habits,12 13 and infant iron-deficiency.14 15
Despite the widely accepted importance of preventive services, mounting evidence suggests that the provision of these services is suboptimal, even for services for which there is ample evidence and broad support. Among a Medicaid population for example, only one-fifth of children received preventive and developmental services that met a basic threshold of quality.16 A national survey of parents found that more than 94% of parents reported one or more unmet needs for parenting guidance, education, or screening by pediatric clinicians of recommended services.17 Overall, rates of delivery for basic preventive services are typically <50%.17 Some would argue that these figures are generous, as studies indicate that most clinicians overestimate the level of preventive care they provide, especially screening tests that they give their patients.18 19
When physicians are asked to describe the challenges in providing preventive services, they are quick to provide a convincing list of barriers commonly faced in pediatric settings.20 In an attempt to provide a framework which categorizes these barriers into groups, Cabana et al performed a meta-analysis on 76 articles which described one or more problems physicians faced while adhering to care guidelines.21 Three barriers most consistently identified in this study relate to time constraints, problems inherent in case finding and prioritization, and a lack of clinician self-efficacy.
Much of the time required to provide preventive care services is spent attempting to assess risk, as care guidelines are often lengthy and complex, and may not apply to the individual circumstances of each patient. According to the US Preventive Services Task Force, when care givers place the greatest emphasis on understanding each patient's risk profile, they can significantly reduce the number of unnecessary interventions.4 As a result, many guidelines attempt to define an explicit, focused list of historical and demographic risk factors to facilitate rapid recognition of risk. To further reduce provider work burden, many of these risk factors are bundled into standardized screening instruments which can be completed by patients or their families.22–25 However, providers are still left to determine who receives each of these instruments, and must also score and/or interpret the results.
A great deal of research indicates that clinical reminders provided to physicians and other care givers at the point of patient care are superior to other methods of affecting clinical practice. However, reminders are typically delivered today by computerized physician order-entry systems or inpatient noting systems.26–31 Unfortunately, for many outpatient preventive services, a reminder at the time of note writing or order entry is often too late, as these events frequently take place after the physician has completed the visit. ‘Just-in-time’ information delivery requires that a reminder be delivered at the time the physician is making a decision, and this is often while they are conversing with a patient. Computers within exam rooms may not be a satisfactory solution, as they can be expensive and susceptible to damage by curious pediatric patients. Computers can also slow the patient encounter and negatively impact the content of physician–patient communications.32 In fact, at our institution, which houses one of the most successful electronic medical record systems in the world, pediatricians have long been resistant to the introduction of computers in their clinics for these reasons.
Child Health Improvement through Computer Automation (CHICA) system
CHICA is a decision-support and electronic medical record system for pediatric health maintenance and disease management. CHICA also serves as a front end for data exchange with the Regenstrief Medical Record System.33 It can also work as a standalone application or be coupled with any other clinical information system. CHICA's primary user interface consists of two sheets of paper that collect handwritten responses to dynamically generated questions and clinical reminders while easily integrating into clinic workflow.34 35 These forms are created by CHICA and tailored to the individual patient. The forms, called adaptive turnaround documents (ATDs), are scanned, and data are read by optical character recognition and optical mark recognition software. CHICA uses a library of Arden Syntax36 rules that utilize data from the RMRS and CHICA record systems to determine what information should be printed on each ATD. CHICA also uses a global prioritization scheme to determine which information is most relevant for inclusion on the printouts.37 This effectively constrains the number of topics that CHICA recommends be addressed to a feasible number for any given patient encounter.
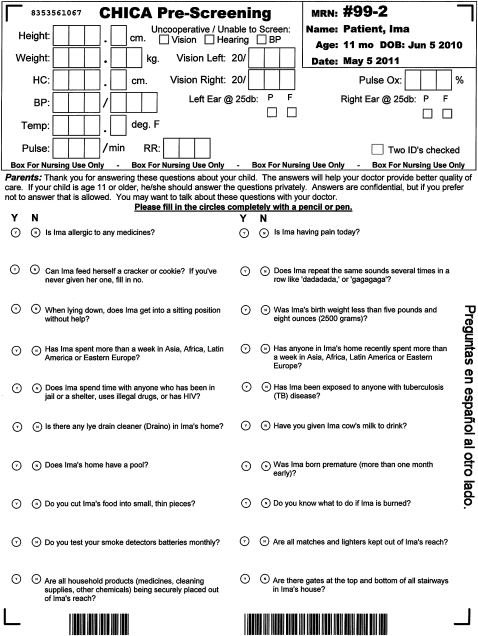
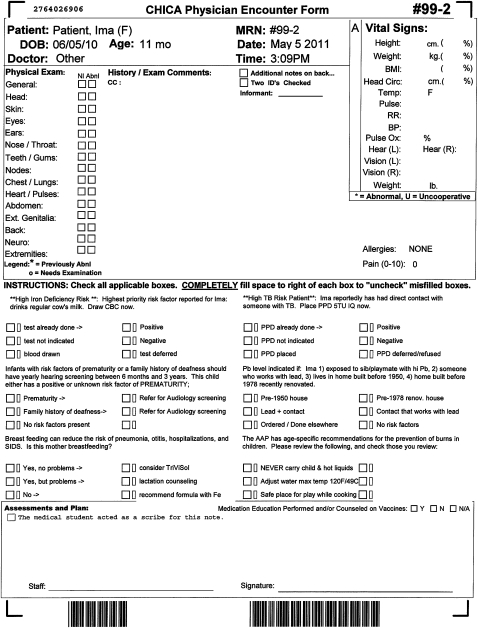
When a patient checks into the clinic, our clinic appointment system sends a registration HL7 message to alert CHICA to begin generating the first of two ATDs. This first form, called the ‘prescreening’ form (PSF), captures data from both clinic staff and parents before the patient is seen by a physician. This form has a section for nurses to enter morphometrics, vital signs, and other data. In addition, the PSF contains 20 questions selected by CHICA to ask a child's family (figure 1). These 20 questions are typically answered by the patient's parent while in the waiting room of the clinic. Data obtained through this form are merged with previously existing data and analyzed to generate a second ATD, known as the ‘provider worksheet’ (PWS). This PWS is tailored to the individual patient and contains up to six reminders which are based on the merged data of the PSF and existing electronic medical records (figure 2). Each reminder contains a ‘stem,’ introducing the reason for the prompt and between one and six ‘leaves’ which consist of check boxes allowing the physician to document their responses to the prompt. Some answers to questions on the PSF might also result in CHICA printing a ‘just in time’ handout (JIT). These JITs can provide additional advice to the physician (eg, a depression screening tool) or to the patient (eg, an asthma action plan or educational handout). The JIT prints at the same time as the PWS and is placed on the chart for the physician's use during the encounter.
Figure 1.
Child Health Improvement through Computer Automation (CHICA) prescreening form (tuberculosis screening questions have been highlighted).
Figure 2.
Child Health Improvement through Computer Automation (CHICA) physician worksheet.
Objective
The purpose of this study was to explore CHICA's ability to implement screening guidelines. We specifically wanted to see how CHICA would affect the screening of patients for the following two conditions:
-
Iron-deficiency anemia38
–The American Academy of Pediatrics recommends blood hemoglobin or hematocrit tests for all 9–15-month-old children who demonstrate nutritional risk factors such as drinking cow's milk before 1 year of age, consuming a low-iron diet, drinking low-iron formula, or being born prematurely.39
-
Tuberculosis40
–Guidelines also exist for the placement of purified protein derivative tests for children. Risk factors indicating that a child should be screened include the following: exposure to someone with tuberculosis (TB), travel to a high risk country, someone else in the home having traveled to a high risk country, or exposure to anyone who is themselves at high risk for TB.41
Methods
Our first step was to modify the CHICA system in order to add in rules for both iron-deficiency anemia and tuberculosis screening. A specific rule set was created for the CHICA system to improve screening for both iron-deficiency anemia and tuberculosis. We adapted questions from American Academy of Pediatrics guidelines for the PSF that asked parents about potential risk factors for these conditions (table 1). These questions were coded in such a way that they would appear a specified number of times over a certain age period (table 1). We also created three PWS prompts for each condition. The first was generic, and would appear if no information was available from the PSF or if no PSF questions were asked of the parents. The generic prompt asked the physician to assess the child's risk factors for iron-deficiency or TB. The physician could indicate identified risk(s) by checking the corresponding box(es) on the prompt. The second was tailored, and would fire if a risk factor was identified on the PSF. The ‘stem’ of the prompt would include the risk factor identified on the PSF. The third was a follow-up prompt and would remind or instruct the physician to follow-up on screening steps taken in previous visits.
Table 1.
Prescreening form prompting for intervention groups
| Condition | Prompt text | Age range | How often |
| Iron-deficiency anemia | Have you given Jenny cow's milk to drink? | 0–12 months | Once every 60 days |
| Iron-deficiency anemia | Are you feeding Jenny low-iron formula? | 0–12 months | Once every 30 days |
| Iron-deficiency anemia | Does Jenny get fed two or more servings of the following foods daily: iron-fortified cereals, fruits, vegetables, juices, or puréed meats? | 6–15 months | Once every 60 days |
| Iron-deficiency anemia | Was Jenny's birth weight less than five pounds and eight ounces (2500 g)? | 0–2 years | Once |
| Tuberculosis | Has Jenny spent more than a week in Asia, Africa, Latin America, or Eastern Europe? | 11 months–11 years | Once a year |
| Tuberculosis | Has Jenny been exposed to anyone with tuberculosis disease? | 11 months–11 years | Once a year |
| Tuberculosis | Has anyone in Jenny's home recently spent more than a week in Asia, Africa, Latin America, or Eastern Europe? | 11 months–11 years | Once a year |
| Tuberculosis | Does Jenny spend time with anyone who has been in jail or a shelter, uses illegal drugs, or has HIV? | 11 months–11 years | Once a year |
Randomization
All children age 0–11 years seen in our main primary care clinic between February 1, 2008 and July 15, 2009 were randomized into an intervention and a control group for each of our two modules. Randomization was done automatically by the CHICA system at the time of patient registration. Each year, this clinic sees about 1200 children in that age group. In the control group, parents were not asked screening questions about TB or iron-deficiency risk in the PSF, and the physician received only the generic PWS prompt, as no questions ever appeared on the PSF. Therefore, any screening that occurred would have to be initiated by the physician as part of routine care.
In the intervention group, parents were asked about TB and iron-deficiency risk on the PSF. If risk factors were identified, the physician received the tailored prompt. If the risk factors were absent, no PWS prompt was printed for the physician. If PSF questions were left unanswered, the physician received the generic prompt.
As patients are already familiar with the use of CHICA in our clinic system, no additional instructions were given to patients in the control or intervention group as to how to fill out the forms; nor were physicians oriented to any new prompts for either TB or iron-deficiency anemia. In general, we have found that patients are extremely thorough in completing the PSF questions.42
Analysis
Differences in measurable covariates were independently analyzed using χ2 analysis. If differences between groups were significant, we included them in further multivariable regressions. We hypothesized that the use of the PSF would significantly improve screening practices for both TB and iron-deficiency. We used logistic regression to test these hypotheses. All calculations were performed using the STATA 9.0 statistical package. All methods for this study were approved by the Indiana University School of Medicine Institutional Review Board.
Results
Over the course of this study, 2239 patients were randomized. The control group had 1123 participants, and the intervention group had 1116 participants. Patients enrolled were about equally split between males and females, and were mostly Hispanic (45%) or African–American (36%). Summary statistics on the population are listed in table 2.
Table 2.
Summary statistics study participants
| Control N (%) | Intervention N (%) | Total | p Value | |
| Sex | 0.96 | |||
| Males | 581 (52) | 578 (52) | 1159 | |
| Females | 542 (48) | 538 (48) | 1080 | |
| Race | 0.22 | |||
| American Indian | 0 (0) | 2 (0) | 2 | |
| Asian Pacific | 27 (2) | 21 (2) | 48 | |
| Black | 397 (36) | 394 (36) | 791 | |
| Hispanic | 510 (46) | 497 (45) | 1007 | |
| White | 152 (14) | 182 (16) | 334 | |
| Patients with at least this many visits | 0.94 | |||
| 1 | 1123 (100) | 1116 (100) | 2239 | |
| 2 | 943 (84) | 955 (86) | 1898 | |
| 3 | 798 (71) | 836 (75) | 1634 | |
| 4 | 630 (56) | 670 (60) | 1300 | |
| 5 | 467 (42) | 487 (44) | 954 | |
| 6 | 296 (26) | 321 (29) | 617 | |
| >6 | 177 (16) | 178 (16) | 355 |
Iron-deficiency anemia
Differences in rates of detection of risk factors for iron-deficiency anemia are listed in table 3. Although slightly more people reported early cow's milk use in the control group (1.1% vs 1.5%), more people in the intervention group reported a low birth weight (3.0% vs 1.1%), low-iron diet (3.9% vs 1.0%), and the use of low-iron formula (13.4% vs 1.2%). Overall, significantly more people reported positive risk factors in the intervention group than in the control group (17.5% vs 3.1%, OR 6.6, 95% CI 4.5 to 9.5). Three children in the intervention group (0.3%) had positive CBCs vs none in the control group, although this difference was not statistically significant (p=0.08).
Table 3.
Differences in positive screening rates for iron-deficiency-anemia risk factors
| No | Percentage | p Value | |
| Early cow's milk | <0.36 | ||
| Control | 17 | 1.5 | |
| Intervention | 12 | 1.1 | |
| Low birth weight | <0.007 | ||
| Control | 14 | 1.3 | |
| Intervention | 33 | 3.0 | |
| Low-iron diet | <0.001 | ||
| Control | 11 | 1.0 | |
| Intervention | 43 | 3.9 | |
| Low-iron formula | <0.001 | ||
| Control | 13 | 1.2 | |
| Intervention | 150 | 13.4 | |
| Any risk factor | <0.001 | ||
| Control | 35 | 3.1 | |
| Intervention | 195 | 17.5 |
Tuberculosis
Differences for screening rates for tuberculosis are listed in table 4. In general, far fewer parents reported risk factors for TB than for iron-deficiency anemia. Although slightly more cases of exposure to someone with tuberculosis were detected in the control group (0.2% vs 0.5%), more cases of travel to a high-risk country were found in the intervention group (0.4% vs 0.3%), as were cases of contact with someone who had traveled to a high-risk country (0.8% vs 0.2%), and living with someone who is at high risk (0.9% vs 0.6%). Again, there were significantly higher detection rates of positive risk factors in the intervention group than in the control group (1.8% vs 0.8%, OR 2.3, 95% CI 1.0 to 5.0). Two children in the intervention group (0.2%) had positive purified protein derivatives versus none in the control group, although this difference was not statistically significant (p=0.16).
Table 4.
Differences in positive screening rates for tuberculosis risk factors
| No | Percentage | p Value | |
| High-risk travel | <0.71 | ||
| Control | 3 | 0.3 | |
| Intervention | 4 | 0.4 | |
| Exposure to tuberculosis | <0.18 | ||
| Control | 6 | 0.5 | |
| Intervention | 2 | 0.2 | |
| Contact with high-risk traveler | <0.06 | ||
| Control | 2 | 0.2 | |
| Intervention | 9 | 0.8 | |
| High-risk contact | <0.47 | ||
| Control | 7 | 0.6 | |
| Intervention | 10 | 0.9 | |
| Any risk factor | <0.05 | ||
| Control | 9 | 0.8 | |
| Intervention | 20 | 1.8 |
Conclusions
With so many recommendations and guidelines available, it is impossible for a clinician to adhere to them all. This is especially true with respect to screening. Many screening tests are risk-based and not meant to be universally performed. Instead, physicians are left to determine who should or should not have a further workup or procedure. This can be a time-consuming process and therefore is often neglected. In this study, we demonstrated that the CHICA system performs well in automatically assessing risk directly from parents and patients to determine who should receive risk-based screening for tuberculosis and iron-deficiency anemia.
In previous studies, we showed that the CHICA PSF is accepted well by parents and integrates easily into the primary care workflow.35 In this study, we add to that knowledge by showing that questions aimed at screening for conditions where targeted screening is needed can enhance the process by collecting data directly from parents. Many more patients were picked up in the intervention than the control group for both a more common condition, iron-deficiency anemia, and a rarer one, tuberculosis. When risks are automatically identified, clinicians can then be alerted to the need for a test or procedure in a manner that will increase compliance with recommendations and guidelines.
As with any research, this study has limitations that warrant further consideration. It is possible that patients who screened positive by CHICA might still not warrant further workup. In other words, it is possible that we may have more positive screens without improving outcomes. Assessing these outcomes is beyond the scope of this study. However, the guidelines are based on studies that have evaluated the questions we used as sensitive and specific, and there is no reason to believe that parents misunderstood them. Further, there is no guarantee that screening results end in treatment or cure, which is the ultimate goal. However, screening properly, and in a non-intrusive fashion, is a necessary first step, and this study confirms that the CHICA system performs that task well. A factorial design, or a study that also had a control group where the CHICA system was not utilized at all, may have been a more powerful design. However, feasibility issues concerning randomization at the time the study began prevented us from studying the interventions in this way.
Integrating decision support into clinical care, at the point of care, is a difficult process. The CHICA system is built on an open-source platform, using national health-information technology standards,43 and has been studied and adjusted at various points along its development. At this stage, we have confirmed the success of the PSF, filled out by parents, in screening patients for two conditions. We have added many more conditions and surveillance modules to our system over time, and we believe that CHICA will continue to screen patients well. Moreover, we are engaged in studies of the physician worksheet to determine if it can close the loop properly, by improving the steps clinicians must take to provide care that meets recommendations.
Footnotes
Competing interests: None.
Ethics approval: Indiana University Institutional Review Board.
Provenance and peer review:Not commissioned; externally peer reviewed.
References
- 1.Woodwell DA, Cherry DK. National Ambulatory Medical Care Survey: 2002 summary. Adv Data 2004;346:1–44 [PubMed] [Google Scholar]
- 2.Anonymous. Recommendations for preventive pediatric health care. Committee on Practice and Ambulatory Medicine. Pediatrics 1995;96:373–4 [PubMed] [Google Scholar]
- 3.US Department of Health and Human Services, Health Resources and Services Administration, Maternal and Child Health Bureau Child Health USA 2003. Rockville, Maryland: US Department of Health and Human Services, 2003 [Google Scholar]
- 4.United States. Agency for Healthcare Research and Quality, US Preventive Services Task Force Guide to Clinical Preventive Services: Recommendations of the US Preventive Services Task Force. Washington, DC: Agency for Healthcare Research and Quality, 2005 [Google Scholar]
- 5.United States. Public Health Service Healthy People 2000: National Health Promotion and Disease Prevention Objectives: Full Report, with Commentary. Washington, DC: US Department of Health and Human Services For sale by Supt. of Docs., USG.P.O., 1991 [Google Scholar]
- 6.Zaza S, Briss PA, Harris KW, Task Force on Community Preventive Services (US) The Guide to Community Preventive Services: What Works to Promote Health? Oxford: Oxford University Press, 2005 [Google Scholar]
- 7.Bass JL, Christoffel KK, Widome M, et al. Childhood injury prevention counseling in primary care settings: a critical review of the literature. Pediatrics 1993;92:544–50 [PubMed] [Google Scholar]
- 8.Nansel TR, Weaver N, Donlin M, et al. Baby, Be Safe: the effect of tailored communications for pediatric injury prevention provided in a primary care setting. Patient Educ Couns 2002;46:175–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.CDC Reported tuberculosis in the United States, 2009. Atlanta, GA: US Department of Health and Human Services, October 2010 [Google Scholar]
- 10.Sharif I, Rieber S, Ozuah PO. Exposure to Reach Out and Read and vocabulary outcomes in inner city preschoolers. J Natl Med Assoc 2002;94:171–7 [PMC free article] [PubMed] [Google Scholar]
- 11.Theriot JA, Franco SM, Sisson BA, et al. The impact of early literacy guidance on language skills of 3-year-olds. Clin Pediatr (Phila) 2003;42:165–72 [DOI] [PubMed] [Google Scholar]
- 12.Pinilla T, Birch LL. Help me make it through the night: behavioral entrainment of breast-fed infants' sleep patterns. Pediatrics 1993;91:436–44 [PubMed] [Google Scholar]
- 13.Wolfson A, Lacks P, Futterman A. Effects of parent training on infant sleeping patterns, parents' stress, and perceived parental competence. J Consult Clin Psychol 1992;60:41–8 [DOI] [PubMed] [Google Scholar]
- 14.Taylor H, Leitman R. Most Doctors Report Fear of Malpractice Liability Has Harmed Their Ability to Provide Quality Care. Health Care News 2002;2:1–4 http://www.harrisinteractive.com (accessed 31 Aug 2007). [Google Scholar]
- 15.Bogen DL, Whitaker RC. Anemia screening in the Special Supplemental Nutrition Program for Women, Infants, and Children: time for change? Arch Pediatr Adolesc Med 2002;156:969–70 [DOI] [PubMed] [Google Scholar]
- 16.Bethell C, Commonwealth Fund., Program on Child Development and Pediatric Care (Commonwealth Fund) Partnering with Parents to Promote the Healthy Development of Young Children Enrolled in Medicaid: Results from a Survey Assessing the Quality of Preventive and Developmental Services for Young Children Enrolled in Medicaid in Three States. New York: Commonwealth Fund, 2002 [Google Scholar]
- 17.Bethell C, Reuland CH, Halfon N, et al. Measuring the quality of preventive and developmental services for young children: national estimates and patterns of clinicians' performance. Pediatrics 2004;113(6 Suppl):1973–83 [PubMed] [Google Scholar]
- 18.Dickey LL, Griffith HM, Kamerow DB. Put prevention into practice: implementing preventive care. US Department of Health and Human Services. J Am Acad Nurse Pract 1994;6:257–66 [DOI] [PubMed] [Google Scholar]
- 19.Mangione-Smith R, DeCristofaro AH, Setodji CM, et al. The quality of ambulatory care delivered to children in the United States. N Engl J Med 2007;357:1515–23 [DOI] [PubMed] [Google Scholar]
- 20.American Academy of Pediatrics Pediatricians' Provision of Preventive Care and Use of Health Supervision Guidelies. Periodic Survey of Fellows No. 56. http://www.aap.org/research/periodicsurvey/ps56exs.htm (accessed 29 Sep 2011). [Google Scholar]
- 21.Cabana MD, Rand CS, Powe NR, et al. Why don't physicians follow clinical practice guidelines? A framework for improvement. JAMA 1999;282:1458–65 [DOI] [PubMed] [Google Scholar]
- 22.Brody DS, Hahn SR, Spitzer RL, et al. Identifying patients with depression in the primary care setting: a more efficient method. Arch Intern Med 1998;158:2469–75 [DOI] [PubMed] [Google Scholar]
- 23.Spitzer RL, Kroenke K, Williams JB. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. Primary Care Evaluation of Mental Disorders. Patient Health Questionnaire. JAMA 1999;282:1737–44 [DOI] [PubMed] [Google Scholar]
- 24.Squires J, Bricker D, Potter L. Revision of a parent-completed development screening tool: Ages and Stages Questionnaires. J Pediatr Psychol 1997;22:313–28 [DOI] [PubMed] [Google Scholar]
- 25.Wolraich ML, Lambert W, Doffing MA, et al. Psychometric properties of the Vanderbilt ADHD diagnostic parent rating scale in a referred population. J Pediatr Psychol 2003;28:559–67 [DOI] [PubMed] [Google Scholar]
- 26.Dexter PR, Perkins S, Overhage JM, et al. A computerized reminder system to increase the use of preventive care for hospitalized patients. N Engl J Med 2001;345:965–70 [DOI] [PubMed] [Google Scholar]
- 27.McDonald CJ. Protocol-based computer reminders, the quality of care and the non-perfectability of man. N Engl J Med 1976;295:1351–5 [DOI] [PubMed] [Google Scholar]
- 28.McDonald CJ, Hui SL, Smith DM, et al. Reminders to physicians from an introspective computer medical record. A two-year randomized trial. Ann Intern Med 1984;100:130–8 [DOI] [PubMed] [Google Scholar]
- 29.McDonald CJ, Hui SL, Tierney WM. Effects of computer reminders for influenza vaccination on morbidity during influenza epidemics. MD Comput 1992;9:304–12 [PubMed] [Google Scholar]
- 30.Overhage JM, Tierney WM, Zhou XH, et al. A randomized trial of ‘corollary orders’ to prevent errors of omission. J Am Med Inform Assoc 1997;4:364–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tierney WM, Hui SL, McDonald CJ. Delayed feedback of physician performance versus immediate reminders to perform preventive care. Effects on physician compliance. Med Care 1986;24:659–66 [DOI] [PubMed] [Google Scholar]
- 32.Sullivan F, Mitchell E. Has general practitioner computing made a difference to patient care? A systematic review of published reports. BMJ 1995;311:848–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McDonald CJ, Overhage JM, Tierney WM, et al. The Regenstrief Medical Record System: a quarter century experience. Int J Med Inform 1999;54:225–53 [DOI] [PubMed] [Google Scholar]
- 34.Biondich PG, Downs SM, Anand V, et al. Automating the recognition and prioritization of needed preventive services: early results from the CHICA system. AMIA Annu Symp Proc 2005:51–5 [PMC free article] [PubMed] [Google Scholar]
- 35.Biondich PG, Overhage JM, Dexter PR, et al. A modern optical character recognition system in a real world clinical setting: some accuracy and feasibility observations. Proc AMIA Symp 2002:56–60 [PMC free article] [PubMed] [Google Scholar]
- 36.Jenders RA, Hripcsak G, Sideli RV, et al. Medical decision support: experience with implementing the Arden Syntax at the Columbia-Presbyterian Medical Center. Proc Annu Symp Comput Appl Med Care 1995:169–73 [PMC free article] [PubMed] [Google Scholar]
- 37.Downs SM, Uner H. Expected value prioritization of prompts and reminders. Proc AMIA Symp 2002:215–19 [PMC free article] [PubMed] [Google Scholar]
- 38.Biondich PG, Downs SM, Carroll AE, et al. Shortcomings in infant iron deficiency screening methods. Pediatrics 2006;117:290–4 [DOI] [PubMed] [Google Scholar]
- 39.Green M, Palfrey J, National Center for Education in Maternal and Child Health (US) Bright Futures: Guidelines for Health Supervision of Infants, Children, and Adolescents. 2nd edn. Arlington, VA: National Center for Education in Maternal and Child Health, 2000 [Google Scholar]
- 40.Lazar CM, Sosa L, Lobato MN. Practices and policies of providers testing school-aged children for tuberculosis, Connecticut, 2008. J Community Health 2010;35:495–9 [DOI] [PubMed] [Google Scholar]
- 41.Anonymous. American Academy of Pediatrics. Committee on Infectious Diseases. Red Book: Report of the Committee on Infectious Diseases. Elk Grove Village, IL: American Academy of Pediatrics, 1994:v [Google Scholar]
- 42.Biondich PG, Anand V, Downs SM, et al. Using adaptive turnaround documents to electronically acquire structured data in clinical settings. AMIA Annu Symp Proc 2003:86–90 [PMC free article] [PubMed] [Google Scholar]
- 43.Blumenthal D. Launching HITECH. N Engl J Med 2010;362:382–5 [DOI] [PubMed] [Google Scholar]