Abstract
Introduction
Chronic activation of the β1-adrenergic receptor (β1-AR) signaling can have deleterious effects on the heart, and animal models over-expressing β1-ARs develop a dilated cardiomyopathy and heart failure. In the classical β-AR pathway, receptor occupancy by an agonist results in increased cyclic AMP (cAMP) levels and activation of protein kinase A (PKA). However, the role of PKA dependent signaling in the development and progression of cardiomyopathies and heart failure is controversial because β-AR signal transduction is generally desensitized in the failing heart, and PKA activity is not increased.
Methods and Results
Neonatal Rat Ventricular Myocytes (NRVMs) were acutely (15 minutes) or chronically (48 hours) treated with isoproterenol, and phosphorylation of protein kinase D (PKD) and histone deacetylase 5 (HDAC5) was measured. Acute β1-AR stimulation or expression of constitutively active (CA)-PKA reduced α1-adrenergic-mediated phosphorylation of HDAC5 and PKD by activation of a phosphatase. Over-expression of CA-PKA also reduced α1-adrenergic-mediated increased expression of contractile protein fetal isoforms and promoted repression of adult isoforms, but had no effect on α1-adrenergic-mediated cellular hypertrophy.
Conclusions
These data indicate that the PKA dependent arm of β-AR signaling can be anti-hypertrophic and presumably beneficial, through dephosphorylation of PKD and HDAC5 and reduction of hypertrophic fetal isoform gene expression.
Keywords: Cardiac hypertrophy, fetal gene program, phosphatase, PP2A
INTRODUCTION
While acute stimulation of cardiac β1-adrenergic receptors (β1-ARs) has been shown to have beneficial effects on myocardial performance, prolonged β1-AR activation ultimately impairs cardiac function (1-3). Along with other adverse biologic effects, chronic β1-AR activation leads to activation of a hypertrophic fetal gene program (FGP), resulting in increases in fetal isoforms such as β-myosin heavy chain (βMyHC), skeletal α-actin and A- or B- natriuretic peptide, and decreases in adult isoforms such as αMyHC and sarcoplasmic reticulum (SR)-associated Ca2+ ATPase (SERCA) (4-6). The remodeled/hypertrophied failing human heart exhibits induction of this fetal program of hypertrophic gene expression, and β1-receptor blockade is associated with reversion of gene expression towards an adult pattern in conjunction with reverse remodeling (3). In cultured rat ventricular cardiac myocytes β1-AR induction of the fetal gene program is mediated through Ca2+-calmodulin dependent kinase II (CaMKII) signaling, and is independent of PKA (4).
In the classical description of the β1-AR signaling pathway, β1-AR stimulation activates protein kinase A (PKA) via an increase in adenylyl cyclase generation of cAMP. PKA then phosphorylates several downstream targets, including L-type Ca2+ channel (LTCC), phospholamban, troponin I and myosin binding protein C, leading to a wide range of effects on cellular calcium handling and contractility. LTCC phosphorylation increases Ca2+ influx; phospholamban inactivation by phosphorylation derepresses SERCA and increases SR Ca2+ reuptake as well as subsequent Ca2+ release; and troponin I and myosin binding protein C phosphorylation increase relaxation and contraction (reviewed in (7)). The immediate result of PKA-dependent phosphorylation of these effectors is increased cardiac myocyte contractile function and myocardial systolic and diastolic performance.
The role of PKA in altered myocardial β1-AR signaling in heart failure is controversial. β-AR signal transduction is generally desensitized in the failing human heart, and PKA activity (8) is not increased. Indeed, cAMP levels are actually decreased (9) in human failing ventricular myocardium, and phosphorylation of the PKA target phospholamban is also decreased (10). Desensitization and down-regulation of β1–ARs and up-regulation of Gαi thus result in attenuated cAMP signaling and a decreased response to β-adrenergic stimulation of contractility. However, there is evidence for PKA mediated hyperphosphorylation of ryanodine receptors (RyR) that may contribute to SR Ca2+ leak in the failing heart (11, 12), while other studies suggest that CaMKII is the kinase responsible for the detrimental effects of RyR phosphorylation in the failing heart (13, 14). In addition, unlike PKA dependent signaling, CaMKII signaling is maintained in the failing heart, through up-regulation of CaMKII activity (8, 15). Taken together, the data support a general hypothesis that chronic adrenergic activation in the failing heart results in adverse biologic effects mediated by CaMKII signaling, along with a loss in potential beneficial effects resulting from down-regulation of PKA dependent signaling.
In the work presented here we further investigate the role of PKA in the induction of hypertrophy in cardiac myocytes. The ability of PKA to block α1-adrenergic signaling was recently demonstrated by Haworth et al (16, 17) who showed that PKA activation by agents that increase cAMP reduces endothelin-1 (ET-1)- and phenylephrine (PE)-mediated phosphorylation of PKD, a known mediator of cardiac hypertrophy (16, 17). PKD and its upstream activator Protein Kinase C (PKC) have both been shown to phosphorylate Class II histone deacetylases (HDACs). HDACs act by deacetylating histones. Histone deacetylation results in a tighter interaction of the histones with genomic DNA, limiting the access of transcription factors to gene promoters and causing localized transcriptional silencing (18). In response to hypertrophic agonists such as ET-1 and phenylephrine, Class II HDACs are phosphorylated, resulting in their nuclear export to the cytoplasm and the activation of the hypertrophy-related fetal gene program. Here we show that acute β1-AR signaling resulting in activation of PKA blocks α1-AR-mediated phosphorylation of PKD and HDAC5, and inhibits expression of fetal hypertrophic genes. However, PKA over-expression results in down-regulation of the adult isoforms αMyHC and SERCA. These observations set the stage for a potential anti-hypertrophic strategy involving selective PKA activation, including that delivered through β1AR stimulation or other agents that increase cAMP, combined with inhibition of CaMKII signaling.
METHODS
Materials
Isoproterenol (Sigma) was dissolved in H20 containing 10-3M ascorbic acid and used at 10-7M final concentration. Additional reagents were added to the cells with the following final concentrations: 10-5M final concentration of phenylephrine (Sigma), 10-7M final concentration of okadaic acid (Biomol), 200nM CGP 20712A20712a (Tocris), 300nM ICI 118551 118551 (Tocris).
Antibodies
P-HDAC5 S498 was a gift from Dr. Tim McKinsey (University of Colorado, Denver), P-PKD, Ser-916 (2051) was purchased from Cell Signaling. Calnexin (4731) antibody was purchased from Sigma. PKAα antibody was purchased from Santa Cruz Biotechnology (sc-903). The HRP (115-035-146) anti-mouse and anti-rabbit were purchased from Jackson Laboratories.
DNA constructs
The CA-PKA construct was a gift of Dr. Eric Olson (University of Texas, Southwestern Medical Center).
Cell Culture and Cardiomyocyte transfection
Neonatal rat ventricular myocytes (NRVMs) were prepared according to the method described in Waspe et al (19) and plated in 0.1% gelatin coated plates. Cardiac myocyte transfections were done using the nucleofaction protocol (Amaxa, Inc). This methodology results in ~50% transfection efficiency (20). Briefly, 2×106 cells were transfected with 4 μg of plasmid DNA according to manufacturer's conditions. The investigation conforms with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1985).
Immunofluorescence and cell size measurements
Cell size was measured using Image J software (NIH) as previously described (21). Immunofluorescence was performed essentially as described (20). Images were captured at a 60X magnification with a fluorescence microscope Zeiss LSM 510 META on Axiovert 200M equipped with Zeis Axiovision LE imaging software.
Real Time PCR
Total RNA was extracted by TRIZol (Invitrogen). 0.5 μg of RNA were reverse transcribed into cDNA using I-script (Bio-Rad). Typically, 0.1 ng of cDNA, 12.5 nM of each primers and Power Syber Green PCR Master Mix (ABI) were used in the RT-PCR reactions. Reactions were performed using the ABI7300 system. The primers were described in (20). All RT-PCR experiments were repeated 5-7 times.
Western Blots
Western Blots were performed essentially as described (22). Antibodies were diluted 1:1000 in 1X TBS (20mM Tris 500mM NaCl pH 7.5) containing 3% BSA and 0.1% tween and incubated with the blot overnight at 4°C. All western blot experiments were repeated 3-4 times.
Statistical Analysis
All analyses were performed using ANOVA with Bonferroni post-hoc test, with a p <0.05 in a two-tailed distribution considered to be statistically significant.
RESULTS
β1–AR stimulation reduces phenylephrine (PE)-mediated increase in phosphorylation of PKD and HDAC5
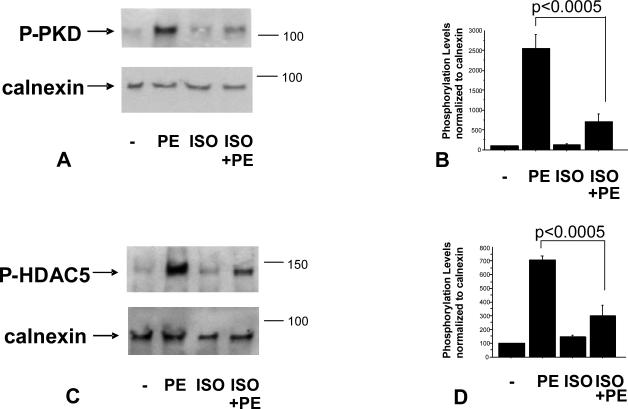
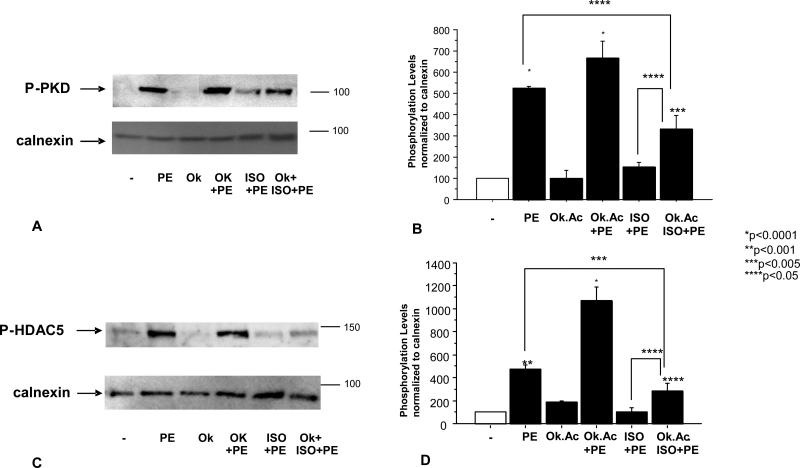
Given that PKA is activated in response to β1–AR stimulation and that PKA activation has recently been shown to block PKD phosphorylation (16, 17), we hypothesized that a β1–AR agonist would act to reduce α1–adrenergic-mediated phosphorylation of PKD. In order to test this hypothesis we treated NRVMs for 15 minutes with the β–AR agonist Isoproterenol (ISO) before adding the α1–AR agonist PE. 15 minutes post PE treatment, NRVMs were harvested and PKD phosphorylation was analyzed by Western Blot. As shown in Figure 1A, ISO treatment significantly reduced PE-mediated phosphorylation of PKD, suggesting that β1–AR stimulation acted to protect the cells from α1–AR-mediated phosphorylation of PKD/HDAC5.
Figure 1.
PE-mediated PKD (A, B) and HDAC5 (C, D) phosphorylation is reduced by ISO treatment. Cells were treated with 10-7M isoproterenol (ISO) for 15 minutes followed by 15 minutes (A) or 1 hour (C) 10-5M phenylephrine (PE) treatment. Phosphorylation levels were determined by western blot using PKD or HDAC5 phospho-specific antibody. Quantification of western blots is shown (B, D).
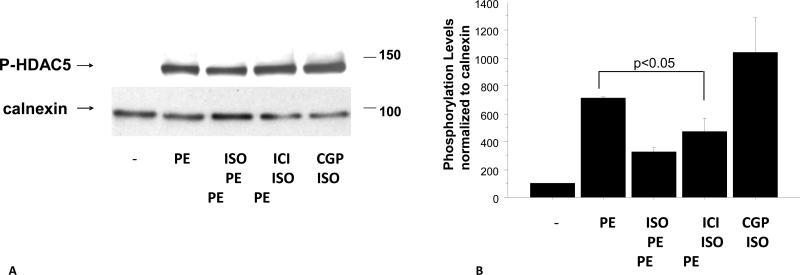
The protective effect of β1–AR stimulation also affected HDAC5 phosphorylation, a downstream target of PKD. As shown in Figure 1C, PE-mediated increases in phospho-HDAC5 were blunted by pretreatment of NRVMs with ISO. To test if this effect was mediated by β1-AR or β2-AR cells were pre-treated with the receptors specific blockers CGP20712a or ICI118551. As shown in Figure 2A and 2B, decreased phosphorylation of HDAC5 is mediated by β1-AR and not β2-AR.
Figure 2.
ISO effect on HDAC5 phosphorylation is mediated by β1-AR and not β2-AR (A, B). Cells were treated with 200nM of the β1-AR CGP 20712A20712a or 300nM of the β2-AR ICI 118551 118551 (Tocris) followed by 10-7M isoproterenol (ISO) for 15 minutes and 1 hour 10-5M phenylephrine (PE) treatment. Phosphorylation levels were determined by western blot using HDAC5 phospho-specific antibody. Quantification of western blots is shown (B).
PKA activity reduces PE-mediated increase in phosphorylation of PKD and HDAC5
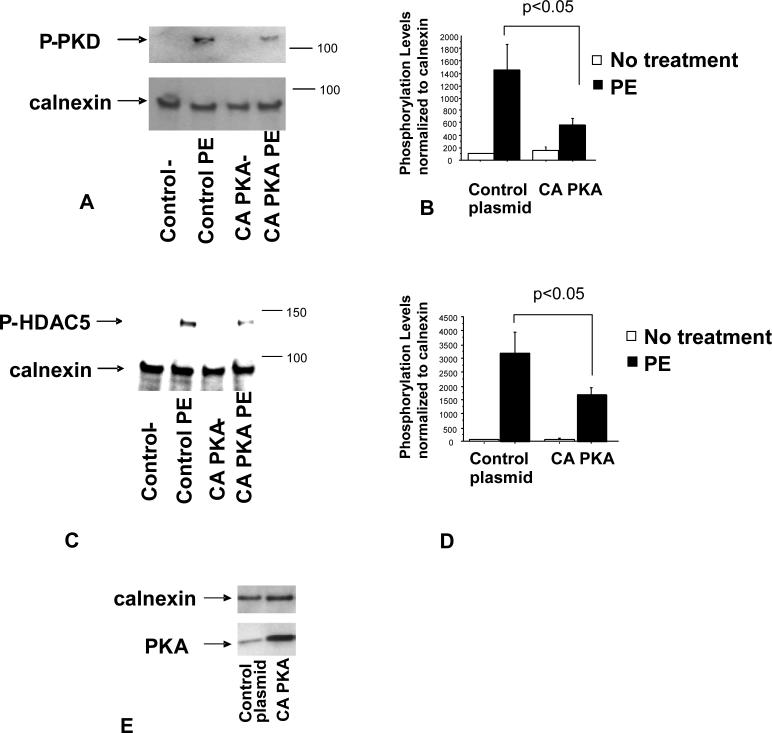
The ability of ISO treatment to reduce α1–AR signaling suggests that PKA activation, a downstream effect of β1–AR stimulation, mediates this response. If so, it would be expected that increased PKA activity without β1–AR stimulation would have a similar effect on α1–AR signaling. In order to increase PKA activity in the absence of ISO treatment, NRVMs were transfected with a DNA construct that over-expresses a constitutively active form of PKA (CA-PKA). As shown in Figure 3A, cells transfected with the CA-PKA construct exhibited increased levels of PKA (Figure 3E), and a significant reduction in PE-related phosphorylation of PKD. Similarly, phosphorylation of HDAC5 was reduced when compared to cells transfected with the control vector (Figure 3B). These data suggest that PKA activation is responsible for β1–AR-mediated effects on PKD and HDAC5 phosphorylation.
Figure 3.
PE-mediated PKD (A, B) and HDAC5 (C, D) phosphorylation is reduced by over-expression of CA-PKA construct. Cells were transfected with a CA-PKA or a control plasmid and treated with 10-5M PE for 15 minutes (A) or 1 hour (C) 24 hours after transfection. Phosphorylation levels were determined by western blot using PKD or HDAC5 phospho-specific antibody. Quantification of western blots is shown (B, D). (E) PKA levels are increased in response to transfection of CA-PKA construct. Cells were transfected using the Amaxa system and PKA levels were measured by western blot.
PKA activity reduces PE-mediated increase of hypertrophic fetal genes
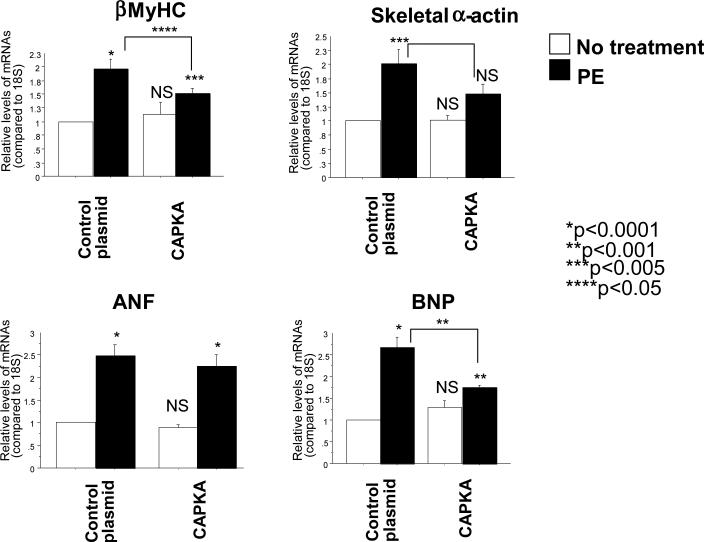
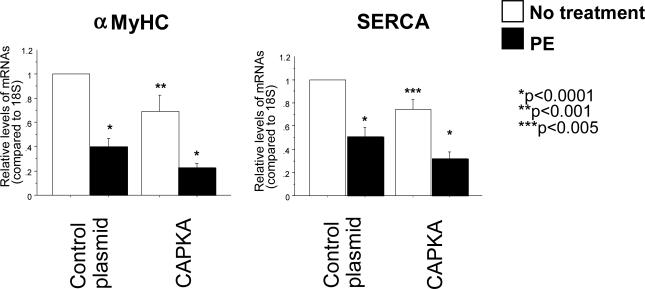
Since HDAC5 is a known transcriptional repressor of genes related to the hypertrophic Fetal Gene Program (FGP), over-expression of CA-PKA and the subsequent protection against PE-mediated phosphorylation of HDAC5 would be expected to attenuate the induction of the FGP in NRVMs treated with PE. In order to test this hypothesis, NRVMs were transfected with the CA-PKA plasmid and 24 hours later treated with PE for an additional 24 hours, with gene expression analyzed by RT-PCR. mRNA abundance analysis showed that CA-PKA significantly reduces PE-mediated increased expression of the FGP markers B-type Natriuretic Peptide (BNP), skeletal α-actin, and β-Myosin Heavy Chain (βMyHC) (Figure 4). Transfection with CA-PKA alone (without PE treatment) appeared to have no effect on these FGP markers. Together, these data further corroborate the protective effects of PKA in the heart in that PKA activity prevents the induction of hypertrophy-related genes in response to α1–AR signaling.
Figure 4.
Over-expression of constitutively active PKA reduces PE-mediated increased gene expression of BNP, βMyHC and skeletal α-actin. Cells were transfected with a CA-PKA construct and treated with 10-5M PE 24 hours after transfection. RNA was extracted 48 hours after treatment and gene expression was analyzed by RT-PCR.
PKA-mediated dephosphorylation of PKD andHDAC5 is likely mediated by PP2A
A possible explanation for the β1–AR-mediated effects on PKD and HDAC5 is that PKA activates a phosphatase that promotes dephosphorylation of PKD and HDAC5. PKA has the ability to activate Phosphatase 2A (PP2A) (23). If PP2A plays a role in the dephosphorylation of PKD or HDAC5, PP2A inhibition would be expected to prevent ISO-mediated dephosphorylation of PKD/HDAC5. In order to determine whether PP2A acts to dephosphorylate PKD/HDAC5, NRVMs were treated with 1nM of okadaic acid (a concentration known to inhibit PP2A (24)) before treatment with ISO. As shown in Figure 5, okadaic acid treatment reduces the protective effect of β1–AR stimulation on PE-mediated increases in PKD and HDAC5 phosphorylation.
Figure 5.
PP2A inhibition reduces ISO-mediated dephosphorylation of PKD (A) or HDAC5 (C). Cells were treated with 100 nM okadaic acid (Ok) 30 minutes prior to 10-7M ISO treatment. Phosphorylation levels were determined by western blot. Treatment with Ok did not affect PKD and HDAC5 phosphorylation levels and are is represented in the graph.
PKA activity down-regulates “adult” contractile protein genes
Although acute β1–AR stimulation appears to have a protective effect on NRVMs, chronic β1–AR activation has been linked to the development of myocardial disease and progression of heart failure. We have previously shown that chronic β1-AR stimulation results in activation of the fetal gene program including down-regulation of αMyHC and SERCA (4). A potential explanation of the adverse effect of chronic adrenergic stimulation lies in the expression of “adult” contractile protein/Ca2+ handling genes whose levels are decreased in pathological states. As shown in Figure 6, transfection of CA-PKA into NRVMs for 24 hours results in decreased expression of two critical adult genes, αMyHC and SERCA. Whereas CA-PKA transfection had no effect on “fetal” genes in NRVMs that were not treated with PE (Figure 4), CA-PKA transfection alone acted to reduce α-MyHC and SERCA levels. As expected, α-MyHC and SERCA levels were also decreased by treatment with PE. Unlike the fetal genes, however, transfection by CA-PKA did not act to block PE-mediated changes. Instead, CA-PKA over-expression and PE treatment had additive effects on the down-regulation of adult genes, suggesting that the regulation of fetal and adult gene expression is mediated by different pathways.
Figure 6.
Over-expression of constitutively active PKA decreases expression of αMyHC and SERCA gene expression. Cells were transfected with a CA-PKA construct and treated with 10-5M PE 24 hours after transfection. RNA was extracted 48 hours after treatment and gene expression was analyzed by RT-PCR.
PKA activity does not prevent cellular hypertrophy
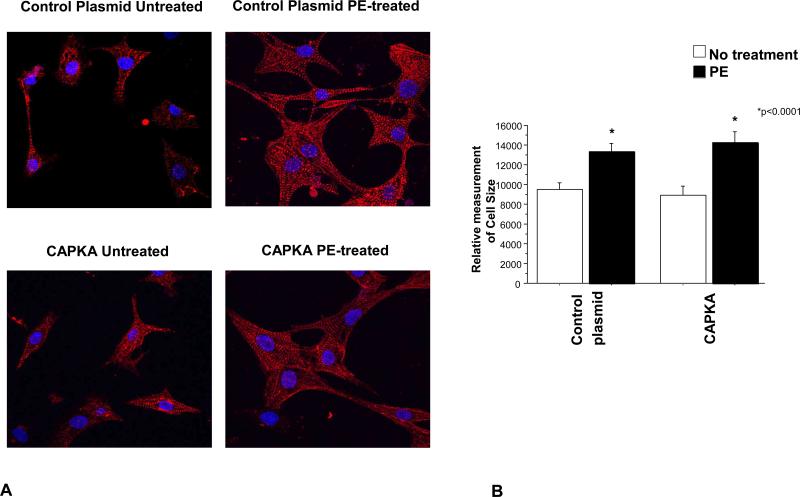
In order to test if the hypertrophic and gene expression changes are mediated by similar pathways we determined cell size in NRVMs expressing CA-PKA and treated with PE. As shown in Figure 7, expression of CA-PKA does not prevent PE-mediated increase in cell size. This result suggests that different signals are involved in promoting changes in gene expression and cellular hypertrophy, and that CA-PKA does not have an effect on PE-mediated cellular hypertrophy.
Figure 7.
Over-expression of constitutively active PKA does not affect PE-mediated cellular hypertrophy. Cells were transfected with a CA-PKA construct and treated with 10-5M PE 24 hours after transfection. Myocytes were immunostained with α-actinin and cell size was measured using ImageJ. A total of 55 cells were measured from 4 separate experiments.
DISCUSSION
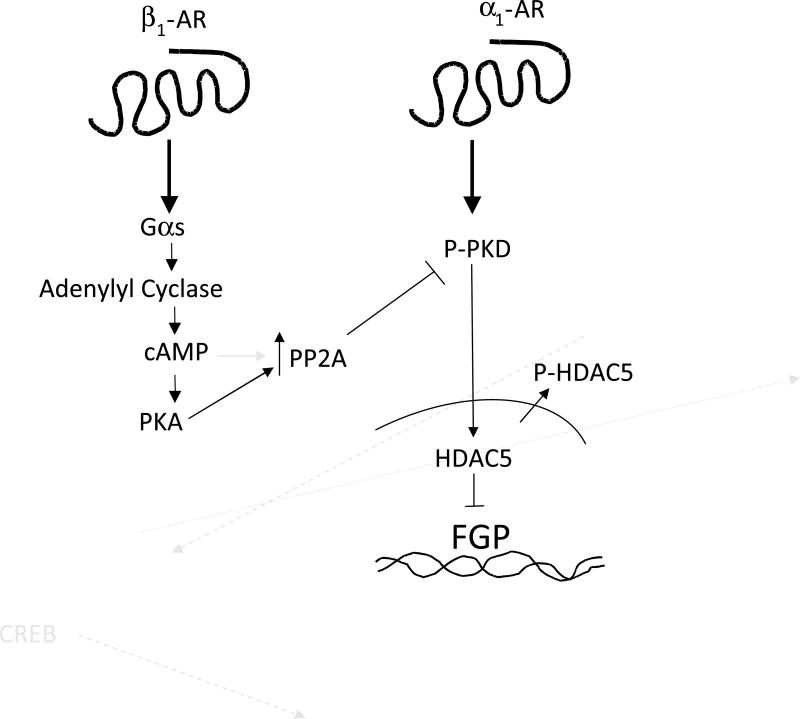
Activation of β1-AR signaling in conditions of stress (such as exercise, traumatic injury and blood loss) results in an elevation of cardiac output that is necessary to meet increased circulatory demand. However, chronic activation of β1-AR signaling is maladaptative and contributes to the progressive natural history of heart failure (25). Recent work by Wang et al (26) demonstrated that acute (10 minutes) β-AR signaling activates PKA, while chronic (24 hours) stimulation results in desensitization of the PKA pathway and increased sensitization of the CaMKII pathway. In our previous work we showed that chronic β1-AR stimulation leads to changes in gene expression and cell size that are associated with a pathologic response dependent on CaMK but not PKA (4). We now show that PKA activation can be protective against the induction of hypertrophy-associated gene expression in an acute setting. PKA activation likely results in activation of a phosphatase that then promotes dephosphorylation of PKD and HDAC5 and reduces PE-mediated phosphorylation of these proteins in response to acute stimulation (Figure 8). Although not shown, NRVMs in culture for 48 h results in HDAC5 phosphorylation independent of PE treatment which preclude long term studies of the effects of PKA on PKD and HDAC5 phosphorylation.
Figure 8.
Schematic representation of the proposed regulatory pathway. Acute stimulation of the β1-AR results in activation of PKA and PP2A. This in turn prevents α1-AR-mediated phosphorylation of PKD and HDAC5 and gene expression activation of fetal isoforms.
Nuclear export of HDACs results in transcription de-repression and activation of gene expression (27). Here we show that β1–AR stimulation reduces PE-mediated phosphorylation of PKD and the Class II HDAC, HDAC5, in NRVMs. This effect is likely a result of β1–AR-mediated activation of PKA since transfection with a CA-PKA construct also reduces PE-mediated phosphorylation of PKD and HDAC5. In addition, CA-PKA reduces PE-mediated activation of the hypertrophic fetal gene program, blocking the up-regulation of BNP, skeletal α-actin, and β-MyHC expression, suggesting that PKA mitigates the deleterious effects of α1-adrenergic signaling. CA-PKA expression in NRVMs also results in down-regulation of the adult isoforms α-MyHC and SERCA, however, indicating that more chronic PKA activation does have negative effects on cardiac myocyte phenotype. Our results suggest that acute activation of the β1–AR can protect against pathologic changes in gene expression through dephosphorylation of PKD, but that chronic activation of PKA is likely detrimental due to down-regulation of α-MyHC and SERCA. The downstream signaling steps that account for the differences between acute and chronic PKA mediated signaling of components of the hypertrophic fetal gene program will need to be defined by additional studies. However, PKA activation of PP2A may be involved in the acute changes. We have previously shown that in H9C2 cells HDAC5 dephosphorylation is mediated by PP2A (28). Another study has shown that PP2A is involved in nuclear import of HDAC5 in endothelial cells (29). Here we show that inhibition of PP2A with okadaic acid blunted the ISO effect on dephosphorylation of PKD or HDAC5. These results suggest that PP2A is involved in the dephosphorylating PKD or HDAC5 in response to β-adrenergic receptor activation.
Recently, another study showed that PKA directly phosphorylates HDAC5 and prevents its translocation to the cytoplasm (30). In this study the authors show that PKA phosphorylation of HDAC5, through cAMP treatment, prevents PE-mediated increase in the expression of fetal isoforms. The authors did not analyze its effect on αMyHC and SERCA expression. Furthermore, the authors showed that cAMP treatment prevented PE-mediated cellular hypertrophy. In our study, CA-PKA did not prevent cellular hypertrophy. It is possible that this effect is mediated by PKA-independent cAMP-mediated activaton of EPAC. EPAC has been shown to have both a hypertrophic effect and to prevent against hypertrophy (31), and at this time, it is unclear if the differences observed upon cAMP treatment and CA-PKA expression are due to other cAMP-activated proteins.
In this work we analyzed the effect of PKA activation in pathologic gene program. We demonstrated that acute (15 minutes) β1-AR stimulation through activation of PKA can protect cardiomyocytes against phosphorylation of proteins involved in promoting changes in gene expression that are associated with pathologic hypertrophy and heart failure. However, chronic (24 hours) PKA activation leads to down-regulation of αMyHC and SERCA, which has been shown to be detrimental to myocardial function. Interestingly, expression of CA-PKA did not block hypertrophic growth in response to PE treatment. This result suggests that independent pathways regulate expression of the fetal and adult isoforms and cell growth, and that HDAC5-mediated gene transcription repression primarily regulates expression of the fetal isoforms. Furthermore, our results underscore the importance of understanding the function of different components of signaling pathways in order to develop therapies that affect specific effects of deregulated pathways.
A limitation of this study is the use of neonatal cardiac myocytes. However, this is an established model to understand the pathways that respond to β-AR stimulation. Moreover, β-AR stimulation of adult cardiac myocytes does not result in changes in the hypertrophic gene program making it an unsuitable model to study pathways involved in changes in gene expression (data not shown). Studies in an animal model of CaMKII inhibition and β-AR stimulation are currently underway in our laboratory, and will properly address the effects of PKA activation in the setting of CaMKII inhibition in an in vivo model.
Acknowledgments
We thank Dr. Eric Olson for the CA-PKA construct and Dr. Peter Mariner for critically reading the manuscript.
This work was supported by the Fondation Leducq and NIH grants 2R01 HL48013 and 1K01HL088708-01.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: Michael R. Bristow: Employee, shareholder, and chairman of the board, ARCA Biopharma. Carmen Sucharov: Equity in Miragen, Inc
REFERENCES
- 1.Bristow MR. Mechanism of action of beta-blocking agents in heart failure. Am J Cardiol. 1997 Dec 4;80(11A):26L–40L. doi: 10.1016/s0002-9149(97)00846-1. [DOI] [PubMed] [Google Scholar]
- 2.Esler M, Kaye D, Lambert G, Esler D, Jennings G. Adrenergic nervous system in heart failure. Am J Cardiol. 1997 Dec 4;80(11A):7L–14L. doi: 10.1016/s0002-9149(97)00844-8. [DOI] [PubMed] [Google Scholar]
- 3.Lowes BD, Gilbert EM, Abraham WT, Minobe WA, Larrabee P, Ferguson D, et al. Myocardial gene expression in dilated cardiomyopathy treated with beta-blocking agents. N Engl J Med. 2002 May 2;346(18):1357–65. doi: 10.1056/NEJMoa012630. [DOI] [PubMed] [Google Scholar]
- 4.Sucharov CC, Mariner PD, Nunley KR, Long C, Leinwand L, Bristow MR. A beta1-adrenergic receptor CaM kinase II-dependent pathway mediates cardiac myocyte fetal gene induction. Am J Physiol Heart Circ Physiol. 2006 Sep;291(3):H1299–308. doi: 10.1152/ajpheart.00017.2006. [DOI] [PubMed] [Google Scholar]
- 5.Mialet Perez J, Rathz DA, Petrashevskaya NN, Hahn HS, Wagoner LE, Schwartz A, et al. Beta 1-adrenergic receptor polymorphisms confer differential function and predisposition to heart failure. Nat Med. 2003 Oct;9(10):1300–5. doi: 10.1038/nm930. [DOI] [PubMed] [Google Scholar]
- 6.Morisco C, Zebrowski DC, Vatner DE, Vatner SF, Sadoshima J. Beta-adrenergic cardiac hypertrophy is mediated primarily by the beta(1)-subtype in the rat heart. J Mol Cell Cardiol. 2001 Mar;33(3):561–73. doi: 10.1006/jmcc.2000.1332. [DOI] [PubMed] [Google Scholar]
- 7.Lohse MJ, Engelhardt S, Eschenhagen T. What is the role of beta-adrenergic signaling in heart failure? Circ Res. 2003 Nov 14;93(10):896–906. doi: 10.1161/01.RES.0000102042.83024.CA. [DOI] [PubMed] [Google Scholar]
- 8.Kirchhefer U, Schmitz W, Scholz H, Neumann J. Activity of cAMP-dependent protein kinase and Ca2+/calmodulin-dependent protein kinase in failing and nonfailing human hearts. Cardiovasc Res. 1999 Apr;42(1):254–61. doi: 10.1016/s0008-6363(98)00296-x. [DOI] [PubMed] [Google Scholar]
- 9.Bristow MR, Feldman AM. Changes in the receptor-G protein-adenylyl cyclase system in heart failure from various types of heart muscle disease. Basic Res Cardiol. 1992;87(Suppl 1):15–35. doi: 10.1007/978-3-642-72474-9_2. [DOI] [PubMed] [Google Scholar]
- 10.Schwinger RH, Munch G, Bolck B, Karczewski P, Krause EG, Erdmann E. Reduced Ca(2+)-sensitivity of SERCA 2a in failing human myocardium due to reduced serin-16 phospholamban phosphorylation. J Mol Cell Cardiol. 1999 Mar;31(3):479–91. doi: 10.1006/jmcc.1998.0897. [DOI] [PubMed] [Google Scholar]
- 11.Marx SO, Reiken S, Hisamatsu Y, Jayaraman T, Burkhoff D, Rosemblit N, et al. PKA phosphorylation dissociates FKBP12.6 from the calcium release channel (ryanodine receptor): defective regulation in failing hearts. Cell. 2000 May 12;101(4):365–76. doi: 10.1016/s0092-8674(00)80847-8. [DOI] [PubMed] [Google Scholar]
- 12.Asai K, Yang GP, Geng YJ, Takagi G, Bishop S, Ishikawa Y, et al. Beta-adrenergic receptor blockade arrests myocyte damage and preserves cardiac function in the transgenic G(salpha) mouse. J Clin Invest. 1999 Sep;104(5):551–8. doi: 10.1172/JCI7418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xiao B, Zhong G, Obayashi M, Yang D, Chen K, Walsh MP, et al. Ser-2030, but not Ser-2808, is the major phosphorylation site in cardiac ryanodine receptors responding to protein kinase A activation upon beta-adrenergic stimulation in normal and failing hearts. Biochem J. 2006 May 15;396(1):7–16. doi: 10.1042/BJ20060116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Benkusky NA, Weber CS, Scherman JA, Farrell EF, Hacker TA, John MC, et al. Intact beta-adrenergic response and unmodified progression toward heart failure in mice with genetic ablation of a major protein kinase A phosphorylation site in the cardiac ryanodine receptor. Circ Res. 2007 Oct 12;101(8):819–29. doi: 10.1161/CIRCRESAHA.107.153007. [DOI] [PubMed] [Google Scholar]
- 15.Calalb MB, McKinsey TA, Newkirk S, Huynh K, Sucharov CC, Bristow MR. Increased phosphorylation-dependent nuclear export of class II histone deacetylases in failing human heart. Clin Transl Sci. 2009 Oct;2(5):325–32. doi: 10.1111/j.1752-8062.2009.00141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haworth RS, Roberts NA, Cuello F, Avkiran M. Regulation of protein kinase D activity in adult myocardium: novel counter-regulatory roles for protein kinase Cepsilon and protein kinase A. J Mol Cell Cardiol. 2007 Dec;43(6):686–95. doi: 10.1016/j.yjmcc.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 17.Haworth RS, Cuello F, Avkiran M. Regulation by phosphodiesterase isoforms of protein kinase A-mediated attenuation of myocardial protein kinase D activation. Basic Res Cardiol. 2011 Jan;106(1):51–63. doi: 10.1007/s00395-010-0116-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thiel G, Lietz M, Hohl M. How mammalian transcriptional repressors work. Eur J Biochem. 2004 Jul;271(14):2855–62. doi: 10.1111/j.1432-1033.2004.04174.x. [DOI] [PubMed] [Google Scholar]
- 19.Waspe LE, Ordahl CP, Simpson PC. The cardiac beta-myosin heavy chain isogene is induced selectively in alpha 1-adrenergic receptor-stimulated hypertrophy of cultured rat heart myocytes. J Clin Invest. 1990 Apr;85(4):1206–14. doi: 10.1172/JCI114554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sucharov CC, Dockstader K, McKinsey TA. YY1 protects cardiac myocytes from pathologic hypertrophy by interacting with HDAC5. Mol Biol Cell. 2008 Oct;19(10):4141–53. doi: 10.1091/mbc.E07-12-1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Emter CA, McCune SA, Sparagna GC, Radin MJ, Moore RL. Low-intensity exercise training delays onset of decompensated heart failure in spontaneously hypertensive heart failure rats. Am J Physiol Heart Circ Physiol. 2005 Nov;289(5):H2030–8. doi: 10.1152/ajpheart.00526.2005. [DOI] [PubMed] [Google Scholar]
- 22.Sucharov CC, Mariner P, Long C, Bristow M, Leinwand L. Yin Yang 1 is increased in human heart failure and represses the activity of the human alpha-myosin heavy chain promoter. J Biol Chem. 2003 Aug 15;278(33):31233–9. doi: 10.1074/jbc.M301917200. [DOI] [PubMed] [Google Scholar]
- 23.Ahn JH, McAvoy T, Rakhilin SV, Nishi A, Greengard P, Nairn AC. Protein kinase A activates protein phosphatase 2A by phosphorylation of the B56delta subunit. Proc Natl Acad Sci U S A. 2007 Feb 20;104(8):2979–84. doi: 10.1073/pnas.0611532104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Singh K, Communal C, Colucci WS. Inhibition of protein phosphatase 1 induces apoptosis in neonatal rat cardiac myocytes: role of adrenergic receptor stimulation. Basic Res Cardiol. 2000 Oct;95(5):389–96. doi: 10.1007/s003950070038. [DOI] [PubMed] [Google Scholar]
- 25.Port JDBM. Richard A, Walsh MD, editors. Adrenergic Receptor Coupling and Uncoupling in Heart Failure. Molecular Mechanisms for Cardiac Hypertrophy and Failure. pp. 323–40.
- 26.Wang W, Zhu W, Wang S, Yang D, Crow MT, Xiao RP, et al. Sustained beta1-adrenergic stimulation modulates cardiac contractility by Ca2+/calmodulin kinase signaling pathway. Circ Res. 2004 Oct 15;95(8):798–806. doi: 10.1161/01.RES.0000145361.50017.aa. [DOI] [PubMed] [Google Scholar]
- 27.Zhang CL, McKinsey TA, Chang S, Antos CL, Hill JA, Olson EN. Class II histone deacetylases act as signal-responsive repressors of cardiac hypertrophy. Cell. 2002 Aug 23;110(4):479–88. doi: 10.1016/s0092-8674(02)00861-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sucharov CC, Langer S, Bristow M, Leinwand L. Shuttling of HDAC5 in H9C2 cells regulates YY1 function through CaMKIV/PKD and PP2A. Am J Physiol Cell Physiol. 2006 Nov;291(5):C1029–37. doi: 10.1152/ajpcell.00059.2006. [DOI] [PubMed] [Google Scholar]
- 29.Illi B, Dello Russo C, Colussi C, Rosati J, Pallaoro M, Spallotta F, et al. Nitric oxide modulates chromatin folding in human endothelial cells via protein phosphatase 2A activation and class II histone deacetylases nuclear shuttling. Circ Res. 2008 Jan 4;102(1):51–8. doi: 10.1161/CIRCRESAHA.107.157305. [DOI] [PubMed] [Google Scholar]
- 30.Ha CH, Kim JY, Zhao J, Wang W, Jhun BS, Wong C, et al. PKA phosphorylates histone deacetylase 5 and prevents its nuclear export, leading to the inhibition of gene transcription and cardiomyocyte hypertrophy. Proc Natl Acad Sci U S A. 2010 Aug 31;107(35):15467–72. doi: 10.1073/pnas.1000462107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gloerich M, Bos JL. Epac: defining a new mechanism for cAMP action. Annu Rev Pharmacol Toxicol. 2010;50:355–75. doi: 10.1146/annurev.pharmtox.010909.105714. [DOI] [PubMed] [Google Scholar]