Abstract
We study the effect of osmotic stress, exerted by salts, on carbohydrate binding to the sugar-specific bacterial channel maltoporin. When the channel is reconstituted into planar lipid bilayers, single events of its occlusion by sugar are seen as transient interruptions in the flow of small ions. We find that, for most salts, changes in the free energy of maltoporin-sugar binding vary linearly with solution osmotic pressure. Such a change in binding with solution osmolarity indicates that for each salt a constant number of salt-excluding water molecules is released upon sugar-maltoporin association at all salt concentrations. We find that larger numbers of water molecules are released upon binding of the cyclic carbohydrate β-cyclodextrin (CD) than upon binding of the corresponding linear homologue maltoheptaose (m7). Remarkably, the extent to which salts affect the binding constant depends sensitively on the type of salt; dehydration in solutions of different anions corresponds to the Hofmeister series. In sodium sulfate solutions, CD and m7 respectively release about 120 and 35 water molecules; in sodium chloride solutions, 35 and 15 waters. No water release is observed with sodium bromide. Finally, by adding adamantane, known to form an inclusion complex with CD, we can infer that CD not only dehydrates but also undergoes a conformational change upon binding to the channel. Our results demonstrate how osmotic stress can improve single-molecule detection of different solutes using protein-based nanopores.
INTRODUCTION
Cells maintain homeostasis by regulating the flux of ions and nutrients across their membranes. In gram-negative bacteria, passive transport of nutrients across the outer membrane is mediated by channel-forming proteins, known as porins (Nakae 1986). Spanning the rigid external membrane, porins allow the diffusion of small water-soluble molecules into and out of the periplasm, with different degrees of specificity toward substrates existing among porins. Bacterial porins are usually divided into general and solute-specific. The former allow non-selective permeation of various water-soluble substrates, with a 600 Dalton cut-off (Schirmer 1998, Delcour 2009). In contrast, solute-specific porins have specialized binding sites that provide more effective transport of rare and depleted substrates (Nikaido 1992, Klebba and Newton, 1998, Schirmer 1998).
Maltoporin is a maltose- and maltodextrin-specific porin found in the outer membranes of gram-negative bacteria, such as Escherichia coli, Schigella sonnei, and others (Boos and Shuman 1998). A rigid homotrimer of maltoporin spans the outer membrane, where three identical units form transmembrane 18-stranded β-barrel pores with maltose-binding domains (Schirmer et al 1995, Dutzler et al 1996). Substantial progress has been made toward understanding the maltoporin-assisted transport of maltose and maltodextrins in bacteria. Initially, maltoporin was identified as a receptor of bacteriophage lambda (Randall-Hazelbauer and Schwartz 1973). Its role in maltose transport was established several years later (Szmelcman and Hofnung 1975), followed by crystal structure determination (Schirmer et al 1995, Dutzler et al 1996, Wang et al 1997), and detailed studies of transport parameters (Benz et al 1986, Benz et al 1987, Nekolla et al 1994, van Gelder et al 2000).
In lipid bilayers, maltoporin forms stable ion-permeable channels with weak cationic selectivity (Benz et al 1987). Introducing maltodextrins into solutions decreases the small-ion current (Benz et al 1986), suggesting that the sugar-binding sites are located within the pores. Reconstituting purified maltoporin from E. coli into planar lipid bilayers allows one to study the sugar transport in real time with single-molecular resolution (Bezrukov et al 2000, Kullman et al 2002, Kullman et al 2006, Gurnev et al 2006). Individual events of sugar binding and translocation can be observed as well-resolved transient interruptions of the ion current through the trimer pores.
Maltoporin recognizes its designated substrate when maltose residues come into contact with two specific sets of aromatic and ionizable residues (“greasy slide” and “polar track”) within the constriction zone of each pore (Dutzler et al 1996, Wang et al 1997). While the aromatic residues of the greasy slide can profit from favorable van der Waals interactions with the aromatic sugar rings, the polar track residues most likely stabilize sugar molecules on the greasy slide by forming hydrogen bonds with the hydroxyl groups of the substrate. The binding of sugars to the channel involves significant restructuring of water of hydration both on the sugar molecule and within the channel (Dutzler et al 2002).
Here we have studied binding of both the cyclic maltooligosaccharide, β-cyclodextrin (CD), and the linear sugar, maltoheptaose (m7), to maltoporin channels reconstituted into planar lipid bilayers. We find that, like its linear homologue maltoheptaose, CD binds to maltoporin and blocks concurrent small-ion flow through the pore. Binding times of CD to maltoporin are substantially longer than those of the linear sugar. Moreover, binding is observed only when CD is applied to the “extracellular” side of maltoporin; the “periplasmic” side of the protein is virtually unable to bind β-cyclodextrin, leading us to conclude that the cyclic molecule, CD, is apparently unable to move along the sugar-transporting pathway in the pore.
Studied at the single-molecule level, the response of the binding reaction to the osmotic stress exerted by different osmolytes probes changes in hydration and, more generally, physical forces governing the binding process (Von Hippel and Wong 1964, Sidorova and Rau 2001, Harries et al 2005, Harries and Rösgen 2008, Gurnev et al 2009). We have previously used a single nanopore sensor to measure the guest-host interaction between cyclodextrin and adamantane (Gurnev et al 2009). This approach allowed us to measure the association kinetics in the presence of several salt species. Here, we extend this methodology to study specific interactions between channel protein and its carbohydrate substrate. Binding of CD and m7 sugars to maltoporin provides a model to investigate molecular hydration upon intermolecular interaction in the crowded environment of a cell. We find that salts acting as co-solutes are able to greatly affect the strength of the binding. For most of the salts studied here, changes in the free energy of maltoporin-sugar equilibrium binding vary linearly with solution osmotic pressure. Such a change with solution osmolarity indicates a constant number of salt-excluding water molecules released upon sugar-maltoporin association. Significantly fewer water molecules are displaced from the interacting surfaces when CD is replaced by its linear analogue, maltoheptaose.
We have established that the changes in the binding constants of CD and maltoheptaose to maltoporin depend sensitively on the anion species: sulfate anion promotes much more dehydration upon binding than does chloride or acetate; no effect is observed with bromide. This trend of anion effect on the strength of sugar-maltoporin association follows the Hofmeister ranking of anions (Hofmeister 1887, Von Hippel and Wong 1964, Collins and Washabaugh 1985). Because we interrogate a single maltoporin trimer in a planar lipid bilayer, we are able to follow separately the changes in underlying rate constants of the sugar binding and unbinding. Remarkably, not only the strength of interaction, as reported by the off-rate, but also the frequency of binding events, seen as the salt-dependent on-rate, is affected by salt.
When adamantane, which forms a rigid inclusion complex with the hollow CD molecule (Cromwell et al 1985), was present in the bulk, the number of blocking events was reduced. The effect was more pronounced when the adamantane concentration was increased. This finding suggests that the inclusion complex cannot interact with maltoporin, and therefore, that CD must undergo a significant structural change in order to be able to bind to the greasy slide. Overall, our results show how the osmotic stress of co-solutes can regulate the transport processes in the cell. They also demonstrate that osmotic stress can be used to probe the details of specific protein/substrate interaction and their modification by the presence of cosolute salts that are preferentially excluded from the interacting surfaces. Extending our previous study of guest-host interactions, here we show that ligand-protein interactions can be modified in the presence of salts in proportion not only to the accessible surface area buried in the process, but also to conformational changes that a ligand undergoes upon binding.
MATERIALS AND METHODS
Planar bilayer lipid membranes were formed by the lipid monolayer opposition technique (Kullman et al 2002, adapted from Montal and Mueller 1972) from 0.1 % solution of diphytanoylphosphatidylcholine (Avanti Polar Lipids) in n-pentane (Burdick and Jackson) on a circular aperture of ~ 50 μm diameter in a Teflon partition separating two (cis- and trans-) compartments of the experimental chamber. Typical diphytanoylphosphatidylcholine membrane capacitance was 20–40 pF with ion current smaller than 0.1 pA under applied voltage of ±200 mV. Wild-type maltoporin was purified from E. coli as described below. Small amounts of maltoporin from a stock solution of 100 μg/ml, containing 1% (v/v) of detergent OctylPOE (Alexis), were added to the cis-compartment of the chamber. Channel insertion was favored by positive transmembrane potential (~200 mV). The applied potential is defined as positive if it is higher on the side of maltoporin addition (cis-side).
If not stated otherwise, β-cyclodextrin (Fluka) at a concentration of 60 μM was added to the cis-side of the membrane, maltoheptaose (Sigma) (30 μM) was added to the cis- and/or trans-side. Adamantane carboxylic acid (Sigma) was admixed to the cis-side from concentrated stock solutions of 20 mM. We reconstituted maltoporin channels in membranes at 0.5 M NaCl and injected aliquots of concentrated NaCl, NaBr, NaCH3COO, NaSCN, and Na2SO4 salts from stock solutions that also contained identical molar fractions of CD or maltoheptaose. All probing salt solutions were buffered with 10 mM Na-phosphate buffer to pH 7. The experiments were performed at room temperature (23 ± 1°C).
Ion current measurements were performed with an Axopatch 200B-2 patch-clamp amplifier (Axon Instruments) in voltage clamp mode using a pair of Ag/AgCl electrodes with 2 M KCl/agarose bridges. Amplifier output signal was filtered by a low-pass 8-pole Butterworth filter (9002, Frequency Devices) at 15 kHz and directly saved into computer memory with a sampling frequency of 50 kHz. Channel amplitude, life-time, and fluctuation analysis were performed using Clampfit 9.2 software (Axon Instruments) as well as software developed in-house.
Maltoporin was isolated from E. coli W3110. Cells were grown overnight in LB broth with 0.2% (w/v) maltose. The cells were harvested by centrifugation, resuspended in 50 mM sodium phosphate buffer, 0.1 M NaCl, 2 mM EDTA, 5% (w/v) sucrose and broken by a French press (10 MPa, two passes). Cell debris were first spun to remove non-broken cells (6000 g, 10 min) and subsequently spun down (100,000 g, 40 min). Maltoporin was extracted from the membrane pellet by octyl-POE with ultracentrifugation at 100,000 g in the buffer containing 20 mM sodium phosphate and 3% octyl-POE detergent (pH 7). Maltoporin was further purified on an affinity column, loaded with amylose resin (New England Biolabs). Maltoporin purity and concentration were analyzed by SDS-PAGE.
RESULTS AND ANALYSIS
Adding picomolar quantities of the protein in detergent to the cis-side of the diphytanoylphosphatidylcholine membrane and applying a transmembrane voltage of +200 mV usually resulted in a single trimer reconstitution within 10 minutes. The present study is based on the analysis of about 200 channel reconstitutions. The channels exhibited a slight asymmetry in conductance with respect to the transmembrane voltage bias: conductance at V = +200 mV was 15–20 % smaller than conductance at V = −200 mV. This difference allowed us to check for channel orientation in the membrane (Gurnev et al 2006). Diphytanoylphosphatidylcholine was chosen as it forms highly stable membranes; substituting diphytanoylphosphatidylcholine with other synthetic or natural lipids did not affect maltoporin conductance and sugar binding in our experiments.
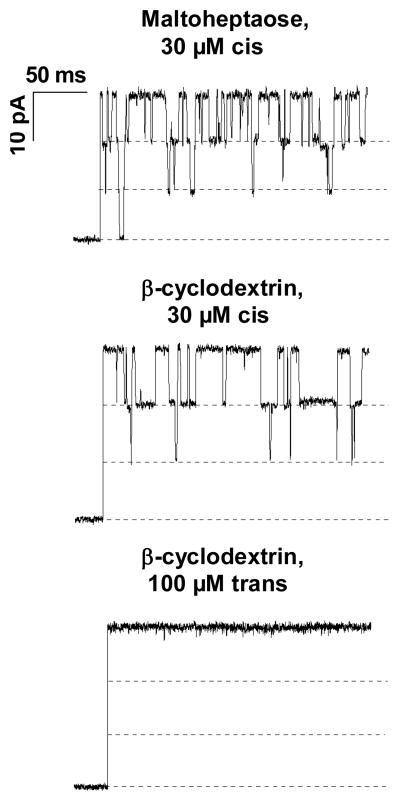
Figure 1 shows typical current traces through maltoporin trimers reconstituted in 1.0 M NaCl solution in the presence of m7 (top) and CD (middle and bottom). The addition of either sugar to the cis-side of the membrane results in discrete current fluctuations equal to multiples of one-third of the initial trimer conductance. These fluctuations reflect transient blockages of small-ion current by the sugar molecules. Any residual conductance of maltoporin in the sugar-bound state was below instrumental resolution. The average duration of the blockage corresponds to the residence time of the sugar molecules in the channel; this time is significantly longer for the cyclic sugar. With the linear maltoheptaose these characteristic fluctuations were observed when sugar was applied to the cis-, trans-, or both sides of the membrane. No such fluctuations were observed when CD was added to the trans-side, even at 100μM (Figure 1, bottom) or higher concentration.
Figure 1.
Addition of sugar molecules to the cis-side of the membrane blocks maltoporin reversibly. Top panel, 30 μM m7, middle panel 30 μM CD. Trans-side addition of 100 μM CD (bottom panel) does not produce any resolvable blockage of ion current. With linear maltohexaose, blockage events are related to channel-facilitated sugar translocation (Kullman et al 2002). Applied voltage was 150 mV.
The current through the fully open channel shown in Figure 1, top panel, is lower than that shown in the middle and bottom panels. This difference reflects intrinsic conductance variation from channel to channel, as previously studied in detail (Kullman et al 2006).
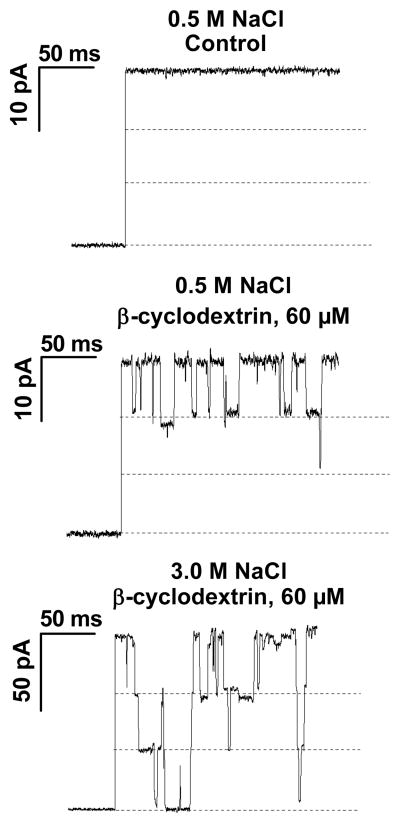
Figure 2 illustrates how the CD-induced channel blockages are transformed by an increase in the bath concentration of NaCl. The current track in the center panel shows the blockage reaction at 0.5 M NaCl, and the bottom panel shows blockage at 3.0 M NaCl. (The current scale is adjusted to facilitate track comparison.) Salt concentration was increased by adding concentrated NaCl aliquots, also containing a constant concentration of CD. At 0.5 M NaCl, Figure 2 shows that the CD-induced blockage events primarily occur within the first one-third level of the channel conductance, corresponding to a situation where only one maltoporin pore is blocked at a time. Increase in salt concentration not only increases the open-channel current but also changes the character of CD binding to the channel. In fact, double and triple blockages, decreasing the current by two or three thirds of the initial current level, can be seen (Figure 2, bottom). Similar experiments were performed for various salts, with maltoporin being first reconstituted in 0.5 M NaCl salt concentration with subsequent addition of the appropriate volumes of the concentrated salt stock to the cis- and trans-compartments.
Figure 2.
Increasing NaCl concentration strengthens the binding of CD molecules to the maltoporin channel. For clarity of presentation, the bottom panel (3.0 M NaCl) is shown with the vertical scale adjusted to match the amplitudes of the 0.5 M traces (top and middle panels). Applied voltage was 150 mV.
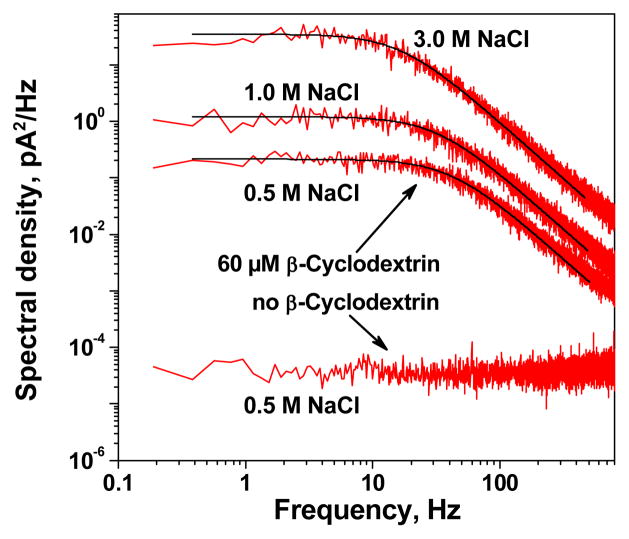
To quantify the kinetic parameters of binding, we used power spectral noise analysis of the channel currents with several examples given in Figure 3. Good Lorentzian fits of the spectra of current fluctuations induced by CD in maltoporin – shown as the solid lines through the power spectra – suggests a two-state Markov process for CD binding to maltoporin, wherein the lifetime distributions of the pore open and the pore blocked are described by single exponentials. The Lorentzian shape of the spectra is maintained at all salt concentrations, suggesting independence of the three pores’ blockages. Similar results were obtained for the linear sugar m7 (data not shown).
Figure 3.
Power spectral densities of noise in the current through a single maltoporin channel, in the absence (lowermost curve) and presence (three upper curves) of 60 μM CD. Clearly, the Lorenzian form is preserved for different NaCl concentrations in the presence of CD, seen as the black curve through the data.
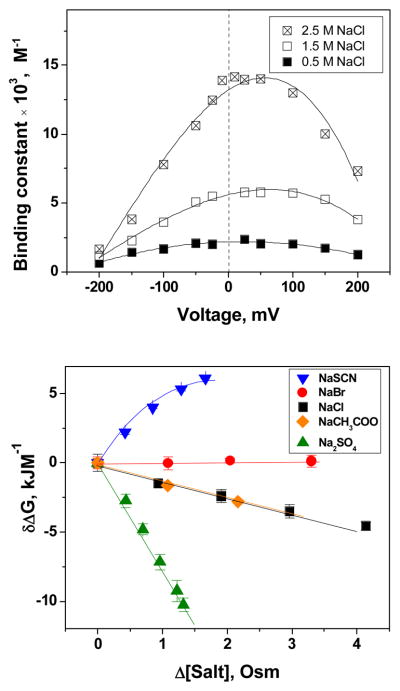
Statistical analysis of the current traces and power spectra allowed us to estimate the equilibrium binding constants as well as the underlying rate constants of CD and maltoheptaose binding to the maltoporin channel. Figure 4, top panel, shows the variation in the equilibrium sugar binding constant, KCD, with applied voltage estimated from the recorded current tracks as
Figure 4.
Top The equilibrium binding constant of CD to maltoporin changes with NaCl concentration and shows pronounced dependence on the applied voltage. Bottom: Salt-induced changes in the free energy of CD binding to maltoporin derived from the binding constant at zero applied voltage are sensitive to salt species. δΔG is the difference between the binding free energy in 0.5 M NaCl solutions, where the measurements were routinely started, and the value of the ΔG at increased salt concentrations.
| Eq. (1) |
Here, [CD] is sugar concentration, and pb is the probability to find a monomer of the trimer in the blocked state pb = 〈I〉/Imax; 〈I〉 and Imax are, respectively, the average current through a trimer in the presence of CD and the current through an unblocked channel. The constant KCD is, in fact, found to be voltage dependent. Though the origin of this voltage sensitivity is not clear, it might be tentatively attributed to the electroosmotic effects (Gu et al 2003) on CD-maltoporin binding kinetics or to the channel’s elastic deformation due to the applied field. To avoid these complications, in our analysis we have considered the values of KCD in the limit of V = 0 mV. The region of small transmembrane voltages also corresponds to the most notable salt-induced changes in binding strength.
The changes in the CD-maltoporin equilibrium binding constant can be translated into changes in binding free energy according to
| Eq. (2) |
where ΔG0 is the free energy of maltoporin/CD association in 0.5 M NaCl, T is the absolute temperature, and R the ideal gas constant. We find that with only one exception, NaSCN, for all salt species probed, namely NaCl, LiCl, NaBr, Na2SO4, and NaCH3COO (sodium acetate), the free energy change exhibits a linear dependence on solution osmolarity (Figure 4, bottom). This linearity indicates that, upon binding, a constant number of hydration waters inaccessible to the salts is displaced from the surfaces of the cyclodextrin molecule and the binding site within the protein pore. Numerically, the equivalent number of displaced water molecules can be estimated from the slopes in Figure 4, bottom, as:
| Eq. (3) |
where μwater is water’s chemical potential, 55.6 is the number of moles of water in 1 Kg of pure water, and mosm is the solute osmolal concentration, all taken at normal conditions.
Similar behavior of the binding free energy vs. solution osmolarity was also found for the linear analogue of β-cyclodextrin, maltoheptaose, in the presence of the same set of probing salts. We find that for both CD and maltoheptaose the number of excluded waters depends on the ionic species, reflecting different extents of exclusion from the sugars and maltoporin interacting surfaces. The extent of exclusion follows the Hofmeister series ranking (Hofmeister 1887, Von Hippel and Wong 1964, Collins and Washabaugh 1985, Harries et al 2005). Results for both CD and its linear counterpart are summarized in Table 1.
Table 1.
The effective number of hydration waters inaccessible to a particular salt that are released upon sugar binding to maltoporin.
| Salt | β-cyclodextrin | Maltoheptaose |
|---|---|---|
| Δ N water | Δ N water | |
| Na2SO4 | 165±8 | 24±8 |
| NaCH3COO | 20±4 | 8±5 |
| NaCl | 22±2 | 8±2 |
| NaBr | 0±2 | −2±1 |
| NaSCN | N/A | N/A |
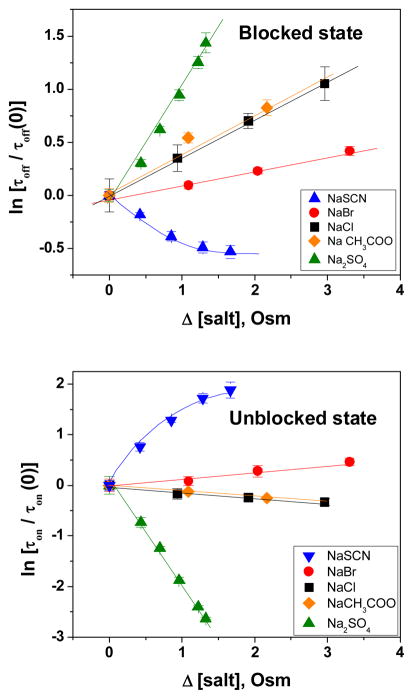
Performing current fluctuation analysis (for details see Kullman et al 2002), we calculated the underlying changes in the rate constants (on- and off-rates), corresponding to CD binding and unbinding to/from maltoporin in the presence of probing salts. The corresponding lifetimes, shown in Figure 5, reveal that not only the off-rates (top panel), which characterize the stability of the sugar-channel association complex in the fluctuating environment, but also the on-rates (bottom panel) are sensitive to the presence of probing co-solute salts. Surprisingly, it is seen that in the case of sulfate ion the salt-induced change in the binding free energy is primarily due to the change in on-rate.
Figure 5.
CD binding kinetics as modified by increasing salt concentrations. Top: logarithm of normalized duration of the blocked state, τoff. Bottom: logarithm of normalized duration of the unblocked state, τon. Values of τ off (0) and τon (0) are measured in 0.5 M NaCl, pH 7, before addition of the probing co-solute salts (see inset).
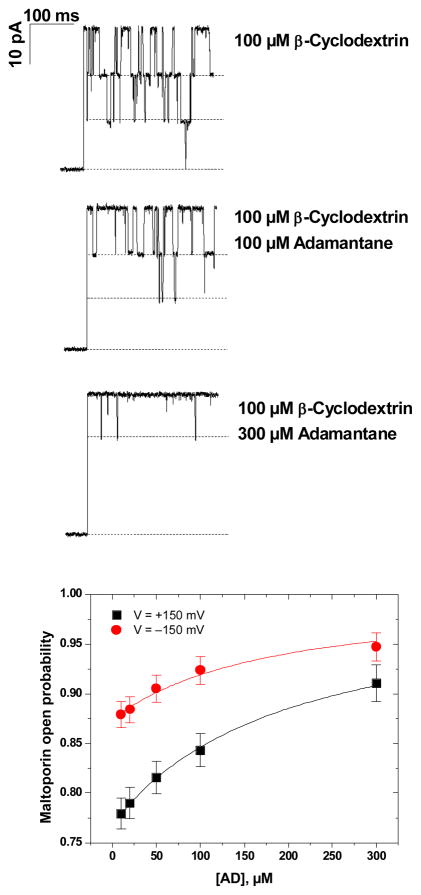
We also investigated CD binding to maltoporin in the presence of adamantane. Considered to be a rigid molecule, adamantane is known to form a tight inclusion complex with the non-polar cavity of cyclodextrin (Cromwell et al 1985, Rekharsky and Inoue 1998). We find that progressive addition of adamantane to CD on the cis-side of the membrane effectively reduces the number of observed current blockage events of maltoporin (Figure 6, current traces), demonstrating a decrease in the pool of CD molecules available for binding. This observation suggests that cyclodextrin not only dehydrates upon binding to the channel but also undergoes a significant conformational change that is prohibited by forming a complex with adamantane (Figure 7). Adding AD alone does not change maltoporin conductance, making it unlikely that AD interacts directly with the pores thereby preventing CD binding; there was also no apparent effect of added AD on the m7 binding kinetics to maltoporin (data not shown).
Figure 6.
Addition of adamantane, known to form a 1:1 inclusion complex with CD molecules (Cromwell et al 1985, Rekharsky and Inoue 1998, Harries et al 2005), reduces the frequency of CD-maltoporin binding events. Membrane-bathing solution was 1.0 M KCl buffered at pH 7.
Figure 7.
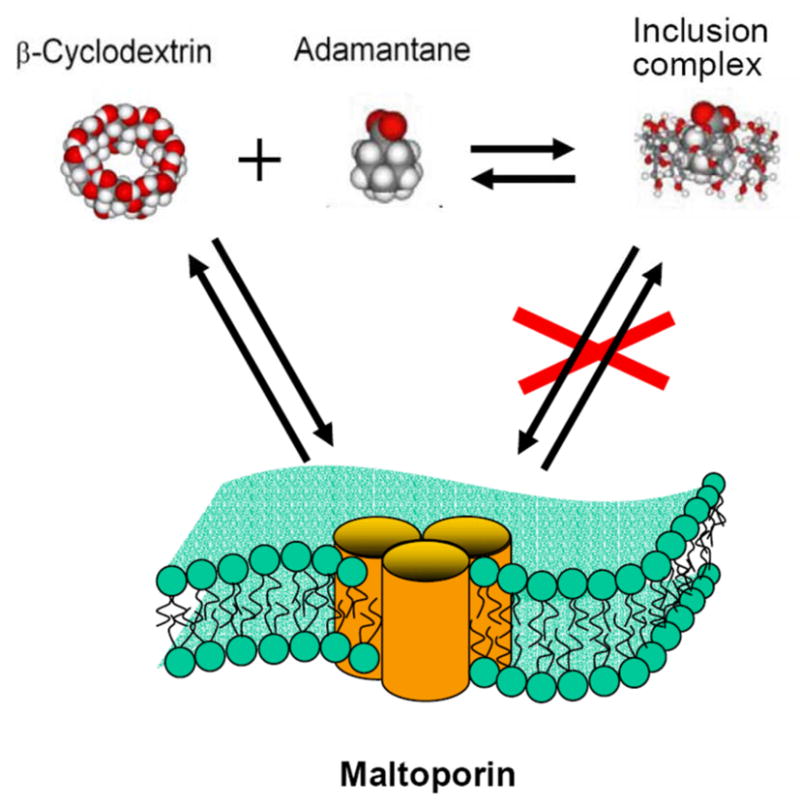
Cartoon representation of the adamantane titration experiment (Figure 6). Formation of the inclusion complex between CD and adamantane decreases the available pool of CD molecules that are able to block the maltoporin channel.
Figure 6, bottom, demonstrates the dependence of the channel open probability on the adamantane concentration [AD] with the concentration of CD held at 100 μM. Solid lines through the data points denote best fits by the following expression, obtained assuming that only a free CD molecule can bind to the channel and that both binding processes are independent of each other:
| Eq. (4) |
Both the association constants of the CD-adamantane inclusion complex, KAD, and of the maltoporin-CD complex, KCD, were used as adjustable parameters. Analysis of the dependencies in a series of experiments yielded KAD = (7.8±3.2)·103 M−1.
DISCUSSION
Interaction of circular β-cyclodextrin with maltoporin binding sites
The strong interaction between the circular truncated-cone-shaped β-cyclodextrin molecule and the maltodextrin-binding site(s) of maltoporin revealed by the present study is remarkable. It turns out that a CD molecule can reach the binding site(s) on the protein, but only when applied from the cis-side of the membrane. Added from that side, CD generates well-resolved current blockages that are not seen after trans-side addition. As mentioned above, judged by the asymmetry of the channel’s current-voltage relationships, maltoporin incorporation is unidirectional. In our earlier study of the binding of bacteriophage lambda to single maltoporin channels (Gurnev et al 2006), we established the link between the asymmetry of channel conductance and channel orientation in the membrane. With the mode of protein addition used here, the channel inserts with its extracellular domain facing the cis-compartment of the experimental cell. From this, we conclude that CD can reach its binding site only from the extracellular side of maltoporin.
Designed by nature as an entry pathway for linear maltooligosaccharides (primarily maltose), each maltoporin unit adopts an hourglass shape with an internal helical twist that follows the primary structure of linear maltodextrins. As proposed by Dutzler et al (2002), translocation of linear maltodextrins through the maltoporin pore can be described as a three-step process that includes: (1) “threading,” during which the sugar molecule moves from the extracellular side into the pore binding sites, (2) a succession of “register shifts,” where the sugar chain moves by one glucosyl unit, shifting each glucosyl moiety from one binding sub-site (of the total three) within the greasy slide to the next sub-site, and (3) “escape” of the sugar into the periplasm. Dutzler and colleagues noted that the “threading” step requires the most significant entropy change, during which the sugar molecule loses many degrees of freedom and undergoes desolvation. This stage also involves large displacement of water molecules from the binding site inside the pore. It is also important to note that the maltoporin entry diameter is relatively large but drops to about 5 Å within the constriction zone provided by loops L1, L3, and partially by L6 folded into the β-barrel pore lumen (Schirmer et al 1995, Dutzler et al 1996).
It is reasonable to assume, therefore, that for circular CD, translocation is stopped at the first (“threading”) step, where the progressive (back and forth) movement of the sugar chain between the binding sub-sites hidden in the 5 Å constriction is impossible. At the same time, we note that CD molecules remain much longer at the binding site, compared with the residence of their linear counterpart, maltoheptaose. The average lifetime of the CD-channel complex is more than three times longer than that of the maltoheptaose-channel complex. For example, in 1.0 M NaCl at 150 mV applied voltage, the respective lifetimes are 7.3±0.5 ms and 2.0±0.3 ms. Surprisingly, this observation suggests that the association of a circular CD molecule with maltoporin binding site(s) is energetically more favorable than for linear sugars, unless some kind of a kinetic trap is involved.
Addition of adamantane reduces the number of CD binding events (Figure 6). This observation indicates that in order to bind to the channel, a cyclodextrin molecule not only has to dehydrate but also to undergo a conformational change. Complexation with adamantane hinders the necessary conformational transition, as is shown in Figure 7, thus decreasing the on-rate of the binding reaction. We note, however, that the value of association constant KAD = (7.8±3.2)·103 M−1 for the CD-adamantane inclusion complex, obtained using Eq. (4), is significantly smaller than the values of the same reaction in bulk, (5.6±0.3) 104 M−1, measured with isothermal titration calorimetry (Harries et al 2005). Among possible explanations of this discrepancy is a modified equilibrium of CD-adamantane complexation next to the channel mouth.
Osmotic stress and salt binding modulate the kinetics of sugar binding
The increase of bulk salt concentration affects the strength of binding of both cyclic and linear forms of maltosugars in a qualitatively similar manner. Both CD and m7 bind more strongly in solutions of increasing concentrations of NaCl, NaCH3COO, and Na2SO4. Among the salts we tested, the largest increase in the equilibrium binding constants for both forms was observed for the sulfate ion, while NaBr did not change the interaction appreciably; thiocyanate even decreased it. Substitution of the different cations (Na, K, and Li) in chloride salts had virtually no effect on binding strength (data not shown).
The anions investigated regulate binding strength according to their position in the classical Hofmeister series: SO4 2−>Cl −~CH3COO −>Br −>SCN −. Importantly, changes in the binding free energy vs. solution osmolarity for SO42−, Cl −, and CH3COO − are linear, indicating that the effect of these ions can be rationalized mostly through the changes in water chemical potential rather than through changes in ion activity. As a result, we are able to characterize the binding sensitivity through the equivalent number of water molecules released from the binding surfaces. The number of released hydration waters was substantially higher for the cyclic sugar, suggesting that CD interaction with the maltoporin channel involves significant conformation change within the structure of the sugar molecule, the channel molecule, or both, that incurs significant dehydration. The ability of added adamantane to decrease CD binding, discussed above, indicates that significant conformational change within the CD molecule is required in order to bind to maltoporin.
The effect of thiocyanate (SCN −) was found to be non-linear, with saturating behavior starting at 1.0 M NaSCN. Furthermore, the effect of SCN is to decrease the binding strength of sugar-maltoprotein complexes. This implies that the direct binding of thiocyanate to the molecular surfaces involved in the sugar-channel complexation reaction dominates reaction equilibrium and kinetics.
One of the major findings of the present study is that not only the off-rates, describing the stability of the sugar-channel complex against thermal fluctuations of the environment, but also the on-rates are modulated by the presence of osmolytes. In particular, the effect of sulfate is mostly due to modifying the on-rate (Figure 5, bottom panel). This should be compared with our earlier observation, made with the CD-adamantane complexation reaction, where the effect of sulfate ions on the equilibrium binding constant was dominated by changes in on-rate (Gurnev et al, 2009). It is important to note that the structures of the complexing surfaces in the present experiments are quite different from those explored in Gurnev et al (2009), where adamantane uses the inner hydrophobic cavity of the CD molecule; in contrast, the association studied here involves the surface of the maltoporin pore and the outer surface of the CD molecule.
In both systems, the effect of sulfate is pronounced and mostly due to the increase in the on-rates of the association reaction. Interestingly, this effect cannot be accounted for by simply assuming that sulfate ions transiently bind or “immobilize” water molecules from the bulk solution thereby effectively increasing the concentration of cyclodextrin. This mechanism would show a nonlinear dependence of ln [τon/τon (0)] on sulfate concentration, contrary to the data shown in Figure 5. Rather, a depletion driving force acting between surfaces would be consistent with this observation (Oosawa and Asakura 1975). Such depletion forces, and the favorable free energy they provide to associated surfaces, are often linear in co-solute concentration. One could expect these forces to affect the on-rate as they “push” surfaces together across a transient gap of solvent cleared of co-solute. It will be instructive to investigate further how ions act through different mechanisms to exert their osmotic action.
References
- Benz R, Schmid A, Nakae T, Vos-Scheperkeuter GH. Pore formation by LamB of Escherichia coli in lipid bilayer membranes. J Bacteriol. 1986;165:978–986. doi: 10.1128/jb.165.3.978-986.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benz R, Schmid A, Vos-Scheperkeuter GH. Mechanism of sugar transport through the sugar-specific LamB channel of Escherichia coli outermembrane. J Membr Biol. 1987;100:21–29. doi: 10.1007/BF02209137. [DOI] [PubMed] [Google Scholar]
- Bezrukov SM, Kullman L, Winterhalter M. Probing sugar translocation through maltoporin at the single channel level. FEBS Letters. 2000;476:224–228. doi: 10.1016/s0014-5793(00)01753-1. [DOI] [PubMed] [Google Scholar]
- Boos W, Shuman H. Maltose/maltodextrin system of Escherichia coli: transport, metabolism, and regulation Microbiol. Mol Biol Rev. 1998;62:204–229. doi: 10.1128/mmbr.62.1.204-229.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins KD, Washabaugh MW. The Hofmeister effect and the behavior of water at interfaces. Q Rev Biophys. 1985;18:323–422. doi: 10.1017/s0033583500005369. [DOI] [PubMed] [Google Scholar]
- Cromwell WC, Bystrom K, Eftink MR. Cyclodextrin-adamantanecarboxylate inclusion complexes: studies of the variation in cavity size. J Phys Chem. 1985;89:326–332. [Google Scholar]
- Delcour AH. Outer membrane permeability and antibiotic resistance. Biochim Biophys Acta. 2009;1794:808–816. doi: 10.1016/j.bbapap.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutzler R, Wang Y-F, Rizkallah PJ, Rosenbusch JP, Schirmer T. Crystal structures of various maltooligosaccharides bound to maltoporin reveal a specific sugar translocation pathway. Structure. 1996;4:127–134. doi: 10.1016/s0969-2126(96)00016-0. [DOI] [PubMed] [Google Scholar]
- Dutzler R, Schirmer T, Karplus M, Fischer S. Translocation mechanism of long sugar chains across the maltoporin membrane channel. Structure. 2002;10(9):1273–1284. doi: 10.1016/s0969-2126(02)00811-0. [DOI] [PubMed] [Google Scholar]
- Gu L-Q, Cheley S, Bayley H. Electroosmotic enhancement of the binding of a neutral molecule to a transmembrane pore. Proc Natl Acad Sci U S A. 2003;100:15498–15503. doi: 10.1073/pnas.2531778100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurnev PA, Oppenheim AB, Winterhalter M, Bezrukov SM. Docking of a single phage lambda to its membrane receptor maltoporin as a time-resolved event. J Mol Biol. 2006;359:1447–1455. doi: 10.1016/j.jmb.2006.04.034. [DOI] [PubMed] [Google Scholar]
- Gurnev PA, Harries D, Parsegian VA, Bezrukov SM. The dynamic side of the Hofmeister effect: a single-molecule nanopore study of specific complex formation. Chem Phys Chem. 2009;10:1445–1449. doi: 10.1002/cphc.200900312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harries D, Rau DC, Parsegian VA. Soultes probe hydration in specific association of cyclodextrin and adamantane. J Am Chem Soc. 2005;127:2184–2190. doi: 10.1021/ja045541t. [DOI] [PubMed] [Google Scholar]
- Harries D, Rösgen J. A Practical Guide on How Osmolytes Modulate Macromolecular Properties Methods. Cell Biol. 2008;84:679–735. doi: 10.1016/S0091-679X(07)84022-2. [DOI] [PubMed] [Google Scholar]
- Hofmeister F. Zur Lehre von der Wirkung der Salze (Über Regelmässigkeiten in der eiweissfällenden Wirkunbg der Salze und ihre Beziehung zum physiologischen Verhalten derselben) Arch Exp Pathol Pharmakol. 1887;24:247–260. [Google Scholar]
- Klebba PE, Newton SM. Mechanisms of solute transport through outer membrane porins: burning down the house. Curr Opin Microbiol. 1998;1:238–247. doi: 10.1016/s1369-5274(98)80017-9. [DOI] [PubMed] [Google Scholar]
- Kullman L, Winterhalter M, Bezrukov SM. Transport of maltodextrins through maltoporin: A single-channel study. Biophys J. 2002;82:803–812. doi: 10.1016/S0006-3495(02)75442-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullman L, Gurnev PA, Winterhalter M, Bezrukov SM. Functional subconformations in protein folding: Evidence from single-channel experiments. Phys Rev Lett. 2006;96:038101. doi: 10.1103/PhysRevLett.96.038101. [DOI] [PubMed] [Google Scholar]
- Nakae T. Outer-membrane permeability of bacteria. Crit Rev Microbiol. 1986;13:1–62. doi: 10.3109/10408418609108734. [DOI] [PubMed] [Google Scholar]
- Nekolla S, Andersen C, Benz R. Noise analysis of ion current through the open and the sugar-induced closed state of the LamB channel of Escherichia coli outer membrane: evaluation of the sugar binding kinetics to the channel interior. Biophys J. 1994;66:1388–1397. doi: 10.1016/S0006-3495(94)80929-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikaido H. Porins and specific channels of bacterial outer membranes. Mol Microbiol. 1992;6:435–442. doi: 10.1111/j.1365-2958.1992.tb01487.x. [DOI] [PubMed] [Google Scholar]
- Oosawa F, Asakura S. Thermodynamics of the polymerization of proteins. Academic Press; New York: 1975. [Google Scholar]
- Randall-Hazelbauer L, Schwartz M. Isolation of the bacteriophage lambda receptor from Escherichia coli. J Bacteriol. 1973;116:1436–1446. doi: 10.1128/jb.116.3.1436-1446.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rekharsky MV, Inoue Y. Complexation thermodynamics of cyclodextrins. Chem Rev. 1998;98:1875–1918. doi: 10.1021/cr970015o. [DOI] [PubMed] [Google Scholar]
- Schirmer T. General and specific porins from bacterial outer membranes. J Struct Biol. 1998;121:101–109. doi: 10.1006/jsbi.1997.3946. [DOI] [PubMed] [Google Scholar]
- Schirmer T, Keller TA, Wang Y-F, Rosenbusch JP. Structural basis for sugar translocation through maltoporin channels at 3.1 Å resolution. Science. 1995;267:512–514. doi: 10.1126/science.7824948. [DOI] [PubMed] [Google Scholar]
- Sidorova NY, Rau DC. Linkage of EcoRI dissociation from its specific DNA recognition site to water activity, salt concentration, and pH: Separating their roles in specific and non-specific binding. J Mol Biol. 2001;310:801–816. doi: 10.1006/jmbi.2001.4781. [DOI] [PubMed] [Google Scholar]
- Szmelcman S, Hofnung M. Maltose transport in Escherichia coli K-12: involvement of the bacteriophage lambda receptor. J Bacteriol. 1975;124:112–118. doi: 10.1128/jb.124.1.112-118.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Gelder P, Dumas F, Rosenbusch JP, Winterhalter M. Oriented channels reveal asymmetric sugar permeation through maltoporin of Escherichia coli. Eur J Biochem. 2000;267:1–7. doi: 10.1046/j.1432-1327.2000.00960.x. [DOI] [PubMed] [Google Scholar]
- Von Hippel PH, Wong K-Y. Neutral salts: the generality of their effects on the stability of macromolecular conformations. Science. 1964;3632:577–580. doi: 10.1126/science.145.3632.577. [DOI] [PubMed] [Google Scholar]
- Wang YF, Dutzler R, Rizkallah PJ, Rosenbusch JP, Schirmer T. Channel specificity: Structural basis for sugar discrimination and differential flux rates in maltoporin. J Mol Biol. 1997;272:56–63. doi: 10.1006/jmbi.1997.1224. [DOI] [PubMed] [Google Scholar]