Abstract
A Neurobiotin injected OFF parasol cell from mid-peripheral macaque retina was studied by reconstruction of serial, ultrathin sections and compared with ON parasol cells studied previously. In most respects, the synaptic inputs to the two subtypes were similar. Only a few of the amacrine cell processes that provided input to the labeled OFF parasol ganglion cell dendrites made or received inputs within the series, and none of these interactions were with the bipolar cells or other amacrine cells presynaptic to the OFF parasol cell. These findings suggest that the direct inhibitory input to OFF parasol cells originates from other areas of the retina. OFF parasol cells were known to receive inputs from two types of diffuse bipolar cells. To identify candidates for the presynaptic amacrine cells, OFF parasol cells were labeled with Lucifer Yellow using a juxtacellular labeling technique, and amacrine cells known to costratify with them were labeled using immunofluorescent methods. Appositions were observed with amacrine cells containing immunoreactive calretinin, parvalbumin, choline acetylatransferase and G6-Gly, a cholecystokinin precursor. These findings suggest that the inhibitory input to parasol cells conveys information about several different attributes of visual stimuli and, particularly, about their global properties.
Keywords: juxtacellular, magnocellular, primate, bipolar, amacrine
INTRODUCTION
Parasol cells are one of the most common types of ganglion cells in primates, comprising approximately 10% of ganglion cells (Perry et al., 1984). These ganglion cells terminate in the magnocellular layers of the lateral geniculate nucleus and give rise to one of the major, parallel processing pathways (Callaway, 2005). There are two subtypes of parasol cells: ON cells, with dendrites in the inner half of the inner plexiform layer (IPL), and OFF cells, with dendrites in the outer half of the IPL (Dacey and Lee, 1994). Both subtypes have relatively large cell bodies and dense dendritic arbors that are restricted to two narrow strata close to the center of the IPL (Watanabe and Rodieck, 1989). Aside from their polarity, the light responses of ON and OFF parasol cells are similar in many respects, but there are also some subtle differences. The kinetics of the OFF responses are slower, and although both subtypes of parasol cells have a nonlinear relationship between stimulus contrast and firing rate, the nonlinearity is more pronounced in the OFF subtype (Chichilnisky and Kalmar, 2002).
ON and OFF parasol cells are very similar in their responses to amino acid neurotransmitters (Zhou et al., 1994) and in the distribution of the receptors for those neurotransmitters (Macri et al., 2000; Lin et al., 2000, 2002). To determine whether there were any differences in the synaptic inputs to the two subtypes of parasol cells, we analyzed an OFF parasol cell in the macaque retina by reconstructing serial, ultrathin sections of a Neurobiotin injected OFF parasol cell, the same technique as we had used previously to study an ON parasol cell (Marshak et al., 2002). OFF parasol cells are known to receive inputs from two types of diffuse bipolar (DB) cells, DB2 and DB3 (Calkins, 1999; Jacoby et al., 2000). To identify candidates for the presynaptic amacrine cells, a juxtacellular labeling method for OFF parasol cells using Lucifer Yellow was developed. Double immunofluorescent labeling techniques were used to label cholinergic and one additional type of amacrine cells, and the triple labeled material was analyzed by confocal laser scanning microscopy.
MATERIALS AND METHODS
Intracellular injection and electron microscopy
An adult female macaque (Macaca mulatta) was euthanized with an overdose of sodium pentobarbital (Nembutal, 50 mg/kg IV) and enucleated at the conclusion of an unrelated experiment at the M.D. Anderson Cancer Center (Houston, TX). The animal protocol was approved by the institution and conformed to institutional animal care and use standards. The eye was cleaned of surrounding tissue, hemisected, and the posterior eyecup was cut into 5 sections. The vitreous and sclera were removed with fine forceps, and the pieces were transferred to oxygenated Ames medium (Sigma Chemical Co., St. Louis, MO). The tissue was first treated with acridine orange (4 min, 10 μM) and then flattened ganglion cell side up on a membrane filter that was positioned in a superfusion chamber on an upright, fixed-stage microscope with epifluorescent illumination. Lucifer Yellow was injected into the cell to confirm that it was a parasol ganglion cell, followed by Neurobiotin, as described previously (Marshak et al., 2002). The tissue was fixed in 2.5% glutaraldehyde/1% paraformaldehyde in 0.1 M phosphate buffer (PB), pH 7.4 at 37°C for approximately 1 hour. After rinsing the tissue in phosphate buffered saline (PBS), the retina was dissected from the choroid and the retinal pigment epithelium and was treated with 1% NaBH4 for 1 hour followed by several rinses of PBS. The retina was then treated with an ascending and descending series of graded ethanol solutions in PBS (10%, 25% and 40% for 10 min each; 50% for 30 min; 40%, 25% and 10% for 10 min each). The retina was rinsed in PBS and incubated in 1:100 avidin-biotin-peroxidase (Standard, Vector Laboratories, Burlingame, CA) overnight, rinsed in PBS and reacted with diaminobenzidine (0.5 mg/ml) and hydrogen peroxide (0.0025%) for 60 minutes. After another PBS rinse, the tissue was treated in 1% OsO4 in 0.1M PB, pH 7.4, for 1 hour, dehydrated and embedded flat in Epon (Ted Pella, Inc., Redding, CA).
Images of the labeled cell were acquired using a Zeiss (Thornwood, NY) 410 confocal laser scanning microscope in the transmitted light mode, and then the piece of retina containing the cell was cut out and re-embedded in Epon for ultrathin sectioning. Serial sections 100 nm thick were cut in the vertical plane and stained with uranyl acetate (2% in 50% methanol, 60 min) and lead citrate (2% aqueous, 1 min). In all, 54 serial sections were analyzed. Labeled dendritic profiles were photographed at 10,000X and, occasionally, at higher or lower magnifications using a JEOL 100 CX transmission electron microscope (EM; Peabody, MA). Most of the synapses in the sample of 99 were onto distal dendrites, 1.0 to 1.5 μm in diameter; 10 synapses were onto proximal dendrites 2.0 to 2.5 μm in diameter. The unlabeled processes were identified using established ultrastructural criteria (Dowling and Boycott, 1966). One dendrite and two of its branches were also graphically reconstructed using Neurolucida 4.3 and NeuroExplorer 3.50 RC3 (MicroBrightField, Inc., Williston, VT). Thus, there were two sets of EM data: one set with all the dendrites studied and a smaller set with only those that were reconstructed graphically. The electron micrographs were digitized, and those used for illustrations were retouched using Photoshop 7.0 (Adobe Systems Inc., San Jose, CA). Digital manipulations of the electron micrographs selected for publication included dodging, burning, and adjusting brightness and contrast. In all instances, the changes were equivalent to the same operations traditionally done in an EM darkroom.
Juxtacellular labeling and confocal microscopy
Eyes from three adult male macaque monkeys (Macaca fascicularis) were obtained from Charles River, BRF (Houston, TX). The vitreous humor, choroid and retinal pigment epithelium were removed with fine forceps in Ames medium (Sigma-Aldrich, Saint Louis, MO) bubbled with 95% O2/5% CO2 at room temperature and placed ganglion cell side up on black membrane filters (GE Nitrocellulose Mixed Esters Black Membrane, 0.8 μm pore size, GE Osmonics, Minnetonka, MN).
Retinas were cut into pieces approximately 7 mm square and placed in a holding chamber, where they were superfused at room temperature with carboxygenated Ames medium at 2 ml/min with a peristaltic pump (Model 1612, Instrumentation Specialties Company, Lincoln, NE). For juxtacellular labeling, pieces of retina were placed in a silicone elastomer (Sylgard 184, Dow Corning, Midland, MI) and plexiglass chamber mounted on an upright, fixed-stage Zeiss standard microscope (Thornwood, NY) at room temperature. Ganglion cell bodies were labeled with 2-3 drops of acridine orange (10 μM) added to the chamber well during superfusion. The ganglion cells were visualized using a 30X dry objective lens and epifluorescent illumination (excitation 395 – 440 nm, emission 470 nm). Parasol ganglion cells were recognized by their large somas. Electrodes were pulled from borosilicate glass with filaments (1.2 mm OD, 0.69 mm ID, Sutter Instruments, Novato, CA) using a P-97 Flaming/Brown Micropipette Puller (Sutter Instruments, Novato, CA). One electrode from each batch was filled with 3M KCl, and under these conditions, the resistance was 100-150 MΩ. The remaining electrodes were filled with 1.5% Lucifer Yellow CH, ammonium salt (Molecular Probes, Eugene, OR) in 50 mM PB (pH 7.4). The electrode was connected to the negative terminal of a Grass-Telefactor SD9 stimulator (West Warwick, RI) with a silver wire, while the Ag/AgCl ground electrode was connected to the positive terminal. The electrode was mounted at a 45 degree angle above the chamber and was lowered straight down onto the surface of the soma without disrupting the membrane. After lowering the electrode, the peristaltic pump was turned off. The juxtacellular labeling procedure was a modification of the method used by Haas et al. (2001 and 2002). The cell was stimulated with a 10-20 V negative pulse 100 msec in duration at 0.5 Hz for 1-2 minutes. The electrode was then gently retracted from the cell, and superfusion was resumed. Pieces of retina were viable for up to 1 hour in the superfusion chamber and 12 hours in the holding chamber. After labeling, the pieces were rinsed in Ames medium for 10 minutes and fixed in 4% paraformaldehyde 0.1 M PB (pH 7.4) at 4°C overnight.
The retinas were rinsed in PBS (Sigma-Aldrich, Saint Louis, MO) and blocked in either 1% or 10% normal donkey serum (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA) with 0.3% Triton-X 100 (Sigma-Aldrich, Saint Louis, MO) in PBS at 4°C for several hours or overnight. Following several PBS rinses, retinas were incubated sequentially in two primary antibodies raised in different species: 1:200 polyclonal goat anti-choline acetyl transferase (ChAT; AB144P, Chemicon International, Temecula, CA) and either 1:1000 polyclonal rabbit anti-calretinin (AB149, Chemicon International, Temecula, CA), or 1:1000 monoclonal mouse anti-parvalbumin (Clone PARV-19, Sigma, Saint Louis, MO), or 1:1000 rabbit anti-G6-Gly (R6B6, a gift from Dr. John Del Valle, University of Michigan, Ann Arbor, MI). The patterns of labeling with these antisera were the same as reported previously (ChAT, Yamada et al., 2003; Calretinin, Mills and Massey, 1999; Parvalbumin, Kolb et al., 2002; G6-Gly, Marshak et al., 1990).
PBS was the buffer used for all rinses and all antibody solutions. Retinas were incubated in the first primary antibody with 0.3% Triton-X 100 for 10 days at 4°C, either with or without 10% normal donkey serum. Retinas were then rinsed in PBS overnight at 4°C. The tissue was incubated in 1:100 biotin conjugated secondary antibody (Jackson ImmunoResearch Laboratories, Inc.), made in donkey and directed against the host species of the first primary antibody, at 4°C for one to two days. After rinsing, tissue was incubated in 1:100 streptavidin conjugated indocarbocyanine (Cy3, Jackson ImmunoResearch Laboratories, Inc.) at 4°C for two to four days. Following a series of rinses, retinas were incubated in the second primary antibody as described above, rinsed in PBS, and incubated in 1:200 indodicarbocyanine (Cy5, Jackson ImmunoResearch Laboratories, Inc.) labeled secondary antibody, made in donkey and directed against the host species of the second primary antibody, at 4°C for two days. Retinas were then mounted ganglion cell side up in Vectashield mounting medium (Vector Laboratories, Burlingame, CA).
ChAT immunoreactive (IR) dendrites were used as depth markers for stratum 4 (S4) and stratum 2 (S2) of the IPL. The majority of labeled ganglion cells were ON parasol cells, but only OFF parasol cells, having dendrites in S2 of the IPL, were used for this study (see Fig. 1 and Fig. 2). Tertiary or higher order dendrites of these OFF parasol cells surrounded by well-labeled amacrine cell processes were selected for further analysis. Images were acquired with a Zeiss 510 META LSM confocal microscope with HeNe/Argon lasers using a 63X oil immersion lens (n.a. = 1.4 ). Images were taken at a zoom of 5.8, a 1024×1024 or 2048×2048 pixel box, and a Z-interval of 0.36 or 0.5 μm. Excitation wavelengths were 458 nm for Lucifer Yellow, 543 nm for Cy3 and 633 nm for Cy5, with BP filters 505-530 and 560-615, and 657-743 in the META setting, respectively. Single optical sections were examined for evidence of appositions between labeled processes. LSM stacks containing appositions were imported into Amira v. 3.0 (ZIB, Mercury Computer Systems, Berlin), and 3D images were made using the Isosurface function, average 2, downsample enabled. Images were rotated to identify spurious appositions due to superposition and also to gain a better understanding of the relationship between the labeled processes in 3 dimensional space. Three dimensional volume renderings were examined from both the vitreal and scleral orientations. The renderings were viewed and captured first from the vitreal orientation, then rotated in the y direction and captured from the scleral orientation. Images of the scleral orientation were turned into mirror images using the “Flip Horizontal” function in PhotoShop v. 6.0.1 (Adobe Systems Incorporated, San Jose, CA) to make comparisons more straightforward. Reconstructed processes were occasionally made transparent to better visualize interactions.
Figure 1.
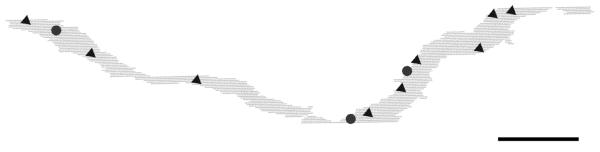
The labeled OFF parasol ganglion cell was imaged digitally in a whole mount preparation before it was re-embedded and sectioned for electron microscopy. It was identified as a parasol ganglion cell by the size of its dendritic field and the density of its dendritic arbor. Parts of the cell body and some proximal dendrites were obscured due to leakage of Neurobiotin at the injection site. Scale bar = 50 μm.
Figure 2.

A low power electron micrograph of the inner plexiform layer (IPL) and parts of the inner nuclear layer (INL) and ganglion cell layer (GCL). Filled triangles denote the borders of the IPL. Labeled OFF parasol ganglion cell dendrites (arrows) ramify at approximately 30% depth, with the lower boundary of the INL being 0% and the upper boundary of the GCL being 100%. Scale bar = 10 μm.
A photomontage of several juxtacellularly labeled ON and OFF parasol ganglion cells was made by first acquiring a low-power image using a 10X Plan-Neofluar lens; this low-power image was used as a guide for the montage. Images of individual cells and portions of cells were then acquired at 40X using an oil immersion lens (n.a. = 1.3), 512 × 512 pixel depth, z-step 0.45 – 0.64 μm, and superimposed on the 10X image to create a high resolution montage. Seven 40X image stacks were used to make the montage, each 1 – 14 sections thick, and surrounding areas were filled with black. Superposition of the images acquired at 40X onto the 10X image insured both correct placement of cells and optimum clarity of neural morphology. Arrangement of images and level adjustments were made using PhotoShop v. 6.0.1 (Adobe Systems Incorporated, San Jose, CA).
RESULTS
The Neurobiotin-injected OFF parasol ganglion cell was located approximately 8 mm from the fovea in the peripheral, temporal retina (Fig. 1). It was identified as a parasol ganglion cell by the size of its perikaryon and dendritic arbor and by its narrowly-stratified and densely-branched dendrites (Watanabe and Rodieck, 1989). In order to verify that this was an OFF cell, the depths of labeled distal dendrites in the IPL were measured using low power electron micrographs, and all were found in the outer half of the IPL (Fig. 2).
The labeled OFF parasol ganglion cell dendrites received 99 chemical synapses in the series. Because the dendritic tree was symmetrical and 234 μm in diameter, approximately 2% of it was sampled. Most of the synapses were located on distal dendrites; only 10 synapses were located on dendrites identified as proximal based on their diameter, 8 from amacrine cell processes and 2 from the same bipolar cell axon terminal. The labeled OFF parasol ganglion cell dendrite had 43 spines in this series, but only 3 received synaptic inputs there, 2 from amacrine cell processes and 1 from a bipolar cell axon.
Inputs from Bipolar Cells
The labeled OFF parasol ganglion cell dendrites received inputs from 23 bipolar cell axons at 27 ribbon synapses in the series (Fig. 3). One axon made two monads, and three made two dyads onto the labeled dendrites. Of the bipolar cell axons presynaptic to the labeled OFF parasol ganglion cell dendrites, 16 could be reconstructed completely enough to be identified. All except one of these had the morphology and ultrastructure characteristic of DB3 axons, and one made a gap junction with another axon terminal of the same type (Fig. 4). Their terminals were large, and they had a regular, roughly ovoid shape, several mitochondria and many synaptic vesicles (Jacoby and Marshak, 2000). The other type of presynaptic bipolar cell axon terminal had an irregular shape and was smaller in diameter. It was relatively electron lucent and contained microtubules but fewer synaptic vesicles (Fig. 5). These are likely to be from axons of DB2 diffuse bipolar cells (Boycott and Wässle, 1991; Calkins, 1999; Jacoby et al., 2000).
Figure 3.
A large, labeled OFF parasol ganglion cell dendrite receives synapses from an amacrine cell (hollow triangles) and a bipolar cell axon at a dyad synapse (arrow). The bipolar cell axon receives a reciprocal synapse (filled triangles) from an electron lucent amacrine cell process. Later in the series, this bipolar cell made another synapse onto the labeled dendrite. Scale bar = 1 μm.
Figure 4.
Serial sections demonstrating a monad synapse onto the labeled OFF parasol ganglion cell dendrite. The diffuse bipolar (DB) 3 bipolar cell axon is filled with vesicles and has a prominent synaptic ribbon (arrow). The bipolar cell also makes a gap junction (triangles) with another bipolar axon of the same type. Scale bar = 0.5 μm.
Figure 5.
Serial sections through a DB2 bipolar cell axon making a monad type ribbon synapse onto the labeled OFF parasol ganglion cell dendrite. The bipolar cell axon is small in diameter, irregularly shaped and contains microtubules. The ribbon (arrow) first appears in the second section, and the third section shows the synaptic ribbon at the presynaptic membrane and the associated postsynaptic density on the membrane of the labeled OFF parasol ganglion cell dendrite. Scale bar = 1 μm.
Inputs from Amacrine Cells
There were 72 synapses from 71 amacrine cell processes onto the dendrites of the labeled OFF parasol ganglion cell. These presynaptic amacrine cells did not engage in many local synaptic interactions. Four amacrine cells presynaptic to the OFF parasol cell received synapses from other amacrine cell processes in this series, two made synapses onto other amacrine cell processes, and one did both. These other amacrine cells did not contact the OFF parasol cell dendrites or the bipolar cells that provided their input. The OFF parasol cell did not receive input from the amacrine cell dendrites found at the same dyads. Of the 11 dendrites of this type, 4 made reciprocal synapses onto the bipolar cells, and 1 of the 4 also contacted another amacrine cell at a gap junction. Most amacrine cell processes presynaptic to the labeled OFF parasol ganglion cell dendrites were electron lucent and contained many vesicles (Fig. 3 and Fig. 6). Many of these, 28% of all presynaptic amacrine cell processes, contained prominent, electron-dense presynaptic specializations surrounded by synaptic vesicles (Fig. 7). The dense bars were approximately 70 nm long and roughly half as wide, with the long axis perpendicular to the synaptic membrane. The OFF parasol ganglion cell also received input from more electron dense amacrine cell processes (Fig. 8), but these were not from AII amacrine cells (Wässle et al., 1995).
Figure 6.
Two electron lucent, vesicle-filled amacrine cell processes make conventional synapses (triangles) onto a labeled OFF parasol ganglion cell dendrite. Neither amacrine cell process made other synaptic contacts within the series. Scale bar = 0.5 μm.
Figure 7.
The labeled OFF parasol ganglion cell receives a synapse from an electron lucent amacrine cell process (triangles). Three presynaptic dense bars are visible there. The dendrite had three spines (asterisks) within the series, two of which are visible in this section. The amacrine cell process did not make other synaptic contacts within the series. Scale bar = 0.5 μm.
Figure 8.

An irregularly-shaped, electron dense amacrine cell dendrite makes a synapse onto the labeled parasol ganglion cell dendrite (triangles). The presynaptic amacrine cell also made and received synapses with other amacrine cells within the series. Scale bar = 0.5 μm.
Graphical Reconstruction
Two branches of a labeled dendrite were graphically reconstructed (Fig. 9); the total length of dendrite reconstructed was 36 μm. There were 12 synapses included, 3 from bipolar cells and 9 from amacrine cells. The proportion of inputs from bipolar cells to inputs from amacrine cells was nearly the same as in the entire sample, a finding suggesting that this dendrite was representative. The synapses from bipolar cells were all located on the side facing the inner nuclear layer (INL), but the amacrine cell synapses were encountered on both sides of the dendrites in approximately equal numbers. The density of ribbon synapses over the entire reconstructed length was 1 every 12 μm, and the density of conventional synapses was 1 every 4 μm.
Figure 9.
Parts of a dendrite of the labeled OFF parasol ganglion cell were reconstructed from serial sections; they are viewed here as if in the whole mount preparation. Soon after the beginning of the series, the dendrite bifurcated, and 12 synapses were found on its branches. The circles represent bipolar cell inputs at ribbon synapses; all 3 were found on the side of the dendrite facing the INL. Triangles represent amacrine cell input at a conventional synapse; these were found on both sides of the reconstructed dendrite. Scale bar = 5 μm.
Light Microscopy
The new juxtacellular labeling technique yielded parasol cells that were well-filled and isolated from one another. Figure 10 is a photomontage showing ON and OFF parasol ganglion cells labeled with Lucifer Yellow. Occasionally, a few perikarya in the vicinity of Lucifer Yellow labeled somas also contained small amounts of the dye, but these were easily distinguished from the parasol cell somas because the intensity of the labeling was much lower, they had no labeled dendrites, and the somas were smaller than those of the labeled parasol ganglion cells.
Figure 10.
A photomontage of juxtacellularly-labeled ON and OFF parasol ganglion cells. ON parasol cells were more commonly labeled. Scale bar = 100 μm.
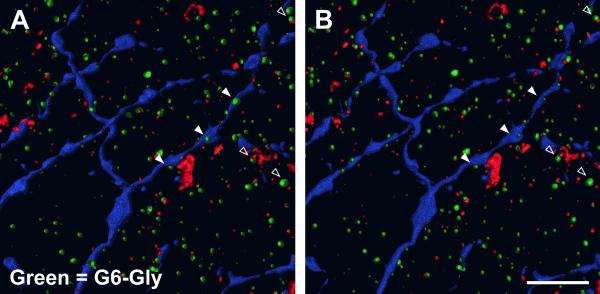
Parvalbumin is a marker for a population of amacrine cells in monkey retinas (Grünert and Wässle, 1996; Kolb et al., 2002; Martin and Grünert, 1992). Many parvalbumin-IR processes had indentations or holes where ChAT-IR or, we presume, processes not labeled in this study invaginate or pass through (not illustrated). Parvalbumin-IR processes apposed labeled OFF parasol ganglion cell dendrites and partially or completely surrounded labeled OFF parasol ganglion cell dendrites along thin stretches of labeled dendrite (Fig. 11 A, B). Under the conditions used in our experiments, the calretinin antibody labeled only AII amacrine cells (Mills and Massey, 1999). Calretinin-IR lobular appendages either completely or partially surrounded passing labeled OFF parasol ganglion cell dendrites (Fig. 11 C, D). G6-Gly is a marker for two populations of amacrine cells in macaque retinas (Marshak et al., 1990). Small G6-Gly-IR processes were apposed to labeled OFF parasol ganglion cell dendrites, either alone or alongside ChAT-IR dendrites (Fig. 12). ChAT was used in all experiments as a depth marker. ChAT-IR dendrites often had indentations in the center, which are likely to be sites of synaptic specializations based on our previous EM study (Yamada et al., 2003). ChAT-IR dendrites were apposed to labeled OFF parasol ganglion cell dendrites, often partially surrounding them. Additional figures are presented in the Supplementary Material section.
Figure 11.
(A) Two parvalbumin-IR processes (green) partially surround labeled OFF parasol ganglion cell dendrites (blue) (filled triangles). A parvalbumin-IR process apposes a terminal of a labeled OFF parasol ganglion cell dendrite (blue) (hollow triangle). A 3D reconstruction showing processes from the vitreal orientation. (B) A 3D reconstruction showing processes from the scleral orientation. Twenty-three single optical sections were used in the 3D reconstruction. Scale bar = 5 μm. (C) A calretinin-IR lobuluar appendage (green) apposes a labeled OFF parasol ganglion cell dendrite (blue) at the base of a spine (filled triangle). A ChAT-IR dendrite (red) apposes another dendrite (hollow triangle). A 3D reconstruction showing processes from the vitreal orientation. (D) A 3D reconstruction showing processes from the scleral orientation. Fourteen single optical sections were used in the 3D reconstruction. Scale bar = 5 μm.
Figure 12.
(A) Three G6-Gly-IR processes (green) appose a labeled OFF parasol ganglion cell dendrite (blue) (filled triangles). A 3D reconstruction showing processes from the vitreal orientation. (B) A 3D reconstruction showing processes from the scleral orientation. Twenty-one single optical sections were used in the 3D reconstruction. Scale bar = 5 μm.
DISCUSSION
Inputs from Bipolar Cells
A Neurobiotin injected OFF parasol ganglion cell from mid-peripheral macaque retina was analyzed in serial, ultrathin sections. There was considerably less bipolar cell input to the peripheral OFF parasol cell in our study, 27%, than was observed in another study of an OFF parasol cell from the central retina. The cell reconstructed from serial sections of the macaque parafovea receives 53% of its input from DB2 and DB3 bipolar cell axons (Calkins, 1999). The same decrease in the proportion of bipolar cell input relative to amacrine cell input with eccentricity has been observed in studies of other primate retinal ganglion cells. Small bistratified cells receive 70% of their input from DB3 and blue cone bipolar cells in the parafovea (Calkins et al., 1998), but in the peripheral retina they receive only 20% of their input from bipolar cells (Ghosh and Grünert, 1999). Midget ganglion cells receive approximately half of their input from midget bipolar cells in the parafovea (Kolb and DeKorver, 1991; Calkins et al., 1994), but a smaller proportion in the peripheral retina (Kolb and Marshak, 2003). Although the proportion of bipolar cell input decreases in the periphery, the same types of bipolar cells are presynaptic to each type of ganglion cell throughout the retina. The higher percentage of input from amacrine cells in peripheral retina may explain, at least in part, the greater responsiveness of all types of ganglion cells in peripheral macaque retina to temporal modulation (Solomon et al., 2005).
The bipolar cells presynaptic to the labeled OFF parasol ganglion cell were partially reconstructed, and most of the bipolar cell axons presynaptic to the OFF parasol ganglion cell were from the DB3 subtype of diffuse bipolar cells, as described previously (Calkins, 1999; Jacoby et al., 2000). DB3 cells have OFF responses to light stimuli in their receptive field centers and responses of opposite polarity to stimulation of the surround (Dacey, 1999). If the receptors for glutamate on their dendrites are similar to those of their homologues in the ground squirrel retina, the DB3 bipolar cells would be particularly well-suited to convey the transient components of the light responses of cones to OFF parasol ganglion cells (DeVries, 2000).
Inputs from Amacrine Cells
The majority of the inputs to the labeled OFF parasol cell, 73%, were from amacrine cell processes. These inputs do not provide the classical receptive field surrounds of parasol cells, however. These responses remain when receptors for the major amacrine cell neurotransmitters are blocked (McMahon et al., 2004). There is physiological evidence suggesting that the inputs to parasol cells from amacrine cells have other functions, instead. Parasol cells respond to stimuli outside their classical receptive fields (Krüger et al., 1975), and studies in other mammalian retinas suggest that these effects are mediated by amacrine cells (Demb et al., 1999). There is psychophysical evidence suggesting that parasol cells are selectively inhibited during saccades in humans (Burr et al., 1994). In rabbit retina, stimuli that mimic the motion of the background produced by eye movements inhibit retinal ganglion cells, and these effects are mediated by polyaxonal amacrine cells via GABA receptors (Roska and Werblin, 2003; Ölveczky et al., 2003). Inputs from amacrine cells may also account for the contrast adaptation observed in the light responses of parasol cells (Chander and Chichilnisky, 2001; Solomon et al., 2002), as proposed in rabbit retina (Hosoya et al., 2005).
A considerable number of the amacrine cells presynaptic to the labeled OFF parasol cell had dense bars in their presynaptic membranes surrounded by synaptic vesicles, structures described previously in amacrine cell processes of the macaque IPL (Raviola and Raviola, 1982). These densities are found in interstitial amacrine cells of teleost fish retinas (Holmgren-Taylor, 1983; Zimmerman, 1983), and a morphologically similar subtype of amacrine cell is found in the macaque retina. These A1 cells have ON-OFF responses to light with action potentials (Stafford and Dacey, 1997). The ultrastructure of these A1 cells has not been studied, but based on their stratification in the central third of the IPL, they might be presynaptic to OFF parasol ganglion cells. This hypothesis could not be tested directly, however, because there is no immunolabeling method selective for A1 amacrine cells.
Aside from the presynaptic dense bars, there were no apparent ultrastructural differences between the amacrine cells presynaptic to the labeled OFF parasol cell and an ON parasol cell studied previously (Marshak et al., 2002). The amacrine cells that made synapses onto the labeled OFF parasol ganglion cell did not engage in many local synaptic interactions. Only 7% of the presynaptic amacrine cells received inputs within the series, and all of these were from amacrine cells that did not interact with the labeled OFF parasol cell in any other way. None of the amacrine cell processes found in dyads with the labeled OFF parasol ganglion cell dendrites made synapses onto the labeled dendrites, and only 36% of the amacrine cell processes in dyads made reciprocal synapses onto the bipolar cell. Thus, the direct inhibitory input and much of the indirect inhibitory input to OFF parasol cells originates from other areas of the retina. Very similar results were found in a study of an ON parasol cell using the same techniques (Marshak et al., 2002). In contrast, central midget ganglion cells receive 58% of their inhibitory synapses from amacrine cells driven by the same midget bipolar cell (Calkins and Sterling, 1996). Only a few of the amacrine cell processes presynaptic to the labeled OFF parasol ganglion cell dendrites made synapses within the series, and none was onto another amacrine cell presynaptic to the OFF parasol cell. The same was true of the amacrine cells presynaptic to an ON parasol cell (Marshak et al., 2002). The amacrine cells presynaptic to midget ganglion cells, on the other hand, frequently contact one another within the same section or nearby (Calkins and Sterling, 1996; Kolb and Marshak, 2003).
To identify possible presynaptic amacrine cells, OFF parasol cells were filled with fluorescent dye, two types of amacrine cells were labeled with antibodies and the triple labeled retinas were analyzed by confocal laser scanning microscopy. All four types of amacrine cells that made appositions with the OFF parasol cells are known to interact with other amacrine cells in macaque retina. The majority of synaptic input to G6-Gly-IR amacrine cells, 72%, is from amacrine cells, and 51% of their output synapses are onto other amacrine cells (Marshak et al., 1990). In central macaque retina, the parvalbumin-IR amacrine cells receive 54% of their input from amacrine cells (Kolb et al., 2002). Calretinin-IR amacrine cells receive 32% of their input from amacrine cells, mainly on lobular appendages in S2 (Wässle et al., 1995). ChAT-IR amacrine cells also receive 32% of their input from amacrine cells and direct at least 28% of their output to amacrine cells (Yamada et al., 2003). Interactions between ChAT-IR and other amacrine cell types were observed directly in our study, as well.
There is also physiological evidence for amacrine cell interactions in the pathway providing input to parasol cells. The GABAA antagonist, picrotoxin, attenuates the light responses of parasol cells, a finding suggesting that GABAergic amacrine cells inhibit other amacrine cells. The other, more subtle effects of picrotoxin on the light responses of parasol cells are also consistent with this interpretation (McMahon et al., 2004). Taken together, these findings suggest that interactions between amacrine cells are important in the pathways providing input to both major types of ganglion cells in primates. In the pathway providing input to midget ganglion cells, these interactions are local and easily appreciated by conventional EM. In the pathway providing input to parasol cells, the interactions between amacrine cells apparently occur on a much larger spatial scale.
Candidates for Presynaptic Amacrine Cell Types
Appositions between ChAT-IR amacrine cell dendrites and OFF parasol cell dendrites were observed in S2 of the IPL, and these are likely to be sites of synapses. Ganglion cell dendrites are the major targets of the ChAT-IR amacrine cells in macaque retina (Mariani and Hersh, 1988). There were concavities in the ChAT-IR amacrine cells at many of the appositions, and some of the appositions were with dendritic spines of the ganglion cells. In an EM study of ChAT-IR amacrine cells in macaque retina, synapses onto ganglion cells were observed at sites like these (Yamada et al., 2003). Cholinergic inputs would be expected to facilitate the responses of the parasol cells to rapidly changing stimuli (Chiao and Masland, 2002). There were also appositions between processes of G6-Gly IR amacrine cells and the dendrites of OFF parasol cells in S2, as observed previously for ON parasol cells in S4 (Jacoby et al., 1996). One G6-Gly-IR amacrine cell type is bistratified (Marshak et al., 1990) and therefore might be an ON amacrine cell that inhibits OFF parasol cells. The increase in the maintained activity of some OFF ganglion cells in the macaque retina after application of the metabotropic glutamate receptor agonist L-2-amino-4-phosphonobutyrate suggests that ON amacrine cells tonically inhibit macaque OFF ganglion cells (Cohen and Miller, 1994). This pathway might account for the differences between ON and OFF parasol cells in maintained firing rates (Chichilnisky and Kalmar, 2002).
Dendrites of parvalbumin-IR amacrine cells also apposed OFF parasol cells, and the specializations in the dendrites at these sites suggest that these were also sites of synapses. These amacrine cells are relatively common and use glycine as their neurotransmitter. They have multiple, thin primary dendrites that are broadly stratified around the center of the IPL (Martin and Grünert, 1992; Grünert and Wässle, 1996). The parvalbumin-IR amacrine cells do not interact exclusively with parasol cells or the neurons that provide their input, however. In the central macaque retina, they also contact midget bipolar and midget ganglion cells (Kolb et al., 2002). Appositions between calretinin-IR lobular appendages of AII amacrine cells and OFF parasol cells were observed by LM. Although processes of AII cells were found in the neuropil in our sample analyzed by EM, they were not presynaptic to the labeled OFF parasol ganglion cell. The most likely explanation for the discrepancy between the two sets of data is that there is a sampling problem in the EM. That is, synapses from AII cells onto OFF parasol cells were not included in our sample analyzed by EM because they are relatively rare or else oriented in such a way that they are difficult to detect using vertical sections. The function of this pathway may be to provide direct, inhibitory input from the rod pathway to OFF parasol cells, augmenting the indirect input via DB3 bipolar cells (Jacoby and Marshak, 2000).
Methodological Considerations
Part of one labeled OFF parasol cell dendrite was also graphically reconstructed. The density of ribbon synapses was 1 per 12 μm of dendritic length, and the density of conventional synapses was 1 per 4 μm, very close to values predicted by LM analysis of a much larger sample of injected cells in the same region of the marmoset retina (Lin et al., 2002). The upper estimate of the density of bipolar inputs to OFF parasol cells was 0.133 puncta per μm of dendrite, the sum of all types of labeled puncta; this is likely to be an overestimate because glutamate receptor subunits may be colocalized. The lower estimate was 0.033 puncta per μm, the density of puncta containing the most abundant subunit; this value would be correct only if all glutamate receptors were colocalized. The density observed using EM, 0.083 ribbon synapses per μm of dendrite, was between the two but closer to the upper limit. In the LM studies of distal parasol cell dendrites, the mean density of glycinergic puncta was 0.156 per μm of dendrite (Lin et al, 2000), and the mean density of GABAergic puncta was 0.109 per μm (Macri et al, 2000). The sum, 0.265, is very close to 0.25 conventional synapses per μm of dendritic length, the value obtained by EM. The good agreement between the two sets of results suggests that, with appropriate EM controls, LM techniques provide an accurate estimate of the density and distribution of synapses onto parasol cells.
The juxtacellular labeling method for filling parasol ganglion cells in this study has two distinct advantages over the original method using biocytin (Pinault, 1996). Using Lucifer Yellow, the yield of filled parasol cells was higher because it was possible to visualize the tips of the electrodes, fill the cells rapidly and obtain immediate feedback about the morphology of the labeled cells. The immunolabeling was also enhanced because it was possible to use avidin and biotin to visualize one of the primary antibodies.
Conclusions
The major input to both types of parasol cells is from amacrine cells, and the variation in ultrastructure of the presynaptic amacrine cells indicated that they are heterogeneous. The LM data also suggested that there is not a single type of amacrine cell specialized to provide input to parasol cells. For example, OFF parasol cells dendrites did not co-fasciculate with amacrine cell processes, as do dendrites of two types of wide-field ganglion cells (Yamada et al., 2005). Instead, amacrine cells containing each of the four of the markers tested in our study made a relatively small number of appositions with the OFF parasol cells. After these experiments were completed, another common type of amacrine cell that costratifies with OFF parasol cells was described. This is a glycinergic amacrine cell that also contains vesicular glutamate transporter 3 (Haverkamp and Wässle, 2004; Jusuf et al., 2005), and it might also make appositions with OFF parasol cells. These amacrine cell types vary in their dendritic field sizes, stratification patterns and, presumably, in their light responses. Thus many types of signals are converging onto parasol ganglion cells from amacrine cells.
The amacrine cells in the pathway providing input to parasol cells engage in very few synaptic interactions within the serial sections that we analyzed by EM. This finding suggests that inhibitory input to parasol cells originates from different areas of the retina than their excitatory input. In this respect, parasol cells are quite different from midget ganglion cells. Many of the amacrine cells presynaptic to midget ganglion cells receive local input, often from the same midget bipolar cells that contact the ganglion cells. These amacrine cells also frequently contact other amacrine cells nearby. The amacrine cells that contact parasol cells are known from previous EM studies to receive input from other amacrine cells, and some interactions were observed in our LM data. Taken together, these findings suggest that the amacrine cells in the pathway providing input to parasol cells interact over relatively long distances. Amacrine cell inputs such as these would be well suited to provide parasol cells with information about the global properties of visual stimuli.
| Antibody | Source, Catalog Number, Lot Number |
Species Raised In |
Immunogen | Monoclonal or Polyclonal |
|---|---|---|---|---|
| ChAT | Chemicon International, AB144P, lot #21100523 |
Goat | Human Placental Enzyme |
Polyclonal |
| Calretinin | Chemicon International, AB149, lot #18040705 |
Rabbit | Guinea Pig Calretinin |
Polyclonal |
| Parvalbumin | Sigma, P3088, Clone PARV-19 |
Mouse | Purified Frog Muscle Parvalbumin |
Monoclonal |
| G6-Gly | Dr. John Del Valle, rabbit #6, bleed #6 |
Rabbit | Tyr-Gly-Trp-Met- Asp-Phe-Gly |
Polyclonal |
Supplementary Material
Table 1.
Bipolar Cells Presynaptic to
| ON Parasol |
OFF Parasol |
|||
|---|---|---|---|---|
| Dyad Composition | n = 25 | n = 23 | ||
| AG | 18 | 72% | 16 | 70% |
| AA | 4 | 16% | 1 | 4% |
| GG | 3 | 12% | 6 | 26% |
| Output to Amacrine | 29/56 | 52% | 19/59 | 32% |
| Input via Reciprocal | 4/13 | 31% | 4/11 | 36% |
ACKNOWLDEGEMENTS
We would like to thank Dr. Roger Price for donating some of the macaque tissue used in this experiment, Mrs. Lillemor Krosby and Mr. John Healy for excellent technical assistance, Mr. Lorenzo Morales for assistance with the figures, and Drs. Roy A. Jacoby and Helga Kolb for valuable discussions.
Supported by grants EY06472 and EY10608 from the National Eye Institute. E.S.Y. is a recipient of CNPq fellowship No. 350117/99-3 and a fellowship from the Latin American Scholars Program of the Pew Charitable Trusts.
LITERATURE CITED
- Boycott BB, Wässle H. Morphological classification of bipolar cells of the primate retina. Eur J Neurosci. 1991;3:1069–1088. doi: 10.1111/j.1460-9568.1991.tb00043.x. [DOI] [PubMed] [Google Scholar]
- Burr DC, Morrone MC, Ross J. Selective suppression of the magnocellular visual pathway during saccadic eye movements. Nature. 1994;371:511–3. doi: 10.1038/371511a0. [DOI] [PubMed] [Google Scholar]
- Calkins DJ. Synaptic orgainization of cone pathways in the primate retina. In: Gegenfurtner KR, Sharpe LT, editors. Color Vision: From Genes to Perception. Cambridge University Press; Cambridge: 1999. pp. 163–179. [Google Scholar]
- Calkins DJ, Schein SJ, Tsukamoto Y, Sterling P. M and L cones in macaque fovea connect to midget ganglion cells by different numbers of excitatory synapses. Nature. 1994;371:70–72. doi: 10.1038/371070a0. [DOI] [PubMed] [Google Scholar]
- Calkins DJ, Sterling P. Absence of spectrally specific lateral inputs to midget ganglion cells in primate retina. Nature. 1996;381:613–615. doi: 10.1038/381613a0. [DOI] [PubMed] [Google Scholar]
- Calkins DJ, Tsukamoto Y, Sterling P. Microcircuitry and mosaic of a blue-yellow ganglion cell in the primate retina. J Neurosci. 1998;18:3373–3385. doi: 10.1523/JNEUROSCI.18-09-03373.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaway EM. Structure and function of parallel pathways in the primate early visual system. J Physiol. 2005;566:13–19. doi: 10.1113/jphysiol.2005.088047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chander D, Chichilnisky EJ. Adaptation to temporal contrast in primate and salamander retina. J Neurosci. 2001;21:9904–16. doi: 10.1523/JNEUROSCI.21-24-09904.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiao CC, Masland RH. Starburst cells nondirectionally facilitate the responses of direction-selective retinal ganglion cells. J Neurosci. 2002;22:10509–13. doi: 10.1523/JNEUROSCI.22-24-10509.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chichilnisky EJ, Kalmar RS. Functional asymmetries in ON and OFF ganglion cells of primate retina. J Neurosci. 2002;22:2737–2747. doi: 10.1523/JNEUROSCI.22-07-02737.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen ED, Miller RF. The role of NMDA and non-NMDA excitatory amino acid receptors in the functional organization of primate retinal ganglion cells. Vis Neurosci. 1994;11:317–332. doi: 10.1017/s0952523800001668. [DOI] [PubMed] [Google Scholar]
- Dacey DM. Primate retina: cell types, circuits and color opponency. Prog Retin Eye Res. 1999;18:737–763. doi: 10.1016/s1350-9462(98)00013-5. [DOI] [PubMed] [Google Scholar]
- Dacey DM, Lee BB. The ‘blue-on’ opponent pathway in primate retina originates from a distinct bistratified ganglion cell type. Nature. 1994;367:731–735. doi: 10.1038/367731a0. [DOI] [PubMed] [Google Scholar]
- Demb JB, Haarsma L, Freed MA, Sterling P. Functional circuitry of the retinal ganglion cell’s nonlinear receptive field. J Neurosci. 1999;19:9756–9767. doi: 10.1523/JNEUROSCI.19-22-09756.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVries SH. Bipolar cells use kainate and AMPA receptors to filter visual information into separate channels. Neuron. 2000;2:847–856. doi: 10.1016/s0896-6273(00)00158-6. [DOI] [PubMed] [Google Scholar]
- Dowling JE, Boycott BB. Organization of the primate retina: electron microscopy. Proc R Soc Lond B. 1966;166:80–111. doi: 10.1098/rspb.1966.0086. [DOI] [PubMed] [Google Scholar]
- Ghosh KK, Grünert U. Synaptic input to small bistratified (blue-ON) ganglion cells in the retina of a new world monkey, the marmoset Callithrix jacchus. J Comp Neurol. 1999;413:417–428. doi: 10.1002/(sici)1096-9861(19991025)413:3<417::aid-cne5>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Grünert U, Wässle H. Glycine receptors in the rod pathway of the macaque monkey retina. Vis Neurosci. 1996;13:101–115. doi: 10.1017/s0952523800007161. [DOI] [PubMed] [Google Scholar]
- Haas K, Sin WC, Javaherian A, Li Z, Cline HT. Single-cell electroporation for gene transfer in vivo. Neuron. 2001;29:583–591. doi: 10.1016/s0896-6273(01)00235-5. [DOI] [PubMed] [Google Scholar]
- Haas K, Jensen K, Sin WC, Foa L, Cline HT. Targeted electroporation in Xenopus tadpoles in vivo—from single cells to the entire brain. Differentiation. 2002;70:148–154. doi: 10.1046/j.1432-0436.2002.700404.x. [DOI] [PubMed] [Google Scholar]
- Haverkamp S, Wässle H. Characterization of an amacrine cell type of the mammalian retina immunoreactive for vesicular glutamate transporter 3. J Comp Neurol. 2004;468:251–263. doi: 10.1002/cne.10962. [DOI] [PubMed] [Google Scholar]
- Holmgren-Taylor I. Synapses of the inner plexiform layer in the retina of cyprinid fish. Cell Tissue Res. 1983;229:337–350. [PubMed] [Google Scholar]
- Hosoya T, Baccus SA, Meister M. Dynamic predictive coding by the retina. Nature. 2005;436:71–77. doi: 10.1038/nature03689. [DOI] [PubMed] [Google Scholar]
- Jacoby R, Stafford D, Kouyama N, Marshak D. Synaptic inputs to ON parasol ganglion cells in the primate retina. J Neurosci. 1996;16:8041–8056. doi: 10.1523/JNEUROSCI.16-24-08041.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacoby RA, Marshak DW. Synaptic connections of DB3 diffuse bipolar cell axons in macaque retina. J Comp Neurol. 2000;416:19–29. doi: 10.1002/(sici)1096-9861(20000103)416:1<19::aid-cne3>3.0.co;2-h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacoby RA, Wiechmann AF, Amara SG, Leighton BH, Marshak DW. Diffuse bipolar cells provide input to OFF parasol ganglion cells in the macaque retina. J Comp Neurol. 2000;416:6–18. doi: 10.1002/(sici)1096-9861(20000103)416:1<6::aid-cne2>3.0.co;2-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jusuf PR, Haverkamp S, Grunert U. Localization of glycine receptor alpha subunits on bipolar and amacrine cells in primate retina. J Comp Neurol. 2005;488:113–128. doi: 10.1002/cne.20555. [DOI] [PubMed] [Google Scholar]
- Kolb H, DeKorver L. Midget ganglion cells of the parafovea of the human retina: a study by electron microscopy and serial section reconstructions. J Comp Neurol. 1991;303:617–636. doi: 10.1002/cne.903030408. [DOI] [PubMed] [Google Scholar]
- Kolb H, Zhang L, Dekorver L, Cuenca N. A new look at calretinin-immunoreactive amacrine cell types in the monkey retina. J Comp Neurol. 2002;453:168–184. doi: 10.1002/cne.10405. [DOI] [PubMed] [Google Scholar]
- Kolb H, Marshak D. The midget pathways of the primate retina. Doc Ophthalmol. 2003;106:67–81. doi: 10.1023/a:1022469002511. [DOI] [PubMed] [Google Scholar]
- Krüger J, Fischer B, Barth R. The shift-effect in retinal ganglion cells of the rhesus monkey. Exp Brain Res. 1975;23:443–446. doi: 10.1007/BF00238025. [DOI] [PubMed] [Google Scholar]
- Lin B, Martin PR, Solomon SG, Grünert U. Distribution of glycine receptor subunits on primate retinal ganglion cells: a quantitative analysis. Eur J Neurosci. 2000;12:4155–4170. [PubMed] [Google Scholar]
- Lin B, Martin PR, Grünert U. Expression and distribution of ionotropic glutamate receptor subunits on parasol ganglion cells in the primate retina. Vis Neurosci. 2002;19:453–465. doi: 10.1017/s0952523802194077. [DOI] [PubMed] [Google Scholar]
- Macri J, Martin PR, Grünert U. Distribution of the alpha1 subunit of the GABA(A) receptor on midget and parasol ganglion cells in the retina of the common marmoset Callithrix jacchus. Vis Neurosci. 2000;17:437–448. doi: 10.1017/s0952523800173109. [DOI] [PubMed] [Google Scholar]
- Mariani AP, Hersh LB. Synaptic organization of cholinergic amacrine cells in the rhesus monkey retina. J Comp Neurol. 1988;267:269–280. doi: 10.1002/cne.902670209. [DOI] [PubMed] [Google Scholar]
- Marshak DW, Aldrich LB, Del Valle J, Yamada T. Localization of immunoreactive cholecystokinin precursor to amacrine cells and bipolar cells of the macaque monkey retina. J Neurosci. 1990;10:3045–3055. doi: 10.1523/JNEUROSCI.10-09-03045.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshak DW, Yamada ES, Bordt AS, Perryman WC. Synaptic input to an ON parasol ganglion cell in the macaque retina: a serial section analysis. Vis Neurosci. 2002;19:299–305. doi: 10.1017/s0952523802192078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin PR, Grünert U. Spatial density and immunoreactivity of bipolar cells in the macaque monkey retina. J Comp Neurol. 1992;323:269–287. doi: 10.1002/cne.903230210. [DOI] [PubMed] [Google Scholar]
- McMahon MJ, Packer OS, Dacey DM. The classical receptive field surround of primate parasol ganglion cells is mediated primarily by a non-GABAergic pathway. J Neurosci. 2004;24:3736–3745. doi: 10.1523/JNEUROSCI.5252-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills SL, Massey SC. AII amacrine cells limit scotopic acuity in central macaque retina: A confocal analysis of calretinin labeling. J Comp Neurol. 1999;411:19–34. [PubMed] [Google Scholar]
- Ölveczky BP, Baccus SA, Meister M. Segregation of object and background motion in the retina. Nature. 2003;423:401–408. doi: 10.1038/nature01652. [DOI] [PubMed] [Google Scholar]
- Perry VH, Oehler R, Cowey A. Retinal ganglion cells that project to the dorsal lateral geniculate nucleus in the macaque monkey. Neuroscience. 1984;12:1101–1123. doi: 10.1016/0306-4522(84)90006-x. [DOI] [PubMed] [Google Scholar]
- Pinault D. A novel single-cell staining procedure performed in vivo under electrophysiological control: morpho-functional features of juxtacellularly labeled thalamic cells and other central neurons with biocytin or Neurobiotin. J Neurosci Methods. 1996;65:113–136. doi: 10.1016/0165-0270(95)00144-1. [DOI] [PubMed] [Google Scholar]
- Raviola E, Raviola G. Structure of the synaptic membranes in the inner plexiform layer of the retina: a freeze-fracture study in monkeys and rabbits. J Comp Neurol. 1982;209:233–248. doi: 10.1002/cne.902090303. [DOI] [PubMed] [Google Scholar]
- Roska B, Werblin F. Rapid global shifts in natural scenes block spiking in specific ganglion cell types. Nat Neurosci. 2003;6:600–608. doi: 10.1038/nn1061. [DOI] [PubMed] [Google Scholar]
- Solomon SG, Martin PR, White AJ, Ruttiger L, Lee BB. Modulation sensitivity of ganglion cells in peripheral retina of macaque. Vision Res. 2002;42:2893–2898. doi: 10.1016/s0042-6989(02)00414-5. [DOI] [PubMed] [Google Scholar]
- Solomon SG, Lee BB, White AJ, Ruttiger L, Martin PR. Chromatic organization of ganglion cell receptive fields in the peripheral retina. J Neurosci. 2005;25:4527–4539. doi: 10.1523/JNEUROSCI.3921-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stafford DK, Dacey DM. Physiology of the A1 amacrine: a spiking, axon-bearing interneuron of the macaque monkey retina. Vis Neurosci. 1997;14:507–522. doi: 10.1017/s0952523800012165. [DOI] [PubMed] [Google Scholar]
- Wässle H, Grünert U, Chun MH, Boycott BB. The rod pathway of the macaque monkey retina: identification of AII-amacrine cells with antibodies against calretinin. J Comp Neurol. 1995;361:537–551. doi: 10.1002/cne.903610315. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Rodieck RW. Parasol and midget ganglion cells of the primate retina. J Comp Neurol. 1989;289:434–454. doi: 10.1002/cne.902890308. [DOI] [PubMed] [Google Scholar]
- Yamada ES, Dmitrieva N, Keyser KT, Lindstrom JM, Hersh LB, Marshak DW. Synaptic connections of starburst amacrine cells and localization of acetylcholine receptors in primate retinas. J Comp Neurol. 2003;461:76–90. doi: 10.1002/cne.10672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada ES, Bordt AS, Marshak DW. Wide-field ganglion cells in macaque retinas. Vis Neurosci. 2005;22:383–393. doi: 10.1017/S095252380522401X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou ZJ, Marshak DW, Fain GL. Amino acid receptors of midget and parasol ganglion cells in primate retina. Proc Natl Acad Sci U SA. 1994;91:4907–4911. doi: 10.1073/pnas.91.11.4907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman RP. Bar synapses and gap junctions in the inner plexiform layer: synaptic relationships of the interstitial amacrine cell of the retina of the cichlid fish, Astronotus ocellatus. J Comp Neurol. 1983;218:471–479. doi: 10.1002/cne.902180410. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.