Abstract
Stem cells derived from adult tissues or from the inner cell mass of blastocyst-stage embryos can self-renew in culture and have the remarkable potential to undergo lineage-specific differentiation. Extensive studies have been devoted to achieving a better understanding of the soluble factors and the mechanism(s) by which they regulate the fate decisions of these cells, but it is only recently that a critical role has been revealed for physical and mechanical factors in controlling self-renewal and lineage specification. This review summarizes selected aspects of current work on stem cell mechanics with an emphasis on the influence of matrix stiffness, surface topography, cell shape and mechanical forces on the fate determination of mesenchymal stem cells and embryonic stem cells.
Keywords: cell mechanics, embryonic stem cells, mechanical factors, mechanical forces, mesenchymal stem cells, stem cell fate
Stem cells have remarkable potential to develop into many different cell types during early life and growth. They are unspecialized cells capable of renewing themselves through cell division, sometimes after long periods of inactivity. In addition, under certain physiological or experimental conditions, they can be induced to become tissue- or organ-specific cells with special functions and thus offer a source of precursor cells to treat degenerative, malignant and genetic diseases, as well as injury caused by inflammation, infection and trauma.
Two kinds of stem cells from animals and humans have been extensively worked on: embryonic stem cells (ESCs) and non embryonic ‘somatic’ or ‘adult’ stem cells. ESCs, derived from the inner cell mass of blastocyst-stage embryos can differentiate into cells of all three germ layers and are thus termed as pluripotent [1,2], while adult stem cells, found among differentiated cells in a tissue or organ, including the brain, bone marrow, peripheral blood, blood vessels, skeletal muscle and skin, are thought to be limited to differentiating into different cell types of their specific tissue of origin. However, in culture some adult stem cells produce more than the cell lineages characteristic of tissues in which they reside. For instance, cultured mesenchymal stem cells (MSCs) isolated from the bone marrow of mammalian species have been demonstrated to be pluripotent, as they are capable of differentiating not only to bone, adipose and cartilage tissue [3], but also to cells with visceral mesoderm, neuroectoderm and endoderm characteristics and their derivatives [3–8]. Thus, ESCs and MSCs are invaluable tools for biomedical research, drug discovery and cell-based therapies.
Recent research has demonstrated that the mechanical properties of the extra cellular environ ment and application of forces to cells trigger cellular responses essential for many aspects of cell structures and functions [8–16]. In addition to studies on cultured cells, accumulating evidence has demonstrated that mechanical forces play a critical role in regulating tissue organization and architecture in vivo [17–21]. While extensive efforts are devoted to the understanding of how soluble factors such as growth factors and cytokines trigger and transduce signals within stem cells, recent studies are beginning to reveal some fascinating details of the mechanical factors that influence the fate determination of these cells. In this review, we will summarize recent advances in the study of matrix stiffness, surface topography, cell shape and mechanical forces, primarily in ESCs and MSCs. Experiments and a future perspective that may further delineate the role of mechanical factors and address the molecular mechanisms of mechanotransduction will also be discussed.
Cellular microenvironment & mechanical stimuli therein
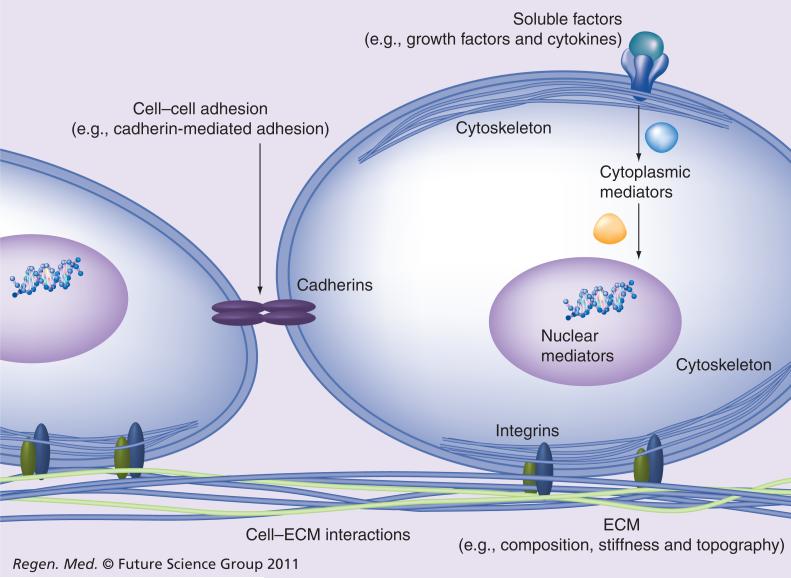
Fate decisions of cells, including stem cells, are influenced by the microenvironment in which they reside. Coordinated interactions with soluble factors, the extracellular matrix (ECM) and neighboring cells provide biochemical and mechanical signals that enable the cells to proliferate, survive, migrate or differentiate. Surface adhesion receptors, such as integrins and cadherins, mediate cell adhesion to the ECM scaffold and to the neighboring cells, respectively (Figure 1).
Figure 1. The cellular microenvironment.
Soluble factors and ECM combine with cell–cell adhesion to control cell fate.
ECM: Extracellular matrix.
As a key component of the extracellular environment, soluble factors have been extensively studied in pluripotent stem cells. For example, basic FGF is essential for undifferentiated growth of human ESCs (hESCs) [22]. The TGF-β superfamily, comprising TGF-β, Activin, Nodal and bone morphogenesis proteins (BMPs), has diverse roles in hESCs [23,24]. TGF-β/Activin/Nodal was shown to co-operate with FGF signaling to maintain pluripotency of hESCs by controlling the expression of the pluripotency factor NANOG. Activation of BMP signaling in hESCs induces mesoderm and trophoectoderm activities depending on the duration of activation [25–27], while activation of the Activin/Nodal pathway can trigger endoderm differentiation [28]. Conversely, inhibition of Activin/Nodal and BMP signaling, alone or in combination, promotes neuroectoderm specification [29–32]. Leukemia inhibitory factor (LIF), can substitute for feeder cells to maintain pluri potency in mouse ESCs (mESCs), but not in hESCs [33]. Furthermore, in contrast to hESCs, activation of the BMP signaling pathway supports self-renewal in combination with LIF in mESCs [34]. Distinction between mESCs and hESCs may be attributed to differences in species divergence and/or temporal origins during development [35]. TGF-b has been identified in global gene expression analyses of MSCs as one of three key growth factor pathways not only sufficient for MSC growth but also influential in differentiation into chondrocytes, osteocytes and adipocytes [36,37].
Mechanical stimuli are increasingly recognized as key regulators of cell structure and function, in addition to soluble factors. The ability of cells to sense forces, transmit them to the interior of the cell interior or to other cells, and transduce them into biochemical signals is essential for a spectrum of cellular responses, including motility of cells, differentiation and regulation of cell proliferation [10,11,15,16,38,39]. Within the cellular microenvironment, passive ECM properties including stiffness, topography and composition can regulate cell behaviors (Figure 1). Furthermore, as cells respond to cues from the microenvironmental cues, they can adopt different shapes, generate traction stress and produce mechanical forces that can be transmitted to neighboring cells. Application of a mechanical stimulus, such as fluid shear stress, to the cell surface activates mechano sensitive ion channels, heterotrimeric G proteins, protein kinases and other membrane-associated signal-transduction molecules; these trigger downstream signaling cascades that lead to force-dependent changes in gene expression [40]. These responses are usually mediated by the distortion of specific adhesion receptors that link to the cytoskeleton, rather than by deformation of the lipid bilayer alone [15].
Mechanical & physical factors determine the fate of MSCs
Substrate stiffness directs MSC fate specification
The importance of sensing the elastic properties of the ECM had been documented in studies with fibroblasts and other cells [14,41]. Engler et al. made the first attempt to evaluate the role of matrix stiffness in modulating the fate of human MSCs (hMSCs) [8], by applying an approach previously developed [42]. They generated polyacrylamide gels coated with collagen as an artificial matrix for cell attachment in vitro. The elastic properties of the matrix ranged from soft to relatively rigid, depending on the extent of chemical crosslinking. The effects of various elasticities on hMSCs were tested while the culture medium was kept the same. They found that the cell fate was dictated by the matrix stiffness: when cells were plated on soft substrates that mimicked the elasticity of brain tissue (0.1–1 kPa), they exhibited a neuronal phenotype. In addition, matrices with intermediate stiffness mimic muscle (8–17 kPa) were myogenic, while comparatively rigid matrices that mimic collagenous bone (25–40 kPa) proved to be osteogenic. Microarray analysis showed that the expression of markers for neurons, muscle, or bone was induced four to sixfold on the corresponding substrate. Immunofluorescence analysis showed that these three particular lineage-specific markers were present at 50% of the levels found in corresponding differentiated cultured cell lines. Therefore, although matrix elasticity is insufficient to induce terminal differentiation, it is very effective in guiding MSCs to develop into an early developmental lineage.
With respect to mechanisms, Engler et al. showed that inhibition of non-muscle myosin (NMM)II blocked all elasticity-directed lineage specification, without strongly perturbing many other aspects of cell function, implying distinct mechanisms whereby matrix stiffness governs directed differentiation [8]. This notion was supported by the observation that regulation by matrix stiffness was complementary to, and even synergistic with, the regulatory effects of specialized soluble factors previously shown to induce directed MSC lineage specification. Zemel et al. recently demonstrated that the alignment of NMMII-based stress fibers in MSCs depended non-monotonically on the matrix rigidity, achieving a maximum value when the cell and matrix rigidity were similar, suggesting mechanical coupling between external environment and internal cyto skeletal organization [43]. The detailed signaling mechanisms by which microenvironmental stiffness controls lineage specification remain to be defined.
The influence of ECM rigidity on MSC lineage commitment was also assessed in more physiologically relevant 3D culture systems. Huebsch et al. encapsulated mouse MSCs into a 3D hydrogel synthetic ECM formed by alginate polymers that present integrin-binding RGD peptides [44]. In this culture model, matrix rigidity had significant effects on MSC phenotype, with osteogenic commitment occurring primarily at intermediate elasticity (11–30 kPa) and adipogenic lineage predominating in softer (2.5–5.0 kPa) microenvironments. While the effects of matrix stiffness were generally in agreement with earlier studies using 2D cultures [8], cell fate in 3D was not correlated with morphology in contrast to previous 2D work [12] (see the following section). Instead, in 3D cultures matrix stiffness regulated integrin binding as well as reorganization of adhesion ligands on the nanoscale, both of which were traction dependent and correlated with osteogenic commitment of MSC populations [8].
Importantly, matrix stiffness not only regulates lineage specifications, but also acts as a potent regulator of self-renewal in adult stem cells. For example, Gilbert et al. showed that, unlike muscle stem cells cultured on rigid plastic dishes (~106 kPa), which lose ‘stemness’, leading to progenitors with greatly diminished regenerative potential, muscle stem cells cultured on soft substrates mimicking the elasticity of muscle (12 kPa) self-renewed and contributed extensively to muscle regeneration when subsequently transplanted into mice [45]. This study again demonstrates that matrix stiffness controls various stem cell fate decisions.
Cell shape regulates commitment of MSCs
Based on previous evidence that cell shape was critical for the regulation of cellular processes such as proliferation, survival and differentiation [10,46,47], McBeath et al. investigated whether changes in cell shape could regulate the commitment of hMSCs to different lineages [12]. By using a micropatterning technique to control cell shape and degree of cell spreading, they demonstrated that hMSCs, when allowed to adhere, flatten and spread, and undergo osteogenesis. By contrast, unspread, round cells became adipocytes. An earlier study suggested that the cell shape could affect the Rho family of GTPases [48], prompting McBeath et al. to assess whether RhoA was involved in lineage specification. They found that the shape-dependent control of lineage commitment of hMSCs was mediated by RhoA activity. Expressing dominant-negative RhoA induced hMSCs to become adipocytes, while constitutively active RhoA caused osteogenesis. This RhoA-controlled commitment signal required actin–myosin-generated tension and completely bypassed the need for soluble differentiation factors. This study demonstrates that cell shape, cytoskeletal mechanics and RhoA signaling are integral to the commitment of stem cell fate.
Topographic changes influence MSC fate
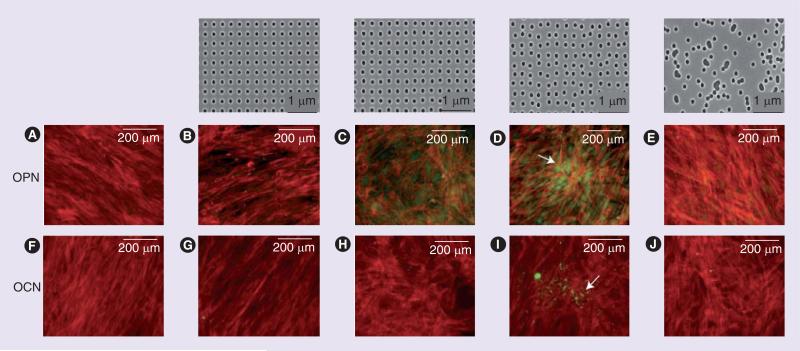
The ECM networks are comprised of a complex mixture of pores, ridges and fibers with sizes in the nanometer range. Physical topography is known to influence cell behavior [49,50]. The development of electron beam lithography (EBL) for the fabrication of ultraprecise nano topographies has greatly facilitated the examination of cell–nanoenvironment interactions. By using a defined EBL, Dalby et al. determined how MSCs responded to nanoscale topographic features [51]. Because nanoscale order in vivo does not typically exhibit the level of organization allowed by EBL, they also used EBL to create not only highly ordered symmetries, but also surfaces with different levels of nano disorder and random surfaces (Figure 2). The substratum was embossed with 120-nm-diameter, 100-nm-deep nanopits from an original pattern, and five different patterns were used, all with either absolute or average center–center spacing of 300 nm: square array (SQ); disordered square array with dots displaced randomly by up to 50 nm on both axes from their position in a true square (DSQ50); disordered square array with dots displaced randomly by up to 20 nm on both axes from their position in a true square (DSQ20); and pits placed randomly over a 150 μm by 150 μm field, repeated to fill a 1 cm2 area (RAND) (Figure 2). After 21 days in culture, MSCs on DSQ50 showed discrete areas of intense cell aggregation and exhibited bone-specific ECM proteins osteopontin and osteocalcin-positive regions, as well as early nodule formation (Figure 2D & I). An extended culture time allowed positive identification of mineralization within the discrete nodules only observed in MSCs cultured on the DSQ50 topography (Figure 2). The use of nanoscale disorder led to similar efficiency of differentiation to that of cells cultured with osteogenic media. But the differentiated cells had a distinct differentiation profile compared with those treated with osteogenic media, suggesting a distinct mechanism for the induction of differentiation.
Figure 2. Osteopontin and osteocalcin staining of mesenchymal stem cells 21 days after cultivation on various nanotopographies.
The top row shows images of nanotopographies fabricated by electron beam lithography. All have 120-nm diameter pits with square array (SQ), disordered square array with dots displaced randomly by up to 50 nm on both axes from their position in a true square (DSQ20), disordered square array with dots displaced randomly by up to 50 nm on both axes from their position in a true square (DSQ50) and pits placed randomly over a 150 × 150 μm field (RAND). Mesenchymal stem cells: on the control (A & F); SQ (B & G); DSQ20 (C & H); DSQ50 (D & I); and RAND (E & J). OPN-positive cells were detected in cells on DSQ20 and DSQ50, and OCN-positive cells were detected in cells on DSQ50. Mature bone nodules containing mineral were also observed in cells on DSQ50 (arrows). Actin: red, OPN/OCN: green.
OCN: Osteocalcin; OPN: Osteopontin.
Adapted with permission from [51].
Mechanical forces control MSC gene expression
To investigate the effects of mechanical forces or strains on MSC differentiation Kurpinski et al. used a micropattened strip to align the MSCs along the direction of the uniaxial strain [52]. They demonstrated that expression of calponin 1, a marker for smooth muscle cells, was increased, whereas cartilage matrix marker expression was decreased. However, when cells were aligned perpendicularly to the direction of the strain, the changes in gene expression were diminished [52]. These results suggest that mechanical strain has profound effects on gene expression and probably the fate of MSCs. In a more recent study, the same group found that TGF-β1 and cyclic mechanical strain, when applied simultaneously, led to a synergistic upregulation of calponin 1 gene expression, indicating that soluble factors and mechanical strain can collaborate to control gene expression in MSCs [53].
Regulation of ESC fate by mechanical & physical factors
Embryonic stem cells can give rise to derivatives of all three embryonic germ layers and therefore have been hailed as a possible means for treating a variety of diseases. Recent studies demonstrated that induced pluripotent stem (iPS) cells can be derived from somatic cells by the use of defined factors [51,54–57]. In comparison with ESCs, iPS cells have the potential advantages to generate genetically diverse and patient-specific differentiated cell populations. To fully realize the potential of ESCs and iPS cells, the conditions and factors that support their long-term self-renewal and efficiently direct cell differentiation must be better defined. As described later, physical and mechanical factors have emerged as key determinants of the cell-fate decisions of ESCs. An improved understanding of these factors and their interplay with biochemical signaling might enable us to better control the fate of ESCs and iPS cells, and therefore facilitate their applications for cell-based therapy and drug discovery.
Mechanical forces control ESC differentiation
Accumulating experimental evidence suggests that mechanical contractile forces have a role in development [58]. However, inability to access animal embryonic cells during early development makes it difficult to determine how important mechanical forces are during early development of animals and how sensitive embryonic cells are to force. Cultured ESCs offer an excellent model for studying biological responses to force by the cells in the inner cell mass. We recently demonstrated that adherent mESCs were softer and much more sensitive to a local cyclic stress than their differentiated counterparts [59]. A local cyclic stress applied through focal adhesions induced spreading in mESCs but not in mESC-derived differentiated cells, which were ten-times stiffer. Cell spreading was accompanied by differentiation of ESCs, as evidenced by downregulation of oct3/4 (pou5f1) gene expression. Traction quantification and pharmacological or shRNA intervention revealed that myosin II contractility, F-actin, Src or cdc42 are essential in the spreading response. These findings demonstrate that cell softness dictates cellular sensitivity to force, suggesting that a local, small, cyclic stress plays a critical role in inducing strong biological responses in soft mESCs that originate from the inner cell mass and in shaping embryogenesis during development. Interestingly, mESCs, when plated as single cells, generated low basal tractions on soft substrates and increased their basal tractions as substrate stiffness increases. However, stiffness at the apical surface of a single mESC did not vary with basal substrate stiffness [60]. By contrast, when mESCs were cultured as aggregates, both apical stiffness and basal tractions of mESC colonies increased with substrate stiffness. This may be attributed to the mechanosensing capacities of E-cadherins at lateral adherens junctions, which could promote mechanical coupling between the apical and the basal cytoskeletal networks in aggregated cells [61]. Future studies are needed to understand the specific mechanisms of stress-induced inhibition of oct3/4 expression in mESCs, to determine whether these findings can be extended to hESCs and iPS cells. It will also be interesting to determine what type of germ-layer cell (endoderm, mesoderm or ectoderm) can be derived from these soft mESCs by what mode of mechanical perturbations. In a separate study, Shimizu et al. demonstrated that cyclic uniaxial strain induced vascular smooth muscle cell differentiation in VEGF receptor 2 (Flk-1)-positive mESCs [62]. Interestingly, fluid shear stress caused the same cells (i.e., Flk-1-positive mESCs) to show vascular endothelial cell activities [63].
Mechanical forces can also direct differentiation of mESC-derived cells. Owing to fluid shear stress, the frictional force generated by viscous flow acting along cells lining blood vessels exerted profound effects on the structure and function of vascular endothelial cells [64]. Adamo et al. assessed its ability to induce hematopoietic commitment [65]. mESCs were induced to form embryoid bodies, in which cells first committed to mesoderm and then produced cells containing the earliest embryonic hematopoietic precursors. The embryoid body-derived cells were further exposed to fluid shear stress, which caused a strong upregulation of Runx1, a master regulator of hematopoietic, and Myb, the prototypical markers of hemogenic sites and concomitantly augmented the hematopoietic colony-forming potential. Consistent with ESC-derived hemato poiesis, shear stress was also found to increase hematopoietic colony-forming potential and expression of hematopoietic markers in the para-aortic splanchnopleura/aorta–gonads–mesonephros of mouse embryos. Abrogation of nitric oxide, a mediator of shear-stress-induced signaling, disrupted hematopoietic potential both in vitro and in vivo.
Effects of matrix stiffness on ESCs
Substrate stiffness was found to also profoundly influence differentiation of ESCs. Evans et al. induced spontaneous differentiation in mESCs by removing LIF, a soluble factor essential for maintaining cells at the undifferentiated stage of mESCs and then assessed the effects of substrate stiffness on lineage specification [66]. They found that while attachment of cells was not affected by the stiffness, cell spreading and cell growth were both increased as a function of substrate stiffness. Similarly, several genes expressed in the primitive streak during gastrulation and implicated in early mesendoderm differentiation, such as Brachyury, Mixl1 and Eomes, were upregulated in cell cultures on stiffer compared with softer substrates. In addition, they also induced mESCs to undergo terminal osteogenic differentiation in the presence of osteogenic supplements and found that differentiation of ESCs was enhanced on stiff substrates compared with soft substrates. These results demonstrated that the mechanical environment can play a role in both early and terminal ESC differentiation. Notably, a high range of substrate stiffness was used in this study (41–2700 kPa). In a more recent study, Chowdhury et al. assessed the effects of lower-stiffness substrates and demonstrated a sharp contrast to the mESCs seeded on the conventional rigid substrates [61]. mESCs cultured on the soft substrates that matched the intrinsic stiffness of the mESCs (~1 kPa) and in the absence of exogenous LIF for 5 days, still generated homogeneous undifferentiated colonies, maintained high levels of pluripotency markers including OCT-4, NANOG and alkaline phosphatase activities, and could be differentiated into cells of all three embryonic germ layers. The soft substrate condition allows mESCs to undergo long-term self-renewal for at least 15 passages in the absence of LIF, suggesting that it can be applied to long-term cultivation of mESCs and potentially to other pluripotent stem cells.
Effects of colony sizes on ESC fate
Human ESCs, like other pluripotent stem cells, proliferate as tight colonies wherein individual cells are strongly adhered to one another. To ask whether colony size and cellular composition played a role in regulating hESC fate and signaling, Peerani et al. used microcontact printing to pattern hESC colonies onto defined adhesive islands with controlled colony diameter and pitch (the distance between colonies) [67]. In these experiments, exogenous cytokines that supported undifferentiated growth of hESCs (e.g., basic FGF and TGF-b) were withdrawn from the culture medium, and the differentiation of hESCs was followed over a 48 h period. This short time window was chosen to track initial changes in colony composition instead of long-term indirect effects. They found that larger colonies with high local cell density promote maintenance of the undifferentiated phenotype in hESCs. This was attributed to increased activity of BMP antagonists such as GDF3, which suppressed BMP signaling and Smad1 activation. Activation of BMP signaling triggers hESC differentiation [25–27]. By contrast, small colonies were found to differentiate into extraembryonic endoderm, which antagonized self-renewal by local secretion of BMP2. These results demonstrated that colony sizes and cellular densities are important physical factors that control critical signaling pathways for hESC fate.
In a more recent study, the same group tested whether combining the control of colony sizes and the treatment of soluble factors would induce directed hESC differentiation. It has been well documented that BMPs and Activin/Nodal are able to induce primitive streak-like differentiation of ESCs [25–28,68,69], but produced a mixture of mesoderm and endoderm cells. They therefore asked whether controlling colony size might also be able to selectively guide these primitive streak-like cells to either mesoderm or definitive endoderm lineages. In the presence of activin A and BMP2, they found that smaller colonies directed hESC differentiation toward definitive endoderm, while larger colonies led to mesoderm differentiation. The functional relevance of controlling colony size and early mesoderm and endoderm differentiation was confirmed through hematopoietic and primitive gut differentiation assays. These results demonstrate the possibility of controlling colony sizes in the presence of inductive factors to guide directed hESC differentiation to either endoderm or mesoderm, together with differentiation-inducing soluble factors.
Putative mechanisms of mechanotransduction
Taken together, a body of observations and experiments demonstrated unequivocally that mechanical factors play significant roles in controlling the fate decisions of pluripotent stem cells, including ESCs and MSCs. However, how individual cells sense these mechanical signals and transduce them into changes in intra cellular biochemical signals and gene expression remains largely unclear. Based on the knowledge learned from other model systems, we propose the following putative mechanisms of mechanotransduction in stem cells. The versatile role of NMMII in mechanical regulation of cell fate is also discussed.
Mechanical regulation of integrin activity & signaling
Accumulating evidence points to the deformation of integrins, focal adhesion proteins and possibly other structural proteins as a key molecular mechanism of strain sensing. Friedland et al. reported that α5β1–integrin could switch between relaxed and tensioned states in response to myosin II-generated cytoskeletal force [70]. In this scenario, force might combine with cell-substrate adhesion to generate tension that activates the integrin molecule mechanically. Using the neutrophil migration model, we also showed that myosin II-dependent cellular contraction was necessary for α5β1-integrin activation in neutrophils during chemotaxis [71]. Furthermore, application of physiologically relevant forces causes stretching of single talin rods that expose cryptic binding sites for vinculin in vitro [72]. In a more recent study, Grashoff et al. used a vinculin tension biosensor and demonstrated that in living cells, both vinculin recruitment to and force transmission across focal adhesions are necessary for the stabilization of focal adhesions under force [73].
Therefore, it is likely that differences in matrix properties could cause changes in strains at the cell–matrix sites, leading to differential regulation of the conformational and/or unfolding state of the proteins at adhesions and at other distant sites (e.g., inside the nucleus). Thus, mechanical regulation of inte grin activity and signaling may prove to be a key mechanism that governs fate decisions in stem cells. In experiments with in situ labeling of sterically shielded cysteines, followed by fluorescence imaging, quantitative mass spectrometry, and sequential two-dye labeling, forced unfolding of proteins such as NMMIIA and vimentin was detected in the ‘tensed’ state of adherent MSCs [74]. This approach may prove useful in mapping out force-induced conformational changes of proteins in various adhesion states.
Mechanical action at a distance
It has become increasingly clear that mechanical action occurs at a distance in living cells (see reviews in [15,40]). This is made possible by the propagation of forces and energy through transmembrane integrins and cadherins, associated focal adhesions and junctional complexes, and cytoskeletal networks that connect to the nucleus, the internal nuclear scaffolds and linked chromatin. The fidelity and speed of this intracellular mechanical signaling response can be modulated by altering cytoskeletal prestress, which controls the stiffness of tensed cytoskeletal filaments, such as actin-based stress fibers and intermediate filaments, which span long distances in the cytoplasm. Forces that act on the nucleus might promote changes in the shape, folding or kinetics of specific load-bearing molecules or might modify higher-order chromatin organization, and thereby alter nuclear protein self-assembly, gene transcription, DNA replication or RNA processing – all of which are crucial for cell behavior [15]. This unique form of mechanical signaling provides a more rapid and efficient way to convey information over long distances in living cells than diffusion-based biochemical signaling. It also helps to explain how mechanical forces simultaneously alter the activities of multiple molecules at various sites in the cytoplasm and nucleus, a response that is crucial for control of cellular behavior, tissue development and stem cell fate.
TGF-β release mediated by mechanical force
TGF-β is a growth factor essential for fate choices in both ESCs and MSCs (see previously). It is expressed as an inactive precursor complexed with latency associate peptide (LAP). Dissociation of LAP from TGF-β is essential for its bind to the receptor. The LAP and TGF-β complex further associates with the large fibrillar latent TGF-β-binding proteins to form a large latent complex. TGF-β can be released via a variety of mechanisms that vary depending on the cell types and physiological context. One mechanism for TGF-β release involves proteases, which cleave LAP or certain types of integrins [75].
Recent studies began to reveal evidence for tension-mediated TGF-β1 release. Wipff et al. reported that both external stretching of myofibroblast cultures and increasing myofibroblast intracellular tension directly activated latent TGF-β1 from the ECM [76]. The activation/release requires α-SMA-positive stress fibers and integrin binding to LAP in the large latent complex, but was independent of proteolysis. Stress-induced TGF-β1 activation occurs on stiffer culture substrates with stiffness similar to that of fibroblast-populated early wound granulation tissue, but does not occur on more compliant substrates. In a later study, Ahamed et al. demonstrated that TGF-β1 released from platelets or fibroblasts underwent dramatic activation when subjected to stirring or shear forces [77]. Activation required the presence of latent TGF-β-binding proteins, as TGF-β1 contained in complex with only LAP could not be activated by the same mechanical perturbations. These interesting studies point to a novel mechanism by which growth factor activity can be regulated by local mechanics. Whether tension-induced TGF-β release plays a role in ESCs and MSCs will be an interesting topic for future experimentation.
Versatile roles of myosin II in the regulation of stem cell fate
Non-muscle myosin II is an actin-binding protein that regulates the contractile functions in cells. Of the three different NMMII isoforms identified, two (NMMIIA and NMMIIB) are found almost ubiquitously in higher organisms. Deletion of NMMIIA or NMMIIB in mice leads to embryonic lethality [78,79]. NMMIIA knockout leads to lethality in peri-implantation stage embryos, whereas NMMIIB null mice die late in gestation. Therefore, NMMIIs are essential for early development.
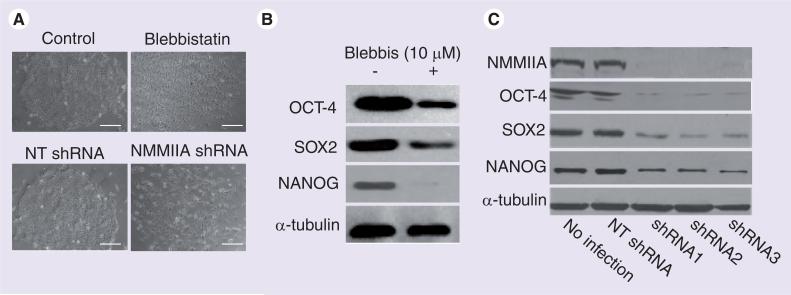
The essential role of NMMII in mechanical regulation in stem cell fate choices has been documented. As depicted earlier in this review, matrix elasticity and cell shape-directed lineage specification of MSCs critically depends on NMMII [8,12], although the detailed molecular mechanisms have not been provided. hESCs, when plated as single cells, exhibited poor viability and cloning efficiency. NMMII-dependent contractility is responsible for the reduced survival: transient inhibition of NMMII or other components of the Rho–ROCK–NMMII cascade prior to/during cell plating markedly improved the viability of dissociated hESCs [80–84], probably by increasing cell spreading and adhesion to the ECM substrates. Interestingly, although hyper-activation of NMMII reduces survival of dissociated hESCs during initial plating, its activity is essential for long-term self-renewal of hESCs: long-term inhibition of NMMII or depletion of NMMIIA impaired the stability of the OCT-4/SOX2/NANOG transcriptional circuitry, prevented colony formation and reduced long-term cell survival (Figure 3) [82]. E-cadherin serves as a major target of NMMIIA in hESCs, as described in some other cell types [78,85]. Inhibition or knockdown of NMMIIA disrupted E-cadherin-mediated adhesion and mechanical activity and reduced the levels of E-cadherin protein [82]. Furthermore, ectopic expression of E-cadherin nearly completely rescued the defects of NMMIIA inhibition and depletion [82]. Taken together, these results suggest that NMMIIA-based contractility does not serve simply as a read-out of intracellular signals but, instead, plays an active role in generating or transmitting signals that affect the fate of stem cells.
Figure 3. Non-muscle myosin IIA is required for colony formation and pluripotency in human embryonic stem cells.
(A) Phase contrast images of human embryonic stem cells (hESCs) with or without the following treatments: blebbistatin (a highly specific inhibitor of NMMII), NT shRNA and NMMIIA-specific shRNAs. shRNA-mediated RNAi was used for depletion of NMMIIA. Depletion or inhibition of NMMII markedly impaired colony formation in hESCs. (B & C) Western blot analysis of OCT-4, SOX2 and NANOG proteins in hESCs treated with or without blebbistatin, or with or without depletion of NMMIIA. Three different shRNAs targeting NMMIIA were used. Depletion or inhibition of NMMII significantly reduced OCT-4, SOX2 and NANOG protein levels. a-tubulin was a loading control.
Scale bar in (A): 100 μm.
NMM: Non-muscle myosin; NT: Nontargeting.
Adapted with permission from [82].
Future perspective
Many questions remain to be explored. First, and probably most important, experiments must be designed to dissect the detailed molecular mechanisms whereby mechanical signals are transduced to govern self-renewal and directed differentiation in the stem cells.
Other questions abound. Can mechanical factors (alone, or in combination) be optimized to induce highly efficient lineage specification of ESCs and MSCs? Similarly, can high-efficiency directed differentiation be accomplished by manipulating mechanical and biochemical signals together? What are the contributions of mechanical factors to late-stage differentiation (e.g., from ESC-derived neural stem cells to mature neurons)? What are the influences of mechanical stimuli on cellular behaviors in the 3D environment, compared with 2D? Do other stem cells, most notably iPS cells, respond similarly to self-renewal or differentiation promoting mechanical signals? New insights into these puzzles should prove valuable in understanding mechanical regulation of stem cell fate and would also facilitate the use of these cells for developmental studies, cell-based therapy and drug discovery.
Executive summary.
■ Two types of stem cells – embryonic stem cells (ESCs) and adult stem cells – have been discussed here.
■ ESCs and mesenchymal stem cells (MSCs) are pluripotent and represent invaluable tools for biomedical research, drug discovery and cell-based therapies.
The cellular microenvironment & the mechanical stimuli therein
■ Soluble factors, the extracellular matrix and neighboring cells are components of cellular microenvironment. Their coordinated interactions provide biochemical and mechanical signals that enable the cells to proliferate, survive, migrate and differentiate.
■ Mechanical stimuli are increasingly recognized as key regulators of cell structure and function in addition to soluble factors. The ability of cells to sense forces, transmit them to the cell interior or to other cells, and transduce them into biochemical signals is essential for a spectrum of cellular responses.
Mechanical & physical factors determine the fate of MSCs
■ Substrate stiffness controls MSC fate decisions, including self-renewal and lineage specifications.
■ Cell shape regulates commitment of MSCs. The shape-dependent control of lineage commitment is mediated by RhoA activity.
■ Topographic changes influence MSC fate via a mechanism distinct from soluble factors.
■ Mechanical forces or strains control MSC gene expression.
Regulation of ESC fate by mechanical & physical factors
■ Mechanical forces control differentiation of ESCs and ESC-derived cells.
■ Matrix stiffness has an effect on ESC self-renewal and directed differentiation.
■ Colony size has an effect on ESC fate.
Potential mechanisms of mechanotransduction
■ Mechanical regulation of integrin activity and signaling may prove to be a key mechanism that governs fate decisions in stem cells.
■ Mechanical action at a distance alters nuclear protein self-assembly, gene transcription, DNA replication and RNA processing.
■ TGF-β release mediated by mechanical force may play a role in ESCs and MSCs.
■ Myosin II has versatile roles in the regulation of stem cell fate.
Acknowledgements
The authors would like to thank the Wang laboratories for helpful discussion.
Financial & competing interests disclosure
The authors’ work is supported by grants from the NIH (GM083812, GM083601 and GM072744), the Illinois Regenerative Medicine Institute (IDPH 2006–05516), NSF CAREER award (0953267), NSF-STC EBICS award, the Beckman award from the University of Illinois and the National Natural Science Foundation of China (30728022). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Bibliography
Papers of special note have been highlighted as:
■ of interest
■■ of considerable interest
- 1.Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292:154–156. doi: 10.1038/292154a0. [DOI] [PubMed] [Google Scholar]
- 2.Thomson JA, Itskovitz-Eldor J, Shapiro SS, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 3.Jiang Y, Jahagirdar BN, Reinhardt RL, et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418:41–49. doi: 10.1038/nature00870. [DOI] [PubMed] [Google Scholar]
- 4.Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 5.Deng J, Petersen BE, Steindler DA, Jorgensen ML, Laywell ED. Mesenchymal stem cells spontaneously express neural proteins in culture and are neurogenic after transplantation. Stem Cells. 2006;24:1054–1064. doi: 10.1634/stemcells.2005-0370. [DOI] [PubMed] [Google Scholar]
- 6.Hofstetter CP, Schwarz EJ, Hess D, et al. Marrow stromal cells form guiding strands in the injured spinal cord and promote recovery. Proc. Natl Acad. Sci. USA. 2002;99:2199–2204. doi: 10.1073/pnas.042678299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kondo T, Johnson SA, Yoder MC, Romand R, Hashino E. Sonic hedgehog and retinoic acid synergistically promote sensory fate specification from bone marrow-derived pluripotent stem cells. Proc. Natl Acad. Sci. USA. 2005;102:4789–4794. doi: 10.1073/pnas.0408239102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8 ■■.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [Matrix stiffness directs mesenchymal stem cell (MSC) lineage specification.] [DOI] [PubMed] [Google Scholar]
- 9.O'Neill C, Jordan P, Ireland G. Evidence for two distinct mechanisms of anchorage stimulation in freshly explanted and 3T3 Swiss mouse fibroblasts. Cell. 1986;44:489–496. doi: 10.1016/0092-8674(86)90470-8. [DOI] [PubMed] [Google Scholar]
- 10.Chen CS, Mrksich M, Huang S, Whitesides GM, Ingber DE. Geometric control of cell life and death. Science. 1997;276:1425–1428. doi: 10.1126/science.276.5317.1425. [DOI] [PubMed] [Google Scholar]
- 11.Engler AJ, Griffin MA, Sen S, Bonnemann CG, Sweeney HL, Discher DE. Myotubes differentiate optimally on substrates with tissue-like stiffness: pathological implications for soft or stiff microenvironments. J. Cell. Biol. 2004;166:877–887. doi: 10.1083/jcb.200405004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12 ■■.McBeath R, Pirone DM, Nelson CM, Bhadriraju K, Chen CS. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev. Cell. 2004;6:483–495. doi: 10.1016/s1534-5807(04)00075-9. [Demonstrates that cell shape and RhoA activity control MSC differentiation.] [DOI] [PubMed] [Google Scholar]
- 13.Georges PC, Miller WJ, Meaney DF, Sawyer ES, Janmey PA. Matrices with compliance comparable to that of brain tissue select neuronal over glial growth in mixed cortical cultures. Biophys. J. 2006;90:3012–3018. doi: 10.1529/biophysj.105.073114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Discher DE, Janmey P, Wang YL. Tissue cells feel and respond to the stiffness of their substrate. Science. 2005;310:1139–1143. doi: 10.1126/science.1116995. [DOI] [PubMed] [Google Scholar]
- 15.Wang N, Tytell JD, Ingber DE. Mechanotransduction at a distance: mechanically coupling the extracellular matrix with the nucleus. Nat. Rev. Mol. Cell. Biol. 2009;10:75–82. doi: 10.1038/nrm2594. [DOI] [PubMed] [Google Scholar]
- 16.Yeung T, Georges PC, Flanagan LA, et al. Effects of substrate stiffness on cell morphology, cytoskeletal structure, and adhesion. Cell Motil. Cytoskeleton. 2005;60:24–34. doi: 10.1002/cm.20041. [DOI] [PubMed] [Google Scholar]
- 17.Pouille PA, Ahmadi P, Brunet AC, Farge E. Mechanical signals trigger Myosin II redistribution and mesoderm invagination in Drosophila embryos. Sci. Signal. 2009;2:ra16. doi: 10.1126/scisignal.2000098. [DOI] [PubMed] [Google Scholar]
- 18.Martin AC, Kaschube M, Wieschaus EF. Pulsed contractions of an actin-myosin network drive apical constriction. Nature. 2009;457:495–499. doi: 10.1038/nature07522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krieg M, Arboleda-Estudillo Y, Puech PH, et al. Tensile forces govern germ-layer organization in zebrafish. Nat. Cell Biol. 2008;10:429–436. doi: 10.1038/ncb1705. [DOI] [PubMed] [Google Scholar]
- 20.Rauzi M, Verant P, Lecuit T, Lenne PF. Nature and anisotropy of cortical forces orienting Drosophila tissue morphogenesis. Nat. Cell Biol. 2008;10:1401–1410. doi: 10.1038/ncb1798. [DOI] [PubMed] [Google Scholar]
- 21.Desprat N, Supatto W, Pouille PA, Beaurepaire E, Farge E. Tissue deformation modulates twist expression to determine anterior midgut differentiation in Drosophila embryos. Dev. Cell. 2008;15:470–477. doi: 10.1016/j.devcel.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 22.Levenstein ME, Ludwig TE, Xu RH, et al. Basic fibroblast growth factor support of human embryonic stem cell self-renewal. Stem Cells. 2006;24:568–574. doi: 10.1634/stemcells.2005-0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vallier L, Alexander M, Pedersen RA. Activin/Nodal and FGF pathways cooperate to maintain pluripotency of human embryonic stem cells. J. Cell Sci. 2005;118:4495–4509. doi: 10.1242/jcs.02553. [DOI] [PubMed] [Google Scholar]
- 24.Xu RH, Sampsell-Barron TL, Gu F, et al. NANOG is a direct target of TGF-b/activin-mediated SMAD signaling in human ESCs. Cell Stem Cell. 2008;3:196–206. doi: 10.1016/j.stem.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chadwick K, Wang L, Li L, et al. Cytokines and BMP-4 promote hematopoietic differentiation of human embryonic stem cells. Blood. 2003;102:906–915. doi: 10.1182/blood-2003-03-0832. [DOI] [PubMed] [Google Scholar]
- 26.Xu RH, Chen X, Li DS, et al. BMP4 initiates human embryonic stem cell differentiation to trophoblast. Nat. Biotechnol. 2002;20:1261–1264. doi: 10.1038/nbt761. [DOI] [PubMed] [Google Scholar]
- 27.Zhang P, Li J, Tan Z, et al. Short-term BMP-4 treatment initiates mesoderm induction in human embryonic stem cells. Blood. 2008;111:1933–1941. doi: 10.1182/blood-2007-02-074120. [DOI] [PubMed] [Google Scholar]
- 28.D'Amour KA, Agulnick AD, Eliazer S, Kelly OG, Kroon E, Baetge EE. Efficient differentiation of human embryonic stem cells to definitive endoderm. Nat. Biotechnol. 2005;23:1534–1541. doi: 10.1038/nbt1163. [DOI] [PubMed] [Google Scholar]
- 29.Chambers SM, Fasano CA, Papapetrou EP, Tomishima M, Sadelain M, Studer L. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat. Biotechnol. 2009;27:275–280. doi: 10.1038/nbt.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pera MF, Andrade J, Houssami S, et al. Regulation of human embryonic stem cell differentiation by BMP-2 and its antagonist noggin. J. Cell Sci. 2004;117:1269–1280. doi: 10.1242/jcs.00970. [DOI] [PubMed] [Google Scholar]
- 31.Smith JR, Vallier L, Lupo G, Alexander M, Harris WA, Pedersen RA. Inhibition of Activin/Nodal signaling promotes specification of human embryonic stem cells into neuroectoderm. Dev. Biol. 2008;313:107–117. doi: 10.1016/j.ydbio.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 32.Zhou J, Su P, Li D, Tsang S, Duan E, Wang F. High-efficiency induction of neural conversion in human ESCs and human induced pluripotent stem cells with a single chemical inhibitor of transforming growth factor-β superfamily receptors. Stem Cells. 2010;28:1741–1750. doi: 10.1002/stem.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Daheron L, Opitz SL, Zaehres H, et al. LIF/STAT3 signaling fails to maintain self-renewal of human embryonic stem cells. Stem Cells. 2004;22:770–778. doi: 10.1634/stemcells.22-5-770. [DOI] [PubMed] [Google Scholar]
- 34.Ying QL, Nichols J, Chambers I, Smith A. BMP induction of Id proteins suppresses differentiation and sustains embryonic stem cell self-renewal in collaboration with STAT3. Cell. 2003;115:281–292. doi: 10.1016/s0092-8674(03)00847-x. [DOI] [PubMed] [Google Scholar]
- 35.Brons IG, Smithers LE, Trotter MW, et al. Derivation of pluripotent epiblast stem cells from mammalian embryos. Nature. 2007;448:191–195. doi: 10.1038/nature05950. [DOI] [PubMed] [Google Scholar]
- 36.Barry F, Boynton RE, Liu B, Murphy JM. Chondrogenic differentiation of mesenchymal stem cells from bone marrow: differentiation-dependent gene expression of matrix components. Exp. Cell Res. 2001;268:189–200. doi: 10.1006/excr.2001.5278. [DOI] [PubMed] [Google Scholar]
- 37.Ng F, Boucher S, Koh S, et al. PDGF, TGF-β, and FGF signaling is important for differentiation and growth of mesenchymal stem cells (MSCs): transcriptional profiling can identify markers and signaling pathways important in differentiation of MSCs into adipogenic, chondrogenic, and osteogenic lineages. Blood. 2008;112:295–307. doi: 10.1182/blood-2007-07-103697. [DOI] [PubMed] [Google Scholar]
- 38.Discher D, Dong C, Fredberg JJ, et al. Biomechanics: cell research and applications for the next decade. Ann. Biomed. Eng. 2009;37:847–859. doi: 10.1007/s10439-009-9661-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Janmey PA, McCulloch CA. Cell mechanics: integrating cell responses to mechanical stimuli. Ann. Rev. Biomed. Eng. 2007;9:1–34. doi: 10.1146/annurev.bioeng.9.060906.151927. [DOI] [PubMed] [Google Scholar]
- 40.Hahn C, Schwartz MA. Mechanotransduction in vascular physiology and atherogenesis. Nat. Rev. Mol. Cell. Biol. 2009;10:53–62. doi: 10.1038/nrm2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Paszek MJ, Zahir N, Johnson KR, et al. Tensional homeostasis and the malignant phenotype. Cancer Cell. 2005;8:241–254. doi: 10.1016/j.ccr.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 42.Pelham RJ, Jr, Wang Y-L. High resolution detection of mechanical forces exerted by locomoting fibroblasts on the substrate. Mol. Biol. Cell. 1999;10:935–945. doi: 10.1091/mbc.10.4.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43 ■■.Zemel A, Rehfeldt F, Brown AE, Discher DE, Safran SA. Optimal matrix rigidity for stress fiber polarization in stem cells. Nat. Phys. 2010;6:468–473. doi: 10.1038/nphys1613. [The alignment of non-muscle myosin (NMMII)-based stress fibers in MSCs depends non-monotonically on the matrix rigidity.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44 ■■.Huebsch N, Arany PR, Mao AS, et al. Harnessing traction-mediated manipulation of the cell/matrix interface to control stem-cell fate. Nat. Mater. 2010;9:518–526. doi: 10.1038/nmat2732. [Assessed the effects of matrix stiffness on MSCs in 3D.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45 ■■.Gilbert PM, Havenstrite KL, Magnusson KE, et al. Substrate elasticity regulates skeletal muscle stem cell self-renewal in culture. Science. 2010;329:1078–1081. doi: 10.1126/science.1191035. [Substrate elasticity mimicking the elasticity of muscle supports self-renewal of muscle stem cells.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Roskelley CD, Desprez PY, Bissell MJ. Extracellular matrix-dependent tissue-specific gene expression in mammary epithelial cells requires both physical and biochemical signal transduction. Proc. Natl Acad. Sci. USA. 1994;91:12378–12382. doi: 10.1073/pnas.91.26.12378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Watt FM, Jordan PW, O'Neill CH. Cell shape controls terminal differentiation of human epidermal keratinocytes. Proc. Natl Acad. Sci. USA. 1988;85:5576–5580. doi: 10.1073/pnas.85.15.5576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ren XD, Kiosses WB, Schwartz MA. Regulation of the small GTP-binding protein Rho by cell adhesion and the cytoskeleton. EMBO J. 1999;18:578–585. doi: 10.1093/emboj/18.3.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Curtis A, Wilkinson C. Nantotechniques and approaches in biotechnology. Trends Biotechnol. 2001;19:97–101. doi: 10.1016/s0167-7799(00)01536-5. [DOI] [PubMed] [Google Scholar]
- 50.Flemming RG, Murphy CJ, Abrams GA, Goodman SL, Nealey PF. Effects of synthetic micro- and nano-structured surfaces on cell behavior. Biomaterials. 1999;20:573–588. doi: 10.1016/s0142-9612(98)00209-9. [DOI] [PubMed] [Google Scholar]
- 51 ■■.Dalby MJ, Gadegaard N, Tare R, et al. The control of human mesenchymal cell differentiation using nanoscale symmetry and disorder. Nat. Mater. 2007;6:997–1003. doi: 10.1038/nmat2013. [Investigated the effects of physical topography on MSC differentiation.] [DOI] [PubMed] [Google Scholar]
- 52 ■■.Kurpinski K, Chu J, Hashi C, Li S. Anisotropic mechanosensing by mesenchymal stem cells. Proc. Natl Acad. Sci. USA. 2006;103:16095–16100. doi: 10.1073/pnas.0604182103. [Mechanical strain controls MSC gene expression.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53 ■■.Kurpinski K, Chu J, Wang D, Li S. Proteomic profiling of mesenchymal stem cell responses to mechanical strain and TGF-β1. Cell. Mol. Bioeng. 2009;2:606–614. doi: 10.1007/s12195-009-0090-6. [Mechanical strain and TGF-β1 co-operate to regulate MSC gene expression.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 55.Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 56.Yu J, Vodyanik MA, Smuga-Otto K, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 57.Park IH, Zhao R, West JA, et al. Reprogramming of human somatic cells to pluripotency with defined factors. Nature. 2008;451:141–146. doi: 10.1038/nature06534. [DOI] [PubMed] [Google Scholar]
- 58.Wozniak MA, Chen CS. Mechanotransduction in development: a growing role for contractility. Nat. Rev. Mol. Cell. Biol. 2009;10:34–43. doi: 10.1038/nrm2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59 ■■.Chowdhury F, Na S, Li D, et al. Material properties of the cell dictate stress-induced spreading and differentiation in embryonic stem cells. Nat. Mater. 2010;9:82–88. doi: 10.1038/nmat2563. [Adherent mouse embryonic stem cells (ESCs) are softer and much more sensitive to a local cyclic stress.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60 ■.Poh YC, Chowdhury F, Tanaka TS, Wang N. Embryonic stem cells do not stiffen on rigid substrates. Biophys. J. 2010;99:L19–L21. doi: 10.1016/j.bpj.2010.04.057. [Single ESCs do not stiffen on rigid substrates.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61 ■.Chowdhury F, Li Y, Poh YC, Yokohama-Tamaki T, Wang N, Tanaka TS. Soft substrates promote homogeneous self-renewal of embryonic stem cells via downregulating cell–matrix tractions. PLoS One. 2010;5:e15655. doi: 10.1371/journal.pone.0015655. [Soft substrates promote homogeneous self-renewal of mouse ESCs.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62 ■.Shimizu N, Yamamoto K, Obi S, et al. Cyclic strain induces mouse embryonic stem cell differentiation into vascular smooth muscle cells by activating PDGF receptor-β. J. Appl. Physiol. 2008;104:766–772. doi: 10.1152/japplphysiol.00870.2007. [Cyclic strain induces mouse ESC differentiation to vascular smooth muscle cells.] [DOI] [PubMed] [Google Scholar]
- 63.Yamamoto K, Sokabe T, Watabe T, et al. Fluid shear stress induces differentiation of Flk-1-positive embryonic stem cells into vascular endothelial cells in vitro. Am. J. Physiol. Heart Circ. Physiol. 2005;288:H1915–H1924. doi: 10.1152/ajpheart.00956.2004. [DOI] [PubMed] [Google Scholar]
- 64.Garin G, Berk BC. Flow-mediated signaling modulates endothelial cell phenotype. Endothelium. 2006;13:375–384. doi: 10.1080/10623320601061599. [DOI] [PubMed] [Google Scholar]
- 65 ■■.Adamo L, Naveiras O, Wenzel PL, et al. Biomechanical forces promote embryonic haematopoiesis. Nature. 2009;459:1131–1135. doi: 10.1038/nature08073. [Fluid shear stress promotes differentiation of ESC-derived hematopoietic precursors.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Evans ND, Minelli C, Gentleman E, et al. Substrate stiffness affects early differentiation events in embryonic stem cells. Eur. Cell Mater. 2009;18:1–13. doi: 10.22203/ecm.v018a01. discussion 13–14. [DOI] [PubMed] [Google Scholar]
- 67 ■■.Peerani R, Rao BM, Bauwens C, et al. Niche-mediated control of human embryonic stem cell self-renewal and differentiation. EMBO J. 2007;26:4744–4755. doi: 10.1038/sj.emboj.7601896. [Assessed the effects of colony sizes on ESC fate.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ng ES, Davis RP, Azzola L, Stanley EG, Elefanty AG. Forced aggregation of defined numbers of human embryonic stem cells into embryoid bodies fosters robust, reproducible hematopoietic differentiation. Blood. 2005;106:1601–1603. doi: 10.1182/blood-2005-03-0987. [DOI] [PubMed] [Google Scholar]
- 69.Nostro MC, Cheng X, Keller GM, Gadue P. Wnt, activin, and BMP signaling regulate distinct stages in the developmental pathway from embryonic stem cells to blood. Cell Stem Cell. 2008;2:60–71. doi: 10.1016/j.stem.2007.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Friedland JC, Lee MH, Boettiger D. Mechanically activated integrin switch controls α5β1 function. Science. 2009;323:642–644. doi: 10.1126/science.1168441. [DOI] [PubMed] [Google Scholar]
- 71.Shin ME, He Y, Li D, et al. Spatiotemporal organization, regulation, and functions of tractions during neutrophil chemotaxis. Blood. 2010;116:3297–3310. doi: 10.1182/blood-2009-12-260851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.del Rio A, Perez-Jimenez R, Liu R, Roca-Cusachs P, Fernandez JM, Sheetz MP. Stretching single talin rod molecules activates vinculin binding. Science. 2009;323:638–641. doi: 10.1126/science.1162912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73 ■.Grashoff C, Hoffman BD, Brenner MD, et al. Measuring mechanical tension across vinculin reveals regulation of focal adhesion dynamics. Nature. 2010;466:263–266. doi: 10.1038/nature09198. [In living cells force transmission across focal adhesions is necessary for the stabilization of focal adhesions under force.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74 ■.Johnson CP, Tang HY, Carag C, Speicher DW, Discher DE. Forced unfolding of proteins within cells. Science. 2007;317:663–666. doi: 10.1126/science.1139857. [Described a technology to detect forced unfolding of proteins within cells.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lyons RM, Gentry LE, Purchio AF, Moses HL. Mechanism of activation of latent recombinant transforming growth factor-β1 by plasmin. J. Cell. Biol. 1990;110:1361–1367. doi: 10.1083/jcb.110.4.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76 ■.Wipff PJ, Rifkin DB, Meister JJ, Hinz B. Myofibroblast contraction activates latent TGF-β1 from the extracellular matrix. J. Cell. Biol. 2007;179:1311–1323. doi: 10.1083/jcb.200704042. [Myofibroblast contraction activates latent TGF-β1.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77 ■.Ahamed J, Burg N, Yoshinaga K, Janczak CA, Rifkin DB, Coller BS. In vitro and in vivo evidence for shear-induced activation of latent transforming growth factor-β1. Blood. 2008;112:3650–3660. doi: 10.1182/blood-2008-04-151753. [Describes shear-induced activation of latent TGF-β1.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Conti MA, Even-Ram S, Liu C, Yamada KM, Adelstein RS. Defects in cell adhesion and the visceral endoderm following ablation of nonmuscle myosin heavy chain II-A in mice. J. Biol. Chem. 2004;279:41263–41266. doi: 10.1074/jbc.C400352200. [DOI] [PubMed] [Google Scholar]
- 79.Tullio AN, Accili D, Ferrans VJ, et al. Nonmuscle myosin II-B is required for normal development of the mouse heart. Proc. Natl Acad. Sci. USA. 1997;94:12407–12412. doi: 10.1073/pnas.94.23.12407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Watanabe K, Ueno M, Kamiya D, et al. A ROCK inhibitor permits survival of dissociated human embryonic stem cells. Nat. Biotechnol. 2007;25:681–686. doi: 10.1038/nbt1310. [DOI] [PubMed] [Google Scholar]
- 81.Xu Y, Zhu X, Hahm HS, et al. Revealing a core signaling regulatory mechanism for pluripotent stem cell survival and self-renewal by small molecules. Proc. Natl Acad. Sci. USA. 2010;107:8129–8134. doi: 10.1073/pnas.1002024107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82 ■■.Li D, Zhou J, Wang L, et al. Integrated biochemical and mechanical signals regulate multifaceted human embryonic stem cell functions. J. Cell. Biol. 2010;191:631–644. doi: 10.1083/jcb.201006094. [NMMIIA controls multifaceted human ESC functions.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83 ■.Chen G, Hou Z, Gulbranson DR, Thomson JA. Actin-myosin contractility is responsible for the reduced viability of dissociated human embryonic stem cells. Cell Stem Cell. 2010;7:240–248. doi: 10.1016/j.stem.2010.06.017. [Actin–myosin contractility is responsible for the reduced viability of dissociated human ESCs.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84 ■.Ohgushi M, Matsumura M, Eiraku M, et al. Molecular pathway and cell state responsible for dissociation-induced apoptosis in human pluripotent stem cells. Cell Stem Cell. 2010;7:225–239. doi: 10.1016/j.stem.2010.06.018. [The Rho–ROCK–NMMII pathway is responsible for dissociation-induced apoptosis in human ESCs.] [DOI] [PubMed] [Google Scholar]
- 85.Shewan AM, Maddugoda M, Kraemer A, et al. Myosin 2 is a key Rho kinase target necessary for the local concentration of E-cadherin at cell–cell contacts. Mol. Biol. Cell. 2005;16:4531–4542. doi: 10.1091/mbc.E05-04-0330. [DOI] [PMC free article] [PubMed] [Google Scholar]