Abstract
The environmental, genetic, and/or age-related changes in proteostasis induce inflammation, oxidative stress, and apoptosis. We quantified the correlation of protein expression of critical proteostasis mediators to severity of chronic lung disease using lung tissue samples from control and chronic obstructive pulmonary disease (COPD) subjects (GOLD stage 0–IV) and cigarette smoke (CS)-induced murine model. The human bronchial epithelial cells, HEK-293, and Beas2B cells were used for in vitro experiments to verify the mechanisms. Our data verifies the correlation of higher expression of valosin-containing protein (VCP) retrograde translocation complex (VCP-Rma1-gp78) with severity of emphysema in COPD lung tissues and over-expression of inflammatory, ER stress and apoptotic mediators like NFκB, GADD-153/CHOP, and p-eIF2α. Moreover, subjects with severe emphysema had a higher accumulation of ubiquitinated proteins and deubiquitinating enzyme, UCHL-1, indicating towards the aggregation of misfolded or damaged proteins. The modulation of both protein degradation and synthesis rates by CS-extract substantiates the pathogenetic role of proteostasis-imbalance in emphysema and COPD. We identified that VCP also mediates proteasomal degradation of HDAC2 and Nrf2, as a potential mechanism for increased oxidative stress and corticosteroid resistance in COPD subjects with emphysema. Next, we confirmed that higher VCP expression associates with increased inflammation and apoptosis using in vitro and murine models. Our data clearly shows aberrant proteostasis in COPD subjects with severe emphysema. In addition, we evaluate therapeutic efficacy of salubrinal (ER stress inhibitor) to correct the proteostasis-imbalance based on its ability to control VCP expression and ubiquitin accumulation. Overall, our data demonstrate for the first time the critical role of proteostasis-imbalance in pathogenesis of severe emphysema.
Keywords: Proteasome, VCP, NFκB, Nrf2, HDAC2
Introduction
Among environmental air pollutants, cigarette smoke (CS) and respiratory infection (bacteria and virus) are the primary risk factors for the pathogenesis of chronic obstructive pulmonary disease (COPD) [1]. CS consists of complex mixture of oxidants or free radicals and different chemical compounds that include reactive aldehydes and semiquinones known to cause oxidative stress in the lungs [2–4]. The pathology of COPD involves persistent inflammation, oxidative stress, impaired lung cell repair, and programmed cell death (apoptosis) leading to emphysematous lung disease [5–7]. Moreover, it is not clear why only few smokers develop COPD or why some non-smokers have COPD. The specific genes and underlying mechanisms of COPD and emphysema pathogenesis remain elusive.
Valosin-containing protein (p97/VCP) is a member of the AAA-ATPase family that is associated with diverse cellular functions comprising nuclear envelope reconstruction, cell cycle, post-mitotic Golgi reassembly, suppression of apoptosis, DNA damage response, and endoplasmic reticulum-associated degradation (ERAD) [8–13]. The over-expression of VCP is implicated in chronic inflammatory diseases like cystic fibrosis, lung cancer, neurological and other age-related disorders [13, 14]. Most of the unwanted proteins in the eukaryotic cell secretory pathway that enter the ER are specifically extracted from the ER and targeted to the cytosol, where they are degraded by ERAD [13]. VCP plays a key role in both protein extraction from the ER and ubiquitin-proteasome mediated protein degradation by ERAD. The ubiquitin-mediated protein modification during ERAD is regulated by a set of three enzymes: an ubiquitin-activating enzyme (E1), an ubiquitin-conjugating enzyme (E2), and an ubiquitin ligase (E3) [15]. Efficient multiubiquitination needed for proteasomal targeting of a model substrate requires an additional conjugation factor, named E4 [16]. VCP is known to associate with E3/E4 ubiquitin ligases like Dorfin [17], gp78/AMFR (autocrine motility factor receptor) [18, 19] and RING finger protein with membrane anchor (Rma1) [20, 21] to promote ERAD. In particular, VCP-gp78-Rma1 interaction is known to enhance both ubiquitination and VCP-polyubiquitin binding during ERAD. This interaction may participate in VCP-mediated inflammatory signaling.
VCP is known to mediate the proteasomal degradation of IκB, an endogenous inhibitor of nuclear factor kappa-light-chain-enhancer of activated B cells (NFκB) [19, 22]. Moreover, VCP expression is known to be induced in response to infection, stress or injury as an endogenous homeostatic mechanism to control chronic inflammation [13]. In addition to NFκB-mediated inflammation, VCP activity and expression may be triggered by oxidative stress induced by CS. We hypothesized that CS-induced oxidative stress and inflammation may modulate VCP and/or proteasomal activity and hence it may be critical for the pathogenesis of severe emphysema in COPD subjects. Moreover, expression of nuclear factor erythroid 2-related factor 2 (Nrf2; oxidative stress response) and histone deacetylase-2 (HDAC2; glucocorticoid resistance) is declined in COPD [23–25]. We tested if VCP regulates these responses by mediating the proteasomal degradation of Nrf2 and HDAC2.
We not only verify here the correlation of VCP, gp78 and Rma1 expression to the pathogenesis of severe emphysema but also provide corroborating evidence that VCP regulates the primary components of COPD pathophysiology, NFκB, Nrf2-(p), and HDAC2. We also show that changes in VCP activity correlates with the levels of CS-induced ubiquitin accumulation and apoptosis. To summarize, we demonstrate the critical role of proteostasis-imbalance in pathogenesis of COPD and severe emphysema. In addition, we evaluate the efficacy of salubrinal (ER stress inhibitor) as a proteostatic modulator to inhibit ubiquitin accumulation and propose its potential therapeutic application in correcting proteostasis-imbalance associated with severe emphysema.
Materials and methods
Human subjects and murine experiments
Frozen lung tissue samples and longitudinal sections were obtained from the NHLBI Lung Tissue Research Consortium (LTRC, NIH). Clinical information, sample size, and classification based on Global initiative for chronic obstructive lung disease (GOLD 0–IV) stages [26] is summarized in Table 1. All the control and COPD subjects were stable, and Gold I–IV COPD subjects had emphysema. Moreover, one patient in each group (Gold I–IV) had first-degree blood relatives with chronic bronchitis. The study protocol was approved by the Institutional Review Board as exempt 4, and subject’s lung function data and other clinical parameters were obtained from each of the LTRC contributing centers without disclosing the subject’s name. The lung samples were analyzed by immunostaining (described below) or immunoblotting (see “Supplementary methods” in the electronic supplementary materials), and statistical correlation of the data was calculated as described below. The results of the human data were verified using cigarette smoke (CS) and Pseudomonas aeruginosa-LPS (Pa-LPS)-induced murine models. All animal experiments were carried out in accordance with the Johns Hopkins University Animal Care and Use Committee approved protocols. We used age-, weight-, and sex-matched (8–10 weeks old) C57BL/6 (NCI Animal Production Program), n=3 in all experiments. All the mice were housed in controlled environment and pathogen-free conditions. Mice were exposed to acute or sub-chronic CS using the TE-2 cigarette smoking machine (Teague Enterprises, Davis, CA) or treated with intratracheal Pa-LPS (see “Supplementary methods” in the electronic supplementary materials). In a separate experiment, the air- and acute CS-exposed mice were treated intra-tracheally with 1 mg/kg salubrinal (electronic supplementary materials).
Table 1.
Patient characteristics
| Parameters | Gold 0 | Gold I | Gold II | Gold III/IV |
|---|---|---|---|---|
| Average age (year (mean±SD)) | 66.9±9.0 | 67.3±8.6 | 68.2±8.7 | 56.7±8.6 |
| Sex | ||||
| M | 10 | 9 | 13 | 11 |
| F | 14 | 6 | 7 | 30 |
| Smoking status | ||||
| Current | 0 | 2 | 2 | 1 |
| Ever | 8 | 11 | 16 | 37 |
| Never | 16 | 2 | 2 | 3 |
| Packs/year (average) | 27.6±35.2 | 40.7±24.8 | 57.7±45.5 | 46.6±31.7 |
| FEV1-% predicted (mean±SD) | 94.3±11.6 | 89.6±11.3 | 64.1±10.4 | 22.4±7.8 |
Immunofluorescence microscopy
The longitudinal tissue sections from human or mouse lungs were immunostained with the primary antibodies (1–2 µg/ml) for VCP (rabbit polyclonal, Santa Cruz Biotechnology (scbt), NFκB (rabbit polyclonal, scbt), gp78 (rabbit polyclonal, scbt), Nrf2 (rabbit polyclonal, scbt), p-Nrf2 (rabbit monoclonal, Abcam), NOS2 (rabbit polyclonal, scbt), p-eIF2α (rabbit polyclonal, scbt), GADD-153 (rabbit polyclonal, scbt), HDAC2 (rabbit polyclonal, scbt), ubiquitin (mouse monoclonal, scbt), and ubiquitin C-terminal hydrolase-L1 (UCH-L1; rabbit polyclonal, scbt), Rma1 (mouse monoclonal, Affinity Bioreagents), carboxyl terminus of Hsp70-interacting protein (CHIP), and KDEL (rabbit polyclonal, Affinity Bioreagents) followed by 1 µg/ml of the secondary antibodies using a previously described protocol [27, 28]. The secondary antibodies used were goat anti-rabbit IgG FITC (scbt), goat anti-mouse IgG/IgM (H+L) Alexa Fluor 488 (Invitrogen), and donkey anti-mouse Dylight 594 (Jackson Immunoresearch). We evaluated the specificity of primary antibodies by first titrating their concentrations. Next, we selected the negative and positive controls from induced (LPS/CS) and non-induced human and/or murine longitudinal lung sections, primary cells or cell lines based on the known literature on the expression of selected protein. The specificity of secondary antibodies was determined by omitting the primary antibodies during immunostaining. In addition to the immunostaining, we also tested these antibodies (like VCP, Ub, p-Nrf2, GADD-153, and HDAC2) by western blotting to make sure they detect the single specific band for these proteins to ascertain the specificity of the antibodies before experimental evaluation as described above. TUNEL staining was used to quantify number of apoptotic cells (electronic supplementary materials). Nuclei were detected by Hoechst (Invitrogen) while hematoxylin and eosin (H&E) was used to evaluate lung morphology and inflammatory state. Images were captured by Axiovert 200 Carl Zeiss Fluorescence microscope using the Zeiss Axiocam HRC camera and Axiovision software. All fluorescent images were captured at room temperature with oil (×63, fluorescence) and air (×20 and ×40) as the imaging medium. The magnifications for the fluorescence microscope were LD Plan- Achroplan (×20/0.40 Korr Phz), Neo Fluar (×40/×0.6 Phz Korr) and Achromat (×63/1.4 oil), respectively with ×1.6 optivar.
Cell culture, transfection and metabolic labeling
The cells were cultured at 37°C with 5% CO2 in MEM [human bronchial epithelial cells (HBE; from Dr. Dieter Gruenert), Beas2b (from ATCC)] or DMEM/F12 (human embryonic kidney 293 (HEK-293) supplemented with 10% fetal bovine serum and 1% penicillin, streptomycin, and amphotericin B from Invitrogen. The cells were transiently transfected with pSM2 vector control or p97/valosin-containing protein short-hairpin RNA (VCPshRNA) using Lipofectamine 2000 as described before [19] or treated overnight with PS-341 (1 µM, Millennium Pharmaceuticals Inc), salubrinal (0.1 & 2 µg/ml or 50µm) or cigarette smoke extract (CSE; 80, 100 or 160 µg/ml, Murty Pharmaceuticals Inc). For VCP over-expression, we transiently transfected HEK-293 cells (as they had lower endogenous levels of VCP compared with Beas2b and HBEs) with VCP-myc plasmid construct for 48 h and the total cell lysates were immunoblotted for VCP, HDAC2, Nrf2, p-Nrf2, and β-actin. The HBE cells were similarly treated with CSE (80 or 160 µg/ml) for 12 h. Protein synthesis was blocked by treating the HBE cells with 50 µg/ml cycloheximide (CHX) for the indicated time points. Metabolic labeling and immunoprecipitation were used to quantify changes in CSE-induced ubiquitin accumulation and protein synthesis (electronic supplementary methods).
Statistical analysis
Data is represented as the mean±SD of at least three experiments. The Student’s t test and ANOVA were used to determine the statistical significance. The murine and human microscopy data were analyzed by densitometry (Matlab R2009b, Mathworks Co.). Percentage fluorescent intensity was obtained by calculating the percent number of pixels over the threshold background intensity using Matlab to quantify the changes in protein expression levels. For this analysis, sections with equal cell density were selected from each sample. For TUNEL and p-Nrf2 staining, the numbers of apoptotic cells and p-Nrf2+ nuclei were counted for statistical analysis. Spearman’s correlation coefficient was used to calculate the significance among the indicated groups.
Results
Increased NFκB activation and ER stress in severe emphysema
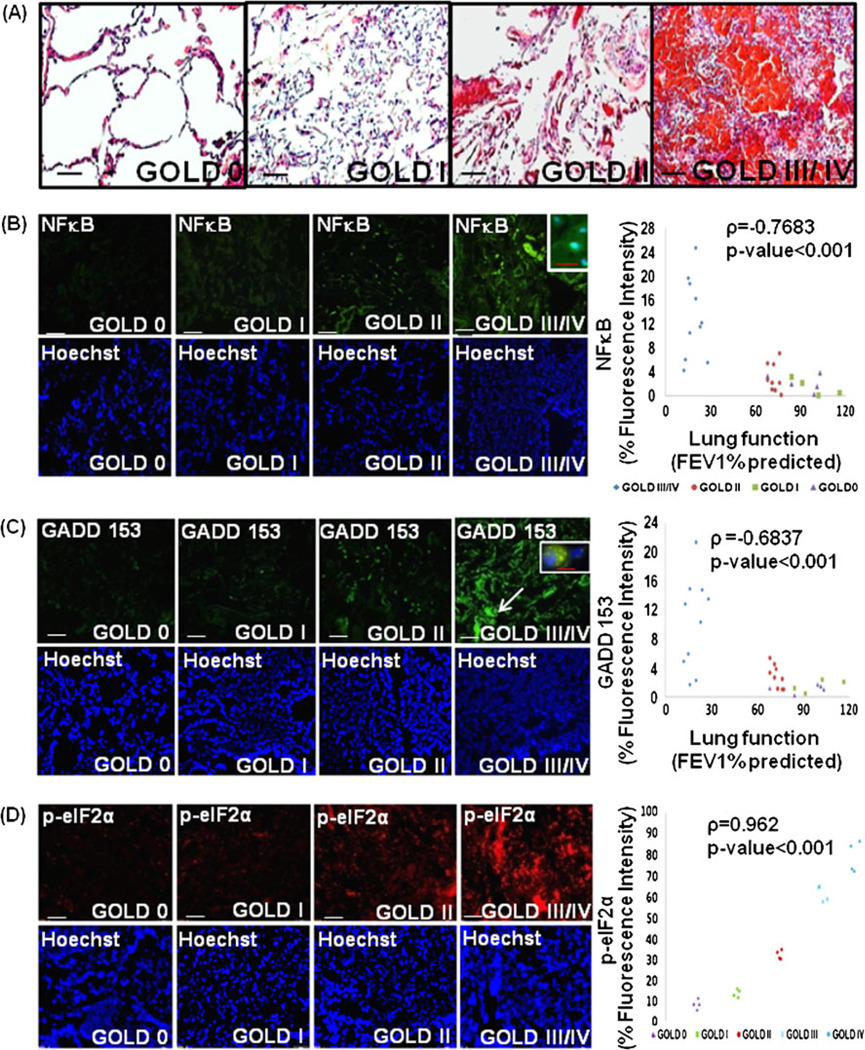
The histological analysis of lung sections from COPD subjects stained with H&E shows a prominent increase in inflammation in the lungs of patients with severe (FEV1% predicted <50%, Gold Stage IV) emphysema compared with the mild emphysema and control subjects (FEV1% predicted ~100%) [26] (Fig. 1a). We next confirmed that NFκB activation is associated with the severity of emphysema (Fig. 1b). The data confirm the correlation of NFκB expression and nuclear localization (inset) with severity of COPD lung disease (ρ=−0.7683, p <0.001). We have recently shown that early age-related changes in proteostasis mediate the pathogenesis of sepsis and acute lung injury [14]. Impaired proteasomal function induces ER stress due to the accumulation of oxidized and/or damaged (misfolded) proteins [29]. ER stress mediates the pathogenesis of several inflammatory disorders, including COPD by inducing cell death and inflammation [30] [24] but the role of ubiquitin-proteasome system (UPS) in COPD is not well studied. We first verified the increased ER stress in severe emphysema by evaluating the expression of transcription factor, C/EBP homologous protein (CHOP) also known as growth arrest and DNA damage-inducible gene 153 (GADD-153), in lung sections from control and COPD subjects by immunostaining. We observed higher expression of GADD-153 in COPD subjects with severe emphysema as compared with mild or controls confirming the role of ER stress in emphysema (Fig. 1c; ρ=−0.6837, p<0.001). We also demonstrate upregulation of p-eIF2α in severe emphysema, further validating ER stress in COPD (Fig. 1d). We postulate based on our recent studies [14, 24] that increased inflammation and apoptosis in emphysema may be a result of proteostasis-imbalance although ER stress may be induced by CS exposure.
Fig. 1.
Elevated nuclear factor kappa-light-chain-enhancer of activated B cells (NFκB) activation and ER stress in severe emphysema. a The paraffin-embedded longitudinal human lung sections of control (GOLD 0) and chronic obstructive pulmonary disease (COPD) lung tissues at GOLD I–IV levels of emphysema (n=8–10 (a, b, c), 4(d) each group), stained with H&E, present significant increase in inflammation in severe (GOLD III/IV) emphysema lung tissues compared with GOLD 0. b These sections were immunostained with primary rabbit polyclonal NFκB/p65 and secondary anti-rabbit-FITC conjugated antibody, and show a significant increase in NFκB expression and nuclear localization at higher severities of emphysema. The higher NFκB levels and nuclear localization with increasing severity of emphysema confirms that progressive lung inflammation is a critical component of COPD pathophysiology. Densitometric analysis (b, right panel) confirms association of NFκB (p<0.001) with severe emphysema. Nuclear (Hoechst) staining of the same area is shown in the bottom panel. c, d To verify the induction of ER stress markers in severe COPD lung tissues, the paraffin-embedded longitudinal sections were immunostained with primary rabbit polyclonal GADD-153, p-eIF2α, and secondary anti-rabbit-FITC and anti-rabbit-TR conjugated antibody, present significant increase in GADD-153 (CHOP) expression and nuclear localization in the tissue sections with severe emphysema. Since GADD-153 is a highly stress inducible gene that is induced in response to ER stress, our data verify the correlation of ER stress with COPD. This is further confirmed with significant increase in p-eIF2α expression with severe emphysema, a more specific ER stress marker correlated with the UPR pathway. Densitometric analysis (right panel) confirms association of increased GADD-153 and p-eIF2α (p<0.001) expression with progressive lung inflammation and severity of emphysema. Hoechst staining of the same area is shown in the bottom panel. Scale: white bar=50 µm and red bar=10 µm
Proteostasis-imbalance leads to accumulation of ubiquitinated proteins in severe emphysema
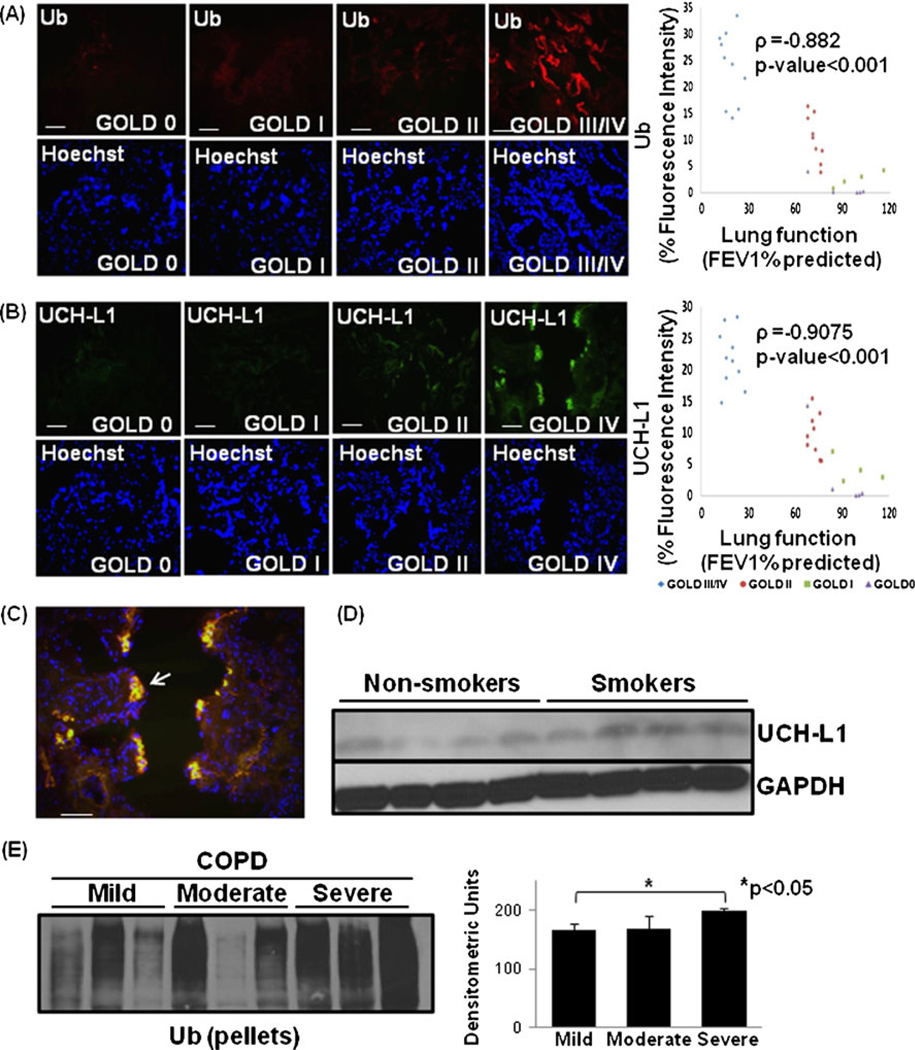
The localization and levels of ubiquitinated proteins in lungs of COPD patients was determined to identify the changes in proteostasis. We detected the accumulation of ubiquitinated proteins with increasing severity of emphysema (Fig. 2a; ρ=−0.882, p<0.001). To confirm the aggregation of poly-ubiquitinated proteins, we verified the expression of deubiquitinating enzyme and aggregation marker, UCH-L1 [31] and observed that its expression correlates with severity of emphysema (Fig. 2b; ρ=−0.9075, p<0.001) similar to the levels of ubiquitinated proteins. Moreover, patient samples with severe emphysema clearly showed the co-localization of ubiquitinated proteins and UCH-L1 (Fig. 2c, arrow) indicating towards their role in chronic inflammation and apoptosis as discussed above. We verified the increase in UCH-L1 expression in smokers with COPD as compared with non-smokers by immunoblotting (Fig. 2d). In support of our data, recent studies have also shown the increased UCH-L1 mRNA expression in ciliated epithelial cells of heavy smokers [32]. Next, we confirmed the aggregation of ubiquitinated proteins by immunoblotting. We observed higher ubiquitin accumulation in the insoluble protein fraction (protein pellets) from COPD subjects with severe emphysema (Fig. 2e). We anticipate that proteostasis-imbalance and cytosolic accumulation of poly-ubiquitinated protein aggregates triggers chronic inflammation and pathogenesis of severe emphysema in COPD.
Fig. 2.
Accumulation of ubiquitinated proteins in severe emphysema. a The paraffin-embedded longitudinal human lung sections of control (GOLD 0) and COPD lung tissues at GOLD I–IV levels of emphysema (n=8–10, each group), immunostained with primary mouse monoclonal Ub (P4D1) and secondary anti-mouse-FITC conjugated antibody, present significant increase in protein levels and accumulation of ubiquitinated proteins in the human lung tissue sections with severe (GOLD III/IV) emphysema. b Lung sections (from a) immunostained with primary rabbit polyclonal UCH-L1 and secondary anti-rabbit-FITC conjugated antibody, show significant increase in its expression and accumulation with increasing severity of emphysema. Nuclear (Hoechst) staining of the same area is shown in the bottom panel. Scale=50 µm. Densitometric analysis confirms association of Ub and UCH-L1 (p<0.001) with progressive lung inflammation and severity of emphysema. c The data show the co-localization of ubiquitin and UCH-L1 in severe COPD lung tissue sections. Scale=50 µm. d This is further confirmed by the up regulation of UCH-L1 in smokers as compared with normal controls. The GAPDH shows the equal loading. e Immunoblot analysis of protein pellets of lung tissues from COPD smokers with mild, moderate and severe emphysema verify the higher accumulation of poly-ubiquitinated insoluble protein aggregates in severe emphysema (p<0.05). The data confirm the accumulation of poly-ubiquitinated proteins in insoluble protein fractions of COPD lungs as a potential mechanism for pathogenesis of severe emphysema
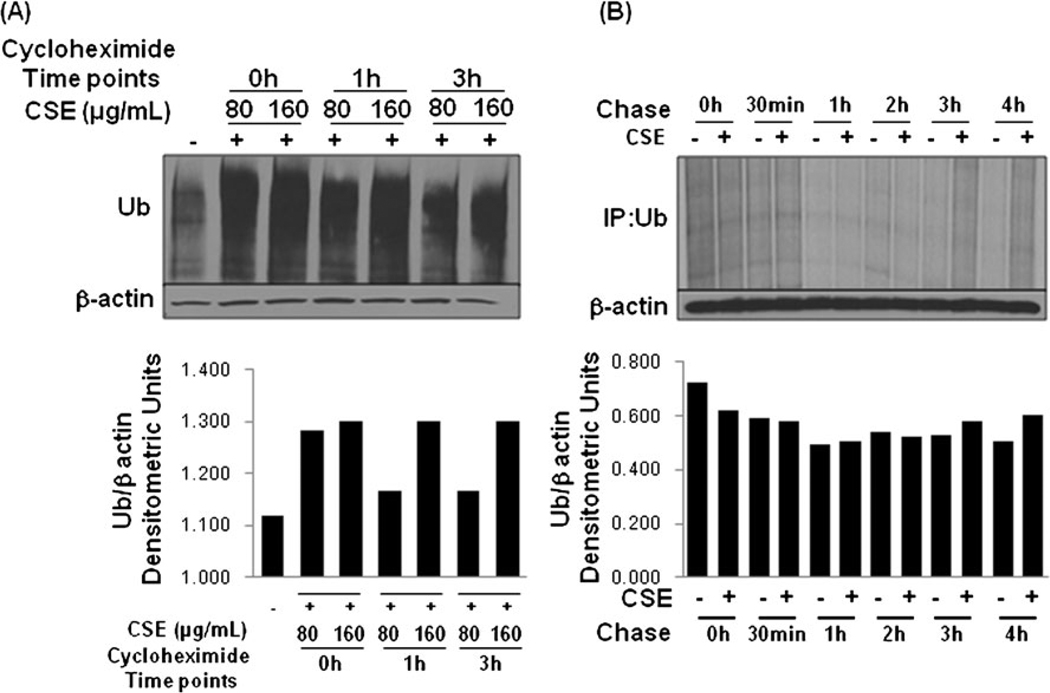
CSE disturbs protein turnover
Similar to the observations in the COPD lung tissues, previous studies have shown that CSE treatment of lung epithelial cells induces ER stress, inflammation and apoptosis [30]. We found that CS exposure or smoking induces VCP expression in murine model and human subjects (discussed below for Figs. 4a,d,f and 7), although the connection between smoke exposure and UPS is unclear. To verify the involvement of CS exposure in proteasome mediated protein turnover, HBE cells were treated with 100 µg/ml CSE for 12 h. Protein synthesis was blocked by treating the cells with 50 µg/ml cycloheximide (CHX) for the indicated time points and the total cell lysate was immunoblotted for ubiquitin (Ub) and β-actin (Fig. 3a). In response to CSE exposure (12 h), the immunoblot revealed a decrease in protein degradation rates by CSE treatment, leading to increase in accumulation of ubiquitinated proteins. CHX treatment leads to a slight decrease in levels of ubiquitinated proteins by 3 h. However, ubiquitinated protein levels are higher in the 160 µg/ml CSE dose indicating that CSE may modulate both protein synthesis and degradation. Since, CS induces VCP activity (discussed below for Figs. 4d and 7) while proteasome mediated protein degradation rates are decreased [24], the accumulation of ubiquitinated proteins is expected that induces chronic inflammation and apoptosis (as discussed above). Next, we verified if CSE affects protein synthesis by chasing synthesis and accumulation of ubiquitinated protein after metabolic labeling. Data shows that CSE modulates protein synthesis (0.5 h) and degradation rates (3–4 h) as seen by accumulation of ubiquitinated proteins (Fig. 3b).
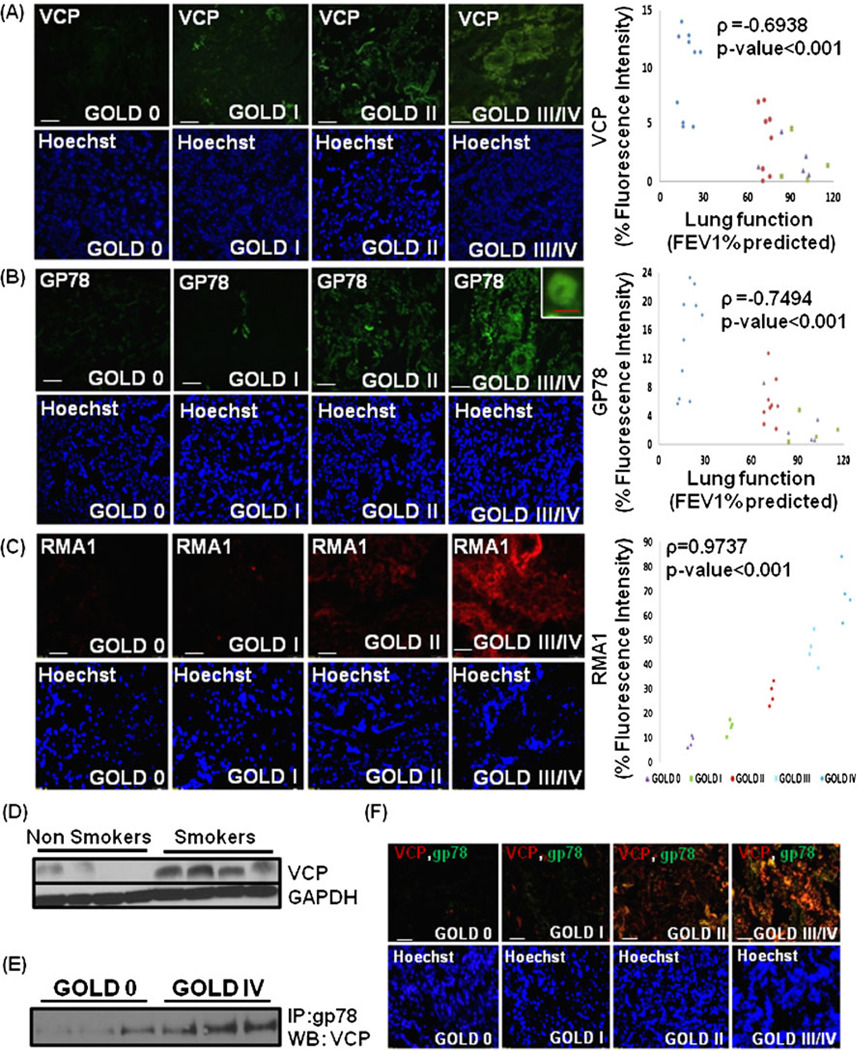
Fig. 4.
Elevated valosin-containing protein (VCP)-gp78-Rma1 expression in human lungs correlates with severity of emphysema. a The paraffin-embedded longitudinal human lung sections of control (GOLD 0) and COPD lung tissues at GOLD I–IV levels of emphysema (n=8–10, each group); were immunostained with primary rabbit polyclonal VCP and secondary anti-rabbit-FITC conjugated antibody. Data shows a significant increase in VCP expression in GOLD III/IV-emphysema lung tissues compared with the GOLD 0-control. Scale=50 µm (b, c). The increase in expression and membrane localization of gp78 (E4) and Rma1 (E3) ubiquitin ligases in the human lung tissue section (n=8–10, b and n=4, c) at higher severities of emphysema is shown. Densitometric analysis (a–c right panels) confirms the association of VCP, gp78, and Rma1 (p<0.001) with progressive lung inflammation. Nuclear (Hoechst) staining of the same area is shown in the bottom panel. Scale: white bar=50 µm and red bar=10 µm. d The immunoblot analysis of total protein extracts of lung tissues from smokers with COPD and non-smoker control human subjects confirm up regulation of VCP in COPD as compared with the normal controls. The GAPDH was used as loading control. (e, f) The elevated interaction of gp78 with VCP is shown in the lung tissue total-protein lysates from severe emphysema (GOLD IV) subjects as compared with control (Gold 0). This is also confirmed by increased co-localization of VCP (red) and gp78 (green) with the severity of emphysema (scale=50 µm). The data clearly demonstrate the induction of VCP-gp78-Rma1 retrograde translocation complex and aberrant proteostasis in emphysema
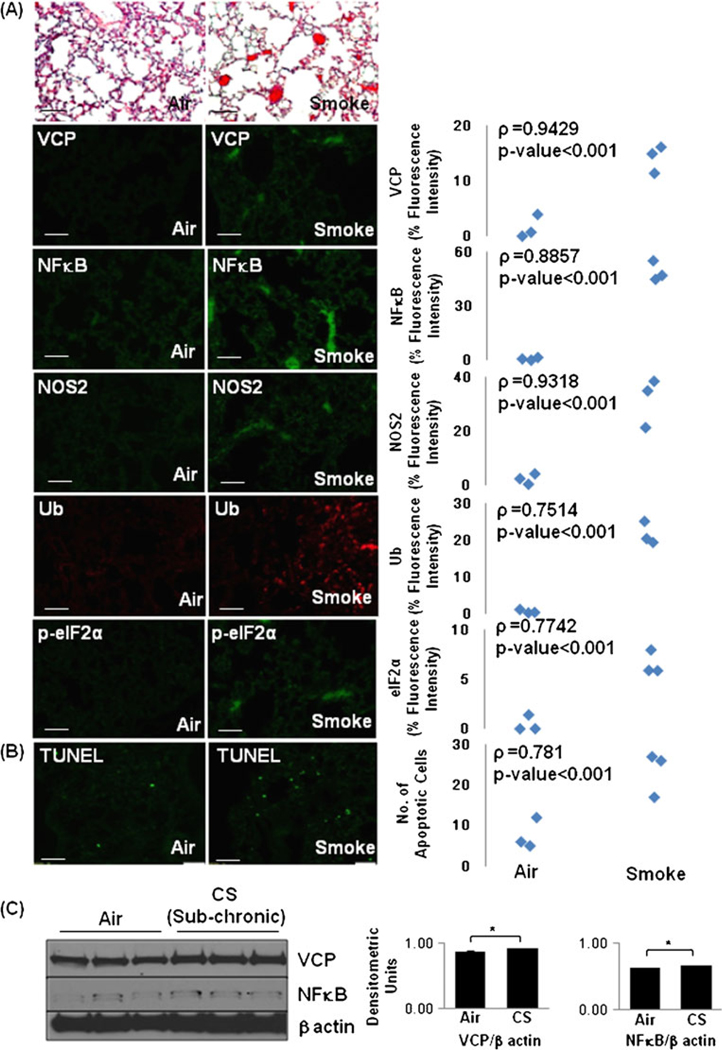
Fig. 7.
Increased VCP expression in cigarette smoke (CS)-exposed murine lungs correlates with stress response and apoptosis. The age- and sex-matched WT mice (n=3, each group) were exposed to 3- (a) or 5-day CS (b). The immunostainings of longitudinal lung sections show a significant increase (p<0.001) in VCP, nuclear factor kappa-light-chain-enhancer of activated B cells (NFκB), NOS2, and p-eIF2α expression levels and accumulation of poly-ubiquitinated (Ub) proteins in lungs of CS-induced mice (a). The extent of inflammation (a) and number of apoptotic cells (b TUNEL staining) were also elevated in the lungs of CS-exposed mice. Scale=50 µm. c The age- and sex-matched WT mice (n=3) were exposed to CS for 4 weeks (sub-chronic exposure). The immunoblotting of lung total-protein lysates demonstrate a significant (p<0.05) increase in VCP and NFκB expression in the sub-chronic CS-exposed murine lungs as compared with air. β-Actin was used as a loading control. The data verify the correlation of VCP expression to ubiquitin accumulation, inflammatory-oxidative stress and apoptosis in response to acute or sub-chronic CS exposure in murine lungs
Fig. 3.
Cigarette smoke extract (CSE) modulates protein turnover rates. a The HBE cells were treated with 80 and 160 µg/ml CSE for 12 h. Protein synthesis was blocked by treating the cells with 50 µg/ml cycloheximide (CHX) for the indicated time points and the total protein lysates were immunoblotted for ubiquitin (Ub) and β-actin. Data indicates that CSE treatment may modulate protein synthesis and degradation leading to increased Ub accumulation. Next, we verified that CSE modulates protein synthesis by chasing protein synthesis and accumulation of ubiquitinated protein after metabolic labeling. b HBE cells were treated with CSE (100 µg/ml) overnight, followed by starvation in Cys/Met-free media for 30 min. The cells were pulsed with 250 µCi/well Trans-35S-cys/met for 30 min. After the 30 min pulse, the cells were washed with ×1 PBS and 1-ml selective media (MEM) was added followed by chase for the indicated time points. The lysates were immuno precipitated with mouse monoclonal Ub antibody and run on SDS-PAGE. The gel was dried and exposed to Kodak BioMax MR film. Data shows that CSE modulates protein synthesis (0.5 h) and degradation rates (3–4 h) as seen by accumulation of ubiquitinated proteins. The densitometric analysis of (a, b) shown in the bottom panels confirms modulation of protein turnover with CSE treatment
VCP, gp78/AMFR and Rma1 expression in human lungs correlates with severity of emphysema
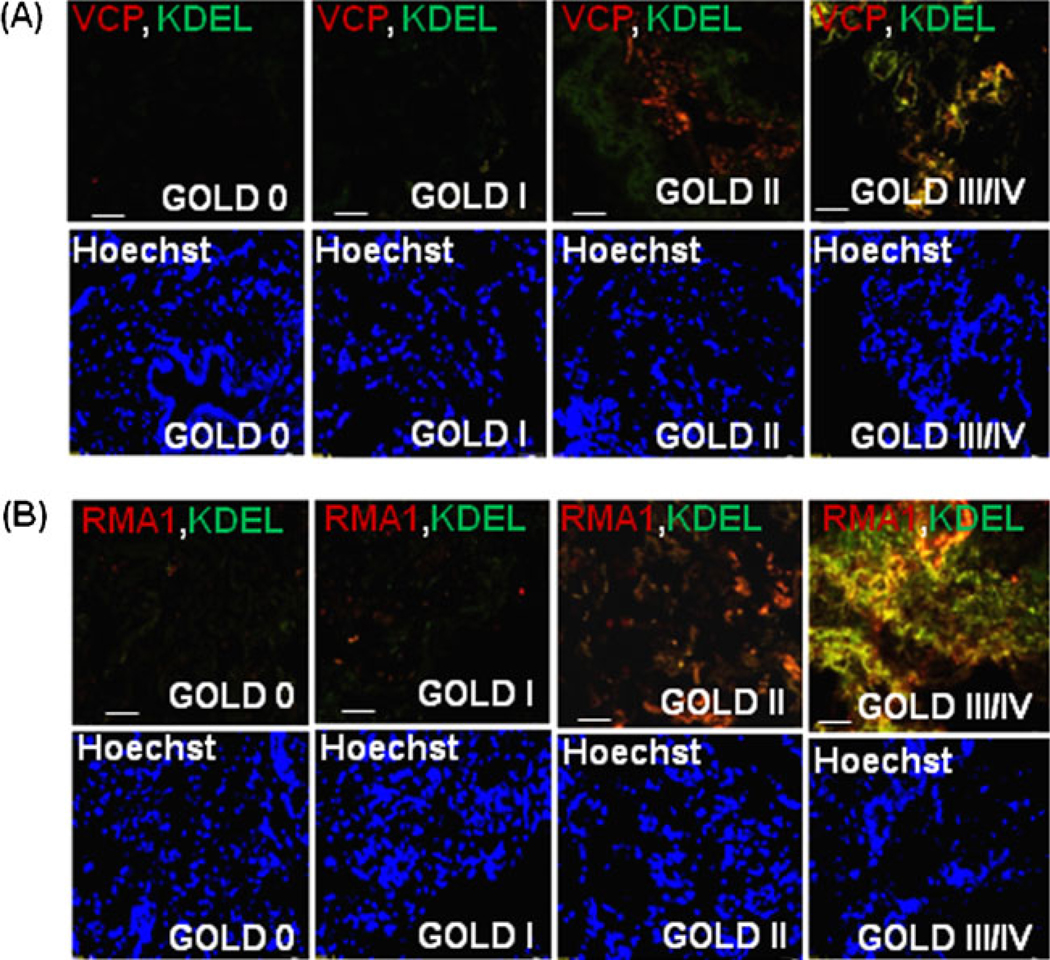
VCP is a major component of the retrograde translocation mechanism for proteasomal degradation of ubiquitinated and/or misfolded proteins [13]. We hypothesized that aberrant regulation of proteasomal activity or proteostasis-imbalance is associated with COPD pathogenesis. To determine, if VCP is involved in proteostasis-imbalance, the COPD patient samples with different severities of emphysema were immunostained for VCP (Fig. 4a). We found that VCP over-expression correlates with the severity of emphysema and chronic lung disease indicating the critical role of VCP in COPD pathogenesis (ρ=−0.6938, p<0.001). We and others have reported that VCP physically interacts with gp78/AMFR (autocrine motility factor receptor) to couple ubiquitination, retrograde-translocation, and proteasomal degradation [13, 18, 33]. Therefore, the COPD patient samples were immunostained for gp78 to confirm the association of VCP-mediated ubiquitination machinery with COPD pathogenesis (Fig. 4b). The gp78 expression was also elevated with increasing severity of emphysema, confirming the deregulation of VCP-mediated ubiquitination machinery in severe emphysema and its association with pathogenesis of chronic lung disease and emphysema (ρ=−0.7494, p<0.001). Since gp78 cooperates with Rma1 (ring finger protein with membrane anchor 1 ubiquitin ligase) in mediating ERAD [20], we immunostained the human COPD lung sections with Rma1. The expression of Rma1 increases with severity of COPD lung disease (Fig. 4c) indicating that the overall activity of VCP-gp78-Rma1 retrograde translocation complex is elevated in COPD and it may play a crucial role in pathogenesis of severe emphysema. In order to verify the specificity of this complex in pathogenesis of severe emphysema, we immunostained the COPD lung tissue samples with CHIP, a common E3 ubiquitin ligase [34] not associated with the VCP-gp78-Rma1 complex. Although the expression of CHIP increases with severity of emphysema, the extent of increase is much lower than the increase in Rma1 or gp78 (Fig. 1 in the electronic supplementary materials). Further confirmation of VCP’s association with pathogenesis of COPD is shown by higher VCP protein expression levels in smokers who developed COPD as compared with nonsmokers (Fig. 4d). GAPDH was used as a loading control. We also demonstrate that VCP and gp78 proteins directly interact (Fig. 4e) and co-localize (Fig. 4f) with increasing severity of COPD lung disease. We evaluated if this increased VCP activity is associated with ER membrane by co-staining with ER marker (KDEL) and observed both VCP and Rma1 co-localization with KDEL (Fig. 5a, b), implying that ER associated VCP-retrograde translocation complex may mediate the proteostasis-imbalance observed in COPD by modulating the ERAD.
Fig. 5.
Valosin-containing protein (VCP) interacts with gp78 and Rma1 on the ER membrane. The paraffin-embedded longitudinal human lung sections of control (GOLD 0) and COPD lung tissues at GOLD I–IV levels of emphysema (n=4–5, each group), were immunostained with primary mouse monoclonal antibodies for VCP (red, a) or Rma1 (red, b). These sections were co-immunostained with rabbit polyclonal KDEL (green, ER membrane marker). a The data show the co-localization of VCP and KDEL in severe (GOLD III/IV) emphysema lung tissues compared with the mild or moderate (GOLD I/II) emphysema and GOLD 0 lung tissues. b The co-localization of Rma1 and KDEL is seen in moderate and severe (GOLD II/III/IV) emphysema lung tissues compared with the mild (GOLD I) emphysema and control (GOLD 0) lung tissues. The data not only verify the increased VCP-Rma1 expression but also demonstrate its localization in or around ER membrane with increasing severity of emphysema in COPD. The nuclear (Hoechst) staining is shown in the bottom panels. Scale=50 µm
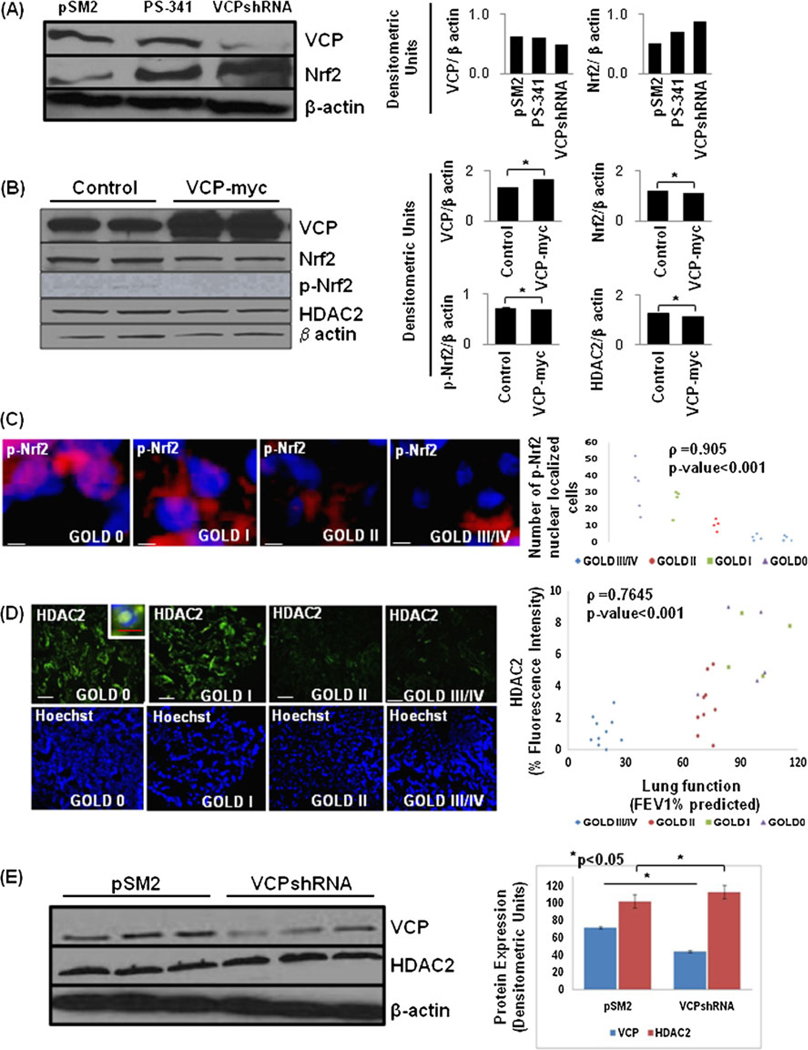
VCP controls expression of Nrf2 and HDAC2 proteins
Nuclear factor erythroid 2-related factor 2 (Nrf2) is a key transcription factor that regulates the cytoprotective transcriptional program in response to oxidative stress [24]. To elucidate that VCP not only controls NFκB levels but also controls Nrf2-mediated anti-oxidant defenses by mediating its proteasomal degradation [35–37], we measured Nrf2 expression in conditions of VCP inhibition (VCPshRNA) as compared with the proteasome inhibition by a therapeutic inhibitor, PS-341 (Velcade). We observed an increase in Nrf2 protein levels by VCP- and proteasome- inhibition (Fig. 6a(left and right panels)). We conclude that rescue of Nrf2 from proteasomal degradation by PS-341 or VCP inhibition induces a protective anti-oxidant response via Nrf2 (unpublished data). Similarly, the inability of corticosteroids to recruit histone deacetylase-2 (HDAC2), due to its proteasomal targeting may result in an abnormal inflammatory response and ineffectiveness of corticosteroid therapy in patients with COPD [38, 39]. We anticipate that higher VCP levels in COPD may lead to decreased Nrf2 and HDAC2 expression and activity based on the recent studies. We verified that VCP over-expression in HEK-293 cells downregulates the expression of Nrf2, p-Nrf2 (activated form), and HDAC2 (Fig. 6b(left and right panels); *p<0.05). Moreover, we also observed a significant decrease in nuclear localization of p-Nrf2 in the lung tissue sections of severe emphysema subjects (Fig. 6c; p<0.001). Our data suggest the role of elevated VCP in mediating the diminished Nrf2 dependent anti-oxidant response in COPD. We also verified the significant reduction of HDAC2 expression in severe emphysema (Gold IV) subjects as compared with mild and moderate emphysema (Gold I and II) and control (GOLD 0) (Fig. 6d(left and right panels); ρ=0.7645, p<0.001). Our data (Figs. 4a, d and 5b, d) suggest that increased VCP activity in COPD subjects may target HDAC2 for proteasomal degradation. We confirmed that VCP inhibition by shRNA increases HDAC2 levels (Fig. 6e(left and right panels)). Our data verify the correlation of increased VCP expression to severe emphysema and HDAC2-mediated glucocorticoid resistance.
Fig. 6.
Valosin-containing protein (VCP) regulates HDAC2 and Nrf2 protein levels. a The Beas2B cells were either transfected with control (pSM2) or pSM2-VCPshRNA (48 h) plasmids or treated with PS-341 (1 µM, overnight). The inhibition of VCP protein levels or proteasomal activity leads to upregulation of Nrf2 protein expression. The efficacy of pSM2-VCPshRNA is shown by VCP immunoblotting. b The HEK-293 cells were transiently transfected with VCP-myc plasmid for 48 h. The data indicate that VCP over-expression decreases Nrf2, p-Nrf2, and HDAC2 protein expression levels as compared with Lipofectamine treated controls. The right panel shows the densitometric analysis for (a, b, *p<0.05). c The paraffin-embedded longitudinal human lung sections of control (GOLD 0) and COPD lung tissue at GOLD I–IV levels of emphysema (n=4–5, each group), were immunostained with primary rabbit monoclonal p-Nrf2 and secondary anti-rabbit TR. The data show a significant decrease in p-Nrf2 (red) nuclear localization (co-localization with nuclear Hoechst, blue staining) with increasing severity of COPD lung disease. Statistical analysis (right panel) confirms the significant decrease in p-Nrf2+ nuclei (p<0.001) with progressive lung inflammation and emphysema. d The control and COPD lung tissue sections (n=8–10) immunostained with polyclonal HDAC2 and secondary anti-rabbit-FITC conjugated antibody, present significant decrease in HDAC2 expression and nuclear localization in the lung tissues with moderate or severe emphysema. Hoechst staining of the same area is shown in the bottom panel. Densitometric analysis (right panel) confirms the significant decrease in HDAC2 expression (p<0.001) with progressive lung inflammation and emphysema. Scale=50 µm. e An increase in HDAC2 protein levels is observed in HBE cells upon shRNA-mediated VCP knockdown. The densitometric analysis (mean±SD, p<0.05) of VCP and HDAC2 normalized to β-actin shows a significant increase in HDAC2 expression in VCP shRNA treated cells. The data verify the role of VCP-mediated proteasomal degradation in controlling activated form of Nrf2 (p-Nrf2) and HDAC2 protein levels
CS exposure induces VCP expression, ER stress, and apoptosis
To identify the effects of CS on VCP expression and its correlation to other mediators of COPD and emphysema pathogenesis, we used an acute CS murine model. We also verified this observation in the sub-chronic CS murine model and found a significant increase in VCP and NFκB expression levels suggesting that CS exposure induces VCP activity in lungs (Fig. 7c). We found that even acute CS exposure induces a significant increase in inflammation, VCP expression (ρ=−0.9429, p<0.001), along with an elevated NFκB and NOS2 expression in the CS-exposed murine lungs as compared with the air (Fig. 7a(panels 1–4)), indicative of CS-induced inflammatory-oxidative stress response. Next, we quantified changes in ubiquitinated proteins, ER stress (p-eIF2α), and number of apoptotic cells (TUNEL). We observed that even acute CS exposure induces a significant increase in accumulation of ubiquitinated proteins (Fig. 7a(panel 5)), ER stress (p-eIF2α; Fig. 7a(panel 6)), and apoptosis (TUNEL staining, Fig. 7b). The data propose the correlation of CS-induced VCP induction to inflammatory-oxidative stress, ubiquitin accumulation, and apoptosis. Similar correlation of VCP expression was seen in murine lungs of intratracheal Pa-LPS-induced acute lung injury model (Fig. 2 in the electronic supplementary materials).
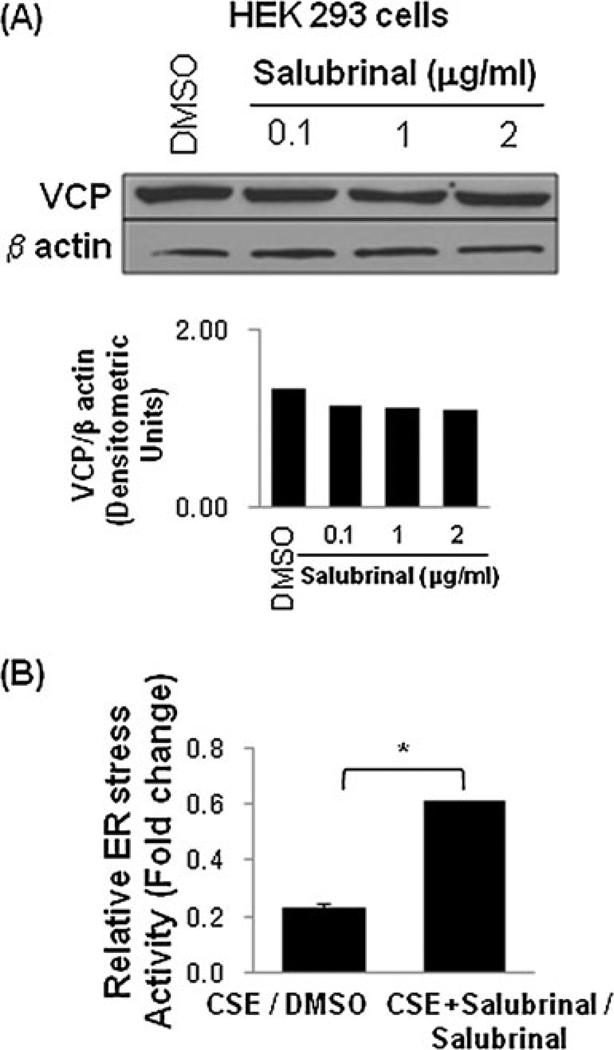
Salubrinal modulates VCP protein levels and controls CSE-induced ER stress activity
We have recently shown the potential of salubrinal in controlling proteostasis-imbalance and inflammation in lung injury [14]. Based on these studies, we tested the efficacy of salubrinal to modulate VCP levels and thereby control the aberrant proteostasis in COPD and emphysema. We observed that salubrinal decreases VCP expression levels in HEK-293 cells (Fig. 8a(upper and lower panels)). We also demonstrate that salubrinal has the potential to rescue the CSE-induced ER stress activity (Fig. 8b; *p<0.05). We anticipate that salubrinal may be inhibiting VCP and ER stress activities (Fig. 9) by two independent mechanisms that need to be evaluated. Next, we tested if salubrinal can control the CS-induced VCP expression in vivo. We observed in our preliminary studies that salubrinal significantly inhibits the acute CS-induced VCP expression (immunoblotting, p=0.04) in the murine lungs that needs to be verified further in chronic CS-exposed mice. Our data suggest the therapeutic potential of salubrinal in correcting the proteostasis-imbalance and treating CS-induced lung injury and emphysema that warrants further evaluation. Hence, we are currently evaluating and standardizing the therapeutic efficacy of salubrinal in sub-chronic and chronic CS-induced murine models of emphysema.
Fig. 8.
Salubrinal modulates VCP expression and controls CSE-induced ER stress. a The HEK-293 cells treated with DMSO (vehicle) or increasing doses of salubrinal show a dose dependent decrease in VCP expression. β-Actin was used as a loading control. The densitometric analysis is shown in the bottom panel. b Salubrinal (50 µM) restores CSE (200 µg/ml) induced decrease in secretory-ER stress reporter activity in HEK-293 cells (*p<0.05). The data suggest the potential efficacy of salubrinal in modulating VCP expression and proteostasis-imbalance in addition to inhibiting ER stress
Fig. 9.
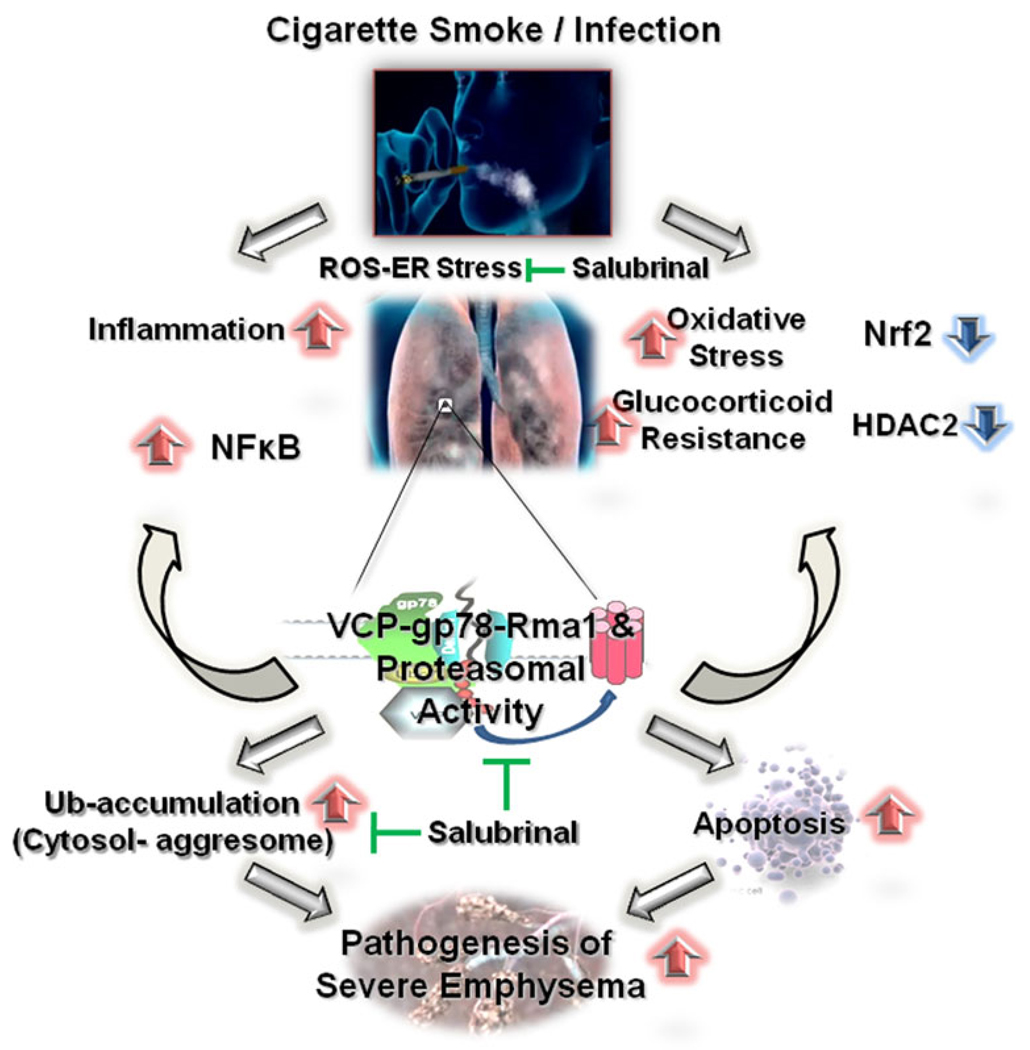
Schematic demonstrating the critical role of proteostasis-imbalance in COPD pathogenesis. Under normal conditions, optimal proteasomal activity balances inflammatory response and oxidative stress. In normal smokers, oxidative stress results in optimal activation of proteasomal pathway resulting in increased NFκB-mediated stress response that is balanced by induction of protective antioxidant response. On the other hand in COPD patients, inherent modification of VCP and proteasomal activity results in cytosolic ubiquitin accumulation, and elevated degradation of IκB, HDAC2, and Nrf2 leading to the pathogenesis of chronic lung inflammation, glucocorticoid resistance, and oxidative stress. Thus, proteostasis-imbalance is critical for pathogenesis of severe emphysema in COPD subjects. This proteostasis-imbalance in COPD lungs may be targeted by a therapeutic compound, salubrinal that controls both VCP activity and ubiquitin accumulation
Discussion
Recent studies suggest that increased inflammation, ER stress and unfolded protein response (UPR) activity contributes to the pathophysiology of COPD (chronic obstructive pulmonary disease) [24, 40, 41]. Although an earlier study showed that lungs of COPD subjects do not have any ER stress compared with the donor (negative control) or IPF (idiopathic pulmonary fibrosis, positive control) lungs [42]. Since this study does not clarify if these subjects had emphysema, we verified the changes in ER stress with increasing severity of emphysema in COPD. We not only verify the increased ER stress (Fig. 1) but also provide here the corroborative evidence in support of our hypothesis that aberrant proteostasis (Fig. 2) contributes to severity of emphysema. We anticipate based on recent studies [24, 40, 41] and current data that ER stress in emphysema may be a result of smoking while aberrant proteostasis contributes to pathogenesis of severe emphysema. We first identified the critical role of VCP in proteostasis-imbalance and pathogenesis of COPD and severe emphysema. VCP is a major retrograde translocation factor that pulls out the proteins from ER membrane that are destined for proteasomal degradation [13]. Since VCP is upregulated while protein turnover is low in COPD, we anticipate this as a mechanism to induce protein aggregation (Fig. 2b) that triggers chronic oxidative stress, inflammation, and apoptosis (Fig. 1c) leading to the pathogenesis of severe emphysema (Fig. 9). We postulate proteostasis-imbalance as a critical mechanism that mediates chronic oxidative stress and inflammation in COPD. The proteostasis-imbalance can be induced by environmental pollutants, age or genetic factors, hence explaining why oxidative stress remains high in COPD patients even after they quit smoking or why some non-smokers develop COPD. We confirmed the correlation of elevated VCP expression with the severity of emphysema in COPD using lung tissues from patients with FEV1-% predicted of >80 (mild, GOLD I), 50%–80% (moderate, GOLD II), and <50% (severe and very severe, GOLD III and IV) (Fig. 4a). In addition, VCP-associated E3/E4 ligases, Rma1, and gp78 are expressed at higher levels in severe emphysema tissue sections (Fig. 4b, c) similar to VCP indicating the role of VCP-gp78-Rma1 retrograde translocation complex in COPD and emphysema pathogenesis. To verify the specificity of the VCP-associated E3–E4 ligases in this process, we evaluated another common E3 ligase, CHIP that is not the part of this complex. We found relatively insignificant change in the expression of this ligase (CHIP) as compared with gp78-Rma1 (Fig. 1a, b in the electronic upplementary materials) indicating the critical role of VCP-gp78-Rma1 retrograde translocation complex in COPD pathogenesis. We also confirmed changes in VCP expression in smokers as compared to control non-smokers (Fig. 4d). We observed that VCP regulates Nrf2-(p) (NF-E2-related factor-2), NFκB (by regulating the degradation of its endogenous inhibitor, IκB) [13], and HDAC2 protein levels indicating towards its critical role in pathogenesis of emphysema and COPD (Fig. 6). The data presented here indicates the overall changes in lung protein expression and further studies are required for the assessment of the protein expression in the specific lung cells.
Our data indicate that proteostasis-imbalance contributes to the pathogenesis of COPD lung disease and severe emphysema by inducing chronic inflammatory response, oxidative stress and apoptosis. We demonstrate here the correlation of VCP-gp78-Rma1-mediated ubiquitination machinery and proteasomal activity [13, 43] with pathogenesis of severe emphysema. We also confirmed the accumulation of ubiquitinated protein by immunostaining and immunoblotting (insoluble protein fraction, pellet) for ubiquitin and aggregation marker, UCH-L1. Our data suggest lower proteasomal and elevated VCP-gp78-Rma1 activity as a potential mechanism that results in cytosolic accumulation of poly-ubiquitinated proteins to initiate chronic inflammatory and apoptotic responses leading to the pathogenesis of severe emphysema in COPD.
CS is the leading cause of emphysema that is known to induce chronic inflammation and oxidative stress as a mechanism to induce severe lung disease in COPD [44, 45]. CS contains approximately 1015 free radicals and electrophiles per puff, and lung cells of smokers are prone to protein and oxidative damage [24]. The increased levels of reactive oxygen species (ROS) have been implicated in initiating the lung inflammatory responses through the activation of redox sensitive transcription factor NFκB [46]. We observed a correlation of VCP expression with NFκB in COPD (Fig. 1) that can be explained by proteasomal degradation of IκB due to elevated VCP levels or activity. We anticipate that VCP protein expression in COPD lungs with severe emphysema is initially triggered as a protective UPR. Our data also suggest that increased or chronic VCP activity not only contributes to NFκB-mediated chronic inflammation but also decreased Nrf2 dependent anti-oxidant response (Fig. 6a–c) due to its degradation via VCP-mediated proteasomal pathway. In addition, these results explain that some cigarette smokers develop less severe lung emphysema compared with other smokers due to optimal UPR. Despite the heightened inflammatory response induced by ROS in CS, this inflammation is subdued in patients with optimal proteasomal activity and VCP-mediated UPR leading to less severe emphysema. However, in patients with significantly higher VCP-dependent ERAD activity, protective anti-inflammatory and oxidative stress response is diminished, leading to the pathogenesis of severe emphysema.
It is apparent that proteostasis-imbalance is a common mechanism that initiates the onset of chronic inflammation and oxidative stress in COPD as it modulates multiple transcription factors and proteins including Nrf2 and NFκB that are known to be associated with pathogenesis of severe emphysema [47, 48]. Previous studies have shown that HDAC2 is negatively regulated by CS via UPS mediated mechanisms [25]. The inability of corticosteroids to recruit HDAC2 or the presence of post-translationally modified HDAC2 may explain the abnormal decrease in its expression levels with increasing COPD severity (Fig. 6d). We suggest here the involvement of VCP in regulating HDAC2 protein levels by controlling its degradation (Fig. 6b, e). The data not only verify the previously documented decrease in HDAC2 levels in COPD [3, 38, 39] but also provide the mechanism for lower HDAC2 activity.
We observed that proteostasis-imbalance can be directly linked to CS exposure, a primary risk factor in COPD pathogenesis [24]. We verified that both acute and sub-chronic CS exposure induces VCP protein levels in murine lungs as compared with the air exposed mice. Moreover, increase in VCP levels correlates with elevated NFκB and NOS2 expression, accumulation of ubiquitinated protein and apoptosis (TUNEL staining) in murine lung tissue sections exposed to acute CS (Fig. 7). It may be possible that CS exposure causes severe damage to proteins in the lungs that triggers VCP activity to avoid CS-induced ER stress and accumulation of ubiquitinated proteins [5, 41]. But since these proteins (especially the high molecular weight proteins) are either misfolded or damaged due to CS exposure, they are poly-ubiquitinated and aggregated as cytosolic aggregates called aggresomes by VCP-dependent mechanisms as we recently discussed [13]. Moreover, we observed that CSE may affect protein synthesis hence protein turnover rates that warrants further verification. In addition, our data on acute CS exposure indicate toward early CS related proteostasis-imbalance that needs to be verified further in chronic CS-exposed murine model to validate the association of CS-mediated proteostasis-imbalance with severe emphysema. Our preliminary data suggest that salubrinal has a potential to correct proteostasis-imbalance based on its ability to control VCP expression, NFκB activation, and ubiquitin accumulation (Fig. 8) [14], in addition to controlling protein turnover [49]. We anticipate that salubrinal modulates p-eIF2α to limit protein synthesis while VCP to control cytosolic ubiquitin accumulation (Fig. 9) by two independent mechanisms that need to be evaluated. Further studies are underway to verify and standardize the therapeutic efficacy of salubrinal in chronic CS-induced murine emphysema model.
We also document a similar correlation of increased VCP expression with pathogenesis of inflammatory lung disease in murine lungs induced by P. aeruginosa-LPS. We found the significant increase in LPS-induced VCP expression, and its correlation to elevated NFκB, NOS2 expression and Nrf2 activity (Fig. 2 in the electronic supplementary materials). We confirmed that VCP induction correlates with accumulation of ubiquitinated proteins (Ub/UCH-L1 localization and expression) and apoptosis (TUNEL staining). The data verify the correlation of elevated VCP expression in response to Pa-LPS or CS-induced injury (discussed above) with inflammatory-oxidative stress and apoptosis. In this study, we focused on the critical component of proteasomal pathway (VCP-gp78-Rma1) that is known to be involved in regulating inflammatory-oxidative stress response and proteasomal degradation of damaged proteins. Our data fill the gap in the knowledge of COPD and emphysema pathogenesis by determining that aberrant proteostasis is a critical step that leads oxidative stress and chronic inflammation in COPD. It also explains why oxidative stress remains high in COPD patients even after they quit smoking as proteostasis-imbalance can be induced by not only CS but also other genetic, environmental or age-related changes. Validation of involvement of such factors that affect proteostasis-imbalance requires further investigation with larger groups of COPD and control subjects.
In conclusion, we for the first time report a thorough analysis of how aberrant regulation of VCP-mediated proteostasis is associated with the severity of COPD lung disease (Fig. 9). Under normal conditions, there is optimal UPS-regulated balance of Nrf2 and IκB (endogenous inhibitor of NFκB) levels, thereby preventing the deregulation of anti-oxidant and inflammatory pathways. In normal smokers, oxidative stress leads to optimal UPS activation resulting in increased NFκB-mediated stress response that is balanced by induction of protective Nrf2 or UPR responses. Whereas in COPD patients, the modification of UPS results in elevated degradation rates of COPD associated factors like IκB, HDAC2 and Nrf2. Moreover, our data suggest that increased levels of damaged proteins induce poly-ubiquitination and aggregation of these proteins by VCP-dependent mechanisms that trigger apoptosis and pathogenesis of COPD and severe emphysema. The data from this study suggest that selective modulation of proteostatic pathways can prevent or retard the progression of severe emphysema. In addition, we propose further evaluation of ubiquitin-proteasome activity as a novel biomarker for emphysema as well as a prognosticator of potential utility of the aforementioned therapeutic strategy. To summarize, the identification of molecular mechanism of proteasomal pathway in COPD lung disease not only contributes to our understanding of COPD pathogenesis but also demonstrates the therapeutic potential of VCP as a novel molecular target, or the use of proteostasis modulators like salubrinal, for further preclinical evaluation and translation.
Supplementary Material
Acknowledgments
We are thankful to the Lung Tissue Research Consortium, NHLBI, NIH for human lung tissue samples and Johns Hopkins University histology core for H&E staining and processing of murine lung tissues. The study was supported by FAMRI and NIH (CTSA UL RR 025005 and RHL096931) grants to NV.
Footnotes
Taehong Min and Manish Bodas contributed equally to this work.
Electronic supplementary material The online version of this article (doi:10.1007/s00109-011-0732-8) contains supplementary material, which is available to authorized users.
Contributor Information
Taehong Min, Division of Pediatric Respiratory Sciences, Department of Pediatrics, Johns Hopkins University, 600 N. Wolfe Street, CMSC 3-122, Baltimore, MD 21287, USA.
Manish Bodas, Division of Pediatric Respiratory Sciences, Department of Pediatrics, Johns Hopkins University, 600 N. Wolfe Street, CMSC 3-122, Baltimore, MD 21287, USA.
Steven Mazur, Division of Pediatric Respiratory Sciences, Department of Pediatrics, Johns Hopkins University, 600 N. Wolfe Street, CMSC 3-122, Baltimore, MD 21287, USA.
Neeraj Vij, Email: nvij1@jhmi.edu, Division of Pediatric Respiratory Sciences, Department of Pediatrics, Johns Hopkins University, 600 N. Wolfe Street, CMSC 3-122, Baltimore, MD 21287, USA; Johns Hopkins University School of Medicine, Baltimore, MD, USA.
References
- 1.Rahman I, Adcock IM. Oxidative stress and redox regulation of lung inflammation in COPD. Eur Respir J. 2006;28(1):219–242. doi: 10.1183/09031936.06.00053805. [DOI] [PubMed] [Google Scholar]
- 2.Moodie FM, Marwick JA, Anderson CS, Szulakowski P, Biswas SK, Bauter MR, Kilty I, Rahman I. Oxidative stress and cigarette smoke alter chromatin remodeling but differentially regulate NF-kappaB activation and proinflammatory cytokine release in alveolar epithelial cells. FASEB J. 2004;18(15):1897–1899. doi: 10.1096/fj.04-1506fje. [DOI] [PubMed] [Google Scholar]
- 3.Yang SR, Chida AS, Bauter MR, Shafiq N, Seweryniak K, Maggirwar SB, Kilty I, Rahman I. Cigarette smoke induces proinflammatory cytokine release by activation of NF-kappaB and posttranslational modifications of histone deacetylase in macrophages. Am J Physiol Lung Cell Mol Physiol. 2006;291(1):L46–L57. doi: 10.1152/ajplung.00241.2005. [DOI] [PubMed] [Google Scholar]
- 4.Pryor WA, Stone K. Oxidants in cigarette smoke. Radicals, hydrogen peroxide, peroxynitrate, and peroxynitrite. Ann NY Acad Sci. 1993;686::12–27. doi: 10.1111/j.1749-6632.1993.tb39148.x. discussion 27–8. [DOI] [PubMed] [Google Scholar]
- 5.Yoshida T, Tuder RM. Pathobiology of cigarette smoke-induced chronic obstructive pulmonary disease. Physiol Rev. 87(3):1047–1082. doi: 10.1152/physrev.00048.2006. [DOI] [PubMed] [Google Scholar]
- 6.Eickmeier O, Huebner M, Herrmann E, Zissler U, Rosewich M. Sputum biomarker profiles in cystic fibrosis (CF) and chronic obstructive pulmonary disease (COPD) and association between pulmonary function. Cytokine. 2010;50(2):152–157. doi: 10.1016/j.cyto.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 7.Filosto S, Castillo S, Danielson A, Franzi L, Khan E, Kenyon N, Last J, Pinkerton K, Tuder R, Goldkorn T. Neutral sphingomyelinase 2: a novel target in cigarette smoke-induced apoptosis and lung injury. Am J Respir Cell Mol Biol. 2011 doi: 10.1165/rcmb.2009-0422OC. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hetzer M, Meyer HH, Walther TC, Bilbao-Cortes D, Warren G, Mattaj IW. Distinct AAA-ATPase p97 complexes function in discrete steps of nuclear assembly. Nat Cell Biol. 2001;3(12):1086–1091. doi: 10.1038/ncb1201-1086. [DOI] [PubMed] [Google Scholar]
- 9.Kondo H, Rabouille C, Newman R, Levine TP, Pappin D, Freemont P, Warren G. p47 is a cofactor for p97-mediated membrane fusion. Nature. 1997;388(6637):75–78. doi: 10.1038/40411. [DOI] [PubMed] [Google Scholar]
- 10.Braun RJ, Zischka H. Mechanisms of Cdc48/VCP-mediated cell death—from yeast apoptosis to human disease. Biochim Biophys Acta. 2008;1783(7):1418–1435. doi: 10.1016/j.bbamcr.2008.01.015. [DOI] [PubMed] [Google Scholar]
- 11.Partridge JJ, Lopreiato JO, Jr, Latterich M, Indig FE. DNA damage modulates nucleolar interaction of the Werner protein with the AAA ATPase p97/VCP. Mol Biol Cell. 2003;14(10):4221–4229. doi: 10.1091/mbc.E03-02-0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meyer HH, Shorter JG, Seemann J, Pappin D, Warren G. A complex of mammalian ufd1 and npl4 links the AAA-ATPase, p97, to ubiquitin and nuclear transport pathways. EMBO J. 2000;19(10):2181–2192. doi: 10.1093/emboj/19.10.2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vij N. AAA ATPase p97/VCP: cellular functions, disease and therapeutic potential. J Cell Mol Med. 2008;12(6A):2511–2518. doi: 10.1111/j.1582-4934.2008.00462.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bodas M, Min T, Vij N. Early-age-related changes in proteostasis augment immunopathogenesis of sepsis and acute lung injury. PLoS One. 2010;5(11):e15480. doi: 10.1371/journal.pone.0015480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meusser B, Hirsch C, Jarosch E, Sommer T. ERAD: the long road to destruction. Nat Cell Biol. 2005;7(8):766–772. doi: 10.1038/ncb0805-766. [DOI] [PubMed] [Google Scholar]
- 16.Koegl M, Hoppe T, Schlenker S, Ulrich HD, Mayer TU, Jentsch S. A novel ubiquitination factor, E4, is involved in multi-ubiquitin chain assembly. Cell. 1999;96(5):635–644. doi: 10.1016/s0092-8674(00)80574-7. [DOI] [PubMed] [Google Scholar]
- 17.Ishigaki S, Hishikawa N, Niwa J, Iemura S, Natsume T, Hori S, Kakizuka A, Tanaka K, Sobue G. Physical and functional interaction between Dorfin and Valosin-containing protein that are colocalized in ubiquitylated inclusions in neurodegenerative disorders. J Biol Chem. 2004;279(49):51376–51385. doi: 10.1074/jbc.M406683200. [DOI] [PubMed] [Google Scholar]
- 18.Zhong X, Shen Y, Ballar P, Apostolou A, Agami R, Fang S. AAA ATPase p97/valosin-containing protein interacts with gp78, a ubiquitin ligase for endoplasmic reticulum-associated degradation. J Biol Chem. 2004;279(44):45676–45684. doi: 10.1074/jbc.M409034200. [DOI] [PubMed] [Google Scholar]
- 19.Vij N, Fang S, Zeitlin PL. Selective inhibition of endoplasmic reticulum-associated degradation rescues {Delta} F508-cystic fibrosis transmembrane regulator and suppresses interleukin-8 levels: therapeutic implications. J Biol Chem. 2006;281(25):17369–17378. doi: 10.1074/jbc.M600509200. [DOI] [PubMed] [Google Scholar]
- 20.Morito D, Hirao K, Oda Y, Hosokawa N, Tokunaga F, Cyr DM, Tanaka K, Iwai K, Nagata AK. Gp78 cooperates with RMA1 in endoplasmic reticulum-associated degradation of CFTR {Delta}F508. Mol Biol Cell. 2008;19(4):1328–1336. doi: 10.1091/mbc.E07-06-0601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Younger JM, Chen L, Ren HY, Rosser MF, Turnbull EL, Fan CY, Patterson C, Cyr DM. Sequential quality-control checkpoints triage misfolded cystic fibrosis transmembrane conductance regulator. Cell. 2006;126(3):571–582. doi: 10.1016/j.cell.2006.06.041. [DOI] [PubMed] [Google Scholar]
- 22.Vij N, Amoako MO, Mazur S, Zeitlin PL. CHOP transcription factor mediates IL-8 signaling in cystic fibrosis bronchial epithelial cells. Am J Respir Cell Mol Biol. 2008;38(2):176–184. doi: 10.1165/rcmb.2007-0197OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Malhotra D, Thimmulappa R, Navas-Acien A, Sandford A, Elliott M, Singh A, Chen L, Zhuang X, Hogg J, Pare P, Tuder RM, Biswal S. Decline in NRF2-regulated antioxidants in chronic obstructive pulmonary disease lungs due to loss of its positive regulator, DJ-1. Am J Respir Crit Care Med. 2008;178(6):592–604. doi: 10.1164/rccm.200803-380OC. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 24.Malhotra D, Thimmulappa R, Vij N, Navas-Acien A, Sussan T, Merali S, Zhang L, Kelsen SG, Myers A, Wise R, Tuder R, Biswal S. Heightened endoplasmic reticulum stress in the lungs of patients with chronic obstructive pulmonary disease: the role of Nrf2-regulated proteasomal activity. Am J Respir Crit Care Med. 2009;180(12):1196–1207. doi: 10.1164/rccm.200903-0324OC. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 25.Adenuga D, Yao H, March TH, Seagrave J, Rahman I. Histone deacetylase 2 is phosphorylated, ubiquitinated, and degraded by cigarette smoke. Am J Respir Cell Mol Biol. 2009;40(4):464–473. doi: 10.1165/rcmb.2008-0255OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rabe KFHS, Anzueto A, Barnes PJ. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2007;176(6):527–528. doi: 10.1164/rccm.200703-456SO. [DOI] [PubMed] [Google Scholar]
- 27.Vij N, Roberts L, Joyce S, Chakravarti S. Lumican regulates corneal inflammatory responses by modulating Fas-Fas ligand signaling. Invest Ophthalmol Vis Sci. 2005;46(1):88–95. doi: 10.1167/iovs.04-0833. [DOI] [PubMed] [Google Scholar]
- 28.Bodas M, Min T, Mazur S, Vij N. Critical modifier role of membrane-cystic fibrosis transmembrane conductance regulator-dependent ceramide signaling in lung injury and emphysema. J Immunol. 2011;186(1):602–613. doi: 10.4049/jimmunol.1002850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kardosh AGE, Pyrko P, Uddin J, Hofman FM, Chen TC, Louie SG, Petasis NA, Schonthal AH. Aggravated endoplasmic reticulum stress as a basis for enhanced glioblastoma cell killing by bortezomib in combination with celecoxib or its non-coxib analogue, 2, 5-dimethylcelecoxib. Cancer Res. 2008;68:843–851. doi: 10.1158/0008-5472.CAN-07-5555. [DOI] [PubMed] [Google Scholar]
- 30.Tagawa YHN, Kasai A, Hayakawa K, Okamura M, Yao J, Kitamura M. Induction of apoptosis by cigarette smoke via ROS dependent endoplasmic reticulum stress and CCAAT/enhancer binding protein-homologous protein (CHOP) Free Radic Biol Med. 2008;45:50–59. doi: 10.1016/j.freeradbiomed.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 31.Wang Z, Figueiredo-Pereira ME. Inhibition of sequestosome 1/p62 up-regulation prevents aggregation of ubiquitinated proteins induced by prostaglandin J2 without reducing its neurotoxicity. Mol Cell Neurosci. 2005;29(2):222–231. doi: 10.1016/j.mcn.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 32.Carolan BJ, Heguy A, Harvey BG, Leopold PL, Ferris B, Crystal RG. Up-regulation of expression of the ubiquitin carboxyl-terminal hydrolase L1 gene in human airway epithelium of cigarette smokers. Cancer Res. 2006;66(22):10729–10740. doi: 10.1158/0008-5472.CAN-06-2224. [DOI] [PubMed] [Google Scholar]
- 33.Yang H, Liu C, Zhong Y, Luo S, Monteiro MJ, Fang S. Huntingtin interacts with the cue domain of gp78 and inhibits gp78 binding to ubiquitin and p97/VCP. PLoS One. 2010;5(1):e8905. doi: 10.1371/journal.pone.0008905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jiang J, Ballinger CA, Wu Y, Dai Q, Cyr DM, Hohfeld J, Patterson C. CHIP is a U-box-dependent E3 ubiquitin ligase: identification of Hsc70 as a target for ubiquitylation. J Biol Chem. 2001;276(46):42938–42944. doi: 10.1074/jbc.M101968200. [DOI] [PubMed] [Google Scholar]
- 35.Rangasamy T, Guo J, Mitzner WA, Roman J, Singh A, Fryer AD, Yamamoto M, Kensler TW, Tuder RM, Georas SN, Biswal S. Disruption of Nrf2 enhances susceptibility to severe airway inflammation and asthma in mice. J Exp Med. 2005;202(1):47–59. doi: 10.1084/jem.20050538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goven D, Boutten A, Lecon-Malas V, Marchal-Somme J, Amara N, Crestani B, Fournier M, Leseche G, Soler P, Boczkowski J, Bonay M. Altered Nrf2/Keap1-Bach1 equilibrium in pulmonary emphysema. Thorax. 2008;63(10):916–924. doi: 10.1136/thx.2007.091181. [DOI] [PubMed] [Google Scholar]
- 37.Itoh K, Wakabayashi N, Katoh Y, Ishii T, Igarashi K, Engel JD, Yamamoto M. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev. 1999;13(1):76–86. doi: 10.1101/gad.13.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barnes PJ. Role of HDAC2 in the pathophysiology of COPD. Annu Rev Physiol. 2009;71:451–464. doi: 10.1146/annurev.physiol.010908.163257. [DOI] [PubMed] [Google Scholar]
- 39.Ito KLS, Caramori G, Chung KF, Barnes PJ, Adcock IM. Cigarette smoking reduces histone deacetylase 2 expression, enhances cytokine expression, and inhibits glucocorticoid actions in alveolar macrophages. FASEB J. 2001;15(6):1110–1112. [PubMed] [Google Scholar]
- 40.Barnes PJ. The cytokine network in chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol. 2009;41(6):631–638. doi: 10.1165/rcmb.2009-0220TR. [DOI] [PubMed] [Google Scholar]
- 41.Kelsen SG, Duan X, Ji R, Perez O, Liu C, Merali S. Cigarette smoke induces an unfolded protein response in the human lung: a proteomic approach. Am J Respir Cell Mol Biol. 2008;38(5):541–550. doi: 10.1165/rcmb.2007-0221OC. [DOI] [PubMed] [Google Scholar]
- 42.Korfei M, Ruppert C, Mahavadi P, Henneke I, Markart P, Koch M, Lang G, Fink L, Bohle RM, Seeger W, Weaver TE, Guenther A. Epithelial endoplasmic reticulum stress and apoptosis in sporadic idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2008;178(8):838–846. doi: 10.1164/rccm.200802-313OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vij N, Mazur S, Zeitlin PL. VCP is involved in ERAD and aggresome formation of ΔF508-CFTR. Pediatr Pulmonol. 2006;41(29):209. [Google Scholar]
- 44.Houghton AM, Mouded M, Shapiro SD. Common origins of lung cancer and COPD. Nat Med. 2008;14(10):1023–1024. doi: 10.1038/nm1008-1023. [DOI] [PubMed] [Google Scholar]
- 45.Rajendrasozhan S, Yang SR, Edirisinghe I, Yao H, Adenuga D, Rahman I. Deacetylases and NF-kappaB in redox regulation of cigarette smoke-induced lung inflammation: epigenetics in pathogenesis of COPD. Antioxid Redox Signal. 2008;10(4):799–811. doi: 10.1089/ars.2007.1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yao H, Yang SR, Kode A, Rajendrasozhan S, Caito S, Adenuga D, Henry R, Edirisinghe I, Rahman I. Redox regulation of lung inflammation: role of NADPH oxidase and NF-kappaB signalling. Biochem Soc Trans. 2007;35(Pt 5):1151–1155. doi: 10.1042/BST0351151. [DOI] [PubMed] [Google Scholar]
- 47.Rangasamy T, Cho CY, Thimmulappa RK, Zhen L, Srisuma SS, Kensler TW, Yamamoto M, Petrache I, Tuder RM, Biswal S. Genetic ablation of Nrf2 enhances susceptibility to cigarette smoke-induced emphysema in mice. J Clin Invest. 2004;114(9):1248–1259. doi: 10.1172/JCI21146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rajendrasozhan S, Chung S, Sundar IK, Yao H, Rahman I. Targeted disruption of NF-{kappa}B1 (p50) augments cigarette smoke-induced lung inflammation and emphysema in mice: a critical role of p50 in chromatin remodeling. Am J Physiol Lung Cell Mol Physiol. 2010;298(2):L197–L209. doi: 10.1152/ajplung.00265.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Boyce M, Bryant KF, Jousse C, Long K, Harding HP, Scheuner D, Kaufman RJ, Ma D, Coen DM, Ron D, Yuan J. A selective inhibitor of eIF2alpha dephosphorylation protects cells from ER stress. Science. 2005;307(5711):935–939. doi: 10.1126/science.1101902. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.