Abstract
Cytoreductive conditioning regimens used in the context of allogeneic hematopoietic cell transplantation (HCT) elicit deficits in innate and adaptive immunity, which predispose patients to infections. As such, transplantation outcomes depend vitally on the successful reconstruction of immune competence. Restoration of a normal peripheral T-cell pool after HCT is a slow process that requires the de novo production of naive T cells in a functionally competent thymus. However, there are several challenges to this regenerative process. Most notably, advanced age, the cytotoxic pretransplantation conditioning, and posttransplantation alloreactivity are risk factors for T-cell immune deficiency as they independently interfere with normal thymus function. Here, we discuss preclinical allogeneic HCT models and clinical observations that have contributed to a better understanding of the transplant-related thymic dysfunction. The identification of the cellular and molecular mechanisms that control regular thymopoiesis but are altered in HCT patients is expected to provide the basis for new therapies that improve the regeneration of the adaptive immune system, especially with functionally competent, naive T cells.
Introduction
Allogeneic hematopoietic cell transplantation (HCT) offers an effective treatment for a broad spectrum of malignant and nonmalignant disorders.1,2 The rates for 1-year and disease-free survival have significantly improved over the last few years.3–6 However, transplant-related complications, such as opportunistic infections and GVHD, continue to seriously affect the patients' quality of life.7,8
Why are transplant recipients prone to develop infections? The answer to this question lies, at least in large part, in how allogeneic HCT is performed. Eligible patients are first treated with chemotherapy and/or radiotherapy before they receive the hematopoietic cell graft. This preconditioning can be done at different intensities and results in cytoreduction or ablation.9 The objectives are to reduce the bulk of malignant cells in instances where allogeneic HCT is used for cancer treatment, to decrease the risk of graft rejection via general host immune suppression, and to improve the engraftment of donor hematopoietic stem cells (HSCs) by evacuating the host marrow. As a side effect, the preconditioning also invariably compromises natural and adaptive immune responses of HCT recipients, predisposing them to infectious complications that may contribute to poor clinical outcome.
The universal observation is made that innate immunity is rapidly restored after allogeneic HCT. Although studies are heterogeneous given the divergent clinical situations, the blood counts of myeloid lineage cells of the natural immune system (ie, neutrophils, monocytes, natural killer cells) often normalize in humans within 2 to 4 weeks after myeloablative allogeneic HCT.10–12 Bacterial infections are frequent during this aplastic phase and may be observed at later time points, especially in the context of incomplete myeloid reconstitution.13 The rebuilding of adaptive T cell-mediated immune responses takes much longer; and until that occurs, there in an increased susceptibility for fungal and viral infections, such as reactivated herpes virus (cytomegalovirus, Epstein-Barr virus, and varicella-zoster virus) and candida, aspergillus, and pneumocystis infections.11 Slow or inadequate T-cell regeneration is thus a major risk factor for late opportunistic infections.
A complete rescue of immune competence, including the continued presence of a broad TCR repertoire critically depends on the recovery of de novo T-cell production in the thymus. The productivity of the thymus depends in turn on several risk factors. They include, but are not restricted to, disease status,14 patient age,15 source and composition of the graft,16 type of conditioning,17 and presence of GVHD.18–20 The purpose of this article is to assess our present-day knowledge about the pathways of T-cell regeneration after allogeneic HCT, with particular focus on the impediments to normal thymus function.
Two pathways to T-cell regeneration after lymphodepletion: a brief overview
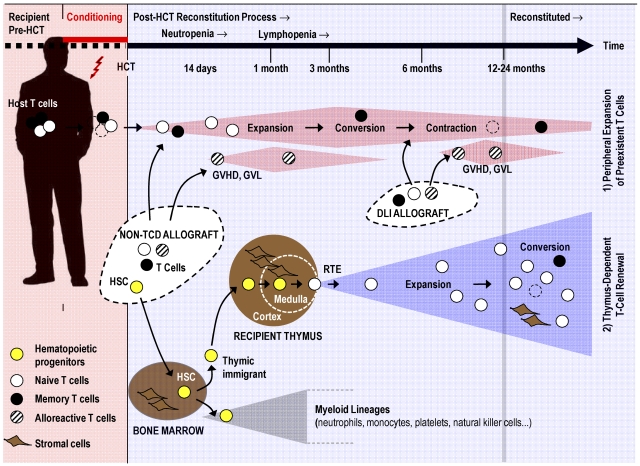
Post-transplantation regeneration of an ablated T-cell compartment is accomplished by thymus-independent and thymus-dependent mechanisms that act in parallel (Figure 1). Blood lymphocyte numbers rise initially because of thymus-independent peripheral expansion of preexisting naive and memory T cells arising from different origins. The 2 separate sources include residual host T cells that survive conditioning and the mature donor T cells transferred via a non–T cell–depleted (non-TCD) stem cell allograft or intentionally given as a donor lymphocyte infusion (DLI).21–24 The kinetics and relative extent to which either cellular source contributes to thymus-independent T-cell expansion early and late after allogeneic HCT may vary depending on the transplantation setting. The dosage of donor T cells and the intensity of conditioning constitute the most important parameters that influence expansion.25 Expansion of donor-derived T cells may dominate after either high-intensity myeloablative HCT or DLI, whereas expansion of host T cells contributes to the T-cell repertoire in patients given nonmyeloablative, reduced intensity conditioning (RIC).9,25,26 However, patterns of T-cell expansion are not always readily predictable, arguing for additional contributory factors determining lymphoid chimerism (eg, underlying disease, type of immune suppression). Moreover, new RIC protocols are rapidly evolving, and early full donor T-cell chimerism has reportedly been achieved with particular nonmyeloablative regimens.25
Figure 1.
T-cell regenerative pathways after allogeneic HCT. Pretransplantation conditioning reduces the patient's existing naive (○) and memory (●) T cells. Post-HCT regeneration of peripheral T cells is accomplished by 2 mechanisms: (1) Cell numbers of residual host T cells and mature donor T cells transferred via a non-TCD stem cell allograft or via DLI expand initially in response to homeostatic signals or cognate antigen. Alloreactive donor T cells (which can mediate GVHD, graft-versus-leukemia, or both; dashed symbols) contribute also to the thymus-independent cell compartment. This T-cell pool is altered as a result of memory T-cell conversion, skewing of the TCR repertoire, and pool size contraction (broken lines indicate senescent T cells). (2) New naive T cells are generated from hematopoietic progenitors (yellow symbols) in a thymus-dependent regenerative mechanism. The stromal cells of the thymus (thymic medullary and cortical compartments are separated from each other by a dashed line) support the differentiation of descendants of thymic immigrants into mature T lymphocytes. Recent thymic emigrants (RTEs) expand in the periphery in response to homeostatic signals and after stimulation by their cognate antigen. Under physiologic conditions, the thymic export assures a constant and life-long supply with naive T cells harboring a diverse TCR repertoire. After allogeneic HCT, the thymus-dependent T-cell renewal is a slow process as it can take up to 12 to 24 months to be completed under favorable conditions. However, advanced age, conditioning, and GVHD impair thymus function and thus interfere with naive T-cell regeneration. The putative mechanisms are discussed in Figure 3.
T-cell expansion is naturally triggered either via stimulation by cognate antigen or through a homeostatic proliferation in response to lymphopenia. The latter process is designated “homeostatic peripheral expansion” (HPE) and depends on low-affinity interactions with self-peptide/major histocompatibility complex (MHC) complexes in conjunction with exposure to high cytokine levels (which accumulate during lymphopenia as a result of reduced consumption).27 This form of T-cell proliferation does not affect TCR diversity in contrast to the skewing of the repertoire, which takes place after activation by cognate antigens.28 Thus, the efficiency of HPE in restoring diversity of the peripheral pool will be limited by the starting repertoire of the mature T cells that serves as the source for expansion. In the absence of newly produced naive T cells exported from the thymus, the peripheral blood T-cell compartment undergoes gradual changes. As a result of frequent interactions with pathogens, the human TCR repertoire is progressively restricted as a consequence of oligoclonal expansions of antigen-specific T cells, which may then convert to memory cells.29
The thymus-independent regenerative pathway will mostly suffice to assure initially the immune competence required for the protection against post-transplantation infections. This capability is better measured by the degree of antigen-specific responsiveness rather than by simply quantifying total T-cell numbers. The conditioning-resistant T cells of the recipient and the transferred donor-derived mature T cells can variably contribute to the adaptive immunity present early after transplantation: First, adoptively transferred naive nonalloreactive donor T cells and naive host T cells that have withstood conditioning may participate in de novo adaptive immune responses against microbial antigens encountered early after HCT. The efficiency and the extent to which these naive T cells contribute to protection from infections appear to be minor because, for example, there is only a low frequency of primary anticytomegalovirus responses early after HCT.30 More commonly, protection against the reexposure to specific antigens may be effected by residual host memory T cells that have survived cytoablative conditioning.30 Donor-derived memory T cells transferred with the stem cell graft or as DLI can also protect against infectious pathogens (eg, cytomegalovirus, Epstein-Barr virus).31 After DLI into significantly lymphopenic patients, donor memory T-cell mediated immunity dominates over the antimicrobial response effected by residual host memory T cells.32 Such capacity of donor T cells to adoptively transmit immunologic competence may be exploited for immunotherapy to supplement allogeneic HCT.24 As reviewed elsewhere in more detail,24,33,34 adoptive cell-based therapies using unmodified or genetically modified T cells has demonstrated clinical efficacy in trials investigating the T-cell response to specific pathogens, tumors, or vaccines. The transfer of unmodified allogeneic T cells can provide a potent tumoricidal effect. The recognition of epitopes expressed on transformed host cells (which may include tumor-specific antigens or normal nonmutated genes, which are “foreign” to the infused T cells) may eventually lead to the elimination of the tumor (in a process referred to as graft-versus-cancer, or in the case of leukemia, a graft-versus-leukemia effect).35 Although such an alloimmune process can cure the underlying disease, it may cause GVHD (see “GVDH”) because of the recognition of antigens that are shared between transformed and normal host cells.
The TCR repertoire of conditioning-resistant host and transferred donor T cells will become increasingly skewed over time. In parallel, the size of this compartment will also progressively contract as a result of replicative senescence and/or activation-induced apoptosis of these cells.22 Because the thymus-independent pathway fails to provide new T cells, the T-cell compartment existing early after transplantation cannot sustain a broad antigenic specificity. In result, immunoprotection is either transient or incomplete as increasingly fewer naive T cells are available to mount a protective immune response. For a functionally complete regeneration of adaptive immunity after allogeneic HCT, it is thus critical that the peripheral T-cell compartment is continuously replenished by naive T cells.
In contrast to the successful development of other hematopoietic lineages, the generation of naive T cells requires in addition to the bone marrow the specialized microenvironment of the thymus (Figure 1).36,37 Mature T cells are continuously exported from the thymus to the periphery where these recent thymic emigrants (RTEs) expand in response to either homeostatic signals or cognate antigens. Under physiologic conditions, a robust thymic export assures a constant, though with age, declining supply of naive T cells recognizing with their TCR repertoire a seemingly unlimited diversity of antigens.38,39
Thymic pathway in allogeneic HCT
The actual size of the peripheral T-cell pool is governed under physiologic conditions, not by the extant intensity of thymic export but by the process of HPE.28 In the special situations of disease-related or iatrogenic lymphodepletion, thymic export becomes important as it singularly contributes to the maintenance of an adequately broad TCR repertoire in a peripheral T-cell pool of regular size. The overall competence of the thymus-dependent regenerative pathway depends on the correct functions of all components involved in T-cell production. These elements involve the quality of the grafted stem cells, the availability of competent bone marrow stromal niches, the regular migration of T-lymphoid progenitors to the thymus, the efficient commitment of the blood-borne hematopoietic cells to a T-cell fate, the subsequent orderly progression of thymocyte maturation, the proficient export of mature thymocytes to the periphery, and the availability of suitable postthymic stromal niches to lodge exported T cells (Figure 1).
Stages of T-cell maturation in mice
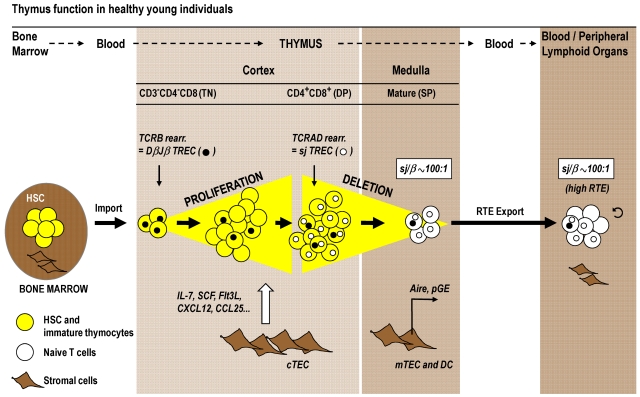
Because hematopoietic stem cells do not reside in the thymus, a steady import of bone marrow-derived progenitors via the blood is required to maintain the de novo generation of T cells.39 The phenotypes of hematopoietic precursor cells and thymocyte subpopulations in humans and mice differ to some extent,38 yet the maturational sequences are analogous. We will focus here on the more readily and intensively studied murine model. On entry into the thymus, the T-cell progenitors initially reside in the cortex. These cells and their immediate progeny are termed triple-negative (TN) because the cells do not express the surface receptors CD3, CD4, or CD8 (Figure 2). Under normal conditions, TN thymocytes undergo a robust expansion as they divide approximately 8 to 9 times during their journey through the thymus cortex.40 The signals that drive cell division are provided by cortical thymic epithelial cells (cTECs), which form a scaffold that, in cooperation with other stromal cells, delivers an array of extrinsic signals to developing T-lymphoid lineage cells.41,42 The soluble mediators (most prominently IL-7, stem cell factor, Fms-like tyrosine kinase 3 ligand, and CCL25) and membrane-bound proteins (eg, chemokine receptors, peptide/MHC complexes) control the import of thymocyte precursors and the survival, division, trafficking, selection, and export of thymocytes. With the acquisition of the cell surface proteins CD4 and CD8, thymocytes are now referred to as double-positive (DP) cells and constitute the most abundant population in the cortex.43 The successful assembly of a complete TCR-αβ complex renders the DP cells subject to positive thymic selection. Thymocytes with a TCR specificity of intermediate affinity for a self-peptide/self-MHC complex expressed by cTECs are chosen to survive and continue their maturation, leaving nonselected thymocytes to undergo death by neglect. Positively selected DP cells differentiate into CD4 and CD8 single-positive (SP) thymocytes according to the restriction of their TCR to recognize either MHC class II and I molecules, respectively. SP thymocytes are typically resident in the medulla where their TCRs recognize self-peptide/MHC complexes presented by medullary thymic epithelial cells (mTECs) and dendritic cells. Interactions of high affinity will mediate intracellular signals that result in programmed cell death and the elimination of self-reactive and thus potentially harmful SP cells. An important prerequisite for this process of negative thymic selection is the expression of peripheral tissue-restricted self-antigens by mTECs (Figure 2). This phenomenon, known as promiscuous gene expression (pGE), is essential for the elimination of autoreactive TCR specificities and is in part controlled by the transcription factor Aire (autoimmune regulator).44 The small percentage of thymocytes (< 5%) that have successfully met the requirements for positive selection and survived negative selection complete a process of post-selection maturation. Under the physiologic steady-state conditions observed in younger mice, a population of 1 to 2 × 106 naive T cells exit the thymus daily to replenish the peripheral lymphoid tissues.45
Figure 2.
Normal thymic T-cell maturation and export. T-cell development requires the import of a bone marrow-derived progenitor population (yellow symbols) via the blood circulation into the thymus cortex. During optimal thymus function in healthy young persons, TN cells proliferate strongly in the cortex in response to signals provided by cTECs (eg, interleukin-7, stem cell factor, chemokines). The subsequent DP stage of thymocyte maturation is subject to thymic-positive selection. In parallel to their migration to the medulla, DP cells differentiate into SP thymocytes, which are subjected to the negative selection process. pGE in Aire-expressing mTECs plays a crucial role in clonal deletion. The mature T cells (white symbols) are exported as RTE via blood circulation to peripheral lymphoid tissues. The magnitude of thymic export can be assessed by TREC analysis. This approach is based on the fact that thymocytes undergo 2 sequential rounds of TCR rearrangements that form 2 families of TREC as byproducts. TCRB rearrangement in TN cells forms several DβJβ TREC species (●), which are diluted among the expanding cell population before the TCRAD is rearranged in DP cells, which generates sjTREC (○). Thymic export can be measured via determination of the sj/β ratio. This signature for RTE indicates the extent of TN cell proliferation, which is in turn a key determinant for the extent of thymic export. The sj/β ratio remains independently of peripheral cell division at approximately 100:1 in young persons. DC indicates dendritic cells; and sj/β, calculated ratio between sjTREC and DβJβTREC.
Investigating thymic export in the clinic
De novo T-cell production after allogeneic HCT is most robust in younger recipients and is detectable from approximately day 100 onwards.18,19,46 Thymic activity has conventionally been monitored using radiologic imaging to verify changes in thymus size. The thymus mass may recover after cytotoxic therapy47 as radiographic signs of rebound hyperplasia can be observed in HCT recipients.29 However, such changes are not thought to represent a rapid response that compensates for the severe loss of T cells after cytoablative conditioning as, at least in mice, the rate of thymic T-cell export is independent of the existing peripheral T cellularity.48 In addition to the time needed for their differentiation in the bone marrow, it takes mouse lymphoid precursors at least 2 to 3 weeks to move through the different thymic compartments and attain a phenotype typical of a mature T cell (Figure 2).45,49 Although a precise time span has so far not been calculated for humans, it is nonetheless correct to assume that it takes, even under favorable conditions, at least weeks to months to produce naive T cells from infused HSCs and that a plateau level of thymic output is reached only after at 1 to 2 years after allogeneic HCT.15 Hence, the repletion of the periphery with adequate numbers of naive T cells is a time-consuming process.
Peripheral naive and memory T cells can be distinguished by their expression patterns of cell surface markers, including CD45RA, CD45RO, CD62L, CCR7, CD27, CD28, CD103, or αEβ7 integrin. Although highly helpful to assess the status of T-cell regeneration, immunophenotyping cannot precisely measure extant thymic output.50 The combined expression of CD45RA and CD31 may be used to approximate the number of RTEs,51 but not every CD31+ naive CD4+ T-cell represents a newly formed T-cell.52 A more precise quantification of RTEs on a cell population level can, however, be achieved when using a polymerase chain reaction-based molecular method that depends on the detection of TCR rearrangement DNA excision circles (TRECs).53 The processes leading to TREC formation occur in mice at precise stages of thymocyte differentiation, whereas the corresponding events in humans take place only within certain developmental windows.38 Despite this difference, TREC detection has been proven informative to assess thymic function both under experimental conditions and in the clinical setting where the impact of aging, HIV infection, and transplantation for hematologic malignancies was investigated.50,54–56 During T-cell maturation, thymocytes undergo 2 sequential rounds of DNA rearrangements that affect the genes encoding the TCR-β and -α chains, respectively, generating 2 separate families of circular DNA. The TCRB locus (which encodes the segments for the TCR-β chain) is recombined first, which produces several DβJβ TREC species in all TN thymocytes (Figure 2 filled circles). Rearrangement of the TCRAD locus (encoding TCR-α) occurs in mice at the subsequent DP maturational stage and generates the signal joint (sj) TREC (Figure 2 open circles). Both DNA families of TREC do not replicate and are thus diluted on cell proliferation both within the thymus and on export to the periphery. TRECs are now routinely measured in either unseparated peripheral blood samples or in the particular T-cell population of interest. TREC frequency (per 105 cells) or counts (per milliliter of blood) are typically determined because both offer congruent measures. The assessment of the 2 TREC families in isolation, DβJβ or sj, does not identify RTEs per se (relevant studies of myeloablative and nonmyeloablative allogeneic HCT are summarized36,37,50). The reason is that DβJβ and sjTRECs, when used individually, also mirror the past proliferative history of the T-cell population under investigation. The dependency of both readouts on division is a confounding factor in situations when thymic output is compared between persons in which the signals that drive or inhibit the expansion of naive T cells are different. However, more conclusive data can be drawn if a refined method is applied that uses the ratio of sjTREC and DβJβ TREC frequencies (sj/β ratio).56 Because the TCRB recombination occurs before TN expansion, DβJβ circles are gradually diluted before TCRAD recombination is initiated in DP cells. The sj/β ratio thus indicates the extent of intrathymic TN cell proliferation and remains under physiologic conditions of healthy young persons constant at approximately 100:1 (Figure 2). The strength of intrathymic proliferation is a key determinant for thymic output because the pool sizes of all subsequent T-cell maturational stages, including RTEs, are strictly proportional to each other.57 Importantly, the sj/β ratio is not altered by cell proliferation in the periphery: Because cell division in the periphery affects the 2 TREC families identically, the sj/β ratio persists at approximately 100:1 in young persons and will only change when intrathymic proliferation is altered (eg, during aging; see “Age, conditioning, and GVHD: the 3 main impediments to normal thymus function in allogeneic HCT”). The sj/β ratio can thus be regarded as a “RTE signature” of the peripheral T-cell pool.20,54–56
The ability to measure precisely thymus function has provided an important diagnostic tool to characterize the dynamics of de novo T-cell development in the context of allogeneic HCT. Although the breadth of studies detailing the usefulness of TREC determinations in clinical and experimental situations cannot be discussed within the scope of this review, there is consensus that TRECs in peripheral T cells are low or undetectable immediately after transplantation. This finding is consistent with a decrease or complete absence of RTEs early after HCT and an emergence of TREC-positive cells once naive T cells are detected in the peripheral blood.11,18
Age, conditioning, and GVHD: the 3 main impediments to normal thymus function in allogeneic HCT
The thymus-dependent regenerative process after lymphoablation and HCT is ostensibly similar to physiologic events during T-cell ontogeny. However, the efficiency of generating naive T cells via this pathway meets additional challenges. For example, T-lymphocyte numbers and quality are particularly compromised in adult recipients of TCD-unrelated donor HCT.18 These observations suggest that advanced age, pretransplantation cytotoxic therapy, and post-transplantation alloreactivity account individually or in combination for clinically relevant defects in T-cell recovery.36,58
Advanced age
Given the decreased toxicity of newly developed RIC protocols, allogeneic HCT is increasingly being used in older patients.2,4,9 Although RIC may directly benefit the generation of naive T cells, the net gain of a decreased conditioning regimen injury to the thymus may nevertheless remain small in view of the dominant effect of age on thymopoiesis. Gradual structural and functional alterations characterize the physiologic process of senescence and are responsible for the observed decline in the adaptive immune response of the elderly59 (Figure 3A). With age, the bone marrow volume decreases and a steady decline in HSC potency occurs in consequence to both changes to the local stem cell niches and HSC intrinsic alterations.60 This latter observation is probably relevant for HCT donor selection because thymus function critically depends on the continued import of lymphoid precursors from the marrow. Thymus productivity itself peaks in the first year of life and declines thereafter. Although the thymus continues to generate functional T cells,53 recovery from lymphoablation is limited in older compared with younger persons. The age-related decline in thymic T-cell export is the consequence of involution as characterized by a steady reduction in intrathymic T-lymphoid cells and a smaller stromal scaffold,61 including a dynamic and significant decline in mTEC numbers.62,63 Although mTECs are instrumental in pGE, it remains unknown whether a contraction of the thymus medulla is at least in part responsible for the enhanced risk of elderly to develop autoimmunity.44,64 Finally, senescence also affects T cells in the periphery. For example, a decline in CD4+ T-cell function has been described and linked in the elderly to impaired humoral immunity.65 CD8+ T cells from older persons display a particular propensity for oligoclonal expansion, which results in a skewing of the TCR repertoire toward antigen specificities previously encountered.59,66 Consequently, an expansion of terminally differentiated memory T cells upholds the cellularity of the peripheral T-cell pool. As a result of this compensatory mechanism and the progressive decline in thymic function, the composition of the peripheral T-cell pool is progressively altered.
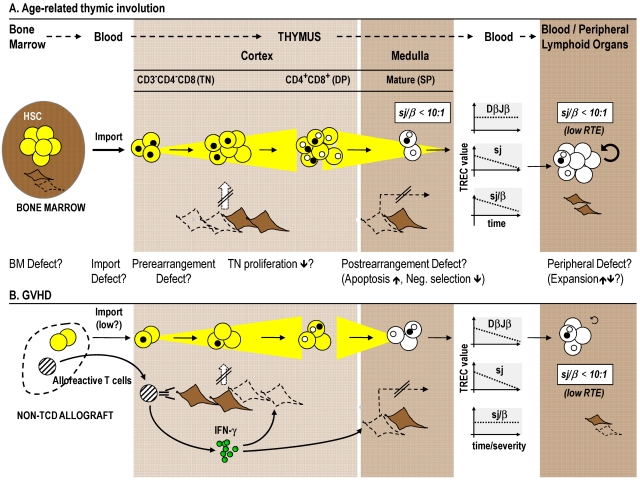
Figure 3.
The effects of age and GVHD on T-cell maturation and export. Age-related changes and GVHD affect T-cell development in prethymic, thymic, and post-thymic compartments. (A) Thymus function itself peaks in the first and second decades of life but then declines in the course of the involution process. During senescence, the sjTREC frequencies decline, whereas DβJβ species remain unchanged. The resultant decrease in the sj/β ratio (which falls below a value of 1:10) indicates lower TN proliferation, which hence explains the decline in RTE export in aged persons. (B) GVHD interference with intrathymic maturation is indicated by parallel decreases in DβJβTREC and sjTREC frequencies. The result is an unchanged sj/β ratio in peripheral blood T cells, which implies that thymopoiesis is affected either before TCRB chain rearrangement and TN proliferation and/or at a time subsequent to TCRAD rearrangement (eg, apoptosis of postrearrangement thymocytes). Acute GVHD therefore impairs thymic function independently from age. Both age-related thymus involution and GVHD are characterized by changes in the thymic stromal microenvironment. Lack of proper stromal function consequently leads to impairment of thymocyte development. In preclinical allogeneic HCT models, the demise of TECs (which may include the death of tolerance-inducing mTECs that express Aire) is linked to alloreactive donor T cells secreting intrathymically IFN-γ in response to activation by host TEC. Abbreviations and symbols are the same as in Figure 2. Broken lines indicate stromal cell death; and strikethrough arrows, deficient TEC function. BM indicates bone marrow.
An inverse correlation between the age of a patient and the onset and maximal productivity of thymopoiesis has been confirmed in allogeneic HCT.15 In a study in children and young adults67 (quoted here as an example of a growing number of clinical reports analyzing immune reconstitution), all recipients of an unrelated TCD-HCT (median, 12 years; range, 2-19 years) generated normal naive CD4+ T-cell numbers within a year after transplantation. In contrast, the rebuilding of the T-cell compartment in adult recipients (median, 38 years; range, 20-59 years) was only completed after 2 to 3 years. In agreement with a delayed normalization of the peripheral T-cell compartment in the elderly, another study reported that de novo generated T cells could only be detected as early as day 100 in HCT recipients who were not older than 60 years.46 Importantly, the rate of recovery of CD4+ T cells in older persons correlated also with an increased risk for life-threatening opportunistic infections.67
Multiparameter TREC analyses have given further insight into the mechanisms effective in a thymus with a declining function. During human thymus senescence, the sj/β signature gradually decreases from the normal value of approximately 100:1 to a number less than 10:1 (Figure 3A).56 The fraction of early intrathymic precursors harboring DβJβ TRECs remains, however, unchanged. These observations not only indicate that the number of divisions per individual TN thymocyte is reduced20,62 but also suggest that the aged thymic microenvironment in transplant recipients composes one of the rate-limiting steps for early post-HCT thymocyte development and thus thymic output. This interpretation is consistent with observations in experimental animal models demonstrating that bone marrow grafts from young mice do not reverse thymus involution in old animals.68 Hence, the intrinsic limitations in lymphohematopoietic progenitor activity may play, in the context of aging, a minor role compared with the restrictions specified by thymic microenvironmental senescence. Consequently, there may only be a limited need for the selection of HCT donors based on age.
Pretransplantation cytotoxic therapy
Myeloablative chemotherapy and total body irradiation are designed to remove disseminated malignant cells. However, historically, the toxicity associated with these regimens has limited their use to younger patients who do not have substantial comorbidities.9 RIC-HCT promises lower damage to the prethymic, thymic, and post-thymic compartments of T-cell development and consequently a faster reconstitution of the adaptive immune system. However, clinical studies are inconclusive regarding the benefits of RIC as both a faster as well as an unchanged recovery of the peripheral T-cell pool have been reported comparing reduced intensity with standard myeloablative conditioning.17 A faster recovery would best be explained as a result of simultaneously improving thymus-independent HPE and thymus-dependent T-cell formation.69 Although the reasons why other studies could not confirm such a beneficial effect for RIC awaits further investigations, the fact that the RIC and myeloablative treatment groups were not matched for several clinical parameters, including age,70 may constitute an important confounding factor in these comparisons.
The precise effects of different intensity conditioning regimens on thymus function are incompletely understood. Irradiation is a commonly used intervention in experimental HCT studies to ablate efficiently host hematopoiesis and to vacate bone marrow niches. It is now well established in mice that the dose of pretransplantation radiation profoundly affects both quantity and quality of posttransplantation thymopoiesis.71–73 These functional deficits may be mostly a consequence of alterations among thymic stromal cells whose diverse functions are reduced or altogether lost. Indeed, the exposure of mice to chemotherapeutic agents used in clinical practice (eg, cyclophosphamide) alters the number and function of TECs, including the Aire-expressing mTECs responsible for pGE.74–77 A failure to produce cytokines and chemokines crucial for TN cell expansion, migration, and differentiation (in particular, IL-7, stem cell factor, Fms-like tyrosine kinase 3 ligand, and CCL25) appears to be a pathogenic mechanism common to thymic insufficiency caused by aging, high-dose chemotherapy, and GVHD (see “GVHD”).78,79 A sophisticated molecular understanding of TEC injury has emerged that links, for example, the epithelial cell response to irradiation to the activation of the highly conserved transforming growth factor-β signaling pathways.80,81 After high-dose chemotherapy, the TEC compartment of young persons is eventually able to fully recover.77,82 Similarly, long-term recovery of regular thymus function after irradiation conditioning has also been observed under experimental conditions.83 Thus, the negative effects of cytotoxic therapy on thymic function, although sometimes prolonged, are usually transient in nature if the recipient is young and commands sufficient thymic functional reserve before therapy.
GVHD
GVHD is a complication of allogeneic HCT and may occur with either an acute or a chronic course. Each form is highlighted by different clinical features and presents with distinct kinetics and pathogenic mechanisms.84–86 The presence of GVHD correlates with a reduced number of naive T cells and an oligoclonal T-cell repertoire conferring a compromised T-cell immune competence18,20,29,87,88 as the host's lymphohematopoietic system serves, in addition to the classic tissues, as a target of antihost alloimmunity.18,20,29,87–89 Indeed, GVHD interferes with T-cell differentiation at all phases of prethymic,90 thymic,91 and post-thymic,92,93 development. In addition, functional defects may also result from immunosuppression as a measure for GVHD prophylaxis or treatment.
Changes in thymus morphology as a consequence of GVHD have been well characterized and are the result of both a reduction in lymphoid cells and alterations in the cellularity and composition of the stromal compartment.94 Impaired thymus export has been demonstrated in GVHD patients using TREC measures.18,36,37 Whereas the long-term effects of chronic GVHD on thymic export had been defined first, it is now also evident that acute GVHD strongly impairs thymic output.20,54 T-cell export usually can recover in younger persons within a year, but such a regenerative process is severely impeded or altogether absent in older patients with acute GVHD.20 Advanced multiparameter TREC analyses revealed that acute GVHD fundamentally interferes with thymocyte maturation. Because both DβJβTREC and sjTREC frequencies decrease in the course of acute GVHD20,54 (Figure 3B), the sj/β ratio among peripheral T cells remains constant, indicating thymopoiesis to be affected before TCRB chain rearrangement and/or at a time after TCRAD rearrangement. Thus, acute GVHD impairs thymic function independent of advanced age (as the latter is marked by a decreased sj/β signature as a result of reduced TN proliferation,56 Figure 3A).
The precise pathophysiologic mechanisms responsible for thymic GVHD in humans remain still largely unresolved. In contrast, preclinical allogeneic HCT models have in many ways delineated cellular and molecular events implicated in thymus stromal injury and its consequences. The morphologic features in models of experimentally induced acute thymic GVHD are similar to those observed in humans. Deficient T-cell development and selection during experimental GVHD are causally linked to alterations in TEC cellular composition and architectural organization (reviewed in Krenger and Höllander36 and Krenger and Höllander37) and are reminiscent of those noted in aging.63 The alloantigen-specific recognition of host TECs by mature donor T cells, which can access the mouse thymus even in the absence of conditioning,95 is now thought to provide the principal injury that limits thymocyte maturation in mice with GVHD (Figure 3B). The unique biology of TECs as antigen-presenting cells authorizes them to prime naive allogeneic T cells in the absence of professional hematopoietic antigen-presenting cells.95 On activation, donor T cells secrete interferon-γ intrathymically, which in turn initiates programmed cell death among cTECs and mTECs. Consequently, immature thymocytes fail to receive the required epithelial survival, expansion, and differentiation signals. The degree of thymopoietic disturbance directly correlates with the in situ number of donor T cells. In contrast, cytokines or glucocorticoids released in the context of acute GVHD as part of the nonspecific inflammatory response do not appear to play a significant role in the pathophysiology of murine thymic GVHD.36,37
It is conceivable that a functional compromise or the loss of specific TEC subpopulations provides a pathogenic link between antihost alloimmunity of acute GVHD and autoimmunity otherwise typically observed in the course of chronic GVHD. This contention is based on the longstanding observation that acute GVHD predisposes to chronic GVHD and that the latter resembles, in certain aspects, autoimmune syndromes.84,96,97 The mechanisms that account for the conversion from an acute to a chronic form of GVHD are incompletely defined. Progressive loss of Aire-expressing mTECs (comparable with the effects of chemotherapy77) and impaired development of thymus-borne regulatory T cells51,98 have been implicated in this transition. A picture thus emerges suggesting that acute GVHD causes thymic injury, which in consequence alters the selection of conventional and regulatory T cells, resulting in a TCR repertoire that mediates the conversion from acute to chronic GVHD.
The knowledge gained from experimental models has now also been used to develop novel preventive and therapeutic strategies. In preclinical models of allogeneic HCT, measures that prevent TEC injury or stimulate the TEC repair exert a beneficial impact on thymic function and the outcome of transplantation.11,12,36,78,99 Once successfully translated to clinical practice, these or comparable therapies may also be able to clarify the current uncertainty whether the increased susceptibility for infections and the incomplete reconstitution of the adaptive immune system in recipients of allogeneic HCT are caused by subclinical GVHD exclusively restricted to the thymus.
In conclusion, advanced age, cytotoxic therapy used for preconditioning, and the development of GVHD are major risk factors for recipients of allogeneic HCT to develop opportunistic infections. As the pool of potential stem cell donors increasingly includes older and genetically disparate, unrelated persons, an increase in the frequency of infectious complications is expected among recipients of allogeneic HCT. From a patient's view and for socioeconomic considerations, it is imperative that post-transplantation T-cell immune deficiency is minimized. A wealth of data from preclinical models and clinical studies has identified the thymus-dependent T-cell regenerative pathway as a target of injurious stimuli stemming from the pretransplantation conditioning regimen and from post-transplantation antihost immunity. Importantly, TECs appear to constitute the common target of these injurious events. The impairment to the thymic stroma in turn negatively affects the maturation, TCR repertoire selection, and export of newly formed T cells. Albeit yet to be fully tested in humans, therapeutic strategies that enhance TEC numbers and function may help to promote a diverse T-cell repertoire and, hence, the generation of a functionally competent adaptive immune system that is expected to improve the outcome of allogeneic HCT.
Acknowledgments
This work was supported by the Swiss National Science Foundation (grant 310030-129838, W.K.; and 310010-122558, G.A.H.), National Institute for Health Research Oxford Biomedical Research Centre Programme (G.A.H.), and the National Institutes of Health (grant R01-AI081918) (B.R.B.).
Authorship
Contribution: W.K., B.R.B., and G.A.H. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Werner Krenger, Department of Biomedicine, University of Basel, Mattenstrasse 28, 4058 Basel, Switzerland; e-mail: werner.krenger@unibas.ch; Georg A. Holländer, Department of Biomedicine, University of Basel, Mattenstrasse 28, 4058 Basel, Switzerland; e-mail: georg-a.hollaender@unibas.ch; and Bruce R. Blazar, Division of Blood and Marrow Transplantation, University of Minnesota, 420 Delaware St SE, Minneapolis, MN 55455; e-mail: blaza001@umn.edu.
References
- 1.Gratwohl A, Baldomero H. Trends of hematopoietic stem cell transplantation in the third millennium. Curr Opin Hematol. 2009;16(6):420–426. doi: 10.1097/MOH.0b013e328330990f. [DOI] [PubMed] [Google Scholar]
- 2.Ballen KK, King RJ, Chitphakdithai P, et al. The national marrow donor program: 20 years of unrelated donor hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2008;14(9 suppl):2–7. doi: 10.1016/j.bbmt.2008.05.017. [DOI] [PubMed] [Google Scholar]
- 3.Gooley TA, Chien JW, Pergam SA, et al. Reduced mortality after allogeneic hematopoietic-cell transplantation. N Engl J Med. 2010;363(22):2091–2101. doi: 10.1056/NEJMoa1004383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deeg HJ, Sandmaier BM. Who is fit for allogeneic transplantation? Blood. 2010;116(23):4762–4770. doi: 10.1182/blood-2010-07-259358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pasquini MC, Wang Z. Current use and outcome of hematopoietic stem cell transplantation: II. CIBMTR Summary Slides 2009. CIBMTR Newsletter. 2009;15(2):7–11. [Google Scholar]
- 6.Pasquini MC, Wang Z. Current use and outcome of hematopoietic stem cell transplantation: I. CIBMTR Summary Slides 2009. CIBMTR Newsletter. 2009;15(1):7–11. [Google Scholar]
- 7.Pidala J, Anasetti C, Jim H. Quality of life after allogeneic hematopoietic cell transplantation. Blood. 2009;114(1):7–19. doi: 10.1182/blood-2008-10-182592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baker KS, Bresters D, Sande JE. The burden of cure: long-term side effects following hematopoietic stem cell transplantation (HSCT) in children. Pediatr Clin North Am. 2010;57(1):323–342. doi: 10.1016/j.pcl.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 9.Turner BE, Collin M, Rice AM. Reduced intensity conditioning for hematopoietic stem cell transplantation: has it achieved all it set out to? Cytotherapy. 2010;12(4):440–454. doi: 10.3109/14653241003709678. [DOI] [PubMed] [Google Scholar]
- 10.Storek J, Zhao Z, Lin E, et al. Recovery from and consequences of severe iatrogenic lymphopenia (induced to treat autoimmune diseases). Clin Immunol. 2004;113(3):285–298. doi: 10.1016/j.clim.2004.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seggewiss R, Einsele H. Immune reconstitution after allogeneic transplantation and expanding options for immunomodulation: an update. Blood. 2010;115(19):3861–3868. doi: 10.1182/blood-2009-12-234096. [DOI] [PubMed] [Google Scholar]
- 12.Cavazzana-Calvo M, André-Schmutz I, Dal Cortivo L, Neven B, Hacein-Bey-Abina S, Fischer A. Immune reconstitution after haematopoietic stem cell transplantation: obstacles and anticipated progress. Curr Opin Immunol. 2009;21(5):544–548. doi: 10.1016/j.coi.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 13.Toubert A. Hematopoietic Stem Cell Transplantation: The EBMT Handbook. 5th ed. Paris: European School of Haematology; 2008. Immune reconstitution after allogeneic HSCT. pp. 296–306. [Google Scholar]
- 14.Saliba RM, Komanduri KV, Giralt S, et al. Leukemia burden delays lymphocyte and platelet recovery after allo-SCT for AML. Bone Marrow Transplant. 2009;43(9):685–692. doi: 10.1038/bmt.2008.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eyrich M, Wollny G, Tzaribaschev N, et al. Onset of thymic recovery and plateau of thymic output are differentially regulated after stem cell transplantation in children. Biol Blood Marrow Transplant. 2005;11(3):194–205. doi: 10.1016/j.bbmt.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 16.Elmaagacli AH, Peceny R, Steckel N, et al. Outcome of transplantation of highly purified peripheral blood CD34+ cells with T-cell add-back compared with unmanipulated bone marrow or peripheral blood stem cells from HLA-identical sibling donors in patients with first chronic phase chronic myeloid leukemia. Blood. 2003;101(2):446–453. doi: 10.1182/blood-2002-05-1615. [DOI] [PubMed] [Google Scholar]
- 17.Jiménez M, Ercilla G, Martínez C. Immune reconstitution after allogeneic stem cell transplantation with reduced-intensity conditioning regimens. Leukemia. 2007;21(8):1628–1637. doi: 10.1038/sj.leu.2404681. [DOI] [PubMed] [Google Scholar]
- 18.Weinberg K, Blazar BR, Wagner JE, et al. Factors affecting thymic function after allogeneic hematopoietic stem cell transplantation. Blood. 2001;97(5):1458–166. doi: 10.1182/blood.v97.5.1458. [DOI] [PubMed] [Google Scholar]
- 19.Jiménez M, Martínez C, Ercilla G, et al. Clinical factors influencing T-cell receptor excision circle (TRECs) counts following allogeneic stem cell transplantation in adults. Transpl Immunol. 2006;16(1):52–59. doi: 10.1016/j.trim.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 20.Clave E, Busson M, Douay C, et al. Acute graft versus host disease transiently impairs thymic output in young patients after allogeneic hematopoietic stem cell transplantation. Blood. 2009;113(25):6477–6484. doi: 10.1182/blood-2008-09-176594. [DOI] [PubMed] [Google Scholar]
- 21.Williams KM, Hakim FT, Gress RE. T cell immune reconstitution following lymphodepletion. Semin Immunol. 2007;19(5):318–330. doi: 10.1016/j.smim.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Muraro PA, Douek DC. Renewing the T cell repertoire to arrest autoimmune aggression. Trends Immunol. 2006;27(2):61–67. doi: 10.1016/j.it.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 23.Hakim FT, Gress RE. Reconstitution of the lymphocyte compartment after lymphocyte depletion: a key issue in clinical immunology. Eur J Immunol. 2005;35(11):3099–3102. doi: 10.1002/eji.200535385. [DOI] [PubMed] [Google Scholar]
- 24.Gress RE, Komanduri KV, Einsele H, Cooper LJ. Lymphoid reconstruction and vaccines. Biol Blood Marrow Transplant. 2007;13(1 suppl 1):17–22. doi: 10.1016/j.bbmt.2006.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baron F, Sandmaier BM. Chimerism and outcomes after allogeneic hematopoietic cell transplantation following nonmyeloablative conditioning. Leukemia. 2006;20(10):1690–1700. doi: 10.1038/sj.leu.2404335. [DOI] [PubMed] [Google Scholar]
- 26.Baron F, Baker JE, Storb R, et al. Kinetics of engraftment in patients with hematologic malignancies given allogeneic hematopoietic cell transplantation after nonmyeloablative conditioning. Blood. 2004;104(8):2254–2262. doi: 10.1182/blood-2004-04-1506. [DOI] [PubMed] [Google Scholar]
- 27.Bourgeois C, Stockinger B. T cell homeostasis in steady state and lymphopenic conditions. Immunol Lett. 2006;107(2):89–92. doi: 10.1016/j.imlet.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 28.Jameson SC. T cell homeostasis: keeping useful T cells alive and live T cells useful. Semin Immunol. 2005;17(3):231–237. doi: 10.1016/j.smim.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 29.Roux E, Dumont-Girard F, Starobinski M, et al. Recovery of immune reactivity after T-cell-depleted bone marrow transplantation depends on thymic activity. Blood. 2000;96(6):2299–2303. [PubMed] [Google Scholar]
- 30.Chalandon Y, Degermann S, Villard J, et al. Pretransplantation CMV-specific T cells protect recipients of T-cell-depleted grafts against CMV-related complications. Blood. 2006;107(1):389–396. doi: 10.1182/blood-2005-07-2746. [DOI] [PubMed] [Google Scholar]
- 31.Storek J, Geddes M, Khan F, et al. Reconstitution of the immune system after hematopoietic stem cell transplantation in humans. Semin Immunopathol. 2008;30(4):425–437. doi: 10.1007/s00281-008-0132-5. [DOI] [PubMed] [Google Scholar]
- 32.Rapoport AP, Stadtmauer EA, Aqui N, et al. Restoration of immunity in lymphopenic individuals with cancer by vaccination and adoptive T-cell transfer. Nat Med. 2005;11(11):1230–1237. doi: 10.1038/nm1310. [DOI] [PubMed] [Google Scholar]
- 33.June CH, Blazar BR, Riley JL. Engineering lymphocyte subsets: tools, trials and tribulations. Nat Rev Immunol. 2009;9(10):704–716. doi: 10.1038/nri2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Casalegno-Garduño R, Schmitt A, Wang X, Xu X, Schmitt M. Targeted cellular immunotherapy for leukemia patients. Transfus Apher Sci. 2010;43(2):207–210. doi: 10.1016/j.transci.2010.07.022. [DOI] [PubMed] [Google Scholar]
- 35.Ringdén O, Karlsson H, Olsson R, Omazic B, Uhlin M. The allogeneic graft-versus-cancer effect. Br J Haematol. 2009;147(5):614–633. doi: 10.1111/j.1365-2141.2009.07886.x. [DOI] [PubMed] [Google Scholar]
- 36.Krenger W, Holländer GA. The role of the thymus in hematopoietic stem cell transplantation. Bone Marrow Transplantation Across Genetic Barriers. In press. [Google Scholar]
- 37.Krenger W, Holländer GA. The immunopathology of thymic GVHD. Semin Immunopathol. 2008;30(4):439–456. doi: 10.1007/s00281-008-0131-6. [DOI] [PubMed] [Google Scholar]
- 38.Vicente R, Swainson L, Marty-Grès S, et al. Molecular and cellular basis of T cell lineage commitment. Semin Immunol. 2010;22(5):270–275. doi: 10.1016/j.smim.2010.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carpenter AC, Bosselut R. Decision checkpoints in the thymus. Nat Immunol. 2010;11(8):666–673. doi: 10.1038/ni.1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Penit C, Lucas B, Vasseur F. Cell expansion and growth arrest phases during the transition from precursor (CD4−8−) to immature (CD4+8+) thymocytes in normal and genetically modified mice. J Immunol. 1995;154(10):5103–5113. [PubMed] [Google Scholar]
- 41.Takahama Y. Journey through the thymus: stromal guides for T-cell development and selection. Nat Rev Immunol. 2006;6(2):127–135. doi: 10.1038/nri1781. [DOI] [PubMed] [Google Scholar]
- 42.Petrie HT, Zúñiga-Pflücker JC. Zoned out: functional mapping of stromal signaling microenvironments in the thymus. Annu Rev Immunol. 2007;25:649–679. doi: 10.1146/annurev.immunol.23.021704.115715. [DOI] [PubMed] [Google Scholar]
- 43.Godfrey DI, Kennedy J, Suda T, Zlotnik A. A developmental pathway involving four phenotypically and functionally distinct subsets of CD3−CD4−CD8− triple-negative adult mouse thymocytes defined by CD44 and CD25 expression. J Immunol. 1993;150(10):4244–4252. [PubMed] [Google Scholar]
- 44.Gardner JM, Fletcher AL, Anderson MS, Turley SJ. AIRE in the thymus and beyond. Curr Opin Immunol. 2009;21(6):582–589. doi: 10.1016/j.coi.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Scollay R, Godfrey DI. Thymic emigration: conveyor belts or lucky dips? Immunol Today. 1995;16(6):268–273. doi: 10.1016/0167-5699(95)80179-0. [DOI] [PubMed] [Google Scholar]
- 46.Castermans E, Hannon M, Dutrieux J, et al. Thymic recovery after allogeneic hematopoietic cell transplantation with nonmyeloablative conditioning is limited to patients younger than 60 years of age. Haematologica. 2011;96(2):298–306. doi: 10.3324/haematol.2010.029702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nasseri F, Eftekhari F. Clinical and radiologic review of the normal and abnormal thymus: pearls and pitfalls. Radiographics. 2010;30(2):413–428. doi: 10.1148/rg.302095131. [DOI] [PubMed] [Google Scholar]
- 48.Berzins SP, Uldrich AP, Sutherland JS, et al. Thymic regeneration: teaching an old immune system new tricks. Trends Mol Med. 2002;8(10):469–476. doi: 10.1016/s1471-4914(02)02415-2. [DOI] [PubMed] [Google Scholar]
- 49.McCaughtry TM, Wilken MS, Hogquist KA. Thymic emigration revisited. J Exp Med. 2007;204(11):2513–2520. doi: 10.1084/jem.20070601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ribeiro RM, Perelson AS. Determining thymic output quantitatively: using models to interpret experimental T-cell receptor excision circle (TREC) data. Immunol Rev. 2007;216:21–34. doi: 10.1111/j.1600-065X.2006.00493.x. [DOI] [PubMed] [Google Scholar]
- 51.Matsuoka K, Kim HT, McDonough S, et al. Altered regulatory T cell homeostasis in patients with CD4+ lymphopenia following allogeneic hematopoietic stem cell transplantation. J Clin Invest. 2010;120(5):1479–1493. doi: 10.1172/JCI41072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kohler S, Thiel A. Life after the thymus: CD31+ and CD31− human naive CD4+ T-cell subsets. Blood. 2009;113(4):769–774. doi: 10.1182/blood-2008-02-139154. [DOI] [PubMed] [Google Scholar]
- 53.Douek DC, McFarland RD, Keiser PH, et al. Changes in thymic function with age and during the treatment of HIV infection. Nature. 1998;396(6712):690–695. doi: 10.1038/25374. [DOI] [PubMed] [Google Scholar]
- 54.Fry TJ. Is a little GVHD a good thing? Blood. 2009;113(25):6274–6275. doi: 10.1182/blood-2009-04-212639. [DOI] [PubMed] [Google Scholar]
- 55.Poulin JF, Sylvestre M, Champagne P, et al. Evidence for adequate thymic function but impaired naive T-cell survival following allogeneic hematopoietic stem cell transplantation in the absence of chronic graft-versus-host disease. Blood. 2003;102(13):4600–4607. doi: 10.1182/blood-2003-05-1428. [DOI] [PubMed] [Google Scholar]
- 56.Dion ML, Poulin JF, Bordi R, et al. HIV infection rapidly induces and maintains a substantial suppression of thymocyte proliferation. Immunity. 2004;21(6):757–768. doi: 10.1016/j.immuni.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 57.Almeida AR, Borghans JA, Freitas AA. T cell homeostasis: thymus regeneration and peripheral T cell restoration in mice with a reduced fraction of competent precursors. J Exp Med. 2001;194(5):591–599. doi: 10.1084/jem.194.5.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Krenger W, Holländer GA. The thymus in GVHD pathophysiology. Best Pract Res Clin Haematol. 2008;21:119–128. doi: 10.1016/j.beha.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 59.Dorshkind K, Montecino-Rodriguez E, Signer RA. The ageing immune system: is it ever too old to become young again? Nat Rev Immunol. 2009;9(1):57–62. doi: 10.1038/nri2471. [DOI] [PubMed] [Google Scholar]
- 60.Conboy IM, Conboy MJ, Wagers AJ, Girma ER, Weissman IL, Rando TA. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature. 2005;433(7027):760–764. doi: 10.1038/nature03260. [DOI] [PubMed] [Google Scholar]
- 61.Lynch HE, Goldberg GL, Chidgey A, Van den Brink MR, Boyd R, Sempowski GD. Thymic involution and immune reconstitution. Trends Immunol. 2009;30(7):366–373. doi: 10.1016/j.it.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Heng TS, Chidgey AP, Boyd RL. Getting back at nature: understanding thymic development and overcoming its atrophy. Curr Opin Pharmacol. 2010;10(4):425–433. doi: 10.1016/j.coph.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 63.Gray D, Seach N, Ueno T, et al. Developmental kinetics, turnover, and stimulatory capacity of thymic epithelial cells. Blood. 2006;108(12):3777–3785. doi: 10.1182/blood-2006-02-004531. [DOI] [PubMed] [Google Scholar]
- 64.Rosato E, Salsano F. Immunity, autoimmunity and autoimmune diseases in older people. J Biol Regul Homeost Agents. 2008;22(4):217–224. [PubMed] [Google Scholar]
- 65.Steinmann GG. Changes in the human thymus during aging. Curr Top Pathol. 1986;75:43–88. doi: 10.1007/978-3-642-82480-7_2. [DOI] [PubMed] [Google Scholar]
- 66.Taub DD, Murphy WJ, Longo DL. Rejuvenation of the aging thymus: growth hormone-mediated and ghrelin-mediated signaling pathways. Curr Opin Pharmacol. 2010;10(4):408–424. doi: 10.1016/j.coph.2010.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Small TN, Papadopoulos EB, Boulad F, et al. Comparison of immune reconstitution after unrelated and related T-cell-depleted bone marrow transplantation: effect of patient age and donor leukocyte infusions. Blood. 1999;93(2):467–480. [PubMed] [Google Scholar]
- 68.Gui J, Zhu X, Dohkan J, Cheng L, Barnes PF, Su DM. The aged thymus shows normal recruitment of lymphohematopoietic progenitors but has defects in thymic epithelial cells. Int Immunol. 2007;19(10):1201–1211. doi: 10.1093/intimm/dxm095. [DOI] [PubMed] [Google Scholar]
- 69.Jiménez M, Martínez C, Ercilla G, et al. Reduced-intensity conditioning regimen preserves thymic function in the early period after hematopoietic stem cell transplantation. Exp Hematol. 2005;33(10):1240–1248. doi: 10.1016/j.exphem.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 70.Maris M, Boeckh M, Storer B, et al. Immunologic recovery after hematopoietic cell transplantation with nonmyeloablative conditioning. Exp Hematol. 2003;31(10):941–952. doi: 10.1016/s0301-472x(03)00201-7. [DOI] [PubMed] [Google Scholar]
- 71.Kelly RM, Highfill SL, Panoskaltsis-Mortari A, et al. Keratinocyte growth factor and androgen blockade work in concert to protect against conditioning regimen-induced thymic epithelial damage and enhance T-cell reconstitution after murine bone marrow transplantation. Blood. 2008;111(12):5734–5744. doi: 10.1182/blood-2008-01-136531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Min D, Taylor PA, Panoskaltsis-Mortari A, et al. Protection from thymic epithelial cell injury by keratinocyte growth factor: a new approach to improve thymic and peripheral T-cell reconstitution after bone marrow transplantation. Blood. 2002;99(12):4592–4600. doi: 10.1182/blood.v99.12.4592. [DOI] [PubMed] [Google Scholar]
- 73.Chung B, Barbara-Burnham L, Barsky L, Weinberg K. Radiosensitivity of thymic interleukin-7 production and thymopoiesis after bone marrow transplantation. Blood. 2001;98(5):1601–1606. doi: 10.1182/blood.v98.5.1601. [DOI] [PubMed] [Google Scholar]
- 74.Williams KM, Mella H, Lucas PJ, Williams JA, Telford W, Gress RE. Single cell analysis of complex thymus stromal cell populations: rapid thymic epithelia preparation characterizes radiation injury. Clin Transl Sci. 2009;2(4):279–285. doi: 10.1111/j.1752-8062.2009.00128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kelly RM, Goren EM, Taylor PA, et al. Short-term inhibition of p53 combined with keratinocyte growth factor improves thymic epithelial cell recovery and enhances T-cell reconstitution after murine bone marrow transplantation. Blood. 2010;115(5):1088–1097. doi: 10.1182/blood-2009-05-223198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Goldberg GL, Dudakov JA, Reiseger JJ, et al. Sex steroid ablation enhances immune reconstitution following cytotoxic antineoplastic therapy in young mice. J Immunol. 2010;184(11):6014–6024. doi: 10.4049/jimmunol.0802445. [DOI] [PubMed] [Google Scholar]
- 77.Fletcher AL, Lowen TE, Sakkal S, et al. Ablation and regeneration of tolerance-inducing medullary thymic epithelial cells after cyclosporine, cyclophosphamide, and dexamethasone treatment. J Immunol. 2009;183(2):823–831. doi: 10.4049/jimmunol.0900225. [DOI] [PubMed] [Google Scholar]
- 78.Weinberg KI. Protection from posttransplantation immune deficiency? Blood. 2007;109(9):3617–3618. [Google Scholar]
- 79.Min D, Panoskaltsis-Mortari A, Kuro OM, Hollander GA, Blazar BR, Weinberg KI. Sustained thymopoiesis and improvement in functional immunity induced by exogenous KGF administration in murine models of aging. Blood. 2007;109(6):2529–2537. doi: 10.1182/blood-2006-08-043794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jeker LT, Barthlott T, Keller MP, et al. Maintenance of a normal thymic microenvironment and T-cell homeostasis require Smad4-mediated signaling in thymic epithelial cells. Blood. 2008;112(9):3688–3695. doi: 10.1182/blood-2008-04-150532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hauri-Hohl MM, Zuklys S, Keller MP, et al. TGF-beta signaling in thymic epithelial cells regulates thymic involution and postirradiation reconstitution. Blood. 2008;112(3):626–634. doi: 10.1182/blood-2007-10-115618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mackall CL, Fleisher TA, Brown MR, et al. Age, thymopoiesis, and CD4+ T-lymphocyte regeneration after intensive chemotherapy. N Engl J Med. 1995;332(3):143–149. doi: 10.1056/NEJM199501193320303. [DOI] [PubMed] [Google Scholar]
- 83.Sykes M, Szot GL, Swenson K, Pearson DA, Wekerle T. Separate regulation of peripheral hematopoietic and thymic engraftment. Exp Hematol. 1998;26(6):457–465. [PubMed] [Google Scholar]
- 84.Martin PJ. Biology of chronic graft-versus-host disease: implications for a future therapeutic approach. Keio J Med. 2008;57(4):177–183. doi: 10.2302/kjm.57.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Socie G, Blazar BR. Acute graft-versus-host disease: from the bench to the bedside. Blood. 2009;114(20):4327–4336. doi: 10.1182/blood-2009-06-204669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ferrara JL, Levine JE, Reddy P, Holler E. Graft-versus-host disease. Lancet. 2009;373(9674):1550–1561. doi: 10.1016/S0140-6736(09)60237-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ochs L, Shu XO, Miller J, et al. Late infections after allogeneic bone marrow transplantations: comparison of incidence in related and unrelated donor transplant recipients. Blood. 1995;86:3979–3986. [PubMed] [Google Scholar]
- 88.Welniak LA, Blazar BR, Murphy WJ. Immunobiology of allogeneic hematopoietic stem cell transplantation. Annu Rev Immunol. 2007;25:139–170. doi: 10.1146/annurev.immunol.25.022106.141606. [DOI] [PubMed] [Google Scholar]
- 89.Ferrara JLM. GVHD: in vivo veritas. Blood. 2006;106(3):772–773. [Google Scholar]
- 90.Aguila HL. Hematopoietic niches: targets of GVHD. Blood. 2010;115(26):5284–5285. doi: 10.1182/blood-2010-04-278531. [DOI] [PubMed] [Google Scholar]
- 91.Beschorner WE, Hutchins GM, Elfenbein GJ, Santos GW. The thymus in patients with allogeneic bone marrow transplants. Am J Pathol. 1978;92:173–181. [PMC free article] [PubMed] [Google Scholar]
- 92.Lin MT, Tseng LH, Frangoul H, et al. Increased apoptosis of peripheral blood T cells following allogeneic hematopoietic cell transplantation. Blood. 2000;95(12):3832–3839. [PubMed] [Google Scholar]
- 93.Dulude G, Roy DC, Perreault C. The effect of graft-versus-host disease on T cell production and homeostasis. J Exp Med. 1999;189(8):1329–1342. doi: 10.1084/jem.189.8.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ghayur T, Seemayer T, Lapp WS. Histologic correlates of immune functional deficits in graft-vs-host disease. In: Burakoff SJ, Deeg HJ, Ferrara J, Atkinson K, et al., editors. Graft-vs.-Host Disease: Immunology, Pathophysiology, and Treatment. New York, NY: Marcel Dekker; 1990. pp. 109–132. [Google Scholar]
- 95.Hauri-Hohl MM, Keller MP, Gill J, et al. Donor T-cell alloreactivity against host thymic epithelium limits T-cell development after bone marrow transplantation. Blood. 2007;109(9):4080–4088. doi: 10.1182/blood-2006-07-034157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chu YW, Gress RE. Murine models of chronic graft-versus-host disease: insights and unresolved issues. Biol Blood Marrow Transplant. 2008;14(4):365–378. doi: 10.1016/j.bbmt.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Daikeler T, Tyndall A. Autoimmunity following haematopoietic stem-cell transplantation. Best Pract Res Clin Haematol. 2007;20(2):349–360. doi: 10.1016/j.beha.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 98.Zhang C, Todorov I, Zhang Z, et al. Donor CD4+ T and B cells in transplants induce chronic graft-versus-host disease with autoimmune manifestations. Blood. 2006;107:2993–3001. doi: 10.1182/blood-2005-09-3623. [DOI] [PubMed] [Google Scholar]
- 99.Holländer GA, Krenger W, Blazar BR. Emerging strategies to boost thymic function. Curr Opin Pharmacol. 2010;10(4):443–453. doi: 10.1016/j.coph.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]