Abstract
Interest in mapping the global distribution of malaria is motivated by a need to define populations at risk for appropriate resource allocation1,2 and to provide a robust framework for evaluating its global economic impact3,4. Comparison of older5–7 and more recent1,4 malaria maps shows how the disease has been geographically restricted, but it remains entrenched in poor areas of the world with climates suitable for transmission. Here we provide an empirical approach to estimating the number of clinical events caused by Plasmodium falciparum worldwide, by using a combination of epidemiological, geographical and demographic data. We estimate that there were 515 (range 300–660) million episodes of clinical P. falciparum malaria in 2002. These global estimates are up to 50% higher than those reported by the World Health Organization (WHO) and 200% higher for areas outside Africa, reflecting the WHO’s reliance upon passive national reporting for these countries. Without an informed understanding of the cartography of malaria risk, the global extent of clinical disease caused by P. falciparum will continue to be underestimated.
The Global Burden of Diseases programme of the WHO has attempted to enumerate the health consequences of malaria infection8,9. Because the African region has a notoriously weak system of reporting infectious diseases, epidemiological evidence from carefully conducted prospective, ‘active’ case-detection studies of malaria morbidity, disability and mortality in populations living under different transmission intensity risks have been compiled to estimate the disease burden10. A different approach was adopted for WHO regions outside Africa, where the burden was computed from ‘passive’ national disease and mortality notifications to WHO regional offices without precisely defining the populations exposed to varied malaria infection risks9,11,12. This use of national disease registration systems to provide accurate reflections of disease rests on three assumptions: that there is complete temporal coverage (every month is reported by a facility), that there is complete spatial coverage (every health facility reports nationwide), and that all disease events present to, and are reported by, health facilities. In reality, passive detection of disease events in most resource-poor countries is incomplete, even outside Africa.
Here we provide a standard global approach to deriving clinical malaria burden by using evidence of the epidemiological risks of disease outcome from active case-detection studies in combination with estimates of populations at risk of various P. falciparum transmission conditions. A comprehensive outline of these procedures is given in Methods. A conservative approach is defined to further account for the confounding of malaria diagnosis efficiency by endemicity (see Supplementary Information A for more detail and original data) and the modifying influence on endemicity of current levels of control and urbanization (see Supplementary Information B).
Our global model suggests that, in 2002, 2.2 billion people were exposed to the threat of P. falciparum malaria, resulting in a conservative estimate of 515 (range 300–660) million clinical attacks attributable to this parasite during that year (Fig. 1 and Table 1). At a regional level, most clinical events attributable to P. falciparum were concentrated in the African region (70%), but the highly populated South East Asia region contributed 25% of the world’s clinical attacks in 2002 (Fig. 2 and Table 2). The WHO suggests that there were 273 million clinical attacks of malaria worldwide in 1998 and that 90% of the global disease incidence is borne by Africa9. Other WHO estimates report that in 1990 the global incidence of malaria was 213 million cases13. Neither of these sources provides sufficient detail on how the estimates were derived. Our models, by contrast, are both data-driven and reproducible. They also indicate that the number of clinical attacks due to P. falciparum might be 50% higher than WHO estimates, and highlight the fact that almost one-third of the global incidence occurs outside Africa.
Figure 1.
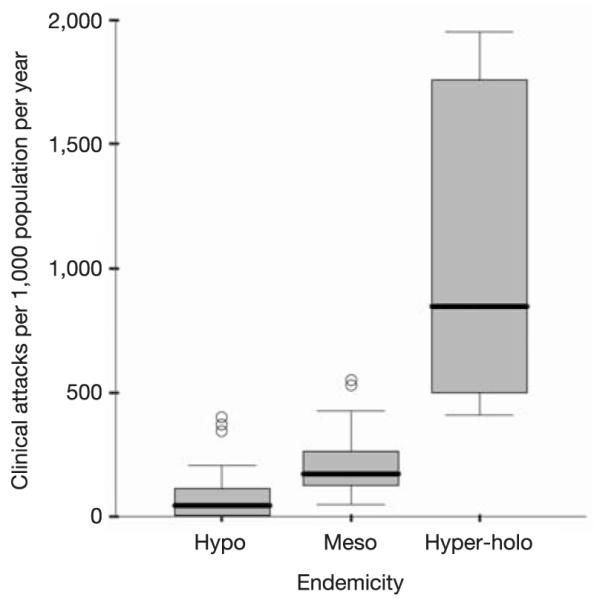
Annual clinical incidence of P. falciparum per 1,000 population according to hypoendemic (n = 39), mesoendemic (n = 25) and combined hyperendemic and holoendemic (n = 8) conditions. The box indicates the inter-quartile range (25% and 75%) and the thick line within the box represents the median. The whiskers represent the 2.5% and 97.5% centiles and outliers are plotted as circles outside this range. Three studies were excluded because they were undertaken in areas of a recorded zero P. falciparum prevalence, and each reported no clinical attacks due to P. falciparum.
Table 1.
Populations (millions) at risk in 2002
| Region | Population in P. falciparum endemicity classes |
||||
|---|---|---|---|---|---|
| Unclassified | Hypoendemic | Mesoendemic | Hyperendemic and holoendemic | Total population at risk | |
| Africa | 13.6 | 39.3 | 67.4 | 414.3 | 521.0 |
| Americas | 3.5 | 43.9 | 10.5 | 0 | 54.5 |
| South East Asia | 47.8 | 827.6 | 486.0 | 0.3 | 1,313.9 |
| Western Pacific | 22.4 | 77.6 | 63.4 | 1.0 | 142.0 |
| Eastern Mediterranean | 32.3 | 143.0 | 33.4 | 0 | 176.4 |
| Europe | 1.1 | 0.3 | 3.2 | 0 | 3.5 |
| Total world | 120.7 | 1,131.7 | 663.9 | 415.6 | 2,211.3 |
Populations were determined according to classifications of malaria risk adjusted for urbanization by WHO region (see Supplementary Information B).
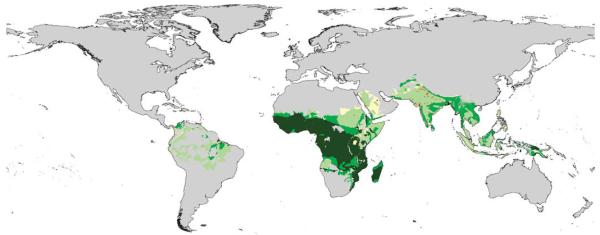
Figure 2.
P. falciparum endemicity distribution within the global limits of risk. Endemicity classes: light green, hypoendemic (areas in which childhood infection prevalence is less than 10%); medium green, mesoendemic (areas with infection prevalence between 11% and 50%); dark green, hyperendemic and holoendemic (areas with an infection prevalence of 50% or more)13. Unclassified areas (yellow) represent only 6% of the global population at risk and are due to discrepancies between the 2002 delineation of risk and the endemicity risk limits developed in refs 6 and 7. Grey areas are a combined mask of areas outside of the transmission limits and areas of population density less than 1 person km−2 (ref. 16).
Table 2.
Estimated data for P. falciparum clinical malaria cases in 2002 (millions)
| Parameter | Hypoendemic | Mesoendemic | Hyperendemic and holoendemic | Total P. falciparum cases |
|---|---|---|---|---|
| Attack rate (per 1,000 population per year) | 43 (6–117) | 171 (125–261) | 849 [500] | – |
| Cases per WHO region (millions) | ||||
| Africa | 1.69 (0.24–4.60) | 11.52 (8.42–17.58) | 351.77 (207.17–351.77) | 364.98 (215.82–373.95) |
| Americas | 1.89 (0.26–5.14) | 1.80 (1.32–2.75) | 0 | 3.69 (1.58–7.89) |
| South East Asia | 35.59 (4.97–96.83) | 83.11 (60.76–126.86) | 0.24 (0.14–0.24) | 118.94 (65.86–223.93) |
| Western Pacific | 3.34 (0.46–9.08) | 10.84 (7.93–16.55) | 0.85 (0.50–0.85) | 15.03 (8.89–26.48) |
| Eastern Mediterranean | 6.15 (0.86–16.73) | 5.71 (4.17–8.71) | 0 | 11.86 (5.03–25.44) |
| Europe | 0.01 (0.00–0.03) | 0.54 (0.40–0.83) | 0 | 0.55 (0.40–0.86) |
| Total world | 48.67 (6.79–132.41) | 113.52 (82.99–173.28) | 352.86 (207.81–352.87) | 515.05 (297.59–658.55) |
Results are medians and IQR (in parentheses) by WHO region and endemicity class, based on urban-adjusted denominators, derived from Table 1; the lower quartile (in square brackets) is presented instead of the IQR for populations living under conditions of hyperendemic and holoendemic transmission (see Methods).
We have not examined mortality attributed directly to P. falciparum, because of the paucity of prospective epidemiological descriptions of cause-specific mortality outside Africa14. The risk of death after a clinical attack of P. falciparum seems much higher in Africa than in South East Asia and the western Pacific. The incidence of severe, life-threatening complications of P. falciparum malaria in Africa15 is at least tenfold that in similar malaria endemic areas in India16 and Vanuatu17. Reasons for this are unclear but might include better access to prompt treatment18 and some cross-Plasmodium species protection against severe disease outcomes19.
We had estimated previously from epidemiological data that there were 221 million P. falciparum attacks in Africa in 1995 (ref. 10). Our 2002 estimate for Africa of 365 million clinical cases derives from a more specific, urban-adjusted endemicity map than that developed specifically for Africa in 1995, which was not structured according to levels of parasite prevalence. It was estimated12 from national statistics that there were 51.2 million P. falciparum cases outside Africa in 1995; our estimate of 150 million cases is considerably higher. There are several possible explanations for this disparity, including our assigning populations at risk of different transmission conditions on the basis of an endemicity map constructed in 1968. We have used this map in its original form because there is no modern equivalent but have taken a very conservative approach to reclassifying areas at risk in 2002 by stepping down endemicity risks in all areas outside Africa and allowing for the rapid increases in urbanization since 1968. Furthermore, the clinical data on active detection of cases were derived from a wide range of malaria endemicities (see Supplementary Information A) to create plausible endemicity-specific median estimates of disease. It seems unlikely that we have overestimated the clinical risks when reapplied to the global distributions of the three broad endemicity classes.
The most obvious explanation is the dependence on national statistics derived from passive detection of cases for the WHO’s present global disease estimates outside Africa. In our analysis we were able to compare WHO reports of clinical incidence from 12 administrative units with survey reports of data on active case detection in the same areas. These limited comparisons demonstrated the scale of under-reporting by passive detection, varying from a threefold difference in Brazil to a 1,000-fold difference in Pakistan.
The global Roll Back Malaria (RBM) initiative aims at halving the burden of malaria within the next six years9. The Millennium Development Goal’s target is to halt the rising incidence of malaria by 2015 (ref. 20). To achieve this, international priorities and resources must be targeted using different information sources, including national economic capacities, evidence-based cost-effective strategies and disease burdens. Inadequate descriptions of the global distribution of disease risk make it impossible to determine priorities and advise funding agencies appropriately. Redressing these deficiencies with robust data must be a priority if international agencies are to understand the size of the challenge set by their targets over the next ten years.
Methods
To identify reports of P. falciparum morbidity risks defined through epidemiological studies, an electronic data search was undertaken through PubMed (http://www.ncbi.nlm.nih.gov/entrez/query.fcgi) using the keyword ‘malaria’ in conjunction with each country name in all WHO regions. Abstracts were reviewed to identify reports of malaria morbidity incidence, and full papers were obtained for all original reports, or cross-referenced sources, that met the following selection criteria: first, that the report covered the period after 1985; second, that the study involved active detection of cases with the use of clinical or epidemiological morbidity definitions; third, that it was possible to compute the numbers of cases confirmed microscopically and the numbers of person-years of observation; and last, that data were reported for all age groups to capture the cumulative incidence from birth until adulthood in communities with different age-specific incidence patterns. Using these criteria for inclusion we identified 83 independent annual incidence estimates of P. falciparum clinical attacks from 22 countries in five WHO regions (see Supplementary Information A). No data were identified from Tajikistan, the only country in which P. falciparum malaria transmission occurs in the European region.
Infection prevalence has been used since the 1950s to describe malaria endemicity categorically21. We therefore identified coincidental cross-sectional measures of P. falciparum infection prevalence within the original morbidity reports, or associated publications, and matched these to 75 communities where rates of clinical attack had been established (see Supplementary Information A). P. falciparum annual rates of clinical attack were summarized as medians and interquartile ranges (IQR) to allow for the ranges and uncertainty of survey estimates within three infection prevalence classes: hypoendemic (parasite prevalence less than 10%), mesoendemic (parasite prevalence between 11% and 50%) and combined hyperendemic and holoendemic (parasite prevalence 50% or more) (Fig. 1). Epidemiological case definitions in areas of hyperendemicity to holoendemicity pose difficulties where fever and infection are common but are not necessarily related causally. We have adopted a working clinical definition in all malaria endemicities of fever in the presence of patent peripheral infection, accepting that this will overestimate the incidence of clinical disease in areas of hyperendemic to holoendemic transmission. To accommodate overestimation in areas of high transmission we have used the lower quartile and median as a more conservative and biologically plausible range of clinical risk.
To define the congruence of human population distribution and P. falciparum transmission we used spatially linked databases of human population, limits of malaria risk and malaria endemicity within a Geographic Information System (GIS) as outlined in detail in Supplementary Information B. In brief, we first defined the spatial extent of P. falciparum risk by using the mapped global limits of malaria risk provided by the WHO22 and modified with contemporary descriptions of spatial risk used to inform antimalarial chemoprophylaxis regimes in travellers22,23, to exclude the following: countries with only P. vivax transmission, areas above anopheline vector-specific altitude limits, and administrative areas defined as risk-free. These boundaries formed the limits of P. falciparum risk and were overlaid on the only available global map of malaria endemicity developed in 1968 (refs 6, 7). This map was part of a major synthesis of historical records, documents and maps of malaria endemicity (using the hypoendemic to holoendemic classifications) interpolated globally for malaria at the peak of its assumed historical distribution. We have assumed that this endemicity map is consistent with contemporary malaria risks in Africa, but development and intervention will have substantially reduced malaria risk elsewhere11. Outside the African region we consider the historical (1968) hyperendemic to holoendemic areas as contemporary (2002) mesoendemic conditions, historically mesoendemic areas as hypoendemic, and hypoendemic risk areas at their historical descriptions within the revised 2002 spatial limits of risk.
Data from Gridded Population of the World (GPW3) version 3.0 beta (http://sedac.ciesin.colombia.edu/gpw) were projected to 2002 by using national inter-censal growth rates from the UN Population Prospects database (http://esa.un.org/unpp). Population totals were extracted by country for those residing in hypoendemic, mesoendemic and hyperendemic-to-holoendemic settings (Table 1, Fig. 2). These population totals were further adjusted for the suppressive effects of urbanization on malaria transmission24 by identifying all urban areas with populations of more than 1 million. Urban population totals within these pixels were reclassified to the risk class below their original classification; thus, those located in hypoendemic areas were regarded as being at no infection risk. Populations in 2002 residing in the different urban-adjusted, P. falciparum endemicity risk zones are shown in Table 1. The endemicity-specific morbid risks were then applied to populations within their respective endemicity classes to estimate numbers of clinical events in 2002 (Table 2).
Supplementary Material
Acknowledgements
This research was funded by the Wellcome Trust, UK. Part of the work on mapping population risk distributions was funded by the World Health Organization Roll Back Malaria Department. The World Health Organization/RBM Department Monitoring and Evaluation Team and staff from WHO regional offices provided passive case detection data, the WHO Evidence for Information and Policy Department provided SALB data and the WHO Public Health Mapping Group provided other geographical boundary files. We thank K. Marsh, D. Forster, C. Macintosh, K. Maitland and D. Rogers for comments on earlier drafts of the manuscript, D. Balk and G. Yetman for supplying alpha versions of GPW 3.0, and A. Tatem for the urban extractions. R.W.S. is a Wellcome Trust Senior Research Fellow and acknowledges the support of the Kenyan Medical Research Institute (KEMRI). S.I.H. is funded by a Research Career Development Fellowship from the Wellcome Trust. C.A.G. is partially funded by the Fundación para la Ciencia y Tecnología (FUNDACYT).
Footnotes
Supplementary Information accompanies the paper on www.nature.com/nature.
Competing interests statement The authors declare competing financial interests: details accompany the paper on www.nature.com.
References
- 1.Hay SI, Guerra CA, Tatem AJ, Noor AM, Snow RW. The global distribution and population at risk of malaria: past, present and future. Lancet Infect. Dis. 2004;4:327–336. doi: 10.1016/S1473-3099(04)01043-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Snow RW, Marsh K, le Sueur D. The need for maps of transmission intensity to guide malaria control in Africa. Parasitol. Today. 1996;12:455–457. [Google Scholar]
- 3.Gallup JL, Sachs JD. The economic burden of malaria. Am. J. Trop. Med. Hyg. 2001;64:85–96. doi: 10.4269/ajtmh.2001.64.85. [DOI] [PubMed] [Google Scholar]
- 4.Kiszewski A, et al. A global index representing the stability of malaria transmission. Am. J. Trop. Med. Hyg. 2004;70:486–498. [PubMed] [Google Scholar]
- 5.Russell PF. World-wide malaria distribution, prevalence and control. Am. J. Trop. Med. Hyg. 1956;5:937–956. doi: 10.4269/ajtmh.1956.5.937. [DOI] [PubMed] [Google Scholar]
- 6.Lysenko AJ, Semashko IN. In: Itogi Nauki: Medicinskaja Geografija. Lebedew AW, editor. Academy of Sciences; Moscow: 1968. pp. 25–146. [Google Scholar]
- 7.Lysenko AJ, Beljaev AE. An analysis of the geographical distribution of Plasmodium ovale. Bull. World Health Organ. 1969;40:383–394. [PMC free article] [PubMed] [Google Scholar]
- 8.Murray CJL, Lopez AD. Mortality by cause for eight regions of the world: global burden of disease study. Lancet. 1997;349:1269–1276. doi: 10.1016/S0140-6736(96)07493-4. [DOI] [PubMed] [Google Scholar]
- 9.World Health Organization . The World Health Report 1999: Making a Difference. World Health Organization; Geneva: 1999. [Google Scholar]
- 10.Snow RW, Craig MH, Deichmann U, Marsh K. Estimating mortality, morbidity and disability due to malaria among Africa’s non-pregnant population. Bull. W.H.O. 1999;77:624–640. [PMC free article] [PubMed] [Google Scholar]
- 11.Carter R, Mendis KN. Evolutionary and historical aspects of the burden of malaria. Clin. Microbiol. Rev. 2002;15:564–594. doi: 10.1128/CMR.15.4.564-594.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mendis K, Sina LJ, Marchensini P, Carter R. The neglected burden of Plasmodium vivax malaria. Am. J. Trop. Med. Hyg. 2001;64:97–106. doi: 10.4269/ajtmh.2001.64.97. [DOI] [PubMed] [Google Scholar]
- 13.Murray CJ, Lopez AD. Global Health Statistics: a Compendium of Incidence, Prevalence and Mortality Estimates for over 200 Countries. Harvard School of Public Health, Boston/World Health Organization; Geneva: 1996. [Google Scholar]
- 14.International Network for the Continuous Demographic Evaluation of Populations and Their Health in Developing Countries. khttp://www.INDEPTH-network.orgl.
- 15.Snow RW, et al. Relation between severe malaria morbidity in children and level of Plasmodium falciparum transmission in Africa. Lancet. 1997;349:1650–1654. doi: 10.1016/S0140-6736(97)02038-2. [DOI] [PubMed] [Google Scholar]
- 16.Prusty SKR, Das BS. Low incidence of the severe complications of malaria and absence of malaria-specific mortality, in Tensa, Sundergarh district, Orissa State, India, an area hyper-endemic for malaria. Ann. Trop. Med. Parasit. 2001;95:133–140. doi: 10.1080/00034980120041080. [DOI] [PubMed] [Google Scholar]
- 17.Maitland K, et al. Absence of malaria-specific mortality in children in an area of hyperendemic malaria. Trans. R. Soc. Trop. Med. Hyg. 1997;91:562–566. doi: 10.1016/s0035-9203(97)90026-2. [DOI] [PubMed] [Google Scholar]
- 18.Alles HK, Mendis KN, Carter R. Malaria mortality rates in South Asia and in Africa: implications for malaria control. Parasitol. Today. 1998;14:369–375. doi: 10.1016/s0169-4758(98)01296-4. [DOI] [PubMed] [Google Scholar]
- 19.Mayfong M, Pukrittayakamee S, Newton PN, White NJ. Mixed species malaria infections in humans. Trends Parasitol. 2004;20:233–240. doi: 10.1016/j.pt.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 20.United Nations Development Programme. Human Development Report 2003. Millennium Development Goals: a Compact among Nations to End Human Poverty. Oxford Univ. Press; Oxford: 2003. [Google Scholar]
- 21.Metselaar D, Van Theil PH. Classification of malaria. Trop. Geogr. Med. 1959;11:157–161. [Google Scholar]
- 22.World Health Organization . International Travel and Health. Situation as on 1 January 2003. World Health Organization; Geneva: 2003. [Google Scholar]
- 23.International Association for Medical Assistance to Travellers World malaria risk chart (including geographical distribution of principal vectors, geographical distribution of P. falciparum malaria, areas where Plasmodium falciparum is resistant to chloroquine and guidelines for suppressive medication by country) Status as of 15 March 2004. ⟨ http://www.iamat.org ⟩ .
- 24.Hay SI, Guerra CA, Tatem AJ, Atkinson PM, Snow RW. Urbanization, malaria transmission and disease burden in Africa. Nature Rev. Microbiol. 2005;3:81–90. doi: 10.1038/nrmicro1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.