Abstract
Obesity has reached pandemic proportions, with bariatric surgery representing the only currently available treatment demonstrating long-term effectiveness. Over 200,000 bariatric procedures are performed each year in the US alone. Given the reliable and singular success of bariatric procedures, increased attention is being paid to identifying the accompanying neurohormonal changes that may contribute to the resulting decrease in energy intake. Numerous investigations of postsurgical changes in gut peptides have been conducted, suggesting greater alterations in endocrine function in combination restrictive and malabsorptive procedures (e.g., Roux-en-Y gastric bypass) as compared to purely restrictive procedures (e.g., gastric banding), which may contribute to the increased effectiveness of combination procedures. However, very few studies have been performed and relatively little is known about changes in neural activation that may result from bariatric procedures, which likely interact with changes in gut peptides to influence postsurgical caloric intake. This review provides a background in the neurohormonal regulation of energy intake and discusses how differing forms of bariatric surgery may affect the neurohormonal network, with emphasis on Roux-en-Y gastric bypass, the most commonly performed procedure worldwide.
The paper represents an invited review by a symposium, award winner or keynote speaker at the Society for the Study of Ingestive Behavior [SSIB] Annual Meeting in Portland, July 2009.
Keywords: Peptide, Food, Overweight, Brain, fMRI, Diet, RYGB, Gastric, Bypass, Banding, Ghrelin, PYY, GLP-1
Currently, bariatric surgery represents the only form of treatment for obesity that demonstrates long-term effectiveness [1–3]. From 1992 to 2006, a ten-fold increase in obesity surgeries was observed [4]. The most common forms of bariatric surgery are Roux-en-Y gastric bypass (RYGB) and adjustable gastric banding (AGB), which together account for approximately 90% of the procedures performed today [5]. Postsurgical reductions in body weight result largely from the restrictive and/or malabsorptive effects of the bariatric procedures. However, these mechanisms alone appear to be insufficient to account for the reductions in caloric intake and body weight seen postsurgically [6–8], even in purely restrictive procedures such as AGB [7,9]. Alterations in the neurohormonal regulation of energy intake have been proposed to contribute to postsurgical reductions in caloric intake, particularly in RYGB [8,10–12].
1. Hormonal influences on energy intake regulation relevant to bariatric surgery
Numerous hormones are involved in controlling appetite and food intake via the activation of brain areas, primarily within the limbic and mesolimbic systems [13]. Hunger and satiety signals from adipose tissue (leptin), the pancreas (insulin) and the gastrointestinal tract (cholecystokinin [CCK], glucagon-like peptide-l [GLP-1], peptide YY3–36 [PYY3–36]) are involved in relaying information about energy status through the neurohormonal gut–brain axis primarily targeting the hypothalamus and brainstem [13]. The major appetite-related peptides, which act through the central nervous system (CNS) to help regulate energy balance and may be affected by bariatric surgery, are introduced below.
1.1. Ghrelin
Secreted mainly by the stomach, ghrelin is an orexigenic peptide that acts on hypothalamic neurons through the bloodstream via vagal afferents containing ghrelin receptors, as well as through direct release within the hypothalamus [14]. In animals, central ghrelin administration also enhances the secretion of the orexigenic neuropeptides neuropeptide-Y (NPY) and agouti-related peptide (AgRP) in the arcuate nucleus (AN) of the hypothalamus [15]. Ghrelin has also been shown to activate dopamine neurons in the ventral tegmental area (VTA) and promote dopamine turnover in the nucleus accumbens (NA) of the ventral striatum [16]. This effect on reward processing in the mesolimbic dopaminergic pathway may be an integral part of ghrelin’s orexigenic action [17,18], supported by evidence that blocking ghrelin receptors in the VTA decreases food intake [16].
1.2. Peptide YY (PYY)
PYY is an anorexigenic lower gut-derived hormone synthesized from L entero-endocrine cells [19]. PYY mediates its actions via Y1–Y5 receptors [19], and the binding of the active component of PYY3–36 to Y2 receptors on vagal afferents leads to activation of the arcuate nucleus to suppress NPY activation [20]. Delayed gastric emptying (via the ileal brake mechanism) also contributes to the inhibitory effect of PYY3–36 on appetite and food intake [21].
1.3. Glucagon-like peptide 1(GLP-1)
Co-released with PYY, GLP-1 is an anorexigenic peptide that is secreted postprandially from the distal gastrointestinal tract. GLP-1 acts as an ileal brake for the upper GI tract and decreases food intake in part by inhibiting gastric emptying, resulting in greater gastric distension [22]. Endogenous GLP-1 affects hypothalamic signaling through vagal afferent neurotransmission [23,24] and by entering the peripheral circulation. Circulating levels of GLP-1 are higher prior to and following food consumption in lean as compared to obese individuals [25].
1.4. Cholecystokinin
An endogenous hormone present in the GI tract and the brain, cholecystokinin (CCK) helps control meal size and duration via a feedback signaling network involving the periphery and the CNS [26]. CCK reaches peak levels within 15–30 min [27,28]. CCK activates receptors on the vagus nerve, which transmit satiety signals to the dorsomedial hypothalamus and minimizes NPY action [27].
1.5. Leptin
An anorexigenic hormone synthesized from adipose tissue, leptin helps control adipose metabolism by stimulation of lipolysis and suppression of lipogenesis [29]. It crosses the blood brain barrier and transmits signals about metabolic status from the periphery to hypothalamic regulatory centers [30]. Once bound to its central receptor, leptin down-regulates appetite stimulating neuropeptides (e.g., NPY, AgRP) while up-regulating anorexigenic alpha-Melano-cyte-stimulating hormone, cocaine-and amphetamine-regulated transcript and corticotropin-releasing hormone [30]. Leptin injections in obese humans have not been efficacious in reducing food intake and weight gain, potentially due to the development of leptin resistance [31]. Studies also suggest that leptin can influence the reward value of food [32,33] and leptin administration can help maintain lost weight [34].
1.6. Insulin
Secreted from the pancreas, insulin varies directly with adiposity, and visceral fat is negatively correlated with insulin sensitivity [35]. Basal and postprandial insulin are greater in obese than in lean individuals [36]. Insulin can penetrate the blood brain barrier and binds to receptors in the AN to decrease food intake [37]. See Table 1 for a brief summary of the major appetite-related peptides relevant to bariatric surgery and their proposed effects on hunger or satiety.
Table 1.
Major appetite-related peptides relevant to bariatric surgery.
| Gut peptides | Central effects on appetite | Alterations due to RYGBa |
|---|---|---|
| Ghrelin | ↑ | ↓ |
| PYY | ↓ | ↑ |
| GLP | ↓ | ↑ |
| CCK | ↓ | → |
| Leptin | ↔ | ↓ |
| Insulin | ↔ | ↓ |
↑= increase. ↓ = decrease. → = no significant change.
↔ = modifies effect depending on energy balance.
According to the majority of study findings to date.
2. Neuroimaging and energy intake regulation in relation to bariatric surgery
Neuroimaging is increasingly becoming more common in obesity research as investigators attempt to understand the biological basis of eating behavior. Positron emission tomography (PET), single photon emission computed tomography (SPECT), and functional magnetic resonance imaging (fMRI) are common neuroimaging techniques used to assess brain activity and provide insight into brain systems linked to feeding behavior (See [38] and [39] for reviews).
The neural systems involved in energy intake regulation are complex but can be roughly divided into two related systems, the homeostatic and hedonic networks [12,17]. The homeostatic system is comprised of the hypothalamus and brainstem, driven mainly by hypothalamic activity [12,17]. The hypothalamus, especially the arcuate nucleus, integrates peripheral hormonal signals and receives inputs from the brainstem, which detects vagal signals related to ingestion [40]. It is responsible for the assimilation of input about internal hunger and satiety signals and the propagation of appropriate output signals to other relevant brain areas (e.g., upward moderation of mesolimbic reward areas to increase the reward salience of food during caloric deprivation) [13,41]. Thus, the homeostatic system drives food intake based on caloric need in order to maintain energy balance and body weight [17,42], which may be determined by a biological settling point [43].
In contrast, the hedonic system drives food intake based on the perceived reward value of food [17,44], operating largely through dopaminergic signaling in the mesolimbic pathway [45]. The mesolimbic pathway initiates behavioral responses based on the predicted reward, or hedonic, value of stimuli encountered in the environment [12,44]. Major components of this complex neural system include the amygdala, hippocampus, VTA, and striatum (caudate, NA, and lentiform nucleus), and these areas have strong functional connections to areas within the prefrontal cortex (PFC; e.g., dorsolateral PFC, orbitofrontal cortex [OFC]) [45]. The PFC is responsible for integrating internal and external sensory information, reward-related information from other cortico-limbic areas and signals from the hypothalamus, which can modulate reward salience [13,17]. The PFC is also connected to other cortical areas involved in motor planning and execution [46].
The relatively limited number of studies that have examined brain activation in relation to food intake or body weight are mainly cross-sectional and focus on the differences in reaction to food stimuli between types of stimuli (e.g., high vs. low-palatability) or body mass index (BMI; e.g., lean vs. obese)[47,48]. The majority of studies in this area coalesce in reporting greater activation in hedonic system activation in response to high- vs. low-palatability stimuli and in obese vs. lean individuals [49,50]. Such findings potentially reflect greater perceived hedonic reward of high (vs. low) palatability food cues and heightened sensitivity to reward in obese (vs. lean) individuals [51,52].
As noted, reward value is largely processed in the PFC, which receives visual, auditory, and orosensory inputs from the thalamus, as well as information from the hypothalamus [12]. There is evidence to suggest that the hypothalamus may modulate reward salience [53–55], which may explain why individuals in a state of caloric need (fasted) show increased sensitivity to, and preference for, sweet and novel tastes [56,57], as well as greater activation of hedonic network brain areas in response to appetitive stimuli [54,58,59]. Conversely, in a fed state (in the presence of postprandial satiety signals), individuals show decreased hedonic network activation in response to appetitive stimuli [54,59]. However, the hedonic system can override attempts by the homeostatic system to maintain energy balance, and drive food intake even in the presence of postprandial satiety signaling [60,61]. Variation in the ease with which satiety signals are overridden by the perceived reward value of food may reflect individual differences in appetitive responsivity, or the desire to eat following exposure to food cues [49].
3. Interactions between gut peptides and brain systems relevant to bariatric surgery
Neurological and hormonal systems are often studied independently, but hunger and satiety signals are mediated by a complex interplay of both systems, the neurohormonal pathway [62]. Ghrelin, PYY, GLP-1, CCK, insulin and leptin are primarily released in the periphery in response to the presence or absence of nutrients in the digestive tract [63,64] but act indirectly on the vagus nerve and/or directly on target areas of the hypothalamus [65], propagating potent hunger and satiety signals to the brain. In addition, gut peptide signaling may have more complex neurobehavioral actions in triggering hypothalamic modulation of the perceived reward value of food via the mesolimbic pathway [66]. See Fig. 1.
Fig. 1.
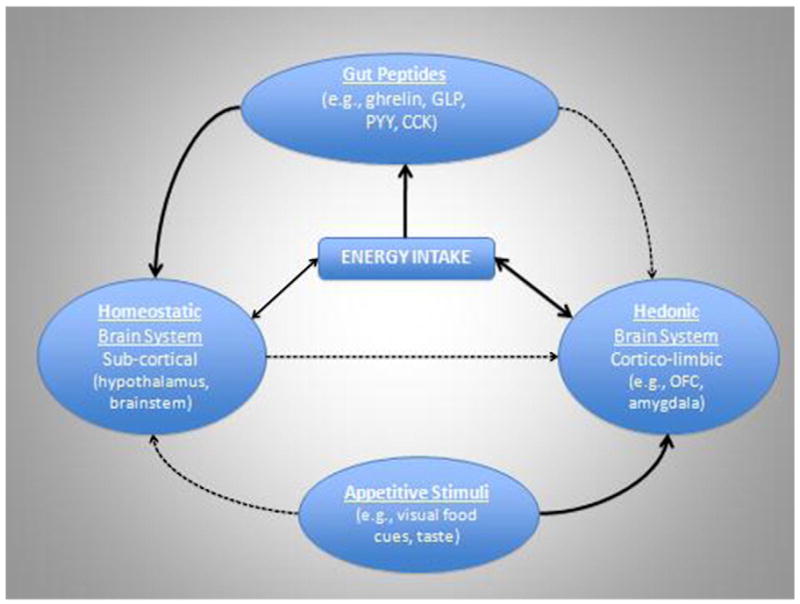
Cartoon representation of the relevant influences on energy intake. The strength of evidence in support of each association is represented by the thickness of the connecting arrows with bolded lines representing strong empirical support and the dotted lines representing more theoretical evidence.
3.1. Neuroimaging and appetite-related hormones
A novel technique for examining in vivo gut–brain interactions in humans is to combine neuroimaging with gut hormone measures or manipulations. Farooqi et al. [67] used fMRI to show that heightened activation in the ventral striatum in response to food cues in two leptin-deficient adolescents was significantly reduced following one week of leptin administration. In other fMRI studies, Rosenbaum et al. [68] showed that a number of changes in brain activation typically associated with increased hunger, which resulted from non-surgical weight loss, were leptin-reversible. Similarly, Baicy et al. [69] reported reduced brain activity in regions linked to hunger (insula, parietal and temporal cortex) in genetically leptin-deficient adults following leptin replacement. These studies are consistent with evidence suggesting that leptin may suppress appetite by down-regulating brain areas associated with drive to eat while up-regulating areas associated with homeostatic control and satiety [33,67].
In an fMRI study of ghrelin infusion, Malik et al. [17] found post-infusion increases in activation in the amygdala, OFC, insula and striatum in response to pictures of highly palatable food in lean males given a 20 min ghrelin infusion, suggesting that some portion of ghrelin’s orexigenic effects may come through the enhancement of the perceived reward value of food. Animal studies [18,70] further implicate an interaction between ghrelin and mesolimbic pathway activation in showing that ghrelin can activate dopamine neurons in the VTA and increase dopamine in the nucleus accumbens, which may stimulate food intake [15,16].
In a study of food-induced hormonal changes, Pannacciulli et al. [71] measured GLP-1 levels and PET activation before and after administered a satiating amount of a liquid formula meal to lean adult subjects. Postprandial increases in GLP-1 were found to correlate positively with increases in activity in the dlPFC and hypothalamus, suggesting that GLP-1 may mediate the purported role of the dlPFC in the cessation of eating (satiety signaling) [71,72]. Finally, in an innovative study of real-time interactions between changes in gut hormone levels and brain activation, Batterham et al. [73] infused PYY3–36 at a rate designed to mimic that which would be expected in response to a meal and assessed brain activation concurrently, using fMRI. These authors showed that increases in PYY3–36 were associated with increased activity in the OFC, parabrachial nucleus, midbrain VTA, insula, anterior cingulate, ventral striatum and posterior hypothalamus. They also reported a PYY3–36-induced shift from homeostatic (hypothalamic) to hedonic (OFC) brain areas in predicting subsequent food intake, which may reflect a switch from homeostatic to hedonic control of appetite with the introduction of a postprandial satiety signal.
4. Surgical interventions for obesity
Surgical interventions are not only more effective than behavioral treatments in the short term but are the only form of obesity intervention with evidence of consistent long-term effectiveness [1,3]. Current surgical interventions for obesity all contain a restrictive component, limiting the amount of food that can enter the stomach pouch as well as the rate at which food can be ingested. Several procedures, most notably RYGB, also contain a malabsorptive part in which the bowel length is shortened, decreasing nutrient and calorie absorption. However, there is controversy regarding the degree and durability of postsurgical malabsorption associated with these procedures [74]. The purely restrictive AGB procedure is gaining popularity, currently representing approximately 25% of procedures [1,75], second only to RYGB, which accounts for approximately 65% of procedures worldwide [1]. Gastric bypass results in more weight loss than gastric banding (GB). The Swedish Obese Subjects [76] study showed that the maximal percent of initial weight lost (1–2 yr post-surgery) was 32%±8 SD in RYBG vs. 20%±10 in GB and this difference was maintained at 10 yr follow up (25% in RYGB vs. 14% in GB). Similarly, at 2-yr follow up, Shah et al. [77] reported reductions of approximately 31% of initial body weight in RYGB vs. 24% in GB. Descriptions of these and other procedures are provided below.
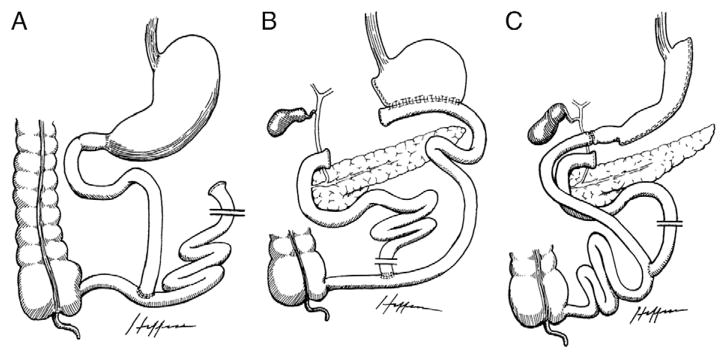
Most purely restrictive procedures create a small gastric pouch with a narrow outlet, limiting the intake of food without disruption of the absorptive function of the small intestine. With VBG, a longitudinal staple line with a tight outlet wrapped by a band or mesh partitions the cardia (proximal part) from the rest of the stomach (Fig. 2A), whereas AGB involves encircling the upper part of the stomach (distal to the gastroesophageal junction) with a tight, adjustable, prosthetic band (Fig. 2B). The amount of restriction in AGB may be altered by the addition or withdrawal of saline solution from the hollow core of the band [78]. Sleeve gastrectomy, intragastric balloon, and endoluminal gastroplasty are other forms of restrictive procedures that are less commonly used. Conventional malabsorptive bariatric operations predominantly circumvent a portion of the small intestine in an effort to reduce nutrient absorption. The jejunoileal bypass (JIB) is an example of a purely malabsorptive technique, which separates the jejunum near the ligament of Treitz and reconnects it near the ileocecal valve, bypassing a long small bowel segment (Fig. 3A). However, this procedure is rarely performed due to significant complications and relatively greater need for revision surgeries [78].
Fig. 2.
Illustrations of restrictive procedures and Roux-en-Y gastric bypass. Reproduced with permission from Dr. Edward C Mun [171] A. Vertical banded gastroplasty; B. Adjustable gastric banding; C. Roux-en-Y gastric bypass.
Fig. 3.
Illustrations of malabsorptive procedures. Reproduced with permission from Dr. Edward C Mun [171] A. Jejunoileal bypass; B. Biliopancreatic diversion; C. Biliopancreatic diversion with duodenal switch.
Combination bariatric procedures incorporate both restrictive and malabsorptive components. Biliopancreatic diversion (BPD) consists of a distal horizontal gastrectomy, where the remnant stomach is anastomosed to the distal small intestine (alimentary limb). The excluded small intestine carries the bile and pancreatic secretions, and is anastomosed to the short bowel channel proximal to the ileocecal valve, leaving a short common limb portion for the mixing of nutrients with secretions (Fig. 3B). The BPD is also limited in use due to adverse health outcomes related to essential nutrient malabsorption [79]. The biliopancreatic diversion with duodenal switch (BPD-DS) includes a sleeve vertical gastrectomy where the gastric fundus is mostly resected, with duodenal closure a few centimeters distal to an intact pylorus facilitating a duodenoileal anastomosis (Fig. 3C). This procedure is chiefly performed on super-obese patients (BMIs >50 kg/m2), as it classically leads to relatively large postsurgical weight loss; however, it is not commonly performed due to adverse health outcomes similar to those seen in BPD [78]. Lastly, RYGB surgery represents the most common bariatric procedure and is considerably more effective than AGB. With RYGB, a small gastric pouch is created and anastomosed to the jejunum with a short Roux-en-Y alimentary limb of distal small bowel, bypassing the majority of the stomach, the entire duodenum and a portion of the jejunum (Fig. 2C).
Despite a rapid increase in the number of studies on bariatric surgery, the precise mechanisms of action are still not well understood, particularly with RYGB [8,80]. While the restricted (15–50 mL) postsurgical pouch size limits ingestive capacity [8] and the bypassing of the upper portion of the small intestine may prevent a small percentage of ingested calories from being absorbed into the body [8,74], these mechanisms account for only a proportion of postsurgical weight loss [8,10,81]. Evidence for this comes from research demonstrating corrective adaptation to the malabsorptive component [74] and limited small meal adaptation [82], as well as subjective reports [83,84] and behavioral data [85,86] suggesting that presurgical preferences for foods high (vs. low) in fat [84], calories [85], and palatability [83,87] are reduced or eliminated following RYGB (but not AGB) surgery. Finally, preliminary data from our laboratory suggest that there may also be a greater reduction in non-specific (to any particular food) desire to eat in response to high-calorie, relative to low-calorie food cues following RYGB. The neurohormonal system has been repeatedly implicated in accounting for this unexplained reduction in postsurgical caloric intake [8,10–12]. Numerous investigations of appetite-related gut peptides have resulted and are described below.
5. Alterations in gut peptides involved in energy intake regulation through bariatric surgery
Ghrelin is produced primarily by the gastric antrum and fundus and, although the anterior section of the stomach is reduced in purely restrictive operations, there is some variation with respect to changes in ghrelin levels after surgical intervention. In some studies, lower fasting ghrelin levels were reported after laparoscopic sleeve gastrectomy (SG) [88,89]; while other findings showed increases in fasting ghrelin following LAGB [90–92]. A rise in ghrelin concentrations following both AGB [93,94] and VGB [92,95,96], have been widely observed, but several studies have noted no change following either procedure [97–100], and two cross-sectional studies found a reduction in ghrelin concentrations following AGB, in comparison to BMI-matched counterparts [101,102].
Prospective and cross-sectional studies of ghrelin levels following RYGB have produced mixed results, with the majority showing a decrease in plasma ghrelin levels post bypass surgery [93,96,103,104]. Cummings et al. [11] in a cross-sectional study showed that ghrelin levels were considerably reduced after RYGB relative to both obese and normal weight controls. Higher levels of ghrelin were also measured in obese persons who had lost weight by dieting than prior to the diet [11], implicating a putative role for ghrelin in the adaptive response that limits the weight loss by dieting and enhances the likelihood of weight regain. Several researchers have since reported significantly lower levels of ghrelin in surgical patients who lost weight from RYGB in both cross-sectional and prospective studies [100,103,105,106]. The first few months following BPD also resulted in a significant reduction in ghrelin concentrations [100,101]. A post-surgical reduction of ghrelin may contribute to the persistent weight loss reported in obese patients following gastric bypass and mixed malabsorptive procedures. However, a number of studies demonstrated no significant change in ghrelin levels following gastric bypass [97,107] and BPD [108], or even higher ghrelin concentrations following both RYGB [109–111] and BPD [112–114].
Discrepancies among these results may be partially attributed to variation in the types of surgical and control groups chosen. In a prospective study, Faraj et al. [115] showed increased ghrelin concentrations in post-RYGB surgical patients undergoing weight loss but did not include a control group. Despite the increase in ghrelin levels observed in the surgical patients, they were still lower than levels reported in normal weight or comparably obese participants from other studies [11,116]. Similarly, another group found an increase in ghrelin at 12 months following gastric bypass that was comparable to BMI-matched controls [117]. A rise in ghrelin may have been anticipated following significant weight loss in these RYGB patients; however, if the postsurgical cohort were compared to BMI-matched controls that had lost weight conventionally, one might have expected a relatively lower ghrelin level in the operative patients.
Variation in surgical technique and hormonal assays may also be contributing factors in the differences reported in the literature. Thaler and Cummings [118] proposed that the inconsistencies across findings may be related to the integrity of autonomic vagal innervation. Vagal innervation influences ghrelin levels [14,82,119], and the degree to which the innervation is preserved is likely to differ between surgeons. In addition, the accuracy of commercial ghrelin assays used in the vast majority of studies has been raised [99]. Although it is not clear what the source of the variance is among studies, the type of surgical procedure seems to have a major influence on ghrelin levels. The overall reduction in postsurgical ghrelin levels in gastric bypass may contribute to the greater weight loss relative to other procedures, such as AGB [11,120].
Changes in PYY levels following bariatric surgery are under further investigation, and although inconsistencies still remain, the majority of evidence to date show that both restrictive [121–123] and malabsorptive [97,105,124] operations lead to a rise in fasting and postprandial PYY. Basal and postprandial PYY levels in morbidly obese surgical patients after VGB, were similar to non-obese participants in cross-sectional studies at 6 months, and were reasonably stable at 12 months post-surgery [123]. Comparable postprandial PYY3–36 levels were also observed in post AGB patients and lean controls in two studies [102,125]. Korner et al. [126] showed an early postprandial rise in PYY concentrations in 12 patients in a cross-sectional study at 15–17 months post-RYGB. Garcia-Fuentes et al. reported that BPD produced a more substantial rise in PYY levels than RYGB in 29 morbidly obese patients [108]. Following RYGB, increases in postprandial PYY have been reported [88,127] and may be due to the absence of a functional pylorus and/or a significant portion of the stomach and pylorus being bypassed resulting in a faster emptying rate into small bowel. Higher PYY concentrations may lead to an early sense of satiety and reduced meal size, and together with reduced ghrelin may contribute to weight loss [88,128]. PYY inhibits ghrelin-sensitive neurons in the arcuate nucleus of the hypothalamus in a dose-dependent manner [129]. A change in the PYY to ghrelin ratio supporting PYY’s anorectic action after bariatric surgery may result in deactivation of appetite centers centrally regulated by ghrelin. Longitudinal neuroimaging studies are needed to examine changes within the same individual pre and post-surgery and across different operations to clarify these points further.
Studies of post-operative changes in GLP-1 suggest that malabsorptive operations lead to a rise in fasting and postprandial GLP-1 concentrations [97,124,130–132]. Two studies have reported no postsurgical change in GLP-1 following AGB [97,98]. In contrast Reinehr et al. [121] reported lower GLP-1 levels in AGB patients at two years post-surgery. On the other hand, an increase in GLP-1 has been reported in one study [122] following SG. GLP-1 levels during an oral glucose tolerance test were increased in VBG and BPD, with a greater increase in BPD relative to VBG [130]. GLP-1 is produced from the distal small intestine; therefore a reduction in stomach size would not be expected to affect circulating levels of GLP-1.
Findings regarding changes in fasting GLP-1 levels after RYGB surgery are also mixed [121,127], but most report higher postprandial levels post-surgery in RYGB [97,105,131,133]. In one study, significant increases in GLP-1 levels were found six weeks following RYGB, when patients were still severely obese [124]. Elevated levels of GLP-1 may contribute to the sustained efficacy of RYGB as well as improve and resolve diabetes, consistent with the incretin effect on weight and glucose metabolism [134]. RYGB restricts the stomach and bypasses the duodenum, which facilitates the rapid delivery of food contents through the GI tract, augmenting the release of GLP-1. It has been hypothesized that the increase in hypothalamic satiety signals (e.g. from GLP-1) may play contribute to the postsurgical weight loss observed after malabsorptive procedures [124,135].
Conflicting postsurgical changes in CCK have also been found in both restrictive and malabsorptive bariatric operations. Kellum et al. [136] measured CCK levels after a glucose or protein meal before and after RYBG and VBG, and the CCK response was not altered by either intervention. However in another study, Foschi at al. [95] evaluated patients before and after VBG surgery with healthy lean volunteer controls and showed that post-VBG patients had a more significant peak CCK response to an acidified meal known to enhance CCK production [137] and a faster time to the peak than controls, without differences between baseline CCK concentrations [95]. While a decline in CCK following RYGB might be predicted due to the diversion of ingested food contents away from the duodenum, the jejunum also secretes CCK [138]. In rats, CCK was not significantly altered after RYGB-induced weight loss [27]. CCK is primarily important for short term control of appetite and satiety [139] and unlike leptin and insulin [140], CCK does not appear to have an autonomous role in the long-term regulation of energy balance and body weight [141]. CCK can work synergistically with leptin to promote short term reduction of food intake in mice [142]. More research is needed to determine CCK’s role in human obesity and the changes following bariatric surgery.
Several studies have demonstrated a significant decline in plasma leptin levels after bariatric surgery in relation to fat loss, irrespective of the surgical intervention [92,97,100,110]. A reduction in postprandial leptin levels were found in post VBG surgical patients in comparison to the baseline pre-operative state [143]. Lower leptin concentrations were seen at 2 and 12 months post BPD as compared to pre-surgical levels [113]. Serum leptin concentrations were also reduced in morbidly obese patients that underwent BPD-DS [144,145]. Rubino et al. [146] measured reduced leptin levels in post-RYGB patients as compared to non-surgical weight loss controls. Recent evidence suggests that leptin replacement therapy may aid in weight loss maintenance [68].
The impact of bariatric surgery on insulin levels and insulin resistance in obese persons is profound. In the bulk of restrictive bariatric operations, insulin tends to fall in postsurgical obese patients [92,97,100,110]. Reduced insulin concentrations were maintained at two years post GB and VBG [92]. Obese patients had decreased insulin levels after LAGB than BMI-matched controls [147]. Weight loss, resulting from gastric bypass and BPD, improves insulin resistance [100,132,148,149]. Insulin levels and resistance were also significantly lowered and improved respectively in post-operative obese patients with and without Night Eating Syndrome five months after RYGB [150]. Metabolic operations are being further investigated as an alternative to pharmacological agents in the treatment of diabetes. See Table 1 for a brief summary of changes in major appetite-related peptides secondary to RYGB surgery.
6. Alterations in neural activation through weight loss and bariatric surgery
The majority of studies in this area are cross-sectional (e.g., lean vs. obese) and show differences in brain activation that suggest obese individuals may experience greater reward-related (predominantly mesolimbic) activation in response to appetitive stimuli [151–153] and food intake (e.g. [68,154]). There is, however, controversy surrounding this hypothesis [153] and it remains unknown whether any such lean vs. obese differences are cause or consequence of an obese state, as very few longitudinal studies have been conducted. Despite radical postsurgical alterations in gut peptide signaling and established interactions between gut peptides and the neural control of food intake, there has been only one study of changes in brain receptor site binding [155] and one investigation of changes in neurological responses to food stimuli following bariatric surgery [156]. The limited available studies of the effect of weight loss and bariatric surgery on brain activation discussed below may provide insight and hypotheses for future investigations.
Although cross-sectional, comparisons between obese and weight-suppressed individuals, who may be prone to weight gain [157,158], may provide some insight into the association between weight loss and neural activation in response to appetitive stimuli and/or food intake. Cornier et al. [159] found greater hypothalamic activation in response to palatable food cues in lean individuals after a controlled five-day eucaloric diet relative to after 2 days of 30% overfeeding, suggesting an association between energy stores and hypothalamic activity in response to appetitive cues. This may reflect down-regulation of hypothalamic activity in lean individuals in response to appetitive cues upon entering a state of positive energy balance. Reduced obese (obesity-prone) individuals, however, did not show the same attenuation of hypothalamic response to food cues as lean individuals upon entering the same overfed state, suggesting an impaired interaction between appetitive stimuli and the homeostatic regulation of energy intake [159]. Although speculative, the absence of a decrease in hypothalamic activation in response to appetitive stimuli upon entering an overfed state may contribute to more or sooner resumption of ‘homeostatic-based’ eating [159]. In addition, impaired attenuation of hypothalamic activity may also leave open the potential for hypothalamic up-regulation of reward-related activation [53,54], which may be consistent with evidence showing greater PFC activation in reduced-obese relative to lean individuals [160]. Although almost certainly an oversimplification, as different subregions within both the hypothalamus [161] and PFC [72,162] play different roles in the regulation of energy intake, this blunt framework may help formulate testable hypotheses regarding the interaction between homeostatic (hypothalamic) and hedonic systems in appetitive responsivity.
Other studies have directly assessed acute responses to food intake, which yield different conclusions than those proposed by Cornier and colleagues [159,160] who examined neural responses to appetitive stimuli, potentially reflecting differences between anticipated and consummatory reward processing [153]. For example, Del Parigi et al. [154] showed differences in reward-related activation in response to a meal between lean and obese participants but no differences between obese and formerly obese participants. The authors suggest that differential or “abnormal” neural responses to meal ingestion in obese relative to lean individuals persists following weight loss. Alternately, in a cross-sectional reanalysis of existing data, Le et al. [72], reported that formerly obese women who successfully achieved weight loss by diet and exercise and maintained their weight loss for 3 months had greater activation of the dorsolateral prefrontal cortex (dlPFC) in response to a meal than did obese women. The authors hypothesize that this difference is related to greater inhibitory or satiety-related activation, for which there is some support [72,154,163], although it is also possible that the increased dlPFC activation reflects greater reward processing [162,164].
In the only available longitudinal study of brain activation pre and post non-surgical weight loss, Rosenbaum et al. [68] reported changes in brain activation in response to food cues following non-surgical weight loss (10% initial body weight) in 6 obese men and women. Following weight loss, leptin-reversible increases in activation were seen in the brainstem, parahippocampal gyrus, culmen, parahippocampal gyrus, inferior and middle frontal gyri, middle temporal gyrus, and lingual gyrus, while leptin-reversible reductions in activation were observed in the hypothalamus, cingulate gyrus, and middle frontal gyrus. Based on these results, the authors suggest that some changes in neural processing that may be associated with hunger and subsequent weight (re)gain may be leptin-reversible [68]. However, the complexity of these findings also illustrate the need for further controlled, hypothesis-driven research of brain activation in response to weight change before conclusions can be drawn about the interaction between brain systems (homeostatic vs. hedonic activation, weight change (gain vs. loss) and state of energy balance (positive vs. negative) in responses to appetitive stimuli.
Finally, two recent studies have examined the effect of bariatric surgery on neural activity. Using PET, Steele et al. [155] recently examined dopamine D2 receptor activity in five obese female subjects pre and six weeks after laparoscopic RYGB surgery. Five regions of interest were studied (ventral striatum, anterior and posterior putamen, anterior and posterior caudate nucleus) and the researchers found that while baseline D2 binding in RYGB patients did not differ from non-obese controls, D2 receptor availability increased from pre to post-surgery, with the increase in receptor availability roughly proportional to the amount of weight lost. This suggests that the correction of diminished D2 binding in obese subjects (due to D2 receptor down-regulation) may play a role in appetite suppression and weight loss after RYGB.
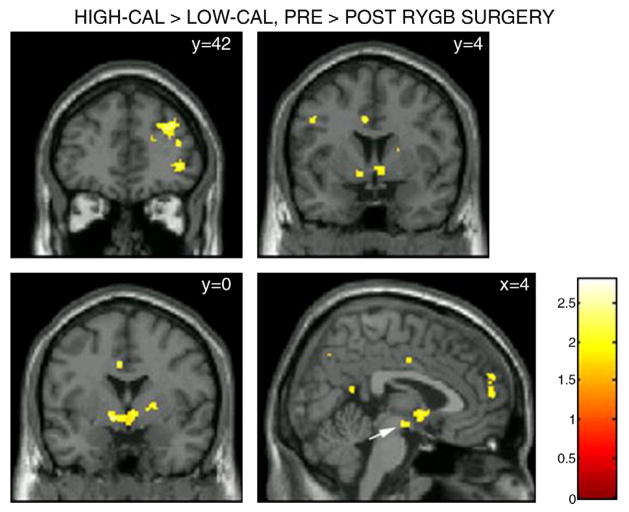
The current authors used fMRI to investigate whether laparoscopic RYGB would result in significant changes in brain activation in response to high- and low-calorie food stimuli in 10 female patients. Given consistent postsurgical reductions in the desire for and intake of food [158,159], it was hypothesized that participants undergoing RYGB would show postsurgical reductions in mesolimbic reward pathway activation in response to food cues. It was also anticipated that postsurgical reductions in mesolimbic activity and desire to eat would be greater in response to high-ED relative to low-ED food cues given that presurgical preferences for foods high (vs. low) in fat and calories are typically reduced or eliminated following RYGB surgery [84,86]. Results revealed postsurgical reductions in brain activation in key areas within the mesolimbic reward pathway in response to food cues, which were significantly more pronounced in response to food cues that were high (vs. low) in caloric density (Figs. 4 and 5). These changes mirrored concurrent postsurgical reductions desire to eat following exposure to food cues that were high (vs. low) in caloric density (Fig. 6). These findings support the contention that RYGB surgery leads to substantial changes in neural responses to food cues encountered in the environment, provide a potential mechanism for the selective reduction in preferences for high-calorie foods, and suggest partial neural mediation of changes in caloric intake seen following RYGB surgery.
Fig. 4.
Glass brain figure depicting brain activation in response to high-calorie relative to low-calorie food stimuli (high-calorie–low-calorie contrast) at p < 0.05 uncorrected. A greater difference between mesolimbic dopaminergic pathway activation in response to high-calorie foods and mesolimbic dopaminergic pathway activation in response to low-calorie foods can be seen pre-relative to post-surgery. For display purposes, activation maps are shown without a cluster extent threshold.
Fig. 5.
Coronal and sagittal slices depicting areas in which the difference between activation in response to high- and low-ED foods (High-ED–Low-ED Contrast) was greater pre-relative to post-surgery. Activation was considered significant at p < 0.05 uncorrected, with an applied cluster extent threshold (k=22). The largest clusters (k>80) were seen in the dlPFC (y=42; top and cluster), ventrolateral PFC (vlPFC; y =42; bottom cluster), ventral striatum (y=4; bottom two clusters), putamen and lentiform nucleus (y =0; bottom cluster), and dmPFC (x=4; rightmost cluster). A nonsignificant cluster (k=20) was also observed in the VTA (x=4; white arrow). MNI coordinates are given in upper left corner of each panel. The color bar represents t values.
Fig. 6.
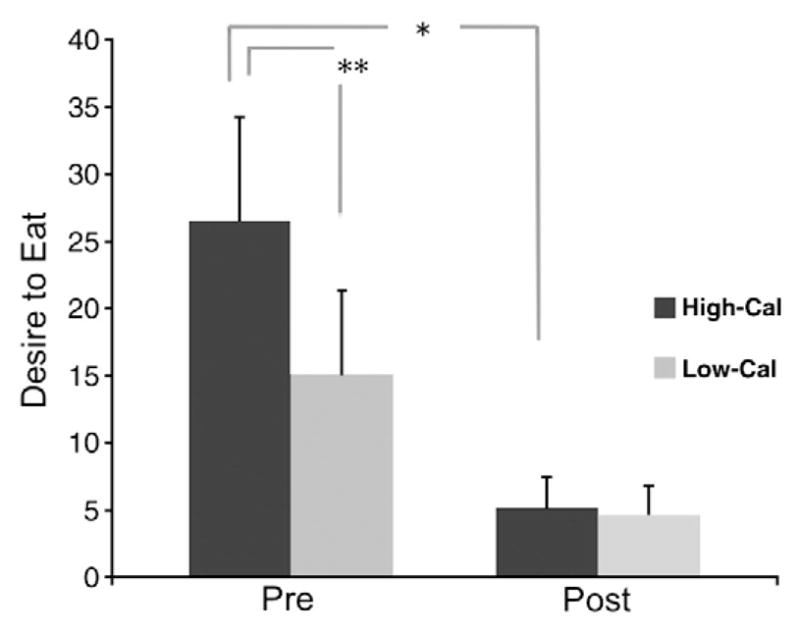
The difference between the desire to eat following exposure to high-calorie relative to low-calorie foods (high-calorie–low-calorie) pre-surgery was greater than the nonsignificant high-calorie–low-calorie difference post-surgery p = 0.007; *p<0.05; **p<0.01.
7. Conclusion
Obesity is linked to significant abnormalities in the neurohormonal control of energy intake, many of which have been elucidated by studies of gut peptides and brain activation in animals and humans. Yet there remains much work to be done in linking gut peptide and brain functions, particularly as they relate to energy balance. Although replication is needed, recent findings suggest that reward-related brain activation caused by exposure to appetizing food cues may be attenuated by elevations in PYY3–36 and GLP-1, simulating a postprandial (fed) state [71,73]. In theory, these postprandial satiety signals are received by the hypothalamus [60], in turn down-regulating hedonic activation in the brain and reducing the likelihood or volume of subsequent food intake via reductions in perceived reward value [53]. However, despite putative attempts by the ‘homeostatic’ system to maintain energy balance, there is substantial evidence (particularly in obese individuals) to suggest that the hedonic system can override the homeostatic system and drive food intake even when sated if the perceived reward value is sufficiently high [41,61].
Surgical interventions for obesity, particularly RYGB, may lead to substantial and simultaneous changes in gut peptides [10], brain activation [155,156], desire to eat [156] and taste preferences [83]. Thus, the effects of bariatric surgery on the neurohormonal regulation of energy intake provides a tantalizing ‘natural’ experiment with which to explore gut–brain interactions [165,166], with an increasing number of studies suggesting that postsurgical changes within the neurohormonal system may account for a proportion of postsurgical weight loss [8,167]. For example, postsurgical reductions in ghrelin and earlier and enhanced postprandial elevations of PYY and GLP-1 may reduce hunger and promote satiety [168]. The success of combination, relative to purely restrictive, procedures suggests that hormonal alterations may contribute to weight loss [167]. Relative to changes in gut peptides, very little is known about changes in brain activation following bariatric procedures. Investigations of non-surgical weight loss support an increase in reward-related/hedonic activation in response to appetitive cues [68,72,154,163], which could help explain weight regain in dieters. In contrast, the absence of an increase in desire to eat following RYGB, even on exposure to highly palatable food cues [84,122], is striking, and consistent with systemic changes in neural responses to food cues.
Interactions between postsurgical changes in gut peptides and neural activity have been widely proposed, but remain to be explicitly tested. Investigations of neurohormonal changes in RYGB may assist in developing non-surgical interventions to treat obesity and related comorbidities, which could serve as a viable treatment alternative for obese individuals who do not have access or do not qualify for bariatric surgery. Pharmacological activation of the hedonic network in animals, already fed beyond satiety, produces hyperphagia and preferentially increases the intake of foods high in fat and sugar [169,170]. Thus, identifying the actions of RYGB on gut–brain signaling may make it possible to develop pharmacological agents to increase satiety, and reduce hunger and preferences for high-ED foods.
References
- 1.Samuel I, Mason EE, Renquist KE, Huang YH, Zimmerman MB, Jamal M. Bariatric surgery trends: an 18-year report from the International Bariatric Surgery Registry. Am J Surg. 2006;192:657–62. doi: 10.1016/j.amjsurg.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 2.Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults—the evidence report. National Institutes of Health. Obes Res. 1998;6(Suppl 2):51S–209S. [PubMed] [Google Scholar]
- 3.Buchwald H, Williams SE. Bariatric surgery worldwide 2003. Obes Surg. 2004;14:1157–64. doi: 10.1381/0960892042387057. [DOI] [PubMed] [Google Scholar]
- 4.American Society for Metabolic and Bariatric Surgery. [Accessed March 2010];Laproscopic adjustable gastric banding. Available at: http://www.asbs.org/html/patients/bypass.html.
- 5.Poves I, Cabrera M, Maristany C, Coma A, Ballesta-Lopez C. Gastrointestinal quality of life after laparoscopic Roux-en-Y gastric bypass. Obes Surg. 2006;16:19–23. doi: 10.1381/096089206775222032. [DOI] [PubMed] [Google Scholar]
- 6.Cummings DE, Shannon MH. Roles for ghrelin in the regulation of appetite and body weight. Arch Surg. 2003;138:389–96. doi: 10.1001/archsurg.138.4.389. [DOI] [PubMed] [Google Scholar]
- 7.Cummings DE, Overduin J, Foster-Schubert KE. Gastric bypass for obesity: mechanisms of weight loss and diabetes resolution. J Clin Endocrinol Metab. 2004;89:2608–15. doi: 10.1210/jc.2004-0433. [DOI] [PubMed] [Google Scholar]
- 8.Tadross JA, le Roux CW. The mechanisms of weight loss after bariatric surgery. Int J Obes (Lond) 2009;33(Suppl 1):S28–32. doi: 10.1038/ijo.2009.14. [DOI] [PubMed] [Google Scholar]
- 9.Brolin RE. Bariatric surgery and long-term control of morbid obesity. JAMA. 2002;288:2793–6. doi: 10.1001/jama.288.22.2793. [DOI] [PubMed] [Google Scholar]
- 10.Vincent RP, le Roux CW. Changes in gut hormones after bariatric surgery. Clin Endocrinol (Oxf) 2008;69:173–9. doi: 10.1111/j.1365-2265.2007.03164.x. [DOI] [PubMed] [Google Scholar]
- 11.Cummings DE, Weigle DS, Frayo RS, Breen PA, Ma MK, Dellinger EP, et al. Plasma ghrelin levels after diet-induced weight loss or gastric bypass surgery. N Engl J Med. 2002;346:1623–30. doi: 10.1056/NEJMoa012908. [DOI] [PubMed] [Google Scholar]
- 12.Berthoud HR, Morrison C. The brain, appetite, and obesity. Annu Rev Psychol. 2008;59:55–92. doi: 10.1146/annurev.psych.59.103006.093551. [DOI] [PubMed] [Google Scholar]
- 13.Lenard NR, Berthoud HR. Central and peripheral regulation of food intake and physical activity: pathways and genes. Obesity (Silver Spring) 2008;16(Suppl 3):S11–22. doi: 10.1038/oby.2008.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Korbonits M, Goldstone AP, Gueorguiev M, Grossman AB. Ghrelin—a hormone with multiple functions. Front Neuroendocrinol. 2004;25:27–68. doi: 10.1016/j.yfrne.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 15.Olszewski PK, Li D, Grace MK, Billington CJ, Kotz CM, Levine AS. Neural basis of orexigenic effects of ghrelin acting within lateral hypothalamus. Peptides. 2003;24:597–602. doi: 10.1016/s0196-9781(03)00105-0. [DOI] [PubMed] [Google Scholar]
- 16.Jerlhag E, Egecioglu E, Dickson SL, Douhan A, Svensson L, Engel JA. Ghrelin administration into tegmental areas stimulates locomotor activity and increases extracellular concentration of dopamine in the nucleus accumbens. Addict Biol. 2007;12:6–16. doi: 10.1111/j.1369-1600.2006.00041.x. [DOI] [PubMed] [Google Scholar]
- 17.Malik S, McGlone F, Bedrossian D, Dagher A. Ghrelin modulates brain activity in areas that control appetitive behavior. Cell Metab. 2008;7:400–9. doi: 10.1016/j.cmet.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 18.Abizaid A, Liu ZW, Andrews ZB, Shanabrough M, Borok E, Elsworth JD, et al. Ghrelin modulates the activity of synaptic input organization of midbrain dopamine nuerons while promoting appetite. J Clin Invest. 2006;116:3229–39. doi: 10.1172/JCI29867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ballantyne GH. Peptide YY(1–36) and peptide YY(3–36): Part I. Distribution, release and actions. Obes Surg. 2006;16:651–8. doi: 10.1381/096089206776944959. [DOI] [PubMed] [Google Scholar]
- 20.le Roux CW, Bloom SR. Peptide YY, appetite and food intake. Proc Nutr Soc. 2005;64(2):213–6. doi: 10.1079/pns2005427. [DOI] [PubMed] [Google Scholar]
- 21.Adrian TE, Savage AP, Sagor GR, Allen JM, Bacarese-Hamilton AJ, Tatemoto K, et al. Effect of peptide YY on gastric, pancreatic, and biliary function in humans. Gastroenterology. 1985;89:494–9. doi: 10.1016/0016-5085(85)90442-1. [DOI] [PubMed] [Google Scholar]
- 22.Tang-Christensen M, Vrang N, Larsen PJ. Glucagon-like peptide containing pathways in the regulation of feeding behaviour. Int J Obes Relat Metab Disord. 2001;25(Suppl 5) doi: 10.1038/sj.ijo.0801912. [DOI] [PubMed] [Google Scholar]
- 23.Kakei M, TYada T, Nakagawa H. Glucagon-like peptide-1 evokes action potenitals and increasescytosolic Ca2+ in rat nodose ganglion neurons. Auton Neurosci. 2002;102:39–44. doi: 10.1016/s1566-0702(02)00182-0. [DOI] [PubMed] [Google Scholar]
- 24.Nakagawa A, Satake H, Nakabayashi H, Nishizawa M, Furuya K, Nakano S, et al. Receptor gene expression of glucagon-like peptide-1, but not glucose-dependent insulinotropic polypeptide, in rat nodose ganglion cells. Auton Neurosci. 2004;110:36–43. doi: 10.1016/j.autneu.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 25.Verdich C, Toubro S, Buemann B, Lysgard-Madsen J, Juul-Holst J, Astrup A. The role of postprandial releases of insulin and incretin hormones in meal-induced satiety-effect of obesity and weight reduction. Int J Obes Relat Metab Disord. 2001;25:1206–14. doi: 10.1038/sj.ijo.0801655. [DOI] [PubMed] [Google Scholar]
- 26.Baile CA, McLaughlin CL, Della-Fera MA. Role of cholecystokinin and opioid peptides in control of food intake. Physiol Rev. 1986;66:172–234. doi: 10.1152/physrev.1986.66.1.172. [DOI] [PubMed] [Google Scholar]
- 27.Suzuki S, Ramos EJ, Goncalves CG, Chen C, Meguid MM. Changes in GI hormones and their effect on gastric emptying and transit times after Roux-en-Y gastric bypass in rat model. Surgery. 2005;138:283–90. doi: 10.1016/j.surg.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 28.Liddle RA, Goldfine ID, Rosen MS, Taplitz RA, Williams JA. Cholecystokinin bioactivity in human plasma. Molecular forms, responses to feeding, and relationship to gallbladder contraction. J Clin Invest. 1985;75:1144–52. doi: 10.1172/JCI111809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fried SK, Ricci MR, Russell CD, Laferrere B. Regulation of leptin production in humans. J Nutr. 2000;130:3127S–31S. doi: 10.1093/jn/130.12.3127S. [DOI] [PubMed] [Google Scholar]
- 30.Arora S, Anubhuti Role of neuropeptides in appetite regulation and obesity—a review. Neuropeptides. 2006;40:375–401. doi: 10.1016/j.npep.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 31.Hukshorn CJ, van Dielen FM, Buurman WA, Westerterp-Plantenga MS, Campfield LA, Saris WH. The effect of pegylated recombinant human leptin (PEG-OB) on weight loss and inflammatory status in obese subjects. Int J Obes Relat Metab Disord. 2002;26:504–9. doi: 10.1038/sj.ijo.0801952. [DOI] [PubMed] [Google Scholar]
- 32.Figlewicz DP, Bennett J, Evans SB, Kaiyala K, Sipols AJ, Benoit SC. Intraventricular insulin and leptin reverse place preference conditioned with high fat diet in rats. Behav Neurosci. 2004;118:479–87. doi: 10.1037/0735-7044.118.3.479. [DOI] [PubMed] [Google Scholar]
- 33.Fulton S, Woodside B, Shizgal P. Modulation of brain reward circuitry by leptin. Science. 2000;287:125–8. doi: 10.1126/science.287.5450.125. [DOI] [PubMed] [Google Scholar]
- 34.Ahima RS. Revisiting leptin’s role in obesity and weight loss. J Clin Invest. 2008;118:2380–3. doi: 10.1172/JCI36284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maffeis C, Manfredi R, Trombetta M, Sordelli S, Storti M, Benuzzi T, et al. Insulin sensitivity is correlated with subcutaneous but not visceral body fat in overweight and obese prepubertal children. J Clin Endocrinol Metab. 2008;93:2122–8. doi: 10.1210/jc.2007-2089. [DOI] [PubMed] [Google Scholar]
- 36.Bjorntorp P. Obesity, atherosclerosis and diabetes mellitus. Verh Dtsch Ges Inn Med. 1987;93:443–8. doi: 10.1007/978-3-642-85460-6_107. [DOI] [PubMed] [Google Scholar]
- 37.Rushing PA, Lutz TA, Seeley RJ, Woods SC. Amylin and insulin interact to reduce food intake in rats. Horm Metab Res. 2000;32:62–5. doi: 10.1055/s-2007-978590. [DOI] [PubMed] [Google Scholar]
- 38.Tataranni PA, DelParigi A. Functional neuroimaging: a new generation of human brain studies in obesity research. Obes Rev. 2003;4:229–38. doi: 10.1046/j.1467-789x.2003.00111.x. [DOI] [PubMed] [Google Scholar]
- 39.Van den Eynde F, Treasure J. Neuroimaging in eating disorders and obesity: implications for research. Child Adolesc Psychiatr Clin N Am. 2009;18:95–115. doi: 10.1016/j.chc.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 40.Obici S, Rossetti L. Minireview: nutrient sensing and the regulation of insulin action and energy balance. Endocrinology. 2003;144:5172–8. doi: 10.1210/en.2003-0999. [DOI] [PubMed] [Google Scholar]
- 41.Berthoud HR. Neural control of appetite: cross-talk between homeostatic and non-homeostatic systems. Appetite. 2004;43:315–7. doi: 10.1016/j.appet.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 42.Gower BA, Weinsier RL, Jordan JM, Hunter GR, Desmond R. Effects of weight loss on changes in insulin sensitivity and lipid concentrations in premenopausal African American and white women. Am J Clin Nutr. 2002;76:923–7. doi: 10.1093/ajcn/76.5.923. [DOI] [PubMed] [Google Scholar]
- 43.Nisbett RE. Eating behavior and obesity in men and animals. Adv Psychosom Med. 1972;7:173–93. doi: 10.1159/000393300. [DOI] [PubMed] [Google Scholar]
- 44.Shin AC, Zheng H, Townsend RL, Sigalet DL, Berthoud HR. Is increased plasma amylin a major player in RGYB-induced reduction of food intake and weight loss? Obesity (Silver Spring) 2009;17:S59. [Google Scholar]
- 45.Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35:4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Balleine BW. The neural basis of choice and decision making. J Neurosci. 2007;27:8159–60. [Google Scholar]
- 47.Spitzer L, Rodin J. Human eating behavior:a critical review of studies in normal weight individuals. Appetite. 1981;2:293–329. [Google Scholar]
- 48.Rodin J. Current status of the internal–external hypothesis for obesity: what went wrong? Am Psychol. 1981;36:361–72. doi: 10.1037//0003-066x.36.4.361. [DOI] [PubMed] [Google Scholar]
- 49.Lowe MR, Butryn ML, Didie ER, Annunziato RA, Thomas JG, Crerand CE, et al. The power of food scale. A new measure of the psychological influence of the food environment. Appetite. 2009;53:114–8. doi: 10.1016/j.appet.2009.05.016. [DOI] [PubMed] [Google Scholar]
- 50.Geliebter A, Ladell T, Logan M, Schneider T, Sharafi M, Hirsch J. Responsivity to food stimuli in obese and lean binge eaters using functional MRI. Appetite. 2006;46:31–5. doi: 10.1016/j.appet.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 51.Gottfried JA, O’Doherty J, Dolan RJ. Encoding predictive reward value in human amygdala and orbitofrontal cortex. Science. 2003;301:1104–7. doi: 10.1126/science.1087919. [DOI] [PubMed] [Google Scholar]
- 52.Kringelbach ML. The human orbitofrontal cortex: linking reward to hedonic experience. Nat Rev Neurosci. 2005;6:691–702. doi: 10.1038/nrn1747. [DOI] [PubMed] [Google Scholar]
- 53.Harris GC, Wimmer M, Aston-Jones G. A role for lateral hypothalamic orexin neurons in reward seeking. Nature. 2005;437:556–9. doi: 10.1038/nature04071. [DOI] [PubMed] [Google Scholar]
- 54.Goldstone AP, de Hernandez CG, Beaver JD, Muhammed K, Croese C, Bell G, et al. Fasting biases brain reward systems towards high-calorie foods. Eur J NeuroSci. 2009;30:1625–35. doi: 10.1111/j.1460-9568.2009.06949.x. [DOI] [PubMed] [Google Scholar]
- 55.Saper CB. Hypothalamic connections with the cerebral cortex. Prog Brain Res. 2000;126:39–48. doi: 10.1016/S0079-6123(00)26005-6. [DOI] [PubMed] [Google Scholar]
- 56.Brunstrom JM, Fletcher HZ. Flavour–flavour learning occurs automatically and only in hungry participants. Physiol Behav. 2008;93:13–9. doi: 10.1016/j.physbeh.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 57.Zverev YP. Effects of caloric deprivation and satiety on sensitivity of the gustatory system. BMC Neurosci. 2004;5:5. doi: 10.1186/1471-2202-5-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hinton EC, Parkinson JA, Holland AJ, Arana FS, Roberts AC, Owen AM. Neural contributions to the motivational control of appetite in humans. Eur J NeuroSci. 2004;20:1411–8. doi: 10.1111/j.1460-9568.2004.03589.x. [DOI] [PubMed] [Google Scholar]
- 59.Fuhrer D, Zysset S, Stumvoll M. Brain activity in hunger and satiety: an exploratory visually stimulated FMRI study. Obesity (Silver Spring) 2008;16:945–50. doi: 10.1038/oby.2008.33. [DOI] [PubMed] [Google Scholar]
- 60.Berthoud HR. Mind versus metabolism in the control of food intake and energy balance. Physiol Behav. 2004;81:781–93. doi: 10.1016/j.physbeh.2004.04.034. [DOI] [PubMed] [Google Scholar]
- 61.Cornell CE, Rodin J, Weingarten H. Stimulus-induced eating when satiated. Physiol Behav. 1989;45:695–704. doi: 10.1016/0031-9384(89)90281-3. [DOI] [PubMed] [Google Scholar]
- 62.Jensen MD. Potential role of new therapies in modifying cardiovascular risk in overweight patients with metabolic risk factors. Obesity (Silver Spring) 2006;14 (Suppl 3):143S–9S. doi: 10.1038/oby.2006.294. [DOI] [PubMed] [Google Scholar]
- 63.Koda S, Date Y, Murakami N, Shimbara T, Hanada T, Toshinai K, et al. The role of the vagal nerve in peripheral PYY3–36-induced feeding reduction in rats. Endocrinology. 2005;146:2369–75. doi: 10.1210/en.2004-1266. [DOI] [PubMed] [Google Scholar]
- 64.Larhammar D. Structural diversity of receptors for neuropeptide Y, peptide YY and pancreatic polypeptide. Regul Pept. 1996;65:165–74. doi: 10.1016/0167-0115(96)00110-3. [DOI] [PubMed] [Google Scholar]
- 65.Date Y, Murakami N, Toshinai K, Matsukura S, Niijima A, Matsuo H, et al. The role of the gastric afferent vagal nerve in ghrelin-induced feeding and growth hormone secretion in rats. Gastroenterology. 2002;123:1120–8. doi: 10.1053/gast.2002.35954. [DOI] [PubMed] [Google Scholar]
- 66.Batterham RL, Ffytche DH, Rosenthal JM, Zelaya FO, Barker GJ, Withers DJ, et al. PYY modulation of cortical and hypothalamic brain areas predicts feeding behaviour in humans. Nature. 2007;450:106–9. doi: 10.1038/nature06212. [DOI] [PubMed] [Google Scholar]
- 67.Farooqi IS, Bullmore E, Keogh J, Gillard J, O’Rahilly S, Fletcher PC. Leptin regulates striatal regions and human eating behavior. Science. 2007;317:1355. doi: 10.1126/science.1144599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rosenbaum M, Sy M, Pavlovich K, Leibel RL, Hirsch J. Leptin reverses weight loss-induced changes in regional neural activity responses to visual food stimuli. J Clin Invest. 2008;118:2583–91. doi: 10.1172/JCI35055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Baicy K, London ED, Monterosso J, Wong ML, Delibasi T, Sharma A, et al. Leptin replacement alters brain response to food cues in genetically leptin-deficient adults. Proc Natl Acad Sci U S A. 2007;104:18276–9. doi: 10.1073/pnas.0706481104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Carlini VP, Varas MM, Cragnolini AB, Schioth HB, Scimonelli TN, de Barioglio SR. Differential role of the hippocampus, amygdala, and dorsal raphe nucleus in regulating feeding, memory, and anxiety-like behavioral responses to ghrelin. Biochem Biophys Res Commun. 2004;313:635–41. doi: 10.1016/j.bbrc.2003.11.150. [DOI] [PubMed] [Google Scholar]
- 71.Pannacciulli N, Le DS, Salbe AD, Chen K, Reiman EM, Tataranni PA, et al. Postprandial glucagon-like peptide-1 (GLP-1) response is positively associated with changes in neuronal activity of brain areas implicated in satiety and food intake regulation in humans. NeuroImage. 2007;35:511–7. doi: 10.1016/j.neuroimage.2006.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Le DS, Pannacciulli N, Chen K, Del Parigi A, Salbe AD, Reiman EM, et al. Less activation of the left dorsolateral prefrontal cortex in response to a meal: a feature of obesity. Am J Clin Nutr. 2006;84:725–31. doi: 10.1093/ajcn/84.4.725. [DOI] [PubMed] [Google Scholar]
- 73.Batterham RL, Cohen MA, Ellis SM, le Roux CW, Withers DJ, Frost GS, et al. Inhibition of food intake in obese subjects by peptide YY3–36. N Engl J Med. 2003;349:941–8. doi: 10.1056/NEJMoa030204. [DOI] [PubMed] [Google Scholar]
- 74.Brolin RE, LaMarca LB, Kenler HA, Cody RP. Malabsorptive gastric bypass in patients with superobesity. J Gastrointest Surg. 2002;6:195–203. doi: 10.1016/s1091-255x(01)00022-1. discussion 4–5. [DOI] [PubMed] [Google Scholar]
- 75.Brethauer SA, Chand B, Schauer PR. Risks and benefits of bariatric surgery: current evidence. Cleve Clin J Med. 2006;73:993–1007. doi: 10.3949/ccjm.73.11.993. [DOI] [PubMed] [Google Scholar]
- 76.Sjostrom L, Narbro K, Sjostrom CD, Karason K, Larsson B, Wedel H, et al. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med. 2007;357:741–52. doi: 10.1056/NEJMoa066254. [DOI] [PubMed] [Google Scholar]
- 77.Shah M, Simha V, Garg A. Review: long-term impact of bariatric surgery on body weight, comorbidities, and nutritional status. J Clin Endocrinol Metab. 2006;91:4223–31. doi: 10.1210/jc.2006-0557. [DOI] [PubMed] [Google Scholar]
- 78.Bult MJ, can Dalen T, Muller AF. Surgical treatment for obesity. Eur J Endocrinol. 2008;158:135–45. doi: 10.1530/EJE-07-0145. [DOI] [PubMed] [Google Scholar]
- 79.Marceau P, Hould FS, Simard S, Lebel S, Bourque RA, Potvin M, et al. Biliopancreatic diversion with duodenal switch. World J Surg. 1998;22:947–54. doi: 10.1007/s002689900498. [DOI] [PubMed] [Google Scholar]
- 80.Cummings DE, Shannon MH. Ghrelin and gastric bypass: is there a hormonal contribution to surgical weight loss? J Clin Endocrinol Metab. 2003;88:2999–3002. doi: 10.1210/jc.2003-030705. [DOI] [PubMed] [Google Scholar]
- 81.Buchwald H, Avidor Y, Braunwald E, Jensen MD, Pories W, Fahrbach K, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292:1724–37. doi: 10.1001/jama.292.14.1724. [DOI] [PubMed] [Google Scholar]
- 82.Williams DL, Grill HJ, Cummings DE, Kaplan JM. Vagotomy dissociates short- and long-term controls of circulating ghrelin. Endocrinology. 2003;144:5184–7. doi: 10.1210/en.2003-1059. [DOI] [PubMed] [Google Scholar]
- 83.Tichansky DS, Boughter JD, Jr, Madan AK. Taste change after laparoscopic Roux-en-Y gastric bypass and laparoscopic adjustable gastric banding. Surg Obes Relat Dis. 2006;2:440–4. doi: 10.1016/j.soard.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 84.Thirlby RC, Bahiraei F, Randall J, Drewnoski A. Effect of Roux-en-Y gastric bypass on satiety and food likes: the role of genetics. J Gastrointest Surg. 2006;10:270–7. doi: 10.1016/j.gassur.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 85.Kenler HA, Brolin RE, Cody RP. Changes in eating behavior after horizontal gastroplasty and Roux-en-Y gastric bypass. Am J Clin Nutr. 1990;52:87–92. doi: 10.1093/ajcn/52.1.87. [DOI] [PubMed] [Google Scholar]
- 86.Halmi KA, Mason E, Falk JR, Stunkard A. Appetitive behavior after gastric bypass for obesity. Int J Obes. 1981;5:457–64. [PubMed] [Google Scholar]
- 87.Burge JC, Schaumburg JZ, Choban PS, DiSilvestro RA, Flancbaum L. Changes in patients’ taste acuity after Roux-en-Y gastric bypass for clinically severe obesity. J Am Diet Assoc. 1995;95:666–70. doi: 10.1016/S0002-8223(95)00182-4. [DOI] [PubMed] [Google Scholar]
- 88.Karamanakos SN, Vagenas K, Kalfarentzos F, Alexandrides TK. Weight loss, appetite suppression, and changes in fasting and postprandial ghrelin and peptide-YY levels after Roux-en-Y gastric bypass and sleeve gastrectomy: a prospective, double blind study. Ann Surg. 2008;247:401–7. doi: 10.1097/SLA.0b013e318156f012. [DOI] [PubMed] [Google Scholar]
- 89.Peterli R, Wolnerhanssen B, Peters T, Devaux N, Kern B, Christoffel-Courtin C, et al. Improvement in glucose metabolism after bariatric surgery: comparison of laparoscopic Roux-en-Y gastric bypass and laparoscopic sleeve gastrectomy: a prospective randomized trial. Ann Surg. 2009;250:234–41. doi: 10.1097/SLA.0b013e3181ae32e3. [DOI] [PubMed] [Google Scholar]
- 90.Schindler K, Prager G, Ballaban T, Kretschmer S, Riener R, Buranyi B, et al. Impact of laparoscopic adjustable gastric banding on plasma ghrelin, eating behaviour and body weight. Eur J Clin Invest. 2004;34:549–54. doi: 10.1111/j.1365-2362.2004.01382.x. [DOI] [PubMed] [Google Scholar]
- 91.Uzzan B, Catheline JM, Lagorce C, Airinei G, Bon C, Cohen R, et al. Expression of ghrelin in fundus is increased after gastric banding in morbidly obese patients. Obes Surg. 2007;17:1159–64. doi: 10.1007/s11695-007-9197-9. [DOI] [PubMed] [Google Scholar]
- 92.Nijhuis J, van Dielen FM, Buurman WA, Greve JW. Ghrelin, leptin and insulin levels after restrictive surgery: a 2-year follow-up study. Obes Surg. 2004;14:783–7. doi: 10.1381/0960892041590980. [DOI] [PubMed] [Google Scholar]
- 93.Fruhbeck G, Rotellar F, Hernandez-Lizoain JL, Gil MJ, Gomez-Ambrosi J, Salvador J, et al. Fasting plasma ghrelin concentrations 6 months after gastric bypass are not determined by weight loss or changes in insulinemia. Obes Surg. 2004;14:1208–15. doi: 10.1381/0960892042386904. [DOI] [PubMed] [Google Scholar]
- 94.Stoeckli R, Chanda R, Langer I, Keller U. Changes of body weight and plasma ghrelin levels after gastric banding and gastric bypass. Obes Res. 2004;12:346–50. doi: 10.1038/oby.2004.43. [DOI] [PubMed] [Google Scholar]
- 95.Foschi D, Corsi F, Pisoni L, Vago T, Bevilacqua M, Asti E, et al. Plasma cholecystokinin levels after vertical banded gastroplasty: effects of an acidified meal. Obes Surg. 2004;14:644–7. doi: 10.1381/096089204323093426. [DOI] [PubMed] [Google Scholar]
- 96.Foschi D, Corsi F, Colombo F, Vago T, Bevilaqua M, Rizzi A, et al. Different effects of vertical banded gastroplasty and Roux-en-Y gastric bypass on meal inhibition of ghrelin secretion in morbidly obese patients. J Invest Surg. 2008;21:77–81. doi: 10.1080/08941930701883624. [DOI] [PubMed] [Google Scholar]
- 97.Korner J, Inabnet W, Febres G, Conwell IM, McMahon DJ, Salas R, et al. Prospective study of gut hormone and metabolic changes after adjustable gastric banding and Roux-en-Y gastric bypass. Int J Obes (Lond) 2009;33:786–95. doi: 10.1038/ijo.2009.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Shak JR, Roper J, Perez-Perez GI, Tseng CH, Francois F, Gamagaris Z, et al. The effect of laparoscopic gastric banding surgery on plasma levels of appetite-control, insulinotropic, and digestive hormones. Obes Surg. 2008;18:1089–96. doi: 10.1007/s11695-008-9454-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ram E, Vishne T, Diker D, Gal-Ad I, Maayan R, Lerner I, et al. Impact of gastric banding on plasma ghrelin, growth hormone, cortisol, DHEA and DHEA-S levels. Obes Surg. 2005;15:1118–23. doi: 10.1381/0960892055002329. [DOI] [PubMed] [Google Scholar]
- 100.Garcia de la Torre N, Rubio MA, Bordiu E, Cabrerizo L, Aparicio E, Hernandez C, et al. Effects of weight loss after bariatric surgery for morbid obesity on vascular endothelial growth factor-A, adipocytokines, and insulin. J Clin Endocrinol Metab. 2008;93:4276–81. doi: 10.1210/jc.2007-1370. [DOI] [PubMed] [Google Scholar]
- 101.Fruhbeck G, Diez-Caballero A, Gil MJ, Montero I, Gomez-Ambrosi J, Salvador J, et al. The decrease in plasma ghrelin concentrations following bariatric surgery depends on the functional integrity of the fundus. Obes Surg. 2004;14:606–12. doi: 10.1381/096089204323093363. [DOI] [PubMed] [Google Scholar]
- 102.Korner J, Inabnet W, Conwell IM, Taveras C, Daud A, Olivero-Rivera L, et al. Differential effects of gastric bypass and banding on circulating gut hormone and leptin levels. Obesity (Silver Spring) 2006;14:1553–61. doi: 10.1038/oby.2006.179. [DOI] [PubMed] [Google Scholar]
- 103.Morinigo R, Casamitjana R, Moize V, Lacy AM, Delgado S, Gomis R, et al. Short-term effects of gastric bypass surgery on circulating ghrelin levels. Obes Res. 2004;12:1108–16. doi: 10.1038/oby.2004.139. [DOI] [PubMed] [Google Scholar]
- 104.Lin E, Gletsu N, Fugate K, McClusky D, Gu LH, Zhu JL, et al. The effects of gastric surgery on systemic ghrelin levels in the morbidly obese. Arch Surg. 2004;139:780–4. doi: 10.1001/archsurg.139.7.780. [DOI] [PubMed] [Google Scholar]
- 105.Rodieux F, Giusti V, D’Alessio DA, Suter M, Tappy L. Effects of gastric bypass and gastric banding on glucose kinetics and gut hormone release. Obesity (Silver Spring) 2008;16:298–305. doi: 10.1038/oby.2007.83. [DOI] [PubMed] [Google Scholar]
- 106.Tritos NA, Mun E, Bertkau A, Grayson R, Maratos-Flier E, Goldfine A. Serum ghrelin levels in response to glucose load in obese subjects post-gastric bypass surgery. Obes Res. 2003;11:919–24. doi: 10.1038/oby.2003.126. [DOI] [PubMed] [Google Scholar]
- 107.Whitson BA, Leslie DB, Kellogg TA, Maddaus MA, Buchwald H, Billington CJ, et al. Entero-endocrine changes after gastric bypass in diabetic and nondiabetic patients: a preliminary study. J Surg Res. 2007;141:31–9. doi: 10.1016/j.jss.2007.02.022. [DOI] [PubMed] [Google Scholar]
- 108.Garcia-Fuentes E, Garrido-Sanchez L, Garcia-Almeida JM, Garcia-Arnes J, Gallego-Perales JL, Rivas-Marin J, et al. Different effect of laparoscopic Roux-en-Y gastric bypass and open biliopancreatic diversion of scopinaro on serum PYY and ghrelin levels. Obes Surg. 2008;18:1424–9. doi: 10.1007/s11695-008-9560-5. [DOI] [PubMed] [Google Scholar]
- 109.Sundbom M, Holdstock C, Engstrom BE, Karlsson FA. Early changes in ghrelin following Roux-en-Y gastric bypass: influence of vagal nerve functionality? Obes Surg. 2007;17:304–10. doi: 10.1007/s11695-007-9056-8. [DOI] [PubMed] [Google Scholar]
- 110.Pardina E, Lopez-Tejero MD, Llamas R, Catalan R, Galard R, Allende H, et al. Ghrelin and apolipoprotein AIV levels show opposite trends to leptin levels during weight loss in morbidly obese patients. Obes Surg. 2009;19:1414–23. doi: 10.1007/s11695-008-9793-3. [DOI] [PubMed] [Google Scholar]
- 111.Vendrell J, Broch M, Vilarrasa N, Molina A, Gomez JM, Gutierrez C, et al. Resistin, adiponectin, ghrelin, leptin, and proinflammatory cytokines: relationships in obesity. Obes Res. 2004;12:962–71. doi: 10.1038/oby.2004.118. [DOI] [PubMed] [Google Scholar]
- 112.Garcia-Unzueta MT, Fernandez-Santiago R, Dominguez-Diez A, Vazquez-Salvi L, Fernandez-Escalante JC, Amado JA. Fasting plasma ghrelin levels increase progressively after biliopancreatic diversion: one-year follow-up. Obes Surg. 2005;15:187–90. doi: 10.1381/0960892053268453. [DOI] [PubMed] [Google Scholar]
- 113.Adami GF, Cordera R, Andraghetti G, Camerini GB, Marinari GM, Scopinaro N. Changes in serum ghrelin concentration following biliopancreatic diversion for obesity. Obes Res. 2004;12:684–7. doi: 10.1038/oby.2004.79. [DOI] [PubMed] [Google Scholar]
- 114.Valera-Mora ME, Manco MCE, Guidone C, Iaconelli A, Gniuli D, Rosa G, et al. Growth hormone and ghrelin secretion in severely obese women before and after bariatric surgery. Obesity (Silver Spring) 2007;15:2012–8. doi: 10.1038/oby.2007.240. [DOI] [PubMed] [Google Scholar]
- 115.Faraj M, Havel PJ, Phelis S, Blank D, Sniderman AD, Cianflone K. Plasma acylation-stimulating protein, adiponectin, leptin, and ghrelin before and after weight loss induced by gastric bypass surgery in morbidly obese subjects. J Clin Endocrinol Metab. 2003;88:1594–602. doi: 10.1210/jc.2002-021309. [DOI] [PubMed] [Google Scholar]
- 116.Cummings DE, Clement K, Purnell JQ, Vaisse C, Foster KE, Frayo RS, et al. Elevated plasma ghrelin levels in Prader Willi syndrome. Nat Med. 2002;8:643–4. doi: 10.1038/nm0702-643. [DOI] [PubMed] [Google Scholar]
- 117.Holdstock C, Engstrom BE, Ohrvall M, Lind L, Sundbom M, Karlsson FA. Ghrelin and adipose tissue regulatory peptides: effect of gastric bypass surgery in obese humans. J Clin Endocrinol Metab. 2003;88:3177–83. doi: 10.1210/jc.2002-021734. [DOI] [PubMed] [Google Scholar]
- 118.Thaler JP, Cummings DE. Minireview: hormonal and metabolic mechanisms of diabetes remission after gastrointestinal surgery. Endocrinology. 2009;150:2518–25. doi: 10.1210/en.2009-0367. [DOI] [PubMed] [Google Scholar]
- 119.le Roux CW, Neary NM, Halsey TJ, Small CJ, Martinez-Isla AM, Ghatei MA, et al. Ghrelin does not stimulate food intake in patients with surgical procedures involving vagotomy. J Clin Endocrinol Metab. 2005;90:4521–4. doi: 10.1210/jc.2004-2537. [DOI] [PubMed] [Google Scholar]
- 120.Cummings DE, Overduin J, Shannon MH, Foster-Schubert KE. Hormonal mechanisms of weight loss and diabetes resolution after bariatric surgery. Surg Obes Relat Dis. 2005;1:358–68. doi: 10.1016/j.soard.2005.03.208. [DOI] [PubMed] [Google Scholar]
- 121.Reinehr T, Roth CL, Schernthaner GH, Kopp HP, Kriwanek S, Schernthaner G. Peptide YY and glucagon-like peptide-1 in morbidly obese patients before and after surgically induced weight loss. Obes Surg. 2007;17:1571–7. doi: 10.1007/s11695-007-9323-8. [DOI] [PubMed] [Google Scholar]
- 122.DePaula AL, Macedo AL, Schraibman V, Mota BR, Vencio S. Hormonal evaluation following laparoscopic treatment of type 2 diabetes mellitus patients with BMI 20–34. Surg Endosc. 2009;23:1724–32. doi: 10.1007/s00464-008-0168-6. [DOI] [PubMed] [Google Scholar]
- 123.Alvarez Bartolome M, Borque M, Martinez-Sarmiento J, Aparicio E, Hernandez C, Cabrerizo L, et al. Peptide YY secretion in morbidly obese patients before and after vertical banded gastroplasty. Obes Surg. 2002;12:324–7. doi: 10.1381/096089202321088084. [DOI] [PubMed] [Google Scholar]
- 124.Morinigo R, Moize V, Musri M, Lacy AM, Navarro S, Marin JL, et al. Glucagon-like peptide-1, peptide YY, hunger, and satiety after gastric bypass surgery in morbidly obese subjects. J Clin Endocrinol Metab. 2006;91:1735–40. doi: 10.1210/jc.2005-0904. [DOI] [PubMed] [Google Scholar]
- 125.le Roux CW, Aylwin SJ, Batterham RL, Borg CM, Coyle F, Prasad V, et al. Gut hormone profiles following bariatric surgery favor an anorectic state, facilitate weight loss, and improve metabolic parameters. Ann Surg. 2006;243:108–14. doi: 10.1097/01.sla.0000183349.16877.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Korner J, Bessler M, Cirilo LJ, Conwell IM, Daud A, Restuccia NL, et al. Effects of Roux-en-Y gastric bypass surgery on fasting and postprandial concentrations of plasma ghrelin, peptide YY, and insulin. J Clin Endocrinol Metab. 2005;90:359–65. doi: 10.1210/jc.2004-1076. [DOI] [PubMed] [Google Scholar]
- 127.Borg CM, le Roux CW, Ghatei MA, Bloom SR, Patel AG, Aylwin SJ. Progressive rise in gut hormone levels after Roux-en-Y gastric bypass suggests gut adaptation and explains altered satiety. Br J Surg. 2006;93:210–5. doi: 10.1002/bjs.5227. [DOI] [PubMed] [Google Scholar]
- 128.Chan JL, Mun EC, Stoyneva V, Mantzoros CS, Goldfine AB. Peptide YY levels are elevated after gastric bypass surgery. Obesity (Silver Spring) 2006;14:194–8. doi: 10.1038/oby.2006.25. [DOI] [PubMed] [Google Scholar]
- 129.Riediger T, Bothe C, Becskei C, Lutz TA. Peptide YY directly inhibits ghrelin-activated neurons of the arcuate nucleus and reverses fasting-induced c-Fos expression. Neuroendocrinology. 2004;79:317–26. doi: 10.1159/000079842. [DOI] [PubMed] [Google Scholar]
- 130.Valverde I, Puente J, Martin-Duce A, Molina L, Lozano O, Sancho V, et al. Changes in glucagon-like peptide-1 (GLP-1) secretion after biliopancreatic diversion or vertical banded gastroplasty in obese subjects. Obes Surg. 2005;15:387–97. doi: 10.1381/0960892053576613. [DOI] [PubMed] [Google Scholar]
- 131.Laferrere B, Teixeira J, McGinty J, Tran H, Egger JR, Colarusso A, et al. Effect of weight loss by gastric bypass surgery versus hypocaloric diet on glucose and incretin levels in patients with type 2 diabetes. J Clin Endocrinol Metab. 2008;93:2479–85. doi: 10.1210/jc.2007-2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Guidone C, Manco M, Valera-Mora E, Iaconelli A, Gniuli D, Mari A, et al. Mechanisms of recovery from type 2 diabetes after malabsorptive bariatric surgery. Diabetes. 2006;55:2025–31. doi: 10.2337/db06-0068. [DOI] [PubMed] [Google Scholar]
- 133.Salinari S, Bertuzzi A, Asnaghi S, Guidone C, Manco M, Mingrone G. First-phase insulin secretion restoration and differential response to glucose load depending on the route of administration in type 2 diabetic subjects after bariatric surgery. Diabetes Care. 2009;32:375–80. doi: 10.2337/dc08-1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Bose M, Olivan B, Teixeira J, Pi-Sunyer FX, Laferrere B. Do Incretins play a role in the remission of type 2 diabetes after gastric bypass surgery: What are the evidence? Obes Surg. 2009;19:217–29. doi: 10.1007/s11695-008-9696-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.de Carvalho CP, Marin DM, de Souza AL, Pareja JC, Chaim EA, de Barros Mazon S, et al. GLP-1 and adiponectin: effect of weight loss after dietary restriction and gastric bypass in morbidly obese patients with normal and abnormal glucose metabolism. Obes Surg. 2009;19:313–20. doi: 10.1007/s11695-008-9678-5. [DOI] [PubMed] [Google Scholar]
- 136.Kellum JM, Kuemmerle JF, O’Dorisio TM, Rayford P, Martin D, Engle K, et al. Gastrointestinal hormone responses to meals before and after gastric bypass and vertical banded gastroplasty. Ann Surg. 1990;211:763–70. doi: 10.1097/00000658-199006000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Konturek JW. Cholecystokinin in the control of gastric acid and plasma gastrin and somatostatin secretion in healthy subjects and duodenal ulcer patients before and after eradication of Helicobacter pylori. J Physiol Pharmacol. 1994;45:3–66. [PubMed] [Google Scholar]
- 138.Katsusuke S, Takeuchi T, Wantabe S, Nishiwaki H. Postprandial plasma cholecystokinin response in patients after gastrectomy and pancreatoduodenectomy. Am J Gastroenterol. 2008;81:1038–42. [PubMed] [Google Scholar]
- 139.Chi MM, Fan G, Fox EA. Increased short-term food satiation and sensitivity to cholecystokinin in neurotrophin-4 knock-in mice. Am J Physiol Regul Integr Comp Physiol. 2004;287:R1044–53. doi: 10.1152/ajpregu.00420.2004. [DOI] [PubMed] [Google Scholar]
- 140.Baskin DG, Figlewicz Lattemann D, Seeley RJ, Woods SC, Porte D, Jr, Schwartz MW. Insulin and leptin: dual adiposity signals to the brain for the regulation of food intake and body weight. Brain Res. 1999;848:114–23. doi: 10.1016/s0006-8993(99)01974-5. [DOI] [PubMed] [Google Scholar]
- 141.Wang L, Barachina MD, Martinez V, Wei JY, Tache Y. Synergistic interaction between CCK and leptin to regulate food intake. Regul Pept. 2000;92:79–85. doi: 10.1016/s0167-0115(00)00153-1. [DOI] [PubMed] [Google Scholar]
- 142.Barrachina MD, Martinez V, Wang L, Wei JY, Tache Y. Synergistic interaction between leptin and cholecystokinin to reduce short-term food intake in lean mice. Proc Natl Acad Sci U S A. 1997;94:10455–60. doi: 10.1073/pnas.94.19.10455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Foschi D, Corsi F, Pisoni L, Vago T, Bevilacqua M, Trabucchi A, et al. Plasma leptin levels after vertical banded gastroplasty for morbid obesity: effects of an acidified meal. Obes Surg. 2003;13:874–8. doi: 10.1381/096089203322618696. [DOI] [PubMed] [Google Scholar]
- 144.Kotidis EV, Koliakos G, Papavramidis TS, Papavramidis ST. The effect of biliopancreatic diversion with pylorus-preserving sleeve gastrectomy and duodenal switch on fasting serum ghrelin, leptin and adiponectin levels: is there a hormonal contribution to the weight-reducing effect of this procedure? Obes Surg. 2006;16:554–9. doi: 10.1381/096089206776944940. [DOI] [PubMed] [Google Scholar]
- 145.Kotidis EV, Koliakos GG, Baltzopoulos VG, Ioannidis KN, Yovos JG, Papavramidis ST. Serum ghrelin, leptin and adiponectin levels before and after weight loss: comparison of three methods of treatment—a prospective study. Obes Surg. 2006;16:1425–32. doi: 10.1381/096089206778870058. [DOI] [PubMed] [Google Scholar]
- 146.Rubino F, Gagner M, Gentileschi P, Kini S, Fukuyama S, Feng J, et al. The early effect of the Roux-en-Y gastric bypass on hormones involved in body weight regulation and glucose metabolism. Ann Surg. 2004;240:236–42. doi: 10.1097/01.sla.0000133117.12646.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Dixon AF, Dixon JB, O’Brien PE. Laparoscopic adjustable gastric banding induces prolonged satiety: a randomized blind crossover study. J Clin Endocrinol Metab. 2005;90:813–9. doi: 10.1210/jc.2004-1546. [DOI] [PubMed] [Google Scholar]
- 148.Lee WJ, Lee YC, Ser KH, Chen JC, Chen SC. Improvement of insulin resistance after obesity surgery: a comparison of gastric banding and bypass procedures. Obes Surg. 2008;18:1119–25. doi: 10.1007/s11695-008-9457-3. [DOI] [PubMed] [Google Scholar]
- 149.Ballantyne GH, Farkas D, Laker S, Wasielewski A. Short-term changes in insulin resistance following weight loss surgery for morbid obesity: laparoscopic adjustable gastric banding versus laparoscopic Roux-en-Y gastric bypass. Obes Surg. 2006;16:1189–97. doi: 10.1381/096089206778392158. [DOI] [PubMed] [Google Scholar]
- 150.Morrow J, Gluck M, Lorence M, Flancbaum L, Geliebter A. Night eating status and influence on body weight, body image, hunger, and cortisol pre- and post-Roux-en-Y Gastric Bypass (RYGB) surgery. Eat Weight Disord. 2008;13:e96–9. doi: 10.1007/BF03327512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Gautier JF, Chen K, Salbe AD, Bandy D, Pratley RE, Heiman M, et al. Differential brain responses to satiation in obese and lean men. Diabetes. 2000;49:838–46. doi: 10.2337/diabetes.49.5.838. [DOI] [PubMed] [Google Scholar]
- 152.Karhunen LJ, Vanninen EJ, Kuikka JT, Lappalainen RI, Tiihonen J, Uusitupa MI. Regional cerebral blood flow during exposure to food in obese binge eating women. Psychiatry Res. 2000;99:29–42. doi: 10.1016/s0925-4927(00)00053-6. [DOI] [PubMed] [Google Scholar]
- 153.Stice E, Spoor S, Ng J, Zald DH. Relation of obesity to consummatory and anticipatory food reward. Physiol Behav. 2009;97:551–60. doi: 10.1016/j.physbeh.2009.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.DelParigi A, Chen K, Salbe AD, Hill JO, Wing RR, Reiman EM, et al. Successful dieters have increased neural activity in cortical areas involved in the control of behavior. Int J Obes (Lond) 2007;31:440–8. doi: 10.1038/sj.ijo.0803431. [DOI] [PubMed] [Google Scholar]
- 155.Steele KE, Prokopowicz GP, Schweitzer MA, Magunsuon TH, Lidor AO, Kuwabawa H, et al. Alterations of central dopamine receptors before and after gastric bypass surgery. Obes Surg. 2007:369–74. doi: 10.1007/s11695-009-0015-4. [DOI] [PubMed] [Google Scholar]
- 156.Ochner CN, Kwok Y, Conceicao E, Pantazatos SP, Puma LM, Carnell S, et al. Selective reducation of neural responses to high calorie foods following gastric bypass surgery. Under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Lowe MR, Davis W, Lucks D, Annunziato R, Butryn M. Weight suppression predicts weight gain during inpatient treatment of bulimia nervosa. Physiol Behav. 2006;87:487–92. doi: 10.1016/j.physbeh.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 158.Carter FA, McIntosh VV, Joyce PR, Bulik CM. Weight suppression predicts weight gain over treatment but not treatment completion or outcome in bulimia nervosa. J Abnorm Psychol. 2008;117:936–40. doi: 10.1037/a0013942. [DOI] [PubMed] [Google Scholar]
- 159.Cornier MA, Von Kaenel SS, Bessesen DH, Tregellas JR. Effects of overfeeding on the neuronal response to visual food cues. Am J Clin Nutr. 2007;86:965–71. doi: 10.1093/ajcn/86.4.965. [DOI] [PubMed] [Google Scholar]
- 160.Cornier MA. The effects of overfeeding and propensity to weight gain on the neuronal responses to visual food cues. Physiol Behav. 2009;97:525–30. doi: 10.1016/j.physbeh.2009.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Rao BS, Prabhakar E. Effects of body weight loss and taste on VMH-LH electrical activity of rats. Physiol Behav. 1992;52:1187–92. doi: 10.1016/0031-9384(92)90480-p. [DOI] [PubMed] [Google Scholar]
- 162.Schur EA, Kleinhans NM, Goldberg J, Buchwald D, Schwartz MW, Maravilla K. Activation in brain energy regulation and reward centers by food cues varies with choice of visual stimulus. Int J Obes (Lond) 2009;33:653–61. doi: 10.1038/ijo.2009.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Gautier JF, Del Parigi A, Chen K, Salbe AD, Bandy D, Pratley RE, et al. Effect of satiation on brain activity in obese and lean women. Obes Res. 2001;9:676–84. doi: 10.1038/oby.2001.92. [DOI] [PubMed] [Google Scholar]
- 164.McBride D, Barrett SP, Kelly JT, Aw A, Dagher A. Effects of expectancy and abstinence on the neural response to smoking cues in cigarette smokers: an fMRI study. Neuropsychopharmacology. 2006;31:2728–38. doi: 10.1038/sj.npp.1301075. [DOI] [PubMed] [Google Scholar]
- 165.Batsis JA, Sarr MG, Collazo-Clavell ML, Thomas RJ, Romero-Corral A, Somers VK, et al. Cardiovascular risk after bariatric surgery for obesity. Am J Cardiol. 2008;102:930–7. doi: 10.1016/j.amjcard.2008.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Mathier MA, Ramanathan RC. Impact of obesity and bariatric surgery on cardiovascular disease. Med Clin North Am. 2007;91:415–31. x–xi. doi: 10.1016/j.mcna.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 167.Cummings DE, Overduin J, Foster-Schubert KE. Gastric bypass for obesity: mechanisms of weight loss and diabetes resolution. J Clin Endocrinol Metab. 2004;89:2608–15. doi: 10.1210/jc.2004-0433. [DOI] [PubMed] [Google Scholar]
- 168.Orlando FA, Goncalves CG, George ZM, Halverson JD, Cunningham PR, Meguid MM. Neurohormonal pathways regulating food intake and changes after Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2005;1:486–95. doi: 10.1016/j.soard.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 169.Barton C, Lin L, York DA, Bray GA. Differential effects of enterostatin, galanin and opioids on high-fat diet consumption. Brain Res. 1995;702:55–60. doi: 10.1016/0006-8993(95)00966-8. [DOI] [PubMed] [Google Scholar]
- 170.Ferrari F, Pelloni F, Giuliani D. B-HT 920-induced effects on rat feeding behaviour. Pharmacol Res. 1992;26:285–92. doi: 10.1016/1043-6618(92)90217-y. [DOI] [PubMed] [Google Scholar]
- 171.Mun EC, Blackburn GL, Matthews JB. Current status of medical and surgical therapy for obesity. Gastroenterology. 2001;120:669–81. doi: 10.1053/gast.2001.22430. [DOI] [PubMed] [Google Scholar]