We established a respiratory syncytial virus (RSV)-infected model in polarized normal human nasal epithelial cells and found that the replication of RSV and the epithelial cell responses including induction of tight junctions were regulated via a protein kinase C δ/hypoxia-inducible factor-1α/nuclear factor-κβ pathway. The control of this pathway may be useful in therapy for RSV-induced respiratory pathogenesis.
Abstract
Respiratory syncytial virus (RSV) is the major cause of bronchitis, asthma, and severe lower respiratory tract disease in infants and young children. The airway epithelium, which has a well-developed barrier regulated by tight junctions, is the first line of defense during respiratory virus infection. In upper airway human nasal epithelial cells (HNECs), however, the primary site of RSV infection, the mechanisms of replication and budding of RSV, and the epithelial cell responses, including the tight junctional barrier, remain unknown. To investigate the detailed mechanisms of replication and budding of RSV in HNECs and the epithelial cell responses, we established an RSV-infected model using human telomerase reverse transcriptase–-transfected HNECs. We first found that the expression and barrier function of tight junction molecules claudin-4 and occludin were markedly induced together with production of proinflammatory cytokines interleukin 8 and tumor necrosis factor-α in HNECs after RSV infection, and the induction of tight junction molecules possibly contributed to budding of RSV. Furthermore, the replication and budding of RSV and the epithelial cell responses in HNECs were regulated via a protein kinase C δ/hypoxia-inducible factor-1α/nuclear factor-κB pathway. The control of this pathway in HNECs may be useful not only for prevention of replication and budding of RSV, but also in therapy for RSV-induced respiratory pathogenesis.
INTRODUCTION
Respiratory syncytial virus (RSV) is a negative-stranded RNA virus in the genus Pneumovirus, family Paramyxoviridae and is the major cause of bronchitis, asthma, and severe lower respiratory tract disease in infants and young children (Bitko and Barik, 1998). The envelope of RSV contains three transmembrane surface proteins, the fusion glycoprotein (F protein), G glycoprotein (G protein), and SH protein. F protein is responsible for fusion of the viral envelope with the plasma membrane of the host target cell, and G protein mediates attachment of the virus particle to the target cell (Levine et al., 1987; Collins et al., 1991). Furthermore, during virus assembly in RSV-infected cells, virus filaments at the cell surface and inclusion bodies in the cytoplasm, which are caused by close physical interaction between the filamentous actin (F-actin) and the virus, were observed by ultrastructural analysis (Jeffree et al., 2007).
RSV infection is limited to the most superficial layer of polarized, ciliated cells in the respiratory tract epithelium, entering through the apical surface (Zhang et al., 2002; Wright et al., 2005). Late steps of the RSV life cycle include assembly and budding of the virus, which also occur at the apical membrane in polarized cells (Roberts et al., 1995). RSV replicates in the airway mucosa, where it may produce uncomplicated upper respiratory infection or spread distally to the lower airways, producing more severe lower respiratory tract infection. The mechanisms of entry, replication, and budding of RSV, however, are still unclear in the upper airway, including in the nasal mucosa.
Many signaling molecules are considered major players in RSV-induced respiratory pathogenesis (Tregoning et al., 2010). RSV activates multiple signaling pathways, including those involving protein kinase C (PKC), mitogen-activated protein kinase (MAPK), and nuclear factor-κB (NF-κB) (Bitko et al., 1997; Bitko and Barik, 1998; Chen et al., 2000; Gower et al., 2001). Activation of PKC plays a role in the early stages of RSV infection (Monick et al., 2001). RSV causes hypoxia-inducible factor-1α (HIF-1α) stabilization, which is important in inflammation and edema formation (Kilani et al., 2004). Furthermore, proinflammatory cytokines and chemokines induced by RSV are regulated via an NF-κB pathway (Yoboua et al., 2010). Therefore, we hypothesized that the PKCδ/HIF-1α/NF-κB pathway might play an important role in RSV-induced respiratory pathogenesis.
The airway epithelium is the first line of defense during respiratory virus infection (Holt et al., 2008). The epithelial barrier is regulated in large part by the apicalmost intercellular junctions, referred to as tight junctions (Schneeberger et al., 1992). Furthermore, tight junctions also separate the apical from the basolateral cell surface domains to establish epithelial cell polarity (Tsukita and Furuse, 1998). Tight junctions are formed by not only the integral membrane proteins claudins, occludin, tricellulin, JAMs (junctional adhesion molecules), and CAR (coxsackie and adenovirus receptor), but also many peripheral membrane proteins, including scaffold PDZ-expression proteins and cell polarity molecules (Tsukita et al., 2001; Sawada et al., 2003; Schneeberger and Lynch, 2004; Ikenouchi et al., 2005). Moreover, it is also known that tight junctions include targets or receptors of viruses and bacteria such as claudin-1 and occludin as coreceptors of hepatitis C virus (HCV), JAM as a reovirus receptor, CAR, and some claudins as Clostridium perfringens enterotoxin receptors (Guttman and Finlay, 2009).
We previously reported that, in human nasal epithelial cells (HNECs) in vivo and in vitro, occludin, JAM-A, ZO-1, ZO-2, claudin-1, -4, -7, -8, -12, -13, -14, and tricellulin were detected together with well-developed tight junction strands (Takano et al., 2005; Kurose et al., 2007; Ohkuni et al., 2009). Furthermore, the human telomerase reverse transcriptase (hTERT)-transfected HNECs with an extended life span that we previously established can be used as an indispensable and stable model for studying the regulation of tight junction proteins in human nasal epithelium (Kuruse et al., 2007; Ohkuni et al., 2009; Kamekura et al., 2010; Ogasawara et al., 2010). In TERT-transfected HNECs, treatment with transforming growth factor-β1 (TGF-β1) markedly induces claudin-4 expression (Kurose et al., 2007). It is possible that tight junctions in HNECs may be useful new molecular targets for defense against respiratory virus infection.
In this study, to investigate the detailed mechanisms of replication and budding of RSV in HNECs and the epithelial cell responses, including production of proinflammatory cytokines and induction of tight junctions, we established an RSV-infected model in HNECs using hTERT-transfected cells, and examined the expression of RSV/G and F proteins and virus filaments. We first found that the expression and function of tight junction molecules were markedly induced together with production of proinflammatory cytokines in HNECs after RSV infection, and the induction of tight junction molecules contributed to budding of RSV. Furthermore, the replication and budding of RSV and the epithelial cell responses in HNECs were regulated via a PKCδ/HIF-1α/NF-κB pathway. Our data provide new insights into the signaling mechanisms that contribute to RSV-induced respiratory pathogenesis.
RESULTS
RSV infects HNECs in vitro
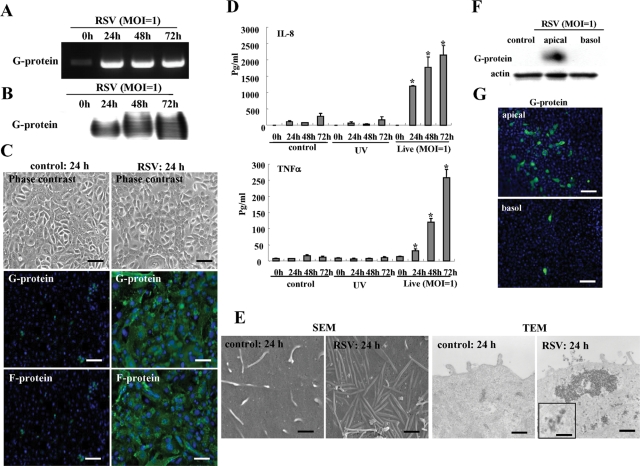
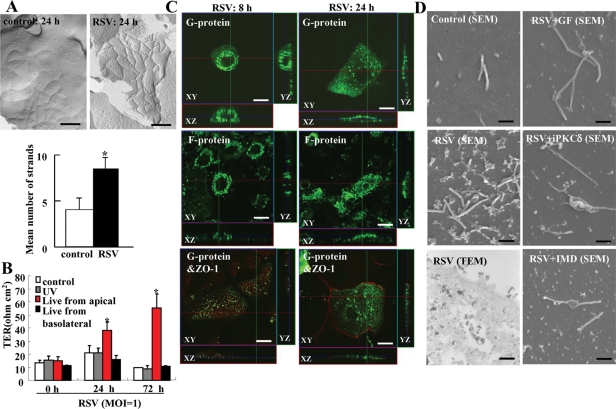
We quantified the susceptibility of HNECs in vitro to RSV infection by using hTERT-transfected HNECs. When the HNECs were infected with RSV at multiplicity of infection (MOI) 1, the mRNA and protein of RSV/G protein were markedly increased from 24 h (Figure 1, A and B). At 24 h after infection by RSV, the expression of RSV/G and F proteins was detected in most cells by immunocytochemistry using specific antibodies (Figure 1C). In an enzyme-linked immunosorbent assay (ELISA) of the medium, the levels of interleukin 8 (IL-8) and tumor necrosis factor-α (TNF-α) were significantly increased in the live virus group compared with the noninfected controls and the group with UV-inactivated RSV (Figure 1D).
FIGURE 1:
RSV-infected HNECs. RT–PCR (A) and Western blot (B) for RSV/G protein in HNECs infected with RSV at an MOI of 1. (C) Phase contrast and immunostaining for RSV/G and F proteins in HNECs at 24 h after infection with RSV at an MOI of 1. Black bar: 40 μM; white bar: 20 μM. (D) ELISA for IL-8 and TNF-α from HNECs infected with RSV at an MOI of 1 or with UV-treated RSV. UV: RSV was inactivated by exposing the virus to UV light at 1 J. Data are means ± SEM, *p < 0.01 compared with 0 h. (E) SEM image and TEM image of HNECs at 24 h after infection with RSV at an MOI of 1. SEM bars: 400 nm; TEM bars: 1 μm. Inset image: 100 nm. Western blot (F) and immunostaining (G) for RSV/G protein in HNECs infected with RSV at an MOI of 1 from apical or basolateral regions by using double-chamber dishes. Bars: 40 μM.
During RSV infection, it is thought that there is a close physical interaction between the filamentous actin and the virus, which is indicated by both the virus filaments and inclusion bodies (Jeffree et al., 2007). In the RSV-infected HNECs, many virus filaments were observed on the cell surface by scanning electron microscopy (SEM), and inclusion bodies were also detected at submembranes by transmission electron microscopy (TEM), but they were not detected in the control (Figure 1E).
To determine whether RSV infected from the apical or basolateral surfaces of HNECs, the cells were infected with RSV from apical or basolateral regions by using double chamber dishes. In Western blots, the RSV/G protein was detected only from the apical region (Figure 1F) and in immunocytochemistry, the expression of RSV/G from the apical group was greater than that from the basolateral group (Figure 1G). Thus HNECs were more susceptible to RSV infection from the apical surface than from the basolateral surface.
Gene expression changes in HNECs infected with RSV
We performed GeneChip analysis of HNECs infected with RSV, and selected gene probes that were up-regulated more than twofold compared with the controls (Table 1). In HNECs infected with RSV, up-regulation of IL-8 and TNF-α was confirmed together with a marked increase of the pattern recognition receptors RIG-I and MDA5. More interestingly, up-regulation of tight junction molecules claudin-2, -4, -7, -9, -14, and -19, occludin, ZO-2, cingulin, and MAGI-1 was observed in HNECs infected with RSV.
TABLE 1:
Gene probes that are up-regulated more than twofold to the control in RSV-infected hTERT-HNECs.
| Gene name | ID | Gene Bank ID | Fold change (>2.0) |
|---|---|---|---|
| CLDN2 | H200002539 | NM_020384 | 2.9 |
| CLDN4 | H300004950 | NM_001305 | 7.3 |
| CLDN7 | H200017305 | NM_001307 | 2.3 |
| CLDN9 | H200009827 | NM_020982 | 3.0 |
| CLDN14 | H200016568 | NM_144492; NM_012130 | 2.8 |
| CLDN19 | H200009873 | NM_148960 | 2.6 |
| OCLN | opHsV0400004868 | NM_002538 | 2.3 |
| TJP2 (ZO-2) | H300020004 | NM_002538 | 4.2 |
| CGN (Cingulin) | H300009163 | 3.4 | |
| BAIAP1 (MAGI-1) | H300019020 | NM_004742 | 2.2 |
| TGFB1 | opHsV0400000071 | NM_002538 | 2.9 |
| PRKCD (PKCδ) | H200014060 | NM_212539; NM_006254 | 2.2 |
| HIF-1α | H300017074 | NM_181054; NM_001530 | 17.2 |
| VEGFC | H200006638 | NM_005429 | 6.3 |
| NT5E | H200013920 | NM_002526 | 6.9 |
| NFKBIA | H200006833 | NM_020529; NM_020529 | 4.1 |
| NFKBIE | H200010505 | NM_004556 | 6.7 |
| NFKBIZ | H300020290 | NM_031419; NM_001005474 | 3.9 |
| IL8 | opHsV0400000818 | NM_000584 | 3.2 |
| TNFA | H200015775 | NM_000594 | 6.0 |
| DDX58 (RIG1) | H200013521 | NM_014314 | 37.1 |
| IFIH1 (MDA5) | H200009565 | NM_022168 | 25.9 |
RSV infection induces tight junction protein claudin-4 in HNECs via a PKCδ/NF-κB signaling pathway
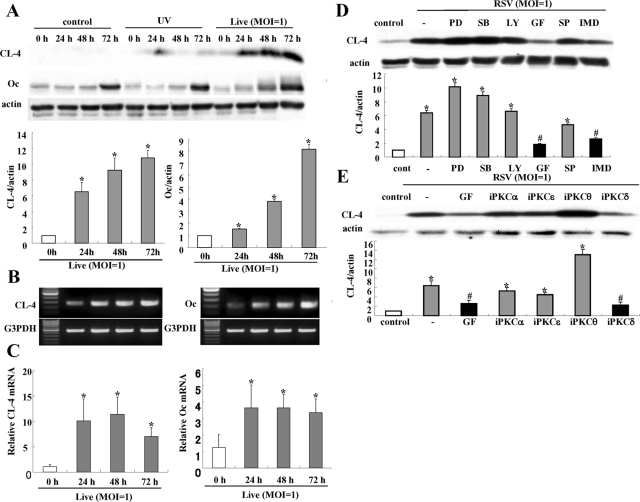
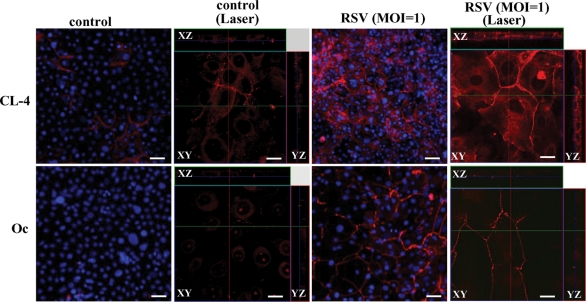
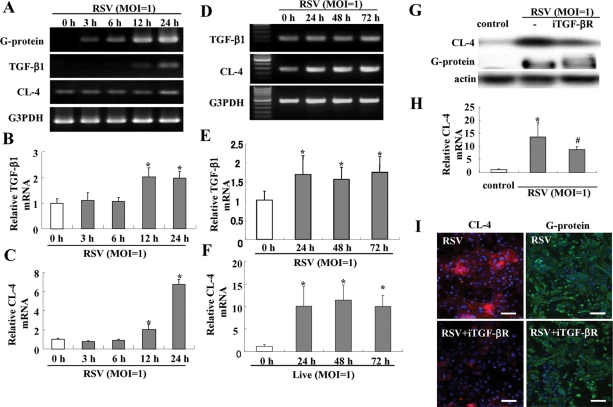
To confirm the up-regulation of tight junction proteins in HNECs infected with RSV, Western blotting, reverse transcription–PCR (RT–PCR), real-time PCR, and immunocytochemistry were performed. In Western blotting, the proteins of claudin-4 and occludin were increased gradually in a time-dependent manner after RSV infection (Figure 2A). In RT–PCR and real-time PCR, the mRNAs of claudin-4 and occludin were increased from 24 h after RSV infection, whereas no changes were observed in the non-infected control and the group with UV-inactivated RSV (Figure 2, B and C; Supplemental Figure 1). In immunocytochemistry at 24 h after RSV infection, claudin-4 and occludin were increased at the membranes of HNECs, compared with the control (Figure 3).
FIGURE 2:
Induction of claudin-4 and occludin in HNECs infected with RSV. (A) Western blot for claudin-4 and occludin in HNECs infected with RSV at an MOI of 1 or with UV-treated RSV. The corresponding expression levels of (A) are shown as bar graphs. Data are means ± SEM, *p < 0.01 compared with 0 h. RT–PCR (B) and real-time PCR (C) for claudin-4 and occludin in HNECs infected with RSV at an MOI of 1. Data are means ± SEM, *p < 0.01 compared with 0 h. (D) Western blot for claudin-4 in HNECs pretreated with inhibitors of signaling pathways 30 min before infection with RSV at an MOI of 1 for 24 h. The corresponding expression levels of (D) are shown as bar graphs. Data are means ± SEM, *p < 0.01 compared with control, #p < 0.01 compared with RSV. PD: MAPK inhibitor PD98059; SB: p38 MAPK inhibitor SB203580; LY: PI3K inhibitor LY294002; GF: pan-PKC inhibitor GF109203X; SP: JNK inhibitor SP600125; IMD: NF-κB inhibitor IMD-0354. (E) Western blot for claudin-4 in HNECs pretreated with inhibitors of PKC isoforms 30 min before infection with RSV at an MOI of 1 for 24 h. GF: pan-PKC inhibitor GF109203X; iPKCα: PKCα inhibitor Gö6976; iPKCδ: PKCδ inhibitor rottlerin; i PKCθ: PKCθ inhibitor myristoylated PKCθ pseudosubstrate peptide inhibitor; iPKCε: PKCε inhibitor PKCε translocation inhibitor. The corresponding expression levels of (E) are shown as bar graphs. Data are means ± SEM, *p < 0.01 compared with control, #p < 0.01 compared with RSV.
FIGURE 3:
Images of inverted microscope and confocal laser scanning microscope using immunostaining for claudin-4 and occludin in HNECs 24 h after infection with RSV at an MOI of 1.
In the control HNECs in vitro, claudin-1 and -7, ZO-1, JAM-A, and E-cadherin were also detected by Western blotting and immunocytochemistry (Kurose et al., 2007). In Western blotting, only claudin-1 was decreased at 72 h after RSV infection, whereas no change of claudin-7, ZO-1, JAM-A, or E-cadherin (Supplemental Figure 2) was observed. In immunocytochemistry at 24 h after RSV infection, ZO-1, JAM-A, and E-cadherin were increased at the membranes compared with the control (Supplemental Figure 3).
To investigate which signaling pathways were associated with induction of claudin-4 by RSV infection, HNECs were pretreated with MAPK inhibitor PD98059, p38 MAPK inhibitor SB203580, phosphoinositide 3-kinase (PI3K) inhibitor LY294002, pan-PKC inhibitor GF109203X, c-Jun N-terminal kinase (JNK) inhibitor SP600125, and NF-κB inhibitor IMD-0354 at 30 min before RSV infection. In Western blotting, the up-regulation of claudin-4 after RSV infection was inhibited by pan-PKC inhibitor GF109203X and NF-κB inhibitor IMD-0354 (Figure 2D).
As the up-regulation of claudin-4 after RSV infection was inhibited by the pan-PKC inhibitor, we investigated the effects of PKC isoforms. When HNECs were pretreated with PKCα inhibitor Gö6976, PKCδ inhibitor rottlerin, PKCθ inhibitor myristoylated PKCθ pseudosubstrate peptide inhibitor, or PKCε inhibitor PKCε translocation inhibitor peptide 30 min before RSV infection, PKCδ inhibitor rottlerin prevented up-regulation of claudin-4 at the same level as pan-PKC inhibitor GF109203X (Figure 2E).
PKCδ inhibitor and NF-κB inhibitor prevent replication of RSV, formation of virus filaments, and production of proinflammatory cytokines in HNECs after RSV infection
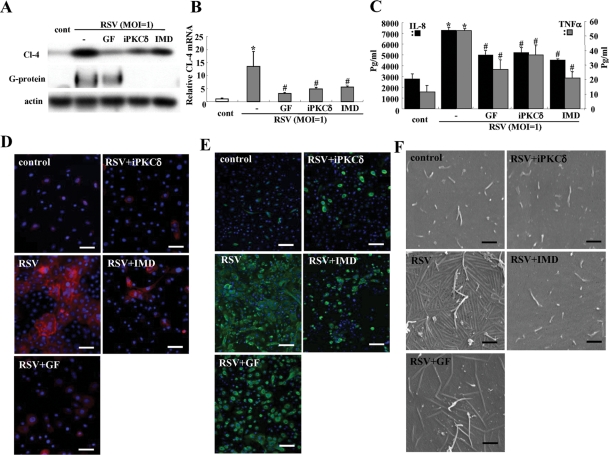
We investigated whether the PKCδ inhibitor and NF-κB inhibitor that prevented up-regulation of claudin-4 in HNECs after RSV infection affected replication of RSV, formation of virus filaments, and expression of proinflammatory cytokines.
In Western blotting and real-time PCR, the up-regulation of claudin-4 after RSV infection was inhibited by pan-PKC inhibitor GF109203X, PKCδ inhibitor rottlerin, and NF-κB inhibitor IMD-0354 (Figure 4, A and B). In Western blots, the expression of RSV/G protein, which indicated the replication of RSV, was inhibited by the PKCδ inhibitor and NF-κB inhibitor (Figure 4A). In the ELISA, the production of IL-8 and TNF-α from HNECs after RSV infection was also inhibited by the PKCδ inhibitor and the NF-κB inhibitor (Figure 4C). In immunocytochemistry, up-regulation of claudin-4 and expression of RSV/G protein after RSV infection were markedly inhibited by the pan-PKC inhibitor, PKCδ inhibitor, and NF-κB inhibitor (Figure 4, D and E). Furthermore, the formation of virus filaments after RSV infection was inhibited by the pan-PKC inhibitor, PKCδ inhibitor, and NF-κB inhibitor (Figure 4F). When we investigated the cytotoxicity of the inhibitors of pan-PKC, PKCδ, and NF-κB in HNEC cells after RSV infection, cytotoxicity was not observed at the concentration of all inhibitors (Supplemental Figure 4).
FIGURE 4:
Inhibitors of pan-PKC, PKCδ, and NF-κB prevent replication of RSV and the epithelial cell responses in HNECs infected with RSV. Western blot (A) for claudin-4 and RSV/G protein, real-time PCR (B) for claudin-4, ELISA (C) for IL-8 and TNF-α, immunostaining for claudin-4 (D) and RSV/G protein (E), and SEM images (F) of HNECs pretreated with inhibitors of pan-PKC, PKCδ, and NF-κB 30 min before infection with RSV at an MOI of 1 for 24 h. GF: pan-PKC inhibitor GF109203X; iPKCδ: PKCδ inhibitor rottlerin; IMD: NF-κB inhibitor IMD-0354. Data are means ± SEM, *p < 0.01 compared with control, #p < 0.01 compared with RSV. Bars of D and E: 40 μm, Bars of F: 1 μm.
RSV infection induces formation of tight junction strands and barrier function via up-regulation of claudin-4 in HNECs
To investigate whether RSV induced the formation of tight junction strands and barrier function of HNECs, freeze-fracture and transepithelial electrical resistance (TER) measurement were performed. In freeze-fracture replicas at 24 h after RSV infection, the mean number of tight junction strands was significantly increased compared with the control and well-developed networks formed by continuous lines of tight junction strands (Figure 5A). For the barrier function, TER values were significantly increased by RSV infection from apical regions of HNECs compared with the control and RSV infection from basolateral regions (Figure 5B).
FIGURE 5:
Structures and barrier function of tight junctions in HNECs infected with RSV. (A) Freeze-fracture replica of HNECs 24 h after infection with RSV at an MOI of 1. Bar: 200 nm. Morphometric analysis of tight junction strands of A is shown as a bar graph. *p < 0.01 compared with control. (B) Barrier function measured as TER in HNECs infected with RSV at an MOI of 1 from apical or basolateral regions by using double-chamber dishes or with UV-treated RSV. UV: RSV was inactivated by exposing the virus to UV light at 1 J. Data are means ± SEM, *p < 0.01 compared with 0 h. (C) Immunostaining for RSV/G and F proteins and ZO-1 in HNECs at 8 h and 24 h after infection with RSV at an MOI of 1. Bars: 10 μm. (D) SEM images and TEM image of HNECs pretreated with inhibitors of pan-PKC, PKCδ, and NF-κB at 30 min before infection with RSV at an MOI of 1 for 24 h. GF: pan-PKC inhibitor GF109203X; iPKCδ: PKCδ inhibitor rottlerin; IMD: NF-κB inhibitor IMD-0354. Bars of SEM: 1 μm; Bar of TEM: 400 nm.
PKCδ inhibitor and NF-κB inhibitor prevent budding of RSV from apical surface of HNECs
In immunocytochemistry at 24 h after RSV infection, RSV/G and /F proteins were detected at submembranes of the HNEC apical surface, whereas they were observed around the nuclei at 8 h after RSV infection (Figure 5C). When RSV/G and /F proteins were detected at submembranes of the HNEC apical surface at 24 h after RSV infection, tight junction protein ZO-1 was observed at cell borders (Figure 5C).
In SEM at 24 h after RSV infection, many small membranous substances were observed at the surfaces of HNECs together with cilia, whereas they were not detected in the control (Figure 5D). It is thought that they indicate budding of RSV, because in TEM at 24 h after RSV infection, a complex of electric, high-density particles is observed in the small membranous substances at the surface and in submembranes of HNECs (Figure 5D). Furthermore, pan-PKC inhibitor GF109203X, PKCδ inhibitor rottlerin, and NF-κB inhibitor IMD-0354 prevented the budding of RSV (Figure 5D).
RSV infection induces expression of claudin-4 but not RSV replication via production of TGF-β1 from HNECs
RSV infection induces the expression of TGF-β in epithelial A594 and PHBE cells (Gibbs et al., 2009). We previously reported that treatment with TGF-β could induce the expression of claudin-4 in HNECs (Kurose et al., 2007). Furthermore, in the present study, when we performed GeneChip analysis of HNECs infected with RSV, up-regulation of TGF-β1 and TGF-β receptor II was observed together with an increase of claudin-4 (Table 2).
TABLE 2:
Antibodies.
| Dilution | ||||
|---|---|---|---|---|
| Antibody | Type | IC | WB | Source |
| Claudin-1 | pAb | 1:1000 | Zymed Laboratories (San Francisco, CA) | |
| Claudin-4 | pAb | 1:100 | 1:1000 | Zymed Laboratories |
| Claudin-7 | pAb | 1:1000 | Zymed Laboratories | |
| Occludin | pAb | 1:100 | 1:1000 | Zymed Laboratories |
| JAM-A | pAb | 1:100 | 1:1000 | Zymed Laboratories |
| ZO-1 | pAb | 1:100 | 1:1000 | Zymed Laboratories |
| E-cadherin | mAb (36/E-cadherin) | 1:200 | 1:1000 | BD Biosciences (San Jose, CA) |
| RSV/G and /F proteins | mAb | 1:100 | 1:4000 | Tsutsumi et al., 1989 |
| HIF-1α | mAb | 1:1000 | Novus Biologicals (Littleton, CO) | |
| Phospho-PKCδ | pAb | 1:1000 | Cell Signaling Technology (Danvers, MA) | |
| PKCδ | mAb | 1:1000 | BD PharMingen (San Diego, CA) | |
| Phospho-pan-PKC | pAb | 1:1000 | Cell Signaling Technology | |
| Pan-PKC | pAb | 1:1000 | Santa Cruz Biotechnology (Santa Cruz, CA) | |
| Phospho-NF-κB | pAb | 1:1000 | Cell Signaling Technology | |
| NF-κB | pAb | 1:1000 | Cell Signaling Technology | |
| Actin | pAb | 1:1000 | Sigma-Aldrich | |
pAb, rabbit polyclonal antibody; mAb, mouse mAb.
We investigated whether up-regulation of claudin-4 in HNECs after RSV infection was caused via TGF-β signaling. In RT–PCR and real-time PCR after RSV infection, mRNAs of RSV/G protein, TGF-β1, and claudin-4 were significantly increased from 3 h, 12 h, and 24 h, respectively (Figure 6, A–C). The up-regulation of mRNAs of TGF-β1 and claudn-4 in HNECs after RSV infection was maintained from 24 h until 72 h, whereas no changes were observed in the non-infected control and the group with UV-inactivated RSV (Figure 6, D–F; Supplemental Figure 1). In Western blotting and real-time PCR, up-regulation of claudin-4 after RSV infection was inhibited by TGF-β receptor I kinase inhibitor (iTGF-βR; Figure 6, G and H), but expression of RSV/G protein, which indicated replication of RSV, was not inhibited by the iTGF-βR (Figure 6, G and H). In immunocytochemistry, the iTGF-βR prevented up-regulation of claudin-4 at the membranes but not expression of RSV/G protein after RSV infection (Figure 6I).
FIGURE 6:
Induction of TGF-β1 in HNECs infected with RSV. RT–PCR (A and D) and real-time PCR (B, C, E, and F) for RSV/G protein, TGF-β1, and claudin-4 in HNECs infected with RSV at an MOI of 1. Data are means ± SEM, *p < 0.01, compared with 0 h. Western blot (G) and real-time PCR (H) for claudin-4, and immunostaining (I) for claudin-4 and RSV/G protein in HNECs pretreated with iTGF-βR 30 min before infection with RSV at an MOI of 1 for 24 h. Data are means ± SEM, *p < 0.01 compared with control, #p < 0.01 compared with RSV. Bars: 40 μm.
Possible regulation of claudin-4 in HNECs after RSV infection via a TGF-β1/PKCδ/HIF-1α/NF-κB signaling pathway
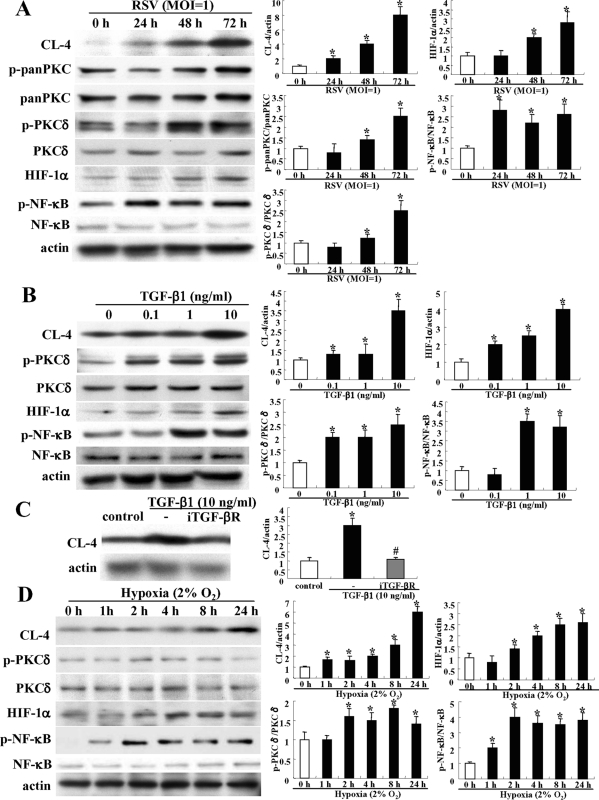
In GeneChip analysis of HNECs infected with RSV, up-regulation of PKCδ, HIF-1α, HIF-1α–associated genes VEGF and NT5, and NF-κB was observed (Table 1). Furthermore, in Western blotting, phospho-PKCδ, HIF-1α, and phospho-NF-κB were increased together with induction of claudin-4 after RSV infection (Figure 7A).
FIGURE 7:
Induction of claudin-4 in HNECs via a TGF-β/PKCδ/HIF-1α/NK-κB pathway. (A) Western blot for claudin-4, phospho-pan-PKC, phospho-PKCδ, HIF-1α, and phospho-NK-κB in HNECs infected with RSV at an MOI of 1. Western blot (B) for claudin-4, phospho-PKCδ, HIF-1α ,and phospho-NK-κB in HNECs at 24 h after treatment with 0.1–10 ng/ml TGF-β1. (C) Western blot for claudin-4 in HNECs pretreated with iTGF-βR 30 min before treatment with 10 ng/ml TGF-β1. Western blot (D) for claudin-4, phospho-PKCδ, and phospho-NK-κB in HNECs 24 h after treatment with hypoxia. The corresponding expression levels of (A–D) are shown as bar graphs. Data are means ± SEM. *p < 0.01 compared with control, #p < 0.01 compared with RSV.
For the mechanisms of the up-regulation of claudin-4 in HNECs after RSV infection, we hypothesized the possible regulation of claudin-4 via a TGF-β1/PKCδ/HIF-1α/NF-κB signaling pathway and confirmed it by using treatment with TGF-β1 and under hypoxia (2% O2).
When HNECs were treated with 0.1–10 ng/ml TGF-β1 for 24 h, up-regulation of claudin-4, phospho-PKCδ, HIF-1α, and phospho-NF-κB was increased from 0.1, 0.1, 0.1, and 1 ng/ml, respectively, in Western blotting (Figure 7B). In RT–PCR, HIF-1α mRNA was increased from 0.1 ng/ml TGF-β1 (Supplemental Figure 5). In Western blotting, up-regulation of claudin-4 after treatment with 10 ng/ml TGF-β was inhibited by iTGF-βR (Figure 7C).
When HNECs were incubated in a 5% CO2/2% O2 incubator balanced by nitrogen for 24 h, claudin-4, phospho-PKCδ, HIF-1α, and phospho-NF-κB were increased from 1, 2, 2, and 1 h after treatment with hypoxia, respectively, in Western blotting (Figure 7D). In RT–PCR, HIF-1α mRNA was increased from 2 h (Supplemental Figure 5).
Possible regulation of claudin-4 in HNECs after RSV infection and replication of RSV via a HIF-1α signaling pathway
To investigate whether HIF-1α directly affected claudin-4 expression and replication of RSV after the infection, HNECs were pretreated with three siRNAs of HIF-1α before RSV infection. Two siRNAs of HIF-1α slightly prevented up-regulation of claudin-4 and expression of RSV/G protein at 24 h after RSV infection in Western blotting (Figure 8A).
FIGURE 8:
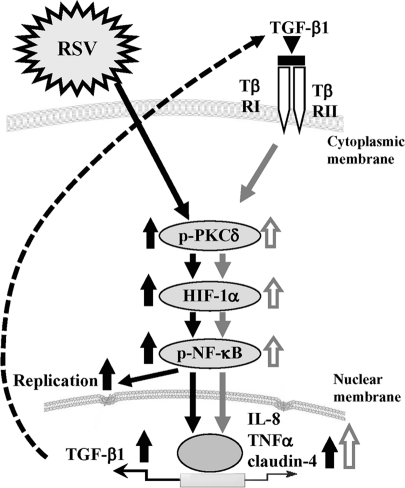
Overview of signal transduction events in HNECs infected with RSV.
DISCUSSION
In the present study, we established an RSV-infected model in HNECs using hTERT-transfected cells and to our knowledge first demonstrated that the replication and budding of RSV and the epithelial cell responses in HNECs were regulated via a PKCδ/HIF-1α/NF-κB pathway.
In the polarized cells of respiratory tract epithelium, RSV enters through the apical surface, and the assembly and budding of the virus occur at the apical membrane (Roberts et al., 1995; Zhang et al., 2002; Wright et al., 2005). In hTERT-transfected HNECs after RSV infection, RSV/G and F proteins were detected in most cells together with production of proinflammatory cytokines IL-8 and TNF-α. Furthermore, RSV entered through the apical surface of the HNEC, and the assembly and budding of the virus, indicated as virus filaments and many small membranous substances, also occurred at the apical membrane or submembrane. These results suggested that hTERT-transfected HNECs might function as an RSV-infected model for HNECs to investigate the regulation of replication and budding of the virus and the epithelial cell responses.
Some claudins are degraded during West Nile virus infection (Medigeshi et al., 2009). In polarized airway epithelial cells infected with rhinoviruses, TER is decreased together with the loss of ZO-1 (Sajjan et al., 2008). Infection with mouse adenovirus type 1 results in reduced expression and cell surface localization of tight junction proteins along with loss of barrier properties (Gralinski et al., 2009). The effects of RSV infection on tight junctions of upper airway HNECs remain known, however.
RSV infection alters the expression of adhesion molecules intercellular adhesion molecule 1 and E-cadherin in A549 cells (Wang et al., 2000). When we performed GeneChip analysis of HNECs infected with RSV compared with the control, we found dramatic up-regulation of tight junction molecules claudin-2, -4, -7, -9, -14, and -19, occludin, ZO-2, cingulin, and MAGI-1, together with up-regulation of proinflammatory cytokines IL-8 and TNF-α, as well as viral double-strand-RNA–induced pattern recognition receptors RIG-I and MAD5. In HNECs infected with live RSV, but not UV-treated RSV, up-regulation of claudin-4 and occludin was confirmed at the levels of protein and mRNA together with up-regulation of the tight junctional barrier function, whereas claudin-1 was decreased at 72 h after RSV infection. In immunocytochemistry at 24 h after RSV infection, not only claudin-4 and occludin but also ZO-1, JAM-A, and E-cadherin were increased at the membranes together with localization of RSV/G and /F proteins at submembranes of the apical surface. These results suggested that the tight junction molecules induced after RSV infection, which also play a crucial role in epithelial cell polarity, might contribute to the budding of the virus from the HNEC apical surface.
It is known that RSV activates multiple signaling pathways, including those involving PKC, MAPK, and NF-κB (Bitko et al., 1997; Bitko and Barik, 1998; Chen et al., 2000; Gower et al., 2001). Activation of PKC plays a role in the early stages of RSV infection (Monick et al., 2001). Previous studies have shown that PKC activation plays a role in the early stages of RSV infection (Sieczkarski et al., 2003) and RSV activates PKCδ at early time points after infection by the virus (Monick et al., 2001). RSV causes HIF-1α stabilization, which is important in inflammation and edema formation (Kilani et al., 2004). Furthermore, proinflammatory cytokines and chemokines induced by RSV are regulated via an NF-κB pathway (Yoboua et al., 2010). In the present study, in HNECs after RSV infection, up-regulation of phospho-PKCδ, HIF-1α, and phospho-NF-κB was observed by Western blotting. Upregulation of claudin-4 in HNECs after RSV infection was prevented by inhibitors of PKCδ and NF-κB. The inhibitors of PKCδ and NF-κB also prevented expression of RSV/G protein, the presence of virus filaments and small membranous substances at the apical membrane or submembrane, and production of proinflammatory cytokines after RSV infection. These results suggest that a PKCδ/HIF-1α/NF-κB pathway plays an important role in the replication and budding of RSV and the epithelial cell responses in HNECs.
RSV infection induces the expression of TGF-β in epithelial A594 and PHBE cells and causes cell-cycle arrest of lung epithelial cells through a TGF-β autocrine pathway (Gibbs et al., 2009). The TGF-β signaling pathway is mediated via PKCδ (Lee et al., 2005). Furthermore, we previously reported that treatment with TGF-β1 could induce claudin-4 expression at the protein and mRNA levels in HNECs (Kurose et al., 2007). We investigated whether TGF-β was closely associated with up-regulation of claudin-4 expression after RSV infection. In HNECs after RSV infection, an increase of TGF-β1 mRNA was observed by GeneChip analysis (Table 1). It was enhanced later than the presence of mRNA of RSV/G protein and earlier than the increase of claudin-4 mRNA, and was maintained, like claudin-4 mRNA, for a long time. iTGF-βR prevented up-regulation of claudin-4 protein but not the presence of RSV/G protein in HNECs after RSV infection. Treatment with TGF-β1 induced not only claudin-4 expression but also expression of HIF-1α, phospho-PKCδ, and phospho-NF-κB in HNECs. These findings indicated that up-regulation of claudin-4 after RSV infection was in part regulated by a TGF-β/PKCδ/HIF-1α/NF-κB pathway in a TGF-β autocrine manner.
PKCδ regulates the stability of HIF-1α under hypoxia (Lee et al., 2007). HIF-1α has been identified as a key regulator of the inflammatory transcription factor NF-κB (Walmsley et al., 2005). In the present study, GeneChip analysis of HNECs after RSV infection showed that expression of PKCδ, HIF-1α, and the HIF-1α-associated genes VEGF and NT5 was increased (Table 1). To investigate whether HIF-1α directly regulated expression of claudin-4 and the replication of RSV in HNECs after the virus infection, HNECs were incubated under hypoxia or treated with siRNAs of HIF-1α. In HNECs cultured under hypoxia, up-regulation of HIF-1α, claudin-4, phospho-PKCδ, and phospho-NF-κB was observed. In HNECs after RSV infection, siRNAs of HIF-1α prevented up-regulation of claudin-4 and the presence of RSV/G protein. These results suggested that HIF-1α directly regulated the replication of RSV and the epithelial cell response in HNECs.
Some tight junction molecules are also receptors of viruses. These receptors include JAM as a reovirus receptor, CAR, and claudin-1 and occludin as coreceptors of HCV (Cohen et al., 2001; Campbell et al., 2005; Evans et al., 2007; Guttman and Finlay, 2009; Ploss et al., 2009). We investigated the possibility that claudin-4 and occludin, which were induced after RSV infection, were receptors of RSV in HNECs. When HNECs were pretreated with siRNAs of claudin-4 or occludin, however, no changes of RSV/G protein, virus filaments, and many small membranous substances were observed in HNECs after RSV infection (Supplemental Figure 6, A and B). These findings indicate that claudin-4 and occludin are not receptors of RSV in HNECs and that there are other mechanisms of the budding of RSV.
In conclusion, we established an RSV-infected model in normal HNECs using hTERT-transfected cells and found that the replication and budding of RSV and the epithelial cell responses in HNECs were regulated via a PKCδ/HIF-1α/NF-κB pathway. In addition, it was thought that the PKCδ/HIF-1α/NF-κB pathway via TGF-β in an autocrine manner might also be important in the epithelial cell responses at the late stage after RSV infection (Figure 8). Inhibition of replication and budding of RSV in upper airway HNECs via these mechanisms might contribute to useful new preventive treatments for severe lower respiratory tract disease in infants and young children.
MATERIALS AND METHODS
Reagents and inhibitors
Recombinant human IL-8, TNF-α, and TGF-β1 were purchased from PeproTech EC (London, UK). A pan-PKC inhibitor (GF109203X), MAPK inhibitor (PD98059), p38 MAPK inhibitor (SB203580), PI3K inhibitor (LY294002), PKCα inhibitor (Gö6976), PKCδ inhibitor (rottlerin), PKCθ inhibitor (myristoylated PKCθ pseudosubstrate peptide inhibitor), PKCε inhibitor (PKCε translocation inhibitor peptide), and iTGF-βR were purchased from Calbiochem-Novabiochem (San Diego, CA). JNK inhibitor SP600125 and NF-κB inhibitor IMD-0354 were purchased from Sigma-Aldrich (St. Louis, MO). Alexa 488 (green)– and Alexa 594 (red)–conjugated anti–mouse and anti–rabbit immunoglobulin G (IgG) antibodies were purchased from Molecular Probes (Eugene, OR). Horseradish peroxidase–conjugated polyclonal goat anti–rabbit IgGs were purchased from Dako (Glostrup, Denmark). The enhanced chemiluminescence Western blotting system was obtained from GE Healthcare UK (Buckinghamshire, UK).
Cell culture and treatments
The cultured HNECs were derived from mucosal tissues of patients with hypertrophic rhinitis or chronic sinusitis who underwent inferior turbinectomy at Sapporo Medical University, the Sapporo Hospital of Hokkaido Railway Company, or the KKR Sapporo Medical Center Tonan Hospital. Informed consent was obtained from all patients, and this study was approved by the ethics committees of the above institutions.
The methods for primary culture of HNECs were as reported previously (Koizumi et al., 2008). Some primary cultured HNECs were transfected with the catalytic component of telomerase, the human catalytic subunit of the hTERT gene, as described previously (Kurose et al., 2007; Kamekura et al., 2009; Ohukuni et al., 2009, Ogasawara et al., 2010). The cells were plated on 35-mm or 60-mm culture dishes (Corning Glass Works, Corning, NY), which were coated with rat tail collagen (500 μg of dried tendon/ml 0.1% acetic acid). The cells were cultured in serum-free bronchial epithelial cell basal medium (Lonza Walkersville, Walkersville, MD) supplemented with bovine pituitary extract (1% vol/vol), 5 μg/ml insulin, 0.5 μg/ml hydrocortisone, 50 μg/ml gentamicin, 50 μg/ml amphotericin B, 0.1 ng/ml retinoic acid, 10 μg/ml transferrin, 6.5 μg/ml triiodothyronine, 0.5 μg/ml epinephrine, 0.5 ng/ml epidermal growth factor (Lonza Walkersville), 100 U/ml penicillin, and 100 μg/ml streptomycin (Sigma-Aldrich) and were incubated in a humidified, 5% CO2/95% air incubator at 37°C. In this experiment, second and third passaged cells were used.
Human RSV was grown in the human laryngeal carcinoma cell line HEp-2. For infection, HNECs at 80% confluence were adsorbed at an RSV MOI of 1 for 60 min at 37°C. After adsorption, the viral solutions were removed and the cells were rinsed twice with growth medium and incubated. The virus titers in the supernatant were determined by a plaque-forming assay with HEp-2 cells. Expression of RSV mRNA was confirmed by RT–PCR.
Some cells were treated with 0.1–10 μg/ml TGF-β1 or incubated in a 2% CO2/2% O2 incubator balanced by nitrogen. They were pretreated with 10 μM PD98059, 10 μM SB203580, 10 μM LY294002, 10 μM GF109203X, 10 μM SP600125, 0.1 μM IMD-0354, 5 μM PKCα inhibitor, 10 μM PKCε inhibitor, 5 μM PKCθ inhibitor, 5 μM PKCδ inhibitor, and iTGF-βR at 30 min before RSV infection or treatment with 10 ng/ml TGF-β1.
siRNA experiment
For knockdown of human HIF-1α, human claudin-4, and human occludin, Stealth Select RNAi against the genes was synthesized by Invitrogen (Carlsbad, CA). The sequences for the sense and antisense strands are shown in Table 3. The hTERT-transfected HNECs at 24 h after plating were transfected with 100 nM siRNA using Lipofectamine RNAiMAX Reagent (Invitrogen). At 48 h after transfection, the cells were infected with RSV for 24 h.
TABLE 3:
siRNAs.
| siRNA | Sense sequence | Antisense sequence |
|---|---|---|
| Claudin-4–siRNA1 | 5′-GCAACAUUGUCACCUCGCAGACCAU-3′ | 5′-AUGGUCUGCGAGGUGACAAUGUUGC-3′ |
| Claudin-4–siRNA2 | 5′-UCCUGUUGGCCGGCCUUAUGGUGAU-3′ | 5′-AUCACCAUAAGGCCGGCCAACAGGA-3′ |
| Occludin–siRNA1 | 5′-UGGAUGACUUCAGGCAGCCUCGUUA-3′ | 5′-UAACGAGGCUGCCUGAAGUCAUCCA-3′ |
| Occludin-siRNA2 | 5′-GGCCUCUUGAAAGUCCACCUCCUUA-3′ | 5′-UAAGGAGGUGGACUUUCAAGAGGCC-3′ |
RNA isolation, RT–PCR, and real-time PCR analysis
Total RNA was extracted and purified using TRIzol (Invitrogen). One microgram of total RNA was reverse-transcribed into cDNA using a mixture of oligo(dT) and Superscript II reverse transcriptase, according to the manufacturer's recommendations (Invitrogen). Synthesis of each cDNA was performed in a total volume of 20 μl for 50 min at 42°C and terminated by incubation for 15 min at 70°C. PCR was performed in a 20 μl total mixture containing 100 pM primer pairs, 1.0 μl of the 20-μl total RT product, PCR buffer, deoxyribonucleotides, and Taq DNA polymerase, according to the manufacturer's recommendations (Takara, Kyoto, Japan). Amplifications were for 25–35 cycles, depending on the PCR primer pair, with cycle times of 15 s at 96°C, 30 s at 55°C, and 60 s at 72°C. Final elongation time was 7 min at 72°C. Seven microliters of the total 20 μl of PCR product was analyzed by 1% agarose gel electrophoresis with ethidium bromide staining and was standardized using a GeneRuler TM 100BP DNA ladder (Fermentas, Ontario, Canada). To provide a quantitative control for reaction efficiency, PCRs were performed with primers coding for the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (G3PDH). Primers used to detect G3PDH and claudin-4, occludin, RSV G protein, TGF-β1, and HIF-1α are indicated in Table 4.
TABLE 4:
Primers of RT–PCR.
| Gene | Forward primer | Reverse primer | Product size (base pairs) |
|---|---|---|---|
| Claudin-4 | AGCCTTCCAGGTCCTCAACT | AGCAGCGAGTCGTACACCTT | 249 |
| Occludin | TCAGGGAATATCCACCTATCACTTCAG | CATCAGCAGCAGCCATGTACTCTTCAC | 189 |
| RSV/G protein | GGGGCAAATGCAAACATGT | GGTATTCTTTTGCATATAGC | 621 |
| HIF-1α | CAGAAGATACAAGTAGCCTC | CTGCTGGAATACTGTAACTG | 673 |
| TGF-β1 | CAGCAACAATTCCTGGCGATA | AAGGCGAAAGCCCTCAATTT | 135 |
| G3PDH | ACCACAGTCCATGCCATCAC | TCCACCACCCTGTTGCTGTA | 452 |
Real-time PCR detection was performed using a TaqMan Gene Expression Assay kit with a StepOnePlus real-time PCR system (Applied Biosystems, Foster City, CA). The amount of 18S rRNA (Hs99999901) mRNA in each sample was used to standardize the quantities of the following mRNAs: claudin-4 (Hs00533616), occludin (Hs00170162), TGF-β1 (Hs00998133), and HIF-1α (Hs00936366). The relative mRNA expression levels between the control and treated samples were calculated by the difference of the threshold cycle (comparative CT [ΔΔCT] method) and presented as the average of triplicate experiments with a 95% confidence interval.
ELISA
The concentrations of human IL-8 and TNF-α in cell culture supernatants of hTERT-transfected HNECs at 24–72 h after treatment with RSV were measured using ELISA kits for human IL-8 and TNF-α (R&D Systems, Minneapolis, MN), according to the manufacturer's instructions.
Western blot analysis
The hTERT-transfected HNECs were scraped from a 60-mm dish containing 300 μl of buffer (1 mM NaHCO3 and 2 mM phenylmethylsulfonyl fluoride), collected in microcentrifuge tubes, and then sonicated for 10 s. The protein concentrations of the samples were determined using a bicinchoninic acid protein assay reagent kit (Pierce Chemical Co., Rockford, IL). Aliquots of 15 μl of protein per lane for each sample were separated by electrophoresis in 4–20% SDS polyacrylamide gels (Daiichi Pure Chemicals, Tokyo, Japan) and electrophoretically transferred to a nitrocellulose membrane (Immobilon; Millipore, Bedford, UK). The membrane was saturated for 30 min at room temperature with blocking buffer (25 mM Tris, pH 8.0, 125 mM NaCl, 0.1% Tween 20, and 4% skim milk) and incubated with anti–phospho-pan-PKC, anti–pan-PKC, anti–phospho-PKCδ, anti-PKCδ, anti–phospho-NFκB, anti-NFκB, anti–JAM-A, anti-actin, anti-occludin, anti-claudin-1, -4, -7, anti–E-cadherin, anti–HIF-1-α, and anti–RSV/G protein antibodies (Table 2) at room temperature for 1 h. Then it was incubated with horseradish peroxidase–conjugated anti–mouse and anti–rabbit IgG antibodies at room temperature for 1 h. The immunoreactive bands were detected using an enhanced chemiluminescence Western blotting system.
Immunocytochemistry
hTERT-transfected HNECs grown in 35-mm glass-coated wells (Iwaki, Chiba, Japan), were fixed with cold acetone and ethanol (1:1 vol:vol) at –20°C for 10 min. After rinsing in phosphate-buffered saline (PBS), the cells were incubated with anti–RSV/G and /F proteins, and anti-occludin, anti–claudin-4, anti–ZO-1, anti–JAM-A, anti–E-cadherin antibodies (Table 2) overnight at 4°C. Alexa Fluor 488 (green)-conjugated anti–rabbit IgG and Alexa Fluor 592 (red)-conjugated anti–mouse IgG (Invitrogen) were used as secondary antibodies. The specimens were examined and photographed with an Olympus IX 71 inverted microscope (Olympus, Tokyo, Japan) and a confocal laser scanning microscope (LSM510; Carl Zeiss, Jena, Germany).
SEM
Cells grown on coated coverslips were fixed with 2.5% glutaraldehyde/0.1 M PBS (pH 7.3) overnight at 4°C. After several rinses with PBS, the cells were postfixed in 1% osmium tetroxide at 4°C for 3 h and then rinsed with distilled water, dehydrated in a graded ethanol series, and freeze-dried. The specimens were sputter-coated with platinum and observed with a scanning electron microscope (S-4300; Hitachi, Tokyo, Japan) operating at 10 kV.
TEM
The cells were fixed in 2.5% glutaraldehyde/0.1 M PBS (pH 7.3) overnight at 4°C, postfixed in 2% osmium tetroxide in the same buffer, dehydrated in a graded ethanol series, and embedded in Epon 812. Ultrathin sections were then cut on a Sorvall Ultramicrotome MT-5000. The sections were stained with uranyl acetate followed by lead citrate and examined at 60 kV with a JEM transmission electron microscope (JEOL, Tokyo, Japan).
Freeze-fracture analysis
For the freeze-fracture technique, the cells grown on 60-mm dishes were centrifuged into pellets and then immersed in 40% glycerin solution after fixation in 2.5% glutaraldehyde/0.1 M PBS. The specimens were fractured at –150°C to –160°C in a JFD-7000 freeze-fracture device (JEOL) and replicated by deposition of platinum/carbon from an electron beam gun positioned at a 45° angle followed by carbon applied from overhead. Replicas were examined at 100 kV with a JEM transmission electron microscope (JEOL). After the replicas were thawed, they were floated on filtered 10% sodium hypochlorite solution for 30 min in Teflon dishes. They were then washed in distilled water for 30 min, mounted on copper grids, and examined at an acceleration voltage of 100 kV with a JEOL-1200EX transmission electron microscope (JEOL). Morphometric analysis was performed on six freeze-fracture replica images of each one, which were printed at a final magnification of 20,000×. The mean number of tight junction strands was determined by doing numerous counts along a line drawn perpendicular to the junctional axis at 200-nm intervals (Stevenson et al., 1988).
Measurement of TER
hTERT-transfected HNECs were cultured to confluence on inner chambers of 12-mm Transwell inserts with 0.4-μm-pore-size filters (Corning Life Sciences). TER was measured using an EVOM voltameter with an ENDOHM-12 (World Precision Instruments, Sarasota, FL) on a heating plate (Fine, Tokyo, Japan) adjusted to 37°C. The values were expressed in standard units of ohms per square centimeter and presented as the mean ± SD. For calculation, the resistance of blank filters was subtracted from that of filters covered with cells.
GeneChip analysis
Microarray slides were scanned using a 3D-Gene human 25k. (TORAY, Tokyo, Japan), and microarray images were automatically analyzed using AROS, version 4.0 (Operon Biotechnologies, Tokyo, Japan).
MTT assay
The cells plated on 24-well tissue culture plates (BD Labware, Franklin Lakes, NJ) were treated with 0.1–2 μg/ml C. perfringens enterotoxin for 1 h. The cell survival was evaluated with the colorimetric assay using an MTT Cell Growth Assay Kit (Millipore, Billerica, MA), according to the manufacturer's recommendations.
Data analysis
Signals were quantified using Scion Image Beta 4.02 Win (Scion, Frederick, MD). Each set of results shown is representative of at least three separate experiments. Results are given as means ± SEM. Differences between groups were tested by analysis of variance followed by a post hoc test and an unpaired two-tailed Student's t test and considered to be significant when p < 0.05.
Supplementary Material
Acknowledgments
We thank Emi Suzuki for her technical support and Yukihiro Somekawa (Sapporo Hospital of Hokkaido Railway Company) and Katsushi Asano (KKR Sapporo Medical Center Tonan Hospital) for their material support. This work was supported by the Suhara Memorial Foundation, the Pancreas Research Foundation of Japan, Grants-in-Aid from the National Project “Knowledge Cluster Initiative” (2nd stage, “Sapporo Biocluster Bio-S”), Program for Developing the Supporting System for Upgrading Education and Research, the Ministry of Education, Culture, Sports, Science, and Technology, and the Ministry of Health, Labor, and Welfare of Japan.
Abbreviations used:
- CAR
coxsackie and adenovirus receptor
- ELISA
enzyme-linked immunosorbent assay
- G3PDH
glyceraldehyde-3-phosphate dehydrogenase
- HCV
hepatitis C virus
- HIF-1α
hypoxia-inducible factor-1α
- HNEC
human nasal epithelial cell
- hTERT
human telomerase reverse transcriptase
- IgG
immunoglobulin G
- IL-8
interleukin 8
- iTGF-βR
TGF-β receptor I kinase inhibitor
- JAM
junctional adhesion molecule
- JNK
c-Jun N-terminal kinase
- MAPK
mitogen-activated protein kinase
- MOI
multiplicity of infection
- NF-κB
nuclear factor-κB
- PBS
phosphate-buffered saline
- PI3K
phosphoinositide 3-kinase
- PKC
protein kinase C
- RSV
respiratory syncytial virus
- RT–PCR
reverse transcription–PCR
- SEM
scanning electron microscopy
- SEM
standard error of the mean
- TEM
transmission electron microscopy
- TER
transepithelial electrical resistance
- TGF-β1
transforming growth factor-β1
- TNF-α
tumor necrosis factor-α.
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E10-11-0875) on May 11, 2011.
REFERENCES
- Bitko V, Velazquez A, Yang L, Yang YC, Barik S. Transcriptional induction of multiple cytokines by human respiratory syncytial virus requires activation of NF-kappa B and is inhibited by sodium salicylate and aspirin. Virology. 1997;232:369–378. doi: 10.1006/viro.1997.8582. [DOI] [PubMed] [Google Scholar]
- Bitko V, Barik S. Persistent activation of RelA by respiratory syncytial virus involves protein kinase C, underphosphorylated IkBb, and sequestration of protein phosphatase 2A by the viral phosphoprotein. J Virol. 1998;72:5610–5618. doi: 10.1128/jvi.72.7.5610-5618.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell JA, Schelling P, Wetzel JD, Johnson EM, Forrest JC, Wilson GA, Aurrand-Lions M, Imhof BA, Stehle T, Dermody TS. Junctional adhesion molecule a serves as a receptor for prototype and field-isolate strains of mammalian reovirus. J Virol. 2005;79:7967–7978. doi: 10.1128/JVI.79.13.7967-7978.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Monick MM, Carter AB, Hunninghake GW. Activation of ERK2 by respiratory syncytial virus in A549 cells is linked to the production of interleukin 8. Exp Lung Res. 2000;26:13–26. doi: 10.1080/019021400269934. [DOI] [PubMed] [Google Scholar]
- Cohen CJ, Shieh JT, Pickles RJ, Okegawa T, Hsieh JT, Bergelson JM. The coxsackievirus and adenovirus receptor is a transmembrane component of the tight junction. Proc Natl Acad Sci USA. 2001;98:15191–15196. doi: 10.1073/pnas.261452898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins PL, Mottet G. Post-translational processing and oligomerization of the fusion glycoprotein of human respiratory syncytial virus. J Gen Virol. 1991;72:3095–3101. doi: 10.1099/0022-1317-72-12-3095. [DOI] [PubMed] [Google Scholar]
- Evans MJ, von Hahn T, Tscherne DM, Syder AJ, Panis M, Wšlk B, Hatziioannou T, McKeating JA, Bieniasz PD, Rice CM. Claudin-1 is a hepatitis C virus co-receptor required for a late step in entry. Nature. 2007;446:801–805. doi: 10.1038/nature05654. [DOI] [PubMed] [Google Scholar]
- Gibbs JD, Ornoff DM, Igo HA, Zeng JY, Imani F. Cell cycle arrest by transforming growth factor beta1 enhances replication of respiratory syncytial virus in lung epithelial cells. J Virol. 2009;83:12424–12431. doi: 10.1128/JVI.00806-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gower TL, Peeples ME, Collins PL, Graham BS. RhoA is activated during respiratory syncytial virus infection. Virology. 2001;283:188–196. doi: 10.1006/viro.2001.0891. [DOI] [PubMed] [Google Scholar]
- Gralinski LE, Ashley SL, Dixon SD, Spindler KR. Mouse adenovirus type 1-induced breakdown of the blood-brain barrier. J Virol. 2009;83:398–410. doi: 10.1128/JVI.00954-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guttman JA, Finlay BB. Tight junctions as targets of infectious agents. Biochim Biophys Acta. 2009;1788:832–841. doi: 10.1016/j.bbamem.2008.10.028. [DOI] [PubMed] [Google Scholar]
- Holt PG, Strickland DH, Wikström ME, Jahnsen FL. Regulation of immunological homeostasis in the respiratory tract. Nat Rev Immunol. 2008;8:142–152. doi: 10.1038/nri2236. [DOI] [PubMed] [Google Scholar]
- Ikenouchi J, Furuse M, Furuse K, Sasaki H, Tsukita S, Tsukita S. Tricellulin constitutes a novel barrier at tricellular contacts of epithelial cells. J Cell Biol. 2005;171:939–945. doi: 10.1083/jcb.200510043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffree CE, Brown G, Aitken J, Su-Yin DY, Tan BH, Sugrue RJ. Ultrastructural analysis of the interaction between F-actin and respiratory syncytial virus during virus assembly. Virology. 2007;369:309–323. doi: 10.1016/j.virol.2007.08.007. [DOI] [PubMed] [Google Scholar]
- Kamekura R, et al. Thymic stromal lymphopoietin induces tight junction protein claudin-7 via NF-kappaB in dendritic cells. Histochem Cell Biol. 2010;133:339–348. doi: 10.1007/s00418-009-0674-1. [DOI] [PubMed] [Google Scholar]
- Kamekura R, et al. Thymic stromal lymphopoietin enhances tight-junction barrier function of human nasal epithelial cells. Cell Tissue Res. 2009;338:283–293. doi: 10.1007/s00441-009-0855-1. [DOI] [PubMed] [Google Scholar]
- Kilani MM, Mohammed KA, Nasreen N, Tepper RS, Antony VB. RSV causes HIF-1alpha stabilization via NO release in primary bronchial epithelial cells. Inflammation. 2004;28:245–251. doi: 10.1007/s10753-004-6047-y. [DOI] [PubMed] [Google Scholar]
- Koizumi J, et al. Protein kinase C enhances tight junction barrier function of human nasal epithelial cells in primary culture by transcriptional regulation. Mol Pharmacol. 2008;74:432–442. doi: 10.1124/mol.107.043711. [DOI] [PubMed] [Google Scholar]
- Kurose M, et al. Induction of claudins in passaged hTERT-transfected human nasal epithelial cells with an extended life span. Cell Tissue Res. 2007;330:63–74. doi: 10.1007/s00441-007-0453-z. [DOI] [PubMed] [Google Scholar]
- Lee JW, et al. Protein kinase C-delta regulates the stability of hypoxia-inducible factor-1 alpha under hypoxia. Cancer Sci. 2007;98:1476–1481. doi: 10.1111/j.1349-7006.2007.00535.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MS, Kim TY, Kim YB, Lee SY, Ko SG, Jong HS, Kim TY, Bang YJ, Lee JW. The signaling network of transforming growth factor beta1, protein kinase Cdelta, and integrin underlies the spreading and invasiveness of gastric carcinoma cells. Mol Cell Biol. 2005;25:6921–6936. doi: 10.1128/MCB.25.16.6921-6936.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine S, Klaiber-Franco R, Paradiso PR. Demonstration that glycoprotein G is the attachment protein of respiratory syncytial virus. J Gen Virol. 1987;68:2521–2524. doi: 10.1099/0022-1317-68-9-2521. [DOI] [PubMed] [Google Scholar]
- Medigeshi GR, Hirsch AJ, Brien JD, Uhrlaub JL, Mason PW, Wiley C, Nikolich-Zugich J, Nelson JA. West Nile virus capsid degradation of claudin proteins disrupts epithelial barrier function. J Virol. 2009;83:6125–6134. doi: 10.1128/JVI.02617-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monick M, Staber J, Thomas K, Hunninghake G. Respiratory syncytial virus infection results in activation of multiple protein kinase C isoforms leading to activation of mitogen-activated protein kinase. J Immunol. 2001;166:2681–2687. doi: 10.4049/jimmunol.166.4.2681. [DOI] [PubMed] [Google Scholar]
- Ogasawara N, et al. PPARgamma agonists upregulate the barrier function of tight junctions via a PKC pathway in human nasal epithelial cells. Pharmacol Res. 2010;61:489–498. doi: 10.1016/j.phrs.2010.03.002. [DOI] [PubMed] [Google Scholar]
- Ohkuni T, Kojima T, Ogasawara N, Masaki T, Ninomiya T, Kikuchi S, Go M, Takano K, Himi T, Sawada N. Expression and localization of tricellulin in human nasal epithelial cells in vivo and in vitro. Med Mol Morphol. 2009;42:204–211. doi: 10.1007/s00795-009-0470-y. [DOI] [PubMed] [Google Scholar]
- Ploss A, Evans MJ, Gaysinskaya VA, Panis M, You H, de Jong YP, Rice CM. Human occludin is a hepatitis C virus entry factor required for infection of mouse cells. Nature. 2009;457:882–886. doi: 10.1038/nature07684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts SR, Compans RW, Wertz GW. Respiratory syncytial virus matures at the apical surfaces of polarized epithelial cells. J Virol. 1995;69:2667–2673. doi: 10.1128/jvi.69.4.2667-2673.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajjan U, Wang Q, Zhao Y, Gruenert DC, Hershenson MB. Rhinovirus disrupts the barrier function of polarized airway epithelial cells. Am J Respir Crit Care Med. 2008;178:1271–1281. doi: 10.1164/rccm.200801-136OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawada N, Murata M, Kikuchi K, Tobioka H, Kojima T, Chiba H. Tight junctions and human disease. Med Electron Microsc. 2003;36:147–156. doi: 10.1007/s00795-003-0219-y. [DOI] [PubMed] [Google Scholar]
- Schneeberger EE, Lynch RD. The tight junction: a multifunctional complex. Am J Physiol Cell Physiol. 2004;286:C1213–C1228. doi: 10.1152/ajpcell.00558.2003. [DOI] [PubMed] [Google Scholar]
- Schneeberger EE, Lynch RD. Structure, function, and regulation of cellular tight junctions. Am J Physiol. 1992;262:L647–L661. doi: 10.1152/ajplung.1992.262.6.L647. [DOI] [PubMed] [Google Scholar]
- Sieczkarski SB, Brown HA, Whittaker GR. Role of protein kinase C ßII in influenza virus entry via late endosomes. J Virol. 2003;77:460–469. doi: 10.1128/JVI.77.1.460-469.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson BR, Anderson JM, Goodenough DA, Mooseker MS. Tight junction structure and ZO-1 content are identical in two strains of Madin-Darby canine kidney cells, which differ in transepithelial resistance. J Cell Biol. 1988;107:2401–2408. doi: 10.1083/jcb.107.6.2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano K, Kojima T, Go M, Murata M, Ichimiya S, Himi T, Sawada N. HLA-DR- and CD11c-positive dendritic cells penetrate beyond well-developed epithelial tight junctions in human nasal mucosa of allergic rhinitis. J Histochem Cytochem. 2005;53:611–619. doi: 10.1369/jhc.4A6539.2005. [DOI] [PubMed] [Google Scholar]
- Tregoning JS, Pribul PK, Pennycook AM, Hussell T, Wang B, Lukacs N, Schwarze J, Culley FJ, Openshaw PJ. The chemokine MIP1alpha/CCL3 determines pathology in primary RSV infection by regulating the balance of T cell populations in the murine lung. PLoS One. 2010;5:e9381. doi: 10.1371/journal.pone.0009381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukita S, Furuse M. Overcoming barriers in the study of tight junction functions: from occludin to claudin. Genes Cells. 1998;3:569–573. doi: 10.1046/j.1365-2443.1998.00212.x. [DOI] [PubMed] [Google Scholar]
- Tsukita S, Furuse M, Itoh M. Multifunctional strands in tight junctions. Nat Rev Mol Cell Biol. 2001;2:285–293. doi: 10.1038/35067088. [DOI] [PubMed] [Google Scholar]
- Tsutsumi H, Nagai K, Suga K, Chiba Y, Chiba S, Tsugawa S, Ogra PL. Antigenic variation of human RSV strains isolated in Japan. J Med Virol. 1989;27:124–130. doi: 10.1002/jmv.1890270211. [DOI] [PubMed] [Google Scholar]
- Walmsley SR, et al. Hypoxia-induced neutrophil survival is mediated by HIF-1alpha-dependent NF-kappaB activity. J Exp Med. 2005;201:105–115. doi: 10.1084/jem.20040624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SZ, Hallsworth PG, Dowling KD, Alpers JH, Bowden JJ, Forsyth KD. Adhesion molecule expression on epithelial cells infected with respiratory syncytial virus. Eur Respir J. 2000;15:358–366. doi: 10.1034/j.1399-3003.2000.15b23.x. [DOI] [PubMed] [Google Scholar]
- Wright PF, Ikizler MR, Gonzales RA, Carroll KN, Johnson JE, Werkhaven JA. Growth of respiratory syncytial virus in primary epithelial cells from the human respiratory tract. J Virol. 2005;79:8651–8654. doi: 10.1128/JVI.79.13.8651-8654.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoboua F, Martel A, Duval A, Mukawera E, Grandvaux N. Respiratory syncytial virus-mediated NF-{kappa}B p65 phosphorylation at serine 536 is dependent on RIG-I, TRAF6, and IKK{beta} J Virol. 2010;84:7267–7277. doi: 10.1128/JVI.00142-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Peeples ME, Boucher RC, Collins PL, Pickles RJ. Respiratory syncytial virus infection of human airway epithelial cells is polarized, specific to ciliated cells, and without obvious cytopathology. J Virol. 2002;76:5654–5666. doi: 10.1128/JVI.76.11.5654-5666.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.