Greatwall kinase is required for M phase maintenance by inhibiting PP2A. Gwl associates with PP2A in G2 oocytes, but the complex dissociates during M phase (meiosis I). Mutating Lys71 to Met (K71M) generates gain-of-function Gwl kinase activity toward endosulfinethat is sufficient to induce oocyte maturation in the absence of progesterone.
Abstract
Greatwall kinase has been identified as a key element in M phase initiation and maintenance in Drosophila, Xenopus oocytes/eggs, and mammalian cells. In M phase, Greatwall phosphorylates endosulfine and related proteins that bind to and inhibit protein phosphatase 2A/B55, the principal phosphatase for Cdk-phosphorylated substrates. We show that Greatwall binds active PP2A/B55 in G2 phase oocytes but dissociates from it when progesterone-treated oocytes reach M phase. This dissociation does not require Greatwall kinase activity or phosphorylation at T748 in the presumptive T loop of the kinase. A mutant K71M Greatwall, also known as Scant in Drosophila, induces M phase in the absence of progesterone when expressed in oocytes, despite its reduced stability and elevated degradation by the proteasome. M phase induction by Scant Greatwall requires protein synthesis but is not associated with altered binding or release of PP2A/B55 as compared to wild-type Greatwall. However, in vitro studies with Greatwall proteins purified from interphase cells indicate that Scant, but not wild-type Greatwall, has low but detectable activity against endosulfine. These results demonstrate progesterone-dependent regulation of the PP2A/B55–Greatwall interaction during oocyte maturation and suggest that the cognate Scant Greatwall mutation has sufficient constitutive kinase activity to promote M phase in Xenopus oocytes.
INTRODUCTION
Entry into mitosis is induced by the activation of M phase–promoting factor (MPF) (cyclin B/Cdk1), which results in the phosphorylation of numerous target substrates that control the events of M phase (Lohka et al., 1987, 1988; Gautier et al., 1990). A complex set of regulatory mechanisms controls the activation of MPF, most notably by altering the activities or levels of the inhibitory Wee1/Myt1 protein kinases or the dual-specificity phosphatase Cdc25 (Dunphy and Kumagai, 1991; Parker and Piwnica-Worms, 1992; Kornbluth et al., 1994; Mueller et al., 1995). These enzymes control the phosphorylation state of Tyr-15 in the ATP-binding site of Cdk1. Phosphorylation/dephosphorylation of this residue is a key step in mitotic initiation and the target of checkpoint controls that prevent MPF activation when DNA is damaged or before DNA replication is complete (Nigg, 2001). Cdk1 is a “proline-directed” kinase, meaning that most of its substrates are phosphorylated at a consensus S/TP site that typically has a basic residue one to three amino acids N-terminal or C-terminal to the phosphorylation site (Maller et al., 1989; Dephoure et al., 2008). Dephosphorylation of such pS/TP sites has been reported to be largely catalyzed by the actions of protein phosphatase 2A (PP2A) (Ferrigno et al., 1993). Various isoforms of PP2A have been reported in which the catalytic subunit is complexed as a heterotrimer with a common scaffold A subunit and one of many specific “B” targeting subunits (Shi, 2009). The PP2A isoforms primarily responsible for the dephosphorylation of pS/TPs appear to be those with targeting subunits of the B55 class, including B55δ in Xenopus egg extracts (Castilho et al., 2009; Mochida et al., 2009) and B55α in human tissue culture cells (Schmitz et al., 2010).
A number of protein kinases are known to contribute to MPF activation. These include p90Rsk in the MAPK pathway, which inhibits Wee1/Myt1 (Palmer and Nebreda, 2000; Tunquist and Maller, 2003), RINGO/CDK complexes that form early in oocyte maturation (Ruiz et al., 2010), and the polo-like kinase Plx1 that phosphorylates and activates Cdc25C (Kumagai and Dunphy, 1996; Qian et al., 1998). Greatwall kinase (Gwl) has been identified as another protein kinase that plays a key role in MPF activation. Gwl itself is activated during Xenopus oocyte maturation, and microinjection of activated wild-type (WT) Gwl purified from okadaic acid (OA)–treated insect cells induces oocyte maturation in the absence of progesterone (Zhao et al., 2008). Immunodepletion of Gwl from cycling egg extracts blocks entry into M phase, and overexpression of Gwl accelerates Cdc25 activation and entry into M phase in oocytes and egg extracts (Yu et al., 2006). Remarkably, the depletion of Gwl from egg extracts results in failure to maintain M phase even when MPF and other mitotic kinases remain fully activated (Castilho et al., 2009; Vigneron et al., 2009). Recently the mechanism of Gwl action has been identified: it inhibits the dephosphorylation of Cdk substrates by phosphorylating endosulfine and a related protein, Arpp19, which bind to and inhibit PP2A/B55 (Gharbi-Ayachi et al., 2010; Mochida et al., 2010). The results of the egg extract depletion experiments indicate that the prevention of Cdk substrate dephosphorylation by PP2A is as important as Cdk activation for maintaining M phase. The mechanism of Gwl activation is not yet clear, although in vitro MPF can phosphorylate Gwl at the T-loop site, T748 (Yu et al., 2006). In Drosophila, an allele of Gwl, K97M Gwl (known as Scant), has been reported to enhance the mitotic defects of reduced polo kinase expression even when expressed at low levels (Archambault et al., 2007).
In this article we show that Gwl forms a complex with PP2A/B55 in G2 (interphase) oocytes but dissociates from the phosphatase in progesterone-treated M phase (GVBD) oocytes. We also analyze the Xenopus homologue of Scant (K71M Gwl) and show that despite reduced stability, its expression is sufficient to promote M phase in oocytes, most likely due to weak constitutive kinase activity against endosulfine.
RESULTS
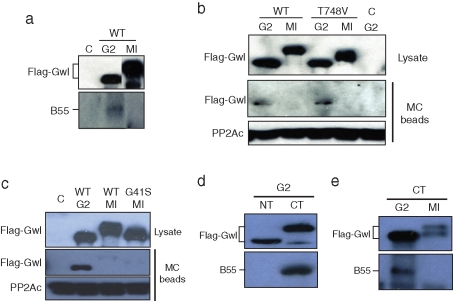
Previous studies of Gwl and PP2A have reported coprecipitation of the two enzymes in cytosol from asynchronous tissue culture cells or in cytostatic factor (CSF) extracts from unfertilized eggs (Vigneron et al., 2009). To investigate possible interactions of Gwl with PP2A/B55 during oocyte maturation, we expressed FLAG-tagged Gwl in oocytes and immunoprecipitated the kinase from G2 and progesterone-treated M phase oocytes (MI, GVBD). Anti-FLAG beads did not coprecipitate PP2A/B55 in M phase; however, coprecipitation of PP2A/B55 with Gwl was evident in G2 oocytes (Figure 1A). Microcystin (MC) is a chemical able to bind with near-equal affinity to the catalytic subunits of both PP1 and PP2A (Swingle et al., 2007), although in Xenopus oocyte extracts it shows high specificity for PP2A holoenzymes (Maton et al., 2005). As shown in Figure 1B, MC-bead precipitation also revealed a complex between PP2A and Gwl in G2/interphase but not in M phase, with both WT and a kinase-dead Gwl mutant in which the T-loop Thr-748 was changed to valine. Greatwall release of PP2A was also evident with a different kinase-dead mutant, G41S Gwl, described previously (Zhao et al., 2008) (Figure 1C). To evaluate which region of Gwl binds to PP2A/B55, mRNA encoding the FLAG-tagged N-terminus (NT) or C-terminus (CT) of Gwl was injected into G2 oocytes, followed by incubation overnight. Immunoprecipitation/Western analysis demonstrated that PP2A/B55 bound to the CT region of Gwl during G2/interphase (Figure 1D), and the phosphatase was released from the CT Gwl fragment in M phase/GVBD oocytes (Figure 1E).
FIGURE 1:
Regulated binding of PP2A/B55 to Greatwall. (A) Greatwall releases PP2A/B55 in M phase. Oocytes were injected with mRNA encoding FLAG-Gwl and incubated overnight as described in Materials and Methods. Some oocytes were then treated with progesterone and monitored for entry into meiosis I (MI, GVBD). Extracts from the control (uninjected), G2, and GVBD oocytes were immunoprecipitated with anti-FLAG antibody beads, and the immunoprecipitates were Western blotted for FLAG and the B55 subunit of PP2A. C, control; WT, wild type. (B) Association of Greatwall with PP2A in G2 phase. Oocytes were injected with mRNA encoding FLAG-WT or T748V Gwl and incubated overnight. Some oocytes were then treated with progesterone and monitored for white-spot formation (GVBD, MI). Lysates from the oocytes were precipitated with microcystin–Sepharose beads (MC), and the precipitates were Western blotted for FLAG and for the catalytic subunit of PP2A (bottom). Lysates for Western blotting were also analyzed before immunoprecipitation (top). (C) Kinase-dead Greatwall releases PP2A in M phase. An experiment like that in B was performed, except that G41S Gwl was analyzed in MI (GVBD) oocytes. (D) The C-terminal region of Greatwall binds PP2A in G2. mRNA encoding FLAG-tagged N-terminal (NT) or C-terminal (CT) Gwl was injected into oocytes, followed by overnight incubation. Oocyte lysates were immunoprecipitated with anti-FLAG antibody beads, and the precipitate was Western blotted with FLAG and B55 antibodies, as indicated. (E) The C-terminal region of Greatwall releases PP2A in M phase. Oocytes were injected with CT Gwl mRNA as in D, except that some were treated with progesterone to stimulate entry into meiosis I (MI), as indicated. At GVBD, FLAG-Gwl was immunoprecipitated on anti-FLAG beads from progesterone-treated (MI) or nontreated (G2) oocytes, and the immunoprecipitates were Western blotted for FLAG and B55.
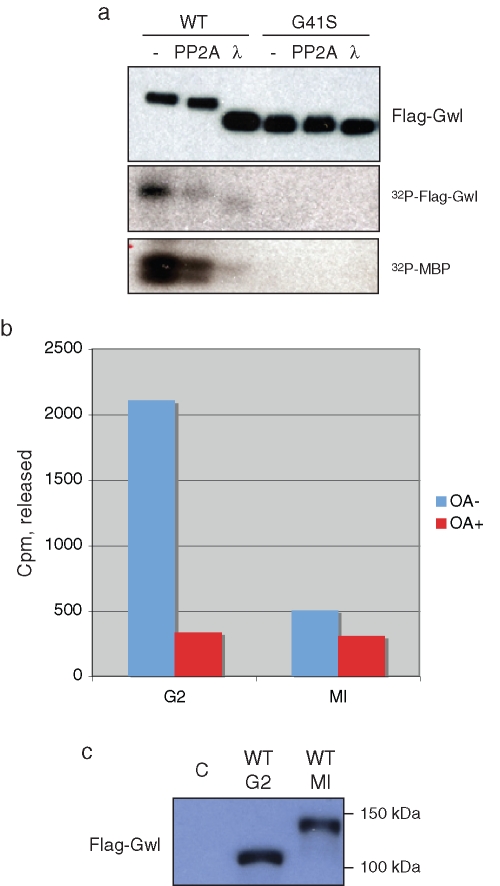
Xenopus Gwl activation during entry into M phase is accompanied by an electrophoretic shift that reflects extensive phosphorylation, most likely including the presumptive T-loop site, which is T748. As shown in Figure 2A, incubation of activated Gwl from GVBD oocytes with either PP2Ac or lambda phosphatase leads to both an increase in Gwl electrophoretic mobility and a decrease in Gwl autophosphorylation and kinase activity toward an in vitro substrate, myelin basic protein (MBP). Therefore an important question is whether PP2A/B55 in the Gwl/PP2A/B55 complex in G2 phase is catalytically active, as this might ensure that Gwl remains inactive during interphase. To assess this possibility, immunoprecipitates of FLAG-tagged Gwl were incubated with 32P-histone H1 phosphorylated by MPF, and phosphatase activity was measured by release of 32P-phosphate as described in Materials and Methods. As shown in Figure 2B, in G2 oocytes significant phosphatase activity was present in the Gwl complex, which could be inhibited by OA. In contrast, little activity was evident with WT Gwl immunoprecipitated from M phase oocytes, which was in the active hyperphosphorylated state as judged by its electrophoretic mobility (Figure 2C), consistent with the release of PP2A/B55 from both WT and kinase-dead (KD) Gwl in M phase (Figure 1).
FIGURE 2:
Greatwall-bound PP2A is catalytically active. (A) Dephosphorylation and deactivation of Gwl. Oocytes were injected with mRNA encoding WT or G41S Gwl. After overnight incubation to allow protein expression, the oocytes were treated with progesterone. At GVBD, oocyte lysates were prepared and anti-FLAG bead immunoprecipitates incubated with either PP2Ac or lambda phosphatase as described in Materials and Methods. The precipitates were then washed and assayed for autophosphorylation or MBP kinase activity (bottom, autoradiographs), or blotted with anti-FLAG antibodies after SDS–PAGE (top), as indicated. (B) Phosphatase activity associated with Gwl. Oocytes were injected with mRNA encoding WT FLAG-tagged Gwl and incubated overnight. Some oocytes were then treated with progesterone to enter M phase. At GVBD, anti-FLAG beads were used to precipitate Gwl, and after washing, the beads were incubated with or without OA and 32P-labeled histone H1 prepared as described in Materials and Methods. The reaction was terminated by addition of 30% trichloracetic acid, and the counts per minute released were quantified by Cerenkov counting in a liquid scintillation counter. Similar results were obtained in several independent experiments. (C) Anti-FLAG immunoblot of the immunoprecipitates in B. C, control oocytes not injected with mRNA but subjected to anti-FLAG bead precipitation and assay. Activity associated with Gwl in B was corrected for background seen with control FLAG bead immunoprecipitates from uninjected oocytes.
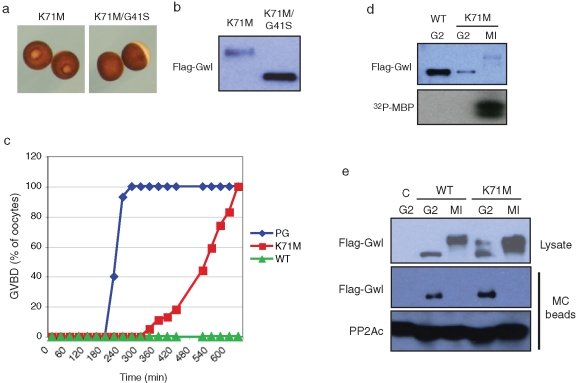
We considered whether known mutants of Gwl differ in association with, or regulation of, B55/PP2A. One such mutant is K71M Gwl, also known as Scant in Drosophila. This form of Gwl was originally described in Drosophila oocytes and embryos as an allele that could enhance defects in mitotic progression caused by reduced polo kinase expression (Archambault et al., 2007). To assess and compare the actions of WT and K71M Gwl in oocyte maturation, mRNAs encoding FLAG-tagged versions of Gwl were injected into oocytes, and progression into M phase was monitored by GVBD (white-spot formation). Figure 3A shows that K71M Gwl could induce white-spot formation. This ability requires kinase activity, as K71M Gwl also containing the KD G41S mutation had no maturation-inducing ability (Figure 3A). As expected, only the K71M-injected oocytes undergoing GVBD contained hyperphosphorylated Gwl (Figure 3B). Figure 3C compares progesterone treatment with the actions of WT and K71M Gwl expressed equally by mRNA injection. The hormone caused 50% GVBD in ∼4 h, whereas K71M Gwl induced 50% GVBD ∼9 h after injection in the absence of hormone. WT Gwl was unable to induce GVBD in any oocytes even 10 h after mRNA injection. Expressed WT Gwl never exhibited elevated kinase activity in the oocyte, as judged by electrophoretic mobility and in vitro kinase activity toward MBP (Figure 3D). Even 4 h after mRNA injection, K71M Gwl was also not activated as a kinase, as judged by its electrophoretic mobility and activity against MBP measured in an immune-complex kinase assay (Figure 3D). However, 7 h after mRNA injection, the activity of Gwl was highly elevated in those K71M-expressing oocytes that underwent GVBD. To assess whether K71M Gwl differs in PP2A/B55 binding and release, PP2A was precipitated with MC beads from K71M Gwl–expressing oocytes in G2 and M phase. As was the case with WT Gwl (Figure 1), K71M Gwl oocytes bound PP2A/B55 in G2 but not in M phase (Figure 3E).
FIGURE 3:
K71M Greatwall induces oocyte maturation in the absence of progesterone. (A) Oocyte morphology. Maturation (GVBD) was assessed by white-spot formation in oocytes incubated overnight after injection of mRNA encoding K71M Gwl or K71M/G41S Gwl, as indicated. (B) Electrophoretic mobility. Lysates from the oocytes in A were Western blotted for FLAG. (C) Germinal vesicle breakdown. Oocytes were treated with progesterone (blue) or microinjected with equal amounts of mRNA encoding either WT Gwl (green) or K71M Gwl (red). Maturation was monitored at the indicated times by the appearance of a white spot signifying GVBD. (D) K71M Gwl kinase activity. Top, the oocytes in (C) expressing WT or K71M Gwl at 240 min (G2) after injection or expressing K71M Gwl at 420 min after injection with a white spot (GVBD, MI) were analyzed by Western blot for Gwl expression. Bottom, the same oocytes were lysed, and Gwl was immunoprecipitated on anti-FLAG beads and analyzed for kinase activity against MBP as described in Materials and Methods. An autoradiograph is shown. (E) K71M Gwl releases PP2A in M phase. Oocytes were injected with mRNA encoding either FLAG-WT or FLAG-K71M Gwl and treated with the proteosome inhibitor MG132. Some FLAG-WT Gwl mRNA-injected oocytes were treated with progesterone to undergo GVBD (MI). G2 and MI lysates were prepared and analyzed by MC bead precipitation and Western blotting as described in Figure 1B.
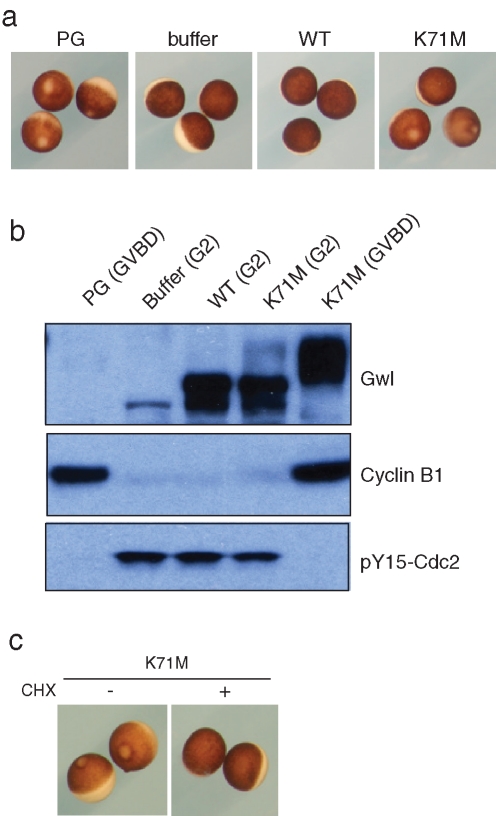
To confirm that the induction of maturation by Gwl mRNA was a direct effect of Gwl, WT or K71M Xenopus Gwl proteins purified from non–OA-treated Sf9 cells were injected into oocytes, which were then monitored for GVBD. As shown in Figure 4A, K71M Gwl protein was able to induce GVBD in some but not all oocytes during an overnight incubation, whereas WT Gwl did not induce maturation in any oocytes. Those K71M Gwl–injected oocytes that did undergo GVBD exhibited increased synthesis of cyclin B1 and dephosphorylation of Tyr-15 in Cdc2, as also seen in progesterone-treated controls (Figure 4B). Increased protein synthesis in response to K71M Gwl is essential for Gwl action because oocytes treated with cycloheximide failed to undergo any GVBD after K71M Gwl injection (Figure 4C).
FIGURE 4:
K71M Gwl protein induces oocyte maturation. (A) Oocyte morphology. Oocytes were treated with progesterone or injected with buffer or WT or K71M Gwl proteins purified from non-OA treated (interphase) Sf9 cells. After incubation overnight, GVBD was assessed by white-spot formation. An oocyte that did not undergo GVBD with K71M Gwl was designated “G2” (e.g., upper oocyte, right panel). (B) Analysis of K71M Gwl expressing oocytes. The oocytes in A were lysed and Western blotted for Gwl, cyclin B1, and pY15 Cdc2, as indicated. At this exposure level, the shifted form of endogenous Gwl in progesterone-treated (GVBD) oocytes is less apparent. (C) Maturation induced by K71M Gwl requires protein synthesis. Active Gwl was purified from OA-treated Sf9 cells as described previously and microinjected into oocytes, followed by incubation in the absence and presence of cycloheximide (CHX, 10 μg/ml). After 6 h, GVBD was assessed by white-spot formation.
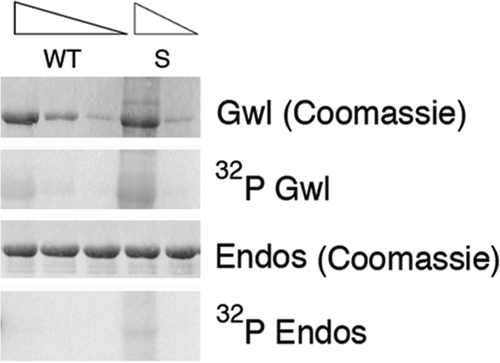
The induction of GVBD by K71M Gwl expressed from injected mRNA (Figure 3) or by Gwl protein purified from non–OA-treated Sf9 cells (Figure 4) reflects hours of the “interphase” action of Gwl before M phase entry. As shown in Figure 3, C and D, immunoprecipitated “interphase” K71M Gwl has no detectable activity against MBP despite having a twofold higher specific activity against MBP in M phase (Supplemental Figure S1). Although the results with MBP assays suggest that K71M Gwl is not a “constitutively active” kinase like CA-Plx1 or CA-Rsk1, two other mutated protein kinases that induce GVBD by virtue of expressing M phase activity levels (Qian et al., 1999; Gross et al., 2001), we considered whether MBP phosphorylation might be a relatively insensitive measure of Gwl activity compared with a physiologically relevant substrate. A precedent for this idea is evident with phosphorylation by G1 Cdks such as cyclin A/Cdk2 (Peeper et al., 1993; Loog and Morgan, 2005). The “hydrophobic patch” in cyclin A containing the MRAIL motif facilitates recognition of substrates that contain an RXLXL sequence, such as the retinoblastoma protein (Schulman et al., 1998). Mutation of the hydrophobic patch impairs Rb phosphorylation but has no effect on the phosphorylation of a nonspecific substrate, histone H1. In light of these considerations, we reinvestigated the activity of WT and Scant Gwl purified from non–OA-treated Sf9 cells for phosphorylation of a physiological substrate, endosulfine, a protein previously found to be important in Drosophila oocyte maturation (Von Stetina et al., 2008). As described earlier, recent reports indicate that Gwl-dependent phosphorylation of endosulfine and related proteins is responsible for the inhibition of PP2A/B55 in M phase (Gharbi-Ayachi et al., 2010; Mochida et al., 2010). As shown in Figure 5, Scant but not WT Gwl had low but detectable dose-dependent activity against endosulfine, as well as autophosphorylating activity. These results suggest that the ability of Scant/K71M Gwl to induce GVBD reflects elevated activity in interphase of the mutant compared with WT Gwl.
FIGURE 5:
Constitutive activity of Scant. Recombinant Drosophila Gwl proteins, either WT or K97M/Scant (equivalent to K71M Xenopus Gwl), were purified from non-OA–treated Sf9 cells and assayed for activity against a fragment of endosulfine fused to maltose-binding protein as described in Materials and Methods. The first and third rows show Coomassie staining of Gwl and endosulfine proteins used in the assay. The amount of Gwl proteins assayed varied from 1 to 50 ng. The autoradiographs in the second and fourth rows show, respectively, autophosphorylation of Gwl and phosphorylation of the endosulfine fragment fused to maltose-binding protein.
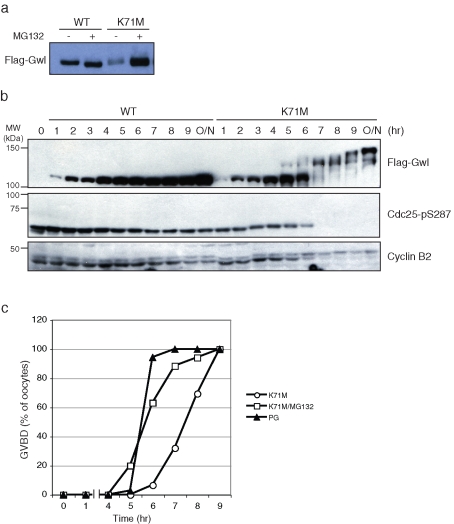
It is remarkable that K71M Gwl is able to induce GVBD even when its expression level is lower than that of WT Gwl (Figure 3D). The lower expression level of K71M Gwl suggested that there might be differences in stability of the mutant compared with WT Gwl. To assess this possibility, oocytes were injected with equal amounts of FLAG-tagged mRNA for WT and K71M Gwl and incubated for 4 h in the presence and absence of MG132, an inhibitor of degradation by the 26S proteasome. As shown in Figure 6A, accumulation of K71M was greatly enhanced by MG132, whereas WT Gwl expression was unaffected. However, whereas enhanced accumulation did accelerate the kinetics of K71M-induced maturation (Figure 6C), it did not lead to immediate activation of expressed Gwl activity as judged by electrophoretic mobility (Figure 6B). WT Gwl was not activated and did not induce GVBD in any oocytes even after 24 h of incubation in the presence of MG132.
FIGURE 6:
K71M Gwl is an unstable protein. (A) Greatwall accumulation. mRNA encoding either WT FLAG-Gwl or FLAG-K71M Gwl was injected into oocytes, and after 4 h of incubation in the absence or presence of the protesome inhibitor MG132 the level of expressed Gwl was assessed by SDS–PAGE and immunoblotting for FLAG-Gwl. (B) Maturation with K71M Gwl and MG132. Oocytes were injected with mRNA encoding FLAG-WT or K71M Gwl and incubated with medium containing 50 μM MG132. At the indicated times, oocyte lysates were prepared and Western blotted for FLAG-Gwl, active Cdc25C (pSer287), and cyclin B2. GVBD (unpublished data) began at 6 h after injection. (C) Acceleration of K71M-induced GVBD by MG132. Oocytes were injected with mRNA encoding K71M Gwl and incubated in the presence or absence of MG132 or treated with progesterone. The time course of GVBD was assessed by white-spot formation at the indicated times.
DISCUSSION
The results identify complex formation between Gwl and PP2A/B55 in Xenopus oocytes by both coimmunoprecipitation and microcystin bead precipitation. Unexpectedly, this interaction only occurs in G2 phase when Gwl has low activity and PP2A/B55 is active (Figures 1 and 2). Although Vigneron et al. (2009) reported complex formation between Gwl and PP2A in asynchronous tissue culture cells, which are largely in interphase, they also reported coimmunoprecipitation of Gwl and PP2A in CSF (M phase) extracts from Xenopus eggs. We do not completely understand the apparent discrepancy between our findings and the latter result. It should be noted that we studied entry into M phase of meiosis I, whereas CSF-arrested oocytes are in metaphase of meiosis II and potentially subject to different regulation; alternatively, because CSF arrest in M phase is unable to be maintained in the absence of Gwl (Yu et al., 2006), it is possible that the CSF extracts examined by Vigneron et al. (2009) did not remain in M phase during the immunoprecipitation.
In any event, given that Gwl activation involves extensive phosphorylation, it seems likely that one function of PP2A/B55 in the G2/interphase complex is to keep Gwl inactive during the long G2/prophase arrest of the immature oocyte. Although inhibition of PP2A by OA in the immune complex with Gwl did not lead to Gwl kinase activation (unpublished data), this result would be expected if other kinases (such as MPF) are also needed for this activation, as has been suggested previously (Yu et al., 2006). The complex dissociates in M phase when Gwl is active and PP2A/B55 activity is inhibited by Gwl-dependent phosphorylation of endosulfine and related proteins (Castilho et al., 2009; Mochida et al., 2009, 2010; Vigneron et al., 2009; Gharbi-Ayachi et al., 2010). KD Gwl also bound PP2A and released it in M phase, suggesting that the kinase activity of Gwl is not necessary for release. Consistent with this, the CT half of Gwl that lacks the complete kinase domain also releases PP2A/B55 in M phase (Figure 1D). It is intriguing that the CT half of Gwl shifts in M phase (Figure 1D), and the shift of Gwl is partially blocked when the T loop (T748) is mutated (Figure 1B). However, phosphorylation of T748 is not involved in the release of PP2A/B55 from the CT region of Gwl in M phase because the phosphatase also dissociated from T748V Gwl in M phase (Figure 1B).
Mutants of a number of constitutively active M phase kinases have previously been reported to cause GVBD when expressed in oocytes. Examples include the polo-like kinase Plx1, p90Rsk, MEK1, and MAPK (Haccard et al., 1995; Huang et al., 1995; Qian et al., 1999; Gross et al., 2001). In all these cases the kinase contained activating mutations resulting in an M phase level of activity before injection or immediately after synthesis in the G2/interphase oocyte. In contrast, the basal kinase activity of Scant against endosulfine is very low but nevertheless appears to involve a constitutive gain of activity toward a key physiological substrate (Figure 5). The time course of GVBD with K71M Gwl requires several hours longer than progesterone treatment without enhanced accumulation in the presence of MG132 (Figure 6C), suggesting that the low level of K71M Gwl constitutive activity is the limiting factor in the action of the mutant.
Studies of Drosophila Scant (K97M Gwl) in cell lines have suggested that the kinase is hyperactive (Archambault et al., 2007). However, comparison of WT and K71M Xenopus Gwl from oocytes at GVBD reveals at most a twofold-higher specific activity (Supplemental Figure S1); similarly, Scant and WT enzymes made in Sf9 cells in the presence of OA do not have greatly different specific activities (unpublished data). We speculate that the massive hyperactivity of Drosophila Scant previously reported may be due to the mitotic arrest of at least some of the cultured cells expressing this protein, leading to the accumulation of active M phase Scant in the preparation. Both fly Scant and frog K71M Gwl appear to be expressed only at very low levels compared with WT Gwl, regardless of whether experiments are done in stably transformed Drosophila tissue culture cells (Archambault et al., 2007), Xenopus oocytes (Figure 3D), or Sf9 insect cells expressing baculovirus constructs (K.B. and M.L.G., unpublished results). This low expression was previously suggested to reflect the toxicity of Scant Gwl (Archambault et al., 2007). However, our studies show that the low expression of K71M is due at least in part to reduced stability, as inhibition of proteasome-mediated degradation led to a dramatic increase in its accumulation and an accelerated rate of M phase entry in oocytes injected with K71M Gwl mRNA (Figure 6). It is unclear why mutating K71 to M confers low constitutive activity to Gwl in oocytes even under low-expression conditions. Mutation of K71 to A, C, E, L, or Q does not enhance M phase promotion by Gwl in oocytes, whereas mutation of K71 to R does (unpublished data). Structural analysis of Gwl will likely be needed in order to understand the basis for enhanced kinase activity by K71M Gwl and its reduced stability compared with WT Gwl. In any case, the results presented here provide new evidence for regulation of Gwl/PP2A interaction during oocyte maturation and employ the Xenopus system to facilitate elucidation of the molecular basis of the effects of Scant/K71M Gwl that appear to be conserved from flies to Xenopus.
MATERIALS AND METHODS
Xenopus oocytes and injections
Xenopus oocytes arrested in G2 were manually dissected from ovarian fragments, and Gwl expression was obtained by microinjecting oocytes with 40 nl of mRNA (0.25 mg/ml) encoding various Gwl constructs, followed by incubation at room temperature. Progesterone (10 μg/ml) was added to some oocytes to induce maturation, and MI (GVBD) oocytes were collected when a well-defined white spot appeared at the animal pole.
Greatwall cloning, mRNA, and protein expression
Full-length Xenopus Gwl cDNA was purchased from Open Biosystems (Huntsville, AL) (MXL1736-9507526; Clone ID, 6637446; Accession Number, BC068968). Full-length, NT (amino acids 1–467) or CT (amino acids 468–887) Gwl was cloned into a pCS-FLAG vector using LIC cloning (Novagen; EMD Chemicals, San Diego, CA). K71M, T748V, and G41S Gwl were generated with a QuikChange Site-Directed Mutagenesis Kit (Agilent, Santa Clara, CA), and mRNA was produced using an mMessage mMachine SP6 Kit (Ambion, Austin, TX). Xenopus His-Gwl protein (WT and K71M) was expressed and purified from baculovirus-infected Sf9 cells with or without OA treatment, as described previously (Yu et al., 2006). The Drosophila Scant (K97M) Gwl protein was generated from a Drosophila WT Gwl cDNA clone with the QuikChange Site-Directed Mutagenesis Kit, and both WT and Scant proteins expressed in Sf9 insect cells were purified without addition of OA as described (Yu et al., 2006).
Immunoprecipitation and Western blotting
For immunoprecipitation of FLAG Gwl, 20 oocytes treated as indicated in the figures were homogenized in 100 μl of EB (80 mM β-glycerophosphate, 20 mM ethylene glycol tetraacetic acid [EGTA], 5 mM MgCl2, 20 mM HEPES, pH 7.5), and the oocyte cytosol was collected by centrifugation at 10,000 × g for 1 min at 4°C. Five microliters of cytosol was mixed with 5 μl of 2× Laemmli sample buffer (Bio-Rad, Hercules, CA) and used as the input fraction for Western blotting. The remaining cytosol was added to 20 μl of anti-FLAG agarose beads (M2 FLAG agarose; Sigma-Aldrich, St. Louis, MO) preequilibrated in EB and incubated for 60 min at 4°C. The beads were then washed three times in EB and resuspended in 20 μl of 2× Laemmli sample buffer. For PP2A precipitation, 50 μl of a 50% slurry of microcystin-agarose beads (Millipore, Billerica, MA) preequilibrated with EB was incubated for 4 h at 4°C with cytosol from 20 oocytes. The beads were washed with EB three times and resuspended in 25 μl of 2× sample buffer. After SDS–PAGE through either a 7.5 or 10% Criterion gel (Bio-Rad), samples were transferred to nitrocellulose membranes (Whatman, Piscataway, NJ) using a semidry Western blotting apparatus and blocked by 5% skim milk before incubation with the indicated primary antibodies. Antibodies against FLAG were obtained from Sigma-Aldrich. Antibodies to pY15 Cdc2 were from Cell Signaling Technology (Beverly, MA), antibodies recognizing all B55 subunit isoforms of PP2A were from Santa Cruz Biotechnology (Santa Cruz, CA), and antibodies to PP2Ac were from Bethyl Laboratories (Montgomery, TX). Antibodies against Xenopus Gwl have been previously described (Yu et al., 2006), and antibodies against Xenopus cyclin B1 and B2 were generated as previously described (Izumi et al., 1992). Antibodies to phospho-ser287 in Xenopus Cdc25C were a kind gift from Joan Ruderman (Harvard Medical School, Boston, MA).
Greatwall kinase assay
The kinase activity of immunoprecipitated Gwl was measured as previously described (Peng et al., 2010). Briefly, oocytes injected with FLAG-Gwl mRNA were homogenized in 5 μl per oocyte of ice-cold EB, and the cytosol collected after centrifugation at 10,000 × g for 1 min at 4°C. Cytosol equivalent to three oocytes was added to 10 μl of anti-FLAG agarose beads (Sigma-Aldrich) preequilibrated with EB and incubated for 60 min at 4°C. The beads were then washed three times in EB and once with kinase buffer (20 mM HEPES, 10 mM MgCl2, 3 mM β-mercaptoethanol, pH 7.6). The kinase reaction was performed by resuspending the beads in 30 μl of kinase buffer supplemented with 10 μg of MBP and 100 μM [γ-32P]ATP (5 μCi per reaction) for 10 min at 30°C, and the reaction was terminated by addition of 8 μl of 5× sample buffer. Half of the reaction was electrophoresed through a 4–20% gradient gel (Criterion; Bio-Rad), and the gel was stained with Imperial Coomassie Blue (Invitrogen, Carlsbad, CA). Autoradiographs were exposed on XRP film (Kodak, Rochester, NY), or the excised radiolabeled MBP bands were counted in a scintillation counter (Beckman Coulter, Brea, CA). For some experiments, immunoprecipitated FLAG-Gwl was pretreated with PP2A or lambda phosphatase as follows. After washing with lambda phosphatase buffer (New England Biolabs, Ipswich, MA), FLAG-Gwl beads were incubated with either 0.2 U of PP2A (Upstate, Millipore) in PP2A buffer or 0.4 U of lambda phosphatase (New England Biolabs) in lambda phosphatase buffer for 30 min at 30°C. The beads were washed with EB three times and once with kinase buffer and used for Western analysis or the kinase assay as described previously. Kinase assays with purified Gwl proteins (1–50 ng) were performed for 10 min at 30ºC in 10 μl of kinase buffer (20 mM HEPES, 10 mM MgCl2, 0.1 mg/ml bovine serum albumin, 3 mM β-mercaptoethanol) supplemented with 100 μM ATP containing 1 mCi of [γ-32P]ATP and using as substrate 1.5 μg of maltose-binding protein fused to a fragment of Xenopus endosulfine containing the phosphorylation site that is required for endosulfine-dependent inhibition of PP2A (56KRLQKGQKYFDSGDYNMAKAK76, Gwl site underlined; Mochida et al., 2010). Reactions were stopped by addition of Laemmli sample buffer and analyzed by SDS–PAGE and autoradiography.
PP2A assay
To generate substrate for PP2A, 20 μg of histone H1 was phosphorylated by MPF in 200 μl of kinase buffer with 100 μM [γ32P]ATP (150 μCi) for 2 h at 30°C. After addition of 1.5 ml of dilution buffer (20 mM Tris HCl, pH 8.0), the reaction was incubated with 200 μl of SP-Sepharose beads for 30 min at room temperature and washed six times with 1 ml of dilution buffer. Histone H1 was eluted from the beads by sequential incubation with 200 μl of dilution buffer containing 400 mM, 600 mM, 800 mM, and 1 M NaCl. Phospho-histone H1 in the 800 mM and 1 M NaCl eluates was pooled, concentrated to 50 μl on a spin column (Vivaspin; GE Healthcare, Piscataway, NJ), and diluted to 200 μl with dilution buffer. Greater than 99% of the radiolabel was precipitable by 30% trichloroacetic acid (TCA). For the phosphatase assay, 10 oocytes expressing FLAG-Gwl were lysed in 50 μl of EB and centrifuged, and the cytosol was incubated with 15 μl of anti-FLAG agarose beads preequilibrated with EB for 1 h at 4°C, followed by washing twice with EB. The FLAG-Gwl beads were resuspended in 20 μl of PP2A buffer (20 mM 3-(N-morpholino)propanesulfonic acid, pH 7.4, 0.1 M NaCl, 60 mM β-mercaptoethanol, 1 mM MgCl2, 1 mM EGTA, 0.1 mM MnCl2, 1 mM dithiothreitol, 10% glycerol, and 0.1 mg/ml serum albumin) containing 3 μg of phospho-histone H1 (0.45 μCi) and incubated for 30 min at 30°C with or without 0.1 μM okadaic acid. The reaction was terminated by addition of 300 μl of ice-cold 30% TCA for 30 min, followed by centrifugation at 20,000 × g for 5 min. Duplicate 100-μl aliquots of the supernatant were quantified by Cerenkov counting in a liquid scintillation counter.
Supplementary Material
Acknowledgments
This work was supported by the Howard Hughes Medical Institute and by a grant from the National Institutes of Health to M.L.G. (GM48430).
Abbreviations used:
- GVBD
germinal vesicle breakdown
- MBP
myelin basic protein
- O/N
overnight
- PG
progesterone
- RINGO
rapid inducer of GZ-M in oocytes
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E11-01-0008) on May 5, 2011.
REFERENCES
- Archambault V, Zhao X, White-Cooper H, Carpenter AT, Glover DM. Mutations in Drosophila Greatwall/Scant reveal its roles in mitosis and meiosis and interdependence with Polo kinase. PLoS Genet. 2007;3:e200. doi: 10.1371/journal.pgen.0030200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castilho PV, Williams BC, Mochida S, Zhao Y, Goldberg ML. The M phase kinase Greatwall (Gwl) promotes inactivation of PP2A/B55δ, a phosphatase directed against CDK phosphosites. Mol Biol Cell. 2009;20:4777–4789. doi: 10.1091/mbc.E09-07-0643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dephoure N, Zhou C, Villen J, Beausoleil SA, Bakalarski CE, Elledge SJ, Gygi SP. A quantitative atlas of mitotic phosphorylation. Proc Natl Acad Sci USA. 2008;105:10762–10767. doi: 10.1073/pnas.0805139105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunphy WG, Kumagai A. The cdc25 protein contains an intrinsic phosphatase activity. Cell. 1991;67:189–196. doi: 10.1016/0092-8674(91)90582-j. [DOI] [PubMed] [Google Scholar]
- Ferrigno P, Langan TA, Cohen P. Protein phosphatase 2A1 is the major enzyme in vertebrate cell extracts that dephosphorylates several physiological substrates for cyclin-dependent protein kinases. Mol Biol Cell. 1993;4:669–677. doi: 10.1091/mbc.4.7.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautier J, Minshull J, Lohka M, Glotzer M, Hunt T, Maller JL. Cyclin is a component of maturation-promoting factor from Xenopus. Cell. 1990;60:487–494. doi: 10.1016/0092-8674(90)90599-a. [DOI] [PubMed] [Google Scholar]
- Gharbi-Ayachi A, Labbe JC, Burgess A, Vigneron S, Strub JM, Brioudes E, Van-Dorsselaer A, Castro A, Lorca T. The substrate of Greatwall kinase, Arpp19, controls mitosis by inhibiting protein phosphatase 2A. Science. 2010;330:1673–1677. doi: 10.1126/science.1197048. [DOI] [PubMed] [Google Scholar]
- Gross SD, Lewellyn AL, Maller JL. A constitutively active form of the protein kinase p90Rsk1 is sufficient to trigger the G2/M transition in Xenopus oocytes. J Biol Chem. 2001;276:46099–46103. doi: 10.1074/jbc.C100496200. [DOI] [PubMed] [Google Scholar]
- Haccard O, Lewellyn A, Hartley RS, Erikson E, Maller JL. Induction of Xenopus oocyte meiotic maturation by MAP kinase. Dev Biol. 1995;168:677–682. doi: 10.1006/dbio.1995.1112. [DOI] [PubMed] [Google Scholar]
- Huang W, Kessler DS, Erikson RL. Biochemical and biological analysis of Mek1 phosphorylation site mutants. Mol Biol Cell. 1995;6:237–245. doi: 10.1091/mbc.6.3.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumi T, Walker DH, Maller JL. Periodic changes in phosphorylation of the Xenopus cdc25 phosphatase regulate its activity. Mol Biol Cell. 1992;3:927–939. doi: 10.1091/mbc.3.8.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornbluth S, Sebastian B, Hunter T, Newport J. Membrane localization of the kinase which phosphorylates p34cdc2 on threonine 14. Mol Biol Cell. 1994;5:273–282. doi: 10.1091/mbc.5.3.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumagai A, Dunphy WG. Purification and molecular cloning of Plx1, a Cdc25-regulatory kinase from Xenopus egg extracts. Science. 1996;273:1377–1380. doi: 10.1126/science.273.5280.1377. [DOI] [PubMed] [Google Scholar]
- Lohka MJ, Hayes MK, Maller JL. Purification of maturation-promoting factor, an intracellular regulator of early mitotic events. Proc Natl Acad Sci USA. 1988;85:3009–3013. doi: 10.1073/pnas.85.9.3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohka MJ, Kyes JL, Maller JL. Metaphase protein phosphorylation in Xenopus laevis eggs. Mol Cell Biol. 1987;7:760–768. doi: 10.1128/mcb.7.2.760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loog M, Morgan DO. Cyclin specificity in the phosphorylation of cyclin-dependent kinase substrates. Nature. 2005;434:104–108. doi: 10.1038/nature03329. [DOI] [PubMed] [Google Scholar]
- Maller J, Gautier J, Langan TA, Lohka MJ, Shenoy S, Shalloway D, Nurse P. Maturation-promoting factor and the regulation of the cell cycle. J Cell Sci. 1989;12((suppl)):53–63. doi: 10.1242/jcs.1989.supplement_12.6. [DOI] [PubMed] [Google Scholar]
- Maton G, Lorca T, Girault JA, Ozon R, Jessus C. Differential regulation of Cdc2 and Aurora-A in Xenopus oocytes: a crucial role of phosphatase 2A. J Cell Sci. 2005;118:2485–2494. doi: 10.1242/jcs.02370. [DOI] [PubMed] [Google Scholar]
- Mochida S, Ikeo S, Gannon J, Hunt T. Regulated activity of PP2A-B55δ is crucial for controlling entry into and exit from mitosis in Xenopus egg extracts. EMBO J. 2009;28:2777–2785. doi: 10.1038/emboj.2009.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochida S, Maslen SL, Skehel M, Hunt T. Greatwall phosphorylates an inhibitor of protein phosphatase 2A that is essential for mitosis. Science. 2010;330:1670–1673. doi: 10.1126/science.1195689. [DOI] [PubMed] [Google Scholar]
- Mueller PR, Coleman TR, Kumagai A, Dunphy WG. Myt1: a membrane-associated inhibitory kinase that phosphorylates Cdc2 on both threonine-14 and tyrosine-15. Science. 1995;270:86–90. doi: 10.1126/science.270.5233.86. [DOI] [PubMed] [Google Scholar]
- Nigg EA. Mitotic kinases as regulators of cell division and its checkpoints. Nat Rev Mol Cell Biol. 2001;2:21–32. doi: 10.1038/35048096. [DOI] [PubMed] [Google Scholar]
- Palmer A, Nebreda AR. The activation of MAP kinase and p34cdc2/cyclin B during the meiotic maturation of Xenopus oocytes. Prog Cell Cycle Res. 2000;4:131–143. doi: 10.1007/978-1-4615-4253-7_12. [DOI] [PubMed] [Google Scholar]
- Parker LL, Piwnica-Worms H. Inactivation of the p34cdc2-cyclin B complex by the human WEE1 tyrosine kinase. Science. 1992;257:1955–1957. doi: 10.1126/science.1384126. [DOI] [PubMed] [Google Scholar]
- Peeper DS, Parker LL, Ewen ME, Toebes M, Hall FL, Xu M, Zantema A, Van Der Eb AJ, Piwnica-Worms H. A- and B-type cyclins differentially modulate substrate specificity of cyclin-cdk complexes. EMBO J. 1993;12:1947–1954. doi: 10.1002/j.1460-2075.1993.tb05844.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng A, Yamamoto TM, Goldberg ML, Maller JL. A novel role for Greatwall kinase in recovery from DNA damage. Cell Cycle. 2010;9:4364–4369. doi: 10.4161/cc.9.21.13632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian YW, Erikson E, Li C, Maller JL. Activated polo-like kinase Plx1 is required at multiple points during mitosis in Xenopus laevis. Mol Cell Biol. 1998;18:4262–4271. doi: 10.1128/mcb.18.7.4262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian YW, Erikson E, Maller JL. Mitotic effects of a constitutively active mutant of the Xenopus polo-like kinase Plx1. Mol Cell Biol. 1999;19:8625–8632. doi: 10.1128/mcb.19.12.8625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz EJ, Vilar M, Nebreda AR. A two-step inactivation mechanism of Myt1 ensures CDK1/cyclin B activation and meiosis I entry. Curr Biol. 2010;20:717–723.. doi: 10.1016/j.cub.2010.02.050. [DOI] [PubMed] [Google Scholar]
- Schmitz MH, et al. Live-cell imaging RNAi screen identifies PP2A-B55α and importin-beta1 as key mitotic exit regulators in human cells. Nat Cell Biol. 2010;12:886–893. doi: 10.1038/ncb2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulman BA, Lindstrom DL, Harlow E. Substrate recruitment to cyclin-dependent kinase 2 by a multipurpose docking site on cyclin A. Proc Natl Acad Sci USA. 1998;95:10453–10458. doi: 10.1073/pnas.95.18.10453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y. Serine/threonine phosphatases: mechanism through structure. Cell. 2009;139:468–484. doi: 10.1016/j.cell.2009.10.006. [DOI] [PubMed] [Google Scholar]
- Swingle M, Ni L, Honkanen RE. Small-molecule inhibitors of ser/thr protein phosphatases: specificity, use and common forms of abuse. Methods Mol Biol. 2007;365:23–38. doi: 10.1385/1-59745-267-X:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunquist BJ, Maller JL. Under arrest: cytostatic factor (CSF)-mediated metaphase arrest in vertebrate eggs. Genes Dev. 2003;17:683–710. doi: 10.1101/gad.1071303. [DOI] [PubMed] [Google Scholar]
- Vigneron S, Brioudes E, Burgess A, Labbe JC, Lorca T, Castro A. Greatwall maintains mitosis through regulation of PP2A. EMBO J. 2009;28:2786–2793. doi: 10.1038/emboj.2009.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Stetina JR, Tranguch S, Dey SK, Lee LA, Cha B, Drummond-Barbosa D. alpha-Endosulfine is a conserved protein required for oocyte meiotic maturation in Drosophila. Development. 2008;135:3697–3706. doi: 10.1242/dev.025114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Zhao Y, Li Z, Galas S, Goldberg ML. Greatwall kinase participates in the Cdc2 autoregulatory loop in Xenopus egg extracts. Mol Cell. 2006;22:83–91. doi: 10.1016/j.molcel.2006.02.022. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Haccard O, Wang R, Yu J, Kuang J, Jessus C, Goldberg ML. Roles of Greatwall kinase in the regulation of cdc25 phosphatase. Mol Biol Cell. 2008;19:1317–1327. doi: 10.1091/mbc.E07-11-1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.