Histone H4 is activated by C/EBPβ in mitotic clonal expansion during adipogenesis. C/EBP-binding sites are identified in histone H4 promoters, and H4 expression is suppressed when C/EBPβ is knocked down or its DNA-binding activity is inhibited by A-C/EBP. These results help in our understanding of how C/EBPβ plays important roles in the proliferation of other cells.
Abstract
CCAAT enhancer binding protein β (C/EBPβ) is required for both mitotic clonal expansion (MCE) and terminal differentiation during the 3T3-L1 adipocyte differentiation program. Whereas the mechanism of C/EBPβ during terminal differentiation is well understood, the mechanism of C/EBPβ in MCE is not. We provide evidence that histone H4, the most conserved cell cycle–related histone, the change of which is strictly correlated with DNA content change during the cell cycle, is transcriptionally activated by C/EBPβ during MCE. Expression of histone H4 is increased at 16 h after induction when 3T3-L1 preadipocytes synchronously reenter S phase, which is correlated with the sequential phosphorylation and activation of C/EBPβ, and expression was partially suppressed when A-C/EBP (dominant negative for C/EBP protein) was overexpressed. One C/EBP-binding site was identified in one of the histone H4 gene promoters (hist4h4), confirmed by both electrophoretic mobility shift assay and chromatin immunoprecipitation assay. C/EBP-binding sites were also found in 9 of 11 other histone H4 promoters, which can also be transactivated by C/EBPβ. Knockdown of C/EBPβ by stealth small interfering RNA partially decreased H4 gene expression and arrested cells in G1 phase as indicated by bromodeoxyuridine incorporation and fluorescence-activated cell sorting analysis of DNA content. This study provides new insights into why C/EBPβ is required for MCE during 3T3-L1 adipocyte differentiation and why C/EBPβ plays important roles in the proliferation of other cell types.
INTRODUCTION
When induced to differentiate, growth-arrested 3T3-L1 preadipocytes synchronously reenter the cell cycle, undergo several rounds of division (mitotic clonal expansion [MCE]), and then express genes that produce adipocyte characteristics (Tang and Lane, 1999; Tang et al., 2003a). CCAAT enhancer binding protein β (C/EBPβ) can bind directly to the promoters and activate the expression of CCAAT enhancer binding protein α (C/EBPα) and peroxisome proliferator–activated receptor γ (PPAR γ), transcriptional activators of most adipocyte genes that lead to the adipocyte phenotype (Christy et al., 1991; Zhu et al., 1995; Fajas et al., 1997; Tang et al., 2003b). Previous studies have indicated that C/EBPβ is potentially required for MCE, which is the necessary step for the terminal differentiation of 3T3-L1 preadipocytes (Tang et al., 2003b; Zhang et al., 2004b). MCE is blocked in C/EBPβ (−/−) mouse embryonic fibroblasts (MEFs), whereas wild-type MEFs can undergo both MCE and adipocyte differentiation upon induction with standard differentiation protocol (Tang et al., 2003b); with overexpression of A-C/EBP, the dominant-negative form of C/EBP protein, which lacks functional DNA-binding and transactivation domains but can form stable inactive heterodimer with C/EBPβ (Greenwel et al., 2000; Zhang et al., 2004a), the S phase entry of MCE is almost totally blocked during the 3T3-L1 adipocyte differentiation program (Zhang et al., 2004b). C/EBPβ also plays important roles in the proliferation of other cell types, such as lobuloalveolar secretory cells (Seagroves et al., 1998), epithelial tumor cells (Zhu et al., 2002), hepatocytes (Trautwein et al., 1996; Greenbaum et al., 1998; Buck et al., 1999), and mammary gland development (Robinson et al., 1998). Although it has been reported that C/EBPβ can activate the promoter of cyclin D1 (Eaton et al., 2001) and that C/EBPβ can also induce the expression of the cyclin-dependent kinase inhibitor p57 (Hirata et al., 2009), the mechanism of its roles in the promotion of G1/S phase transition is not clearly understood.
The critical event for MCE during 3T3-L1 adipocyte differentiation is the transition from G1 phase to S phase. In this transition, histone replication is very important, as is the DNA replication during S phase (Borun et al., 1972). Histone gene transcription occurs constitutively throughout the cell cycle but is up-regulated upon entry of S phase and down-regulated in quiescent cells or at the onset of terminal differentiation (Stein et al., 2006). The changes in histone gene expression occurred concomitantly with modulation of their transcription factors (Pauli et al., 1987). Histone H4, the most highly conserved and strictly cell cycle–regulated nucleosomal protein, has been extensively studied. Both mRNA stability and the transcription rate are involved in histone H4 gene regulation in mammalian cells (Sittman et al., 1983; Heintz, 1991). H4 gene promoter is regulated by four principal protein–DNA interacting domains: two multipartite proximal promoter elements (sites I and II) and with two distal auxiliary domains (sites III and IV). The major cell cycle control proteins that regulate these four domains in histone H4 gene promoters were reported to contain SP1 (Birnbaum et al., 1995), IRF-2 (Aziz et al., 1998a; Vaughan et al., 1998), CDP-cut/HiNF-D (Aziz et al., 1998a, 1998b), HiNF-P (Aziz et al., 1998; Miele et al., 2005, 2007), and HiNF-M (Birnbaum et al., 1995; Aziz et al., 1998; Vaughan et al., 1998).
Our previous findings indicated that C/EBPβ was induced almost immediately after growth-arrested 3T3-L1 preadipocytes were exposed to differentiation inducers. Furthermore, C/EBPβ undergoes sequentially phosphorylation and then becomes activated (Tang et al., 2005). The sequential phosphorylation and activation occurs on the G1/S boundary, which is correlated with histone gene expression, as well as with DNA replication. In this article, we find that C/EBPβ can directly bind to the histone H4 promoters; 10 of 12 mouse histone H4 promoters have potential C/EBP-binding sites and can be transactivated by C/EBPβ. Knockdown of C/EBPβ expression partially decreased the induction of histone H4 expression and subsequently partially blocked MCE.
RESULTS
Inhibition of C/EBPβ by A-C/EBP reduced histone H4 expression and mitotic clonal expansion during 3T3-L1 adipocyte differentiation
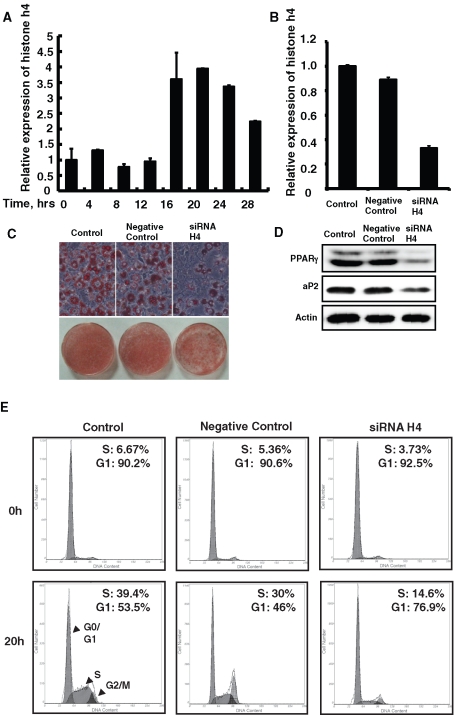
After the induction of differentiation, growth-arrested 3T3-L1 preadipocytes synchronously reentered the cell cycle, underwent several rounds of MCE, and then underwent terminal adipocyte differentiation. Preadipocytes traversed the G1/S check point at ∼16 h after induction, S phase reached maximum at ∼20 h, and the first round cell division finished at ∼28 h; this was evidenced both by the change in the amount of DNA (Supplemental Figure S1) and the expression of histone H4 (Figure 1A), which was consistent with a previous report (Tang et al., 2003a).
FIGURE 1:
The induction of histone H4 expression was required for mitotic clonal expansion and terminal differentiation of 3T3-L1 preadipocytes. (A) Postconfluent 3T3-L1 preadipocytes were induced to differentiate as described, total RNA was isolated at different time points, and quantitative real-time RT-PCR was performed to detect histone H4 expression. (B–E) 3T3-L1 preadipocytes were transfected with histone H4 siRNA, and 24 h after reaching confluence, cells were induced to differentiate as described. Total RNA was isolated at 20 h after induction, the expression of total H4 was detected by real-time RT-PCR, and 18S rRNA was used as loading control (B). After 8 d of induction, fat droplet accumulation was detected by oil red O staining and photographed (C), and the expression of PPAR γ and 422/aP2 was detected by Western blotting; actin was used as loading control (D). DNA content was analyzed by fluorescence-activated cell sorting, and the percentage of cells in S phase or G0/G1 phase was calculated at 0- and 20-h time points (E).
To define the role of histone H4 induction during MCE, the expression of histone H4 was knocked down by specific RNAi. The knockdown effect was confirmed by quantitative real-time PCR (Figure 1B). Although 3T3-L1 preadipocytes treated with histone H4 small interfering RNA (siRNA) grew slowly than those treated with negative control RNAi, they could still reach postconfluence. When treated with histone H4 siRNA, more cells remained in G0/G1 phase and less cells transited to S phase, based on comparison of cells treated with negative control RNAi (Figure 1E). This indicated that the induction of histone H4 expression is required for MCE. We also found that knockdown of histone H4 expression partially suppressed the terminal differentiation of 3T3-L1 as indicated by oil red O staining of fat droplet accumulation as well as adipocyte gene expression (Figure 1, C and D).
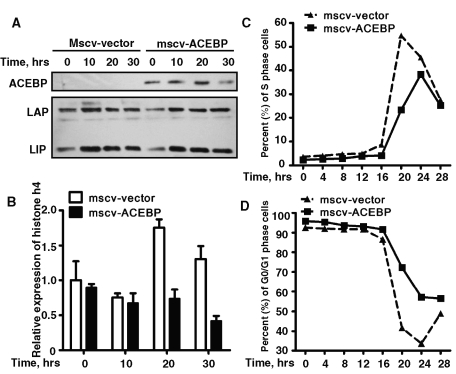
C/EBPβ was previously found to be potentially required for MCE of 3T3-L1 adipocyte differentiation (Tang et al., 2003b; Zhang et al., 2004b). We then explored whether C/EBPβ regulated MCE through histone H4. A-C/EBP, the dominant-negative form of C/EBP protein, which can form a stable inactive heterodimer with C/EBPβ, was overexpressed in 3T3-L1 cells. It has been reported that A-CEBP repressed MCE as well as adipocyte differentiation (Zhang et al., 2004b). Similar experiments were repeated here, and we found that overexpression of A-C/EBP could also partially reduce the induction of histone H4 expression, which indicated that the expression of histone H4 might be regulated by C/EBPβ (Figure 2, A and B). The proportion of cells entering S phase significantly decreased (Figure 2C), whereas more cells stayed in G1 phase (Figure 2D) when A-CEBP was overexpressed.
FIGURE 2:
Overexpression of A-C/EBP partially suppressed the induction of histone H4 expression during MCE of 3T3-L1 adipocyte differentiation. (A, B) 3T3-L1 preadipocytes were infected with retrovirus expression flag–tagged A-C/EBP or with empty vector, cells were induced with differentiation media 2 d after reaching confluence, and the expression of A-C/EBP and C/EBPβ was detected by Western blotting (A); the effect of A-C/EBP on the expression of histone H4 was detected by quantitative real time RT-PCR as indicated in B. (C, D) DNA content was analyzed by fluorescence-activated cell sorting, and the percentage of cells in S phase or G0/G1 phase was analyzed and plotted.
C/EBP-binding site was localized between −167 and −87 on the promoter of Hist4h4
In mammalian cells all histone genes are clustered in several groups in which more than one promoter decides histone expression (including histone H4). Data search from the PubMed website indicated that at least 12 different promoters regulate the transcription of mouse histone H4, and nothing has been reported about which promoters are more important in determining histone H4 expression during the cell cycle.
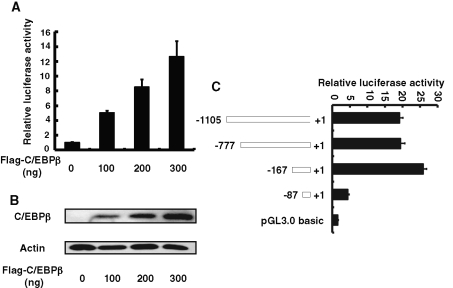
Hist4h4, the only histone H4 in histone cluster 4, was chosen to study the regulation of histones by C/EBPβ during MCE in the following experiments. From −1105 to +1 of hist4h4 promoter was amplified and cloned into pGL3.0 basic plasmid, and the cotransfection experiments indicated that forced expression of C/EBPβ can significantly transactivate the expression of hist4h4 (Figure 3A). Serial truncation assay indicated that the C/EBP response element was located between −167 and −87; the transactivation of hist4h4 by C/EBPβ did not change significantly when the promoter was longer than 167 base pairs, whereas the transactivation dramatically decreased to nearly the basal level when the promoter was truncated to less than 87 base pairs, as indicated in Figure 3C.
FIGURE 3:
The C/EBP response element was localized between −167 and −87 of the hist4h4 promoter. (A) and (B) 3T3-L1 preadipocytes were transiently transfected with hist4h4 promoter reporter construct, along with different amounts of C/EBPβ expression vector, and pRL-TK plasmid was used as internal control; cell extracts were prepared, and luciferase activities were measured and normalized to Renilla activity. (C) 3T3-L1 preadipocytes were transiently transfected with reporter constructs having different truncated hist4h4 promoter, together with C/EBPβ expression vector (300 ng). pRL-TK plasmid was used as internal control, and 48 h later, cell extracts were prepared and luciferase activity was measured and normalized to Renilla activity. Data are presented as means ± SD of at least three independent experiments.
One specific C/EBP-binding site was identified in the hist4h4 promoter
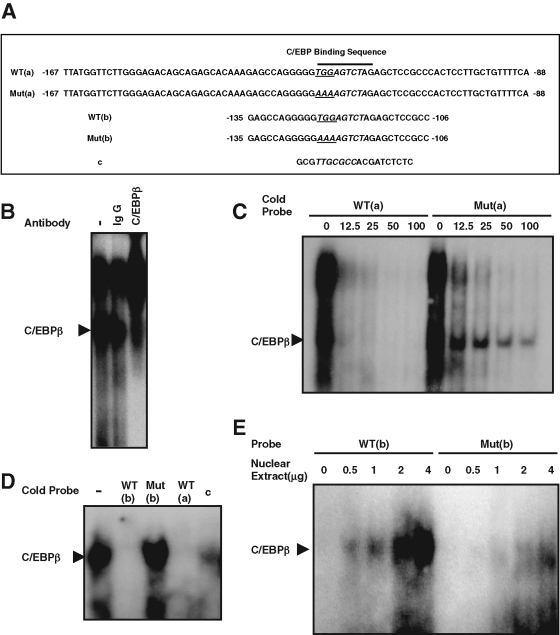
To identify the C/EBP-binding site in the hist4h4 promoter, electrophoretic mobility shift assay (EMSA) was performed with the probe comprising base pairs −167 to −87 (Figure 4A, WT(a)) in the hist4h4 promoter. Two bands were detected. The lower band was significantly supershifted by C/EBPβ antibody but not by nonspecific immunoglobulin G (IgG) (Figure 4B), which indicated that there is a C/EBP-binding site in this 81–base pair probe. Aligning with the classic C/EBP-binding consensus reported before, we found that the region −125 to −117 of hist4h4 was similar to the C/EBP consensus sequence (Figure 4A). Probe Mut(a) was designed with the potential conserved TGG → AAA as underlined and italicized in Figure 4A. Competition experiments indicated that excess unlabeled WT(a) could easily compete with both of the two bands; however, unlabeled Mut(a) with the potential C/EBP-binding site mutated could compete with the upper nonspecific band, while the lower band was very hard to compete away (Figure 4C). To further identify the C/EBP-binding site, a probe WT(b) was designed that only comprised the region −135 to −106 of the hist4h4 promoter, whereas the mutant probe Mut(b) had the same mutation TGG → AAA as Mut(a) as indicated in Figure 4A. Only one band was detected by EMSA with labeled WT(b), and this band can be competed away by unlabeled WT(a) and WT(b) as well as the C/EBP-binding site (Figure 4A, c) in the C/EBPα promoter, but not by Mut(b) (Figure 4D), and the labeled WT(b) could form a specific DNA–protein complex, whereas labeled Mut(b) could not with the same amount of nuclear extract (Figure 4E).
FIGURE 4:
One specific C/EBP-binding site was identified in the hist4h4 promoter. (A) The sequence of five oligonucleotides was presented for following EMSA. Postconfluent 3T3-L1 preadipocytes were induced to differentiate, and nuclear extract was prepared at 20 h. (B) EMSA was performed with probe WT(a); the lower band in lane 1 can be supershifted with specific C/EBPβ antibody in lane 3 but not by nonspecific IgG in lane 2. (C) Both bands (including the lower C/EBPβ band and the upper nonspecific band) were with equal efficiently competed away by cold oligonucleotide WT(a), whereas the lower C/EBPβ band was much more difficult to compete away than the upper nonspecific band by cold mutant oligonucleotide Mut(a), in which the C/EBP-binding site was mutated. (D) WT(b) was used as probe, and in lanes 2–5, 100-fold of cold probe WT(b), Mut(b), WT(a), and c were used for competition. (E) WT(b) and Mut(b) were used as probes, and EMSA was performed with 0–4 μg of nuclear extract EMSA.
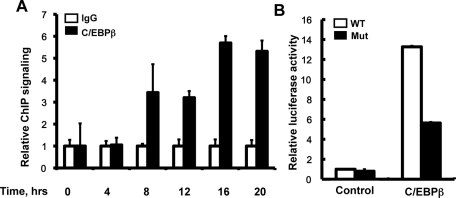
Chromatin immunoprecipitation combined with quantitative PCR (ChIP-qPCR) was performed to further confirm that C/EBPβ could specifically be recruited to hist4h4 promoter before 3T3-L1 preadipocytes synchronously reenter the S phase of MCE (Figure 5A), and the cotransfection experiments indicated that the transactivation by C/EBPβ was significantly decreased (although not totally), whereas the C/EBP-binding site identified earlier was mutated (Figure 5B).
FIGURE 5:
Binding of C/EBPβ to the hist4h4 promoter was confirmed by ChIP. (A) Postconfluent 3T3-L1 preadipocytes were induced to differentiate as described, ChIP-qPCR was performed at different time points after induction with anti-C/EBPβ antibody, and nonspecific IgG was used as negative control. Data are normalized to the IgG controls at each time point. An area of the insulin gene served as a negative control. (B) 3T3-L1 preadipocytes were transiently transfected with wt-1105 pGL3.0-hist4h4 promoter plasmid (open bar) and its mutant plasmid (closed bar) in which the C/EBP-binding site was mutated from TTG to AAA, together with pCDNA3.1-C/EBPβ plasmid. Forty-eight hours later, cell extracts were prepared, and luciferase activity was measured and normalized to Renilla activity. Data are presented as means ± SD of at least three independent experiments.
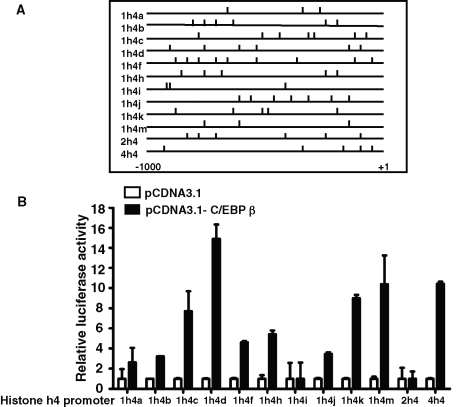
Further analysis indicated that there was more than one potential C/EBP-binding site in the hist4h4 promoter (Figure 6A), and more than one C/EBP-binding site was found in almost all 12 mouse histone H4 promoters as far upstream as 1000 base pairs starting from the transcription start point (Figure 6A). Cotransfection assays indicated that at least 10 of the 12 histone H4 promoters could be significantly transactivated by C/EBPβ (Figure 6B).
FIGURE 6:
Ten of 12 histone H4 promoters could be transactivated by C/EBPβ. (A) Analysis of the potential C/EBP-binding sites and their relative positions in an ∼1000–base pair length of all 12 histone H4 promoters, including hist4h4. (B) 3T3-L1 preadipocytes were transfected with pGL3.0-H4 promoter plasmids and pRL-TK plasmid, together with C/EBPβ expression plasmid (black bars) or empty vector (open bars). Forty-eight hours later, cell extracts were prepared, and luciferase activity was measured and normalized to Renilla activity. Data are presented as means ± SD of at least three independent experiments.
Knockdown of the expression of C/EBPβ reduced histone H4 expression and partially blocked mitotic clonal expansion, whereas overexpression of C/EBPβ promoted the expression of histone H4 and the terminal differentiation of 3T3-L1 cells
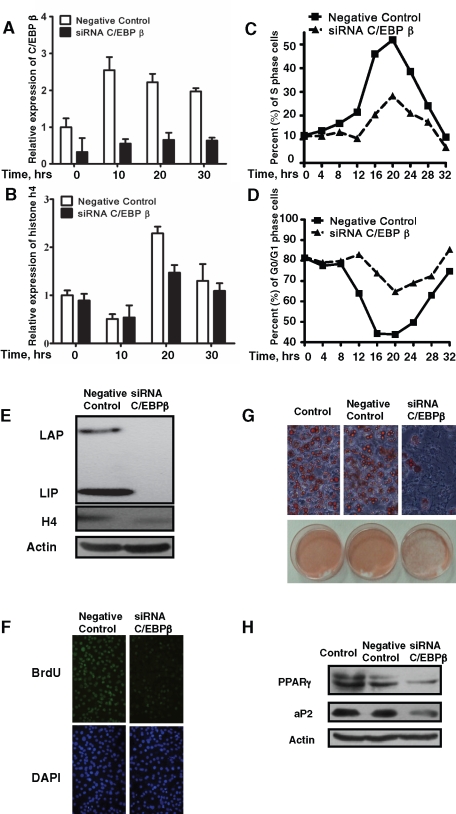
To clearly define the role of C/EBPβ in MCE during the 3T3-L1 adipocyte differentiation program, the expression of C/EBPβ was knocked down by stealth RNAi (Figure 7, A and E). Down-regulation of C/EBPβ reduced the expression of histone H4 both in mRNA level (Figure 7B) and in protein level at 20 h (Figure 7E). Consequently, the proportion of cells entering S phase significantly decreased when C/EBPβ was knocked down (Figure 7C), whereas more cells stayed in G1 phase (Figure 7D), and bromodeoxyuridine (BrdU) labeling further confirmed the results (Figure 7F). Consistent with previous findings, we found that knockdown of C/EBPβ expression partially suppressed 3T3-L1 adipocyte differentiation, as indicated by oil red O staining of fat droplet accumulation (Figure 7G), as well as adipocyte gene expression (Figure 7H).
FIGURE 7:
Knockdown of the expression of C/EBPβ with siRNA partially blocked the induction of histone H4, MCE, and adipocyte differentiation of 3T3-L1 preadipocytes. 3T3-L1 preadipocytes were transfected with C/EBPβ siRNA; 24 h after reaching confluence, cells were induced to differentiate as described. (A, B) Total RNA was isolated at various time points after induction; the expression of C/EBPβ and histone H4 was detected by real-time RT-PCR, with 18S rRNA used as loading control. (C, D) At different time points the DNA content was analyzed by fluorescence-activated cell sorting, and the percentages of cells in S phase or G0/G1 phase were calculated and plotted. (E) The expression of C/EBPβ and histone H4 was detected by Western blotting. (F) At 18 h after induction, a BrdU labeling experiment was performed and visualized with fluorescence microscopy. (G, H) After 8 d of induction, fat droplet accumulation was detected by oil red O staining, and the expression of PPAR γ and 422/aP2 was detected by Western blotting. Actin was used as loading control.
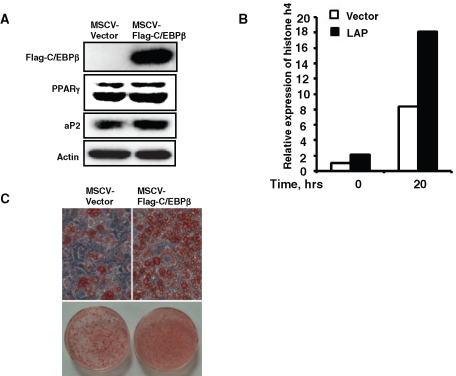
To further confirm the role of C/EBPβ in the induction of histone H4 expression and terminal differentiation of 3T3-L1 preadipocytes, C/EBPβ was overexpressed in 3T3-L1 preadipocytes. We found that overexpression of C/EBPβ promoted the induction of histone H4 expression (Figure 8B) and accelerated adipocyte differentiation, as indicated by oil red O staining of fat droplet accumulation (Figure 8C), as well as adipocyte gene expression (Figure 8A).
FIGURE 8:
Overexpression of C/EBPβ promoted histone H4 expression and accelerated 3T3-L1 adipocyte differentiation. 3T3-L1 preadipocytes were infected with retrovirus expression flag–tagged C/EBPβ or with empty vector; cells were induced with differentiation media 2 d after reaching confluence, and the expression of C/EBPβ was detected by Western blotting (A); the effect of C/EBPβ on the expression of histone H4 was detected by quantitative real-time RT-PCR (B). Eight days after induction, fat droplet accumulation was detected by oil red O staining (C), and the expression of PPAR γ and 422/aP2 was detected by Western blotting; actin was used as loading control (A).
DISCUSSION
C/EBPβ plays an important role in the proliferation of several cell types, including MCE during 3T3-L1 adipocyte differentiation. We found that C/EBPβ was involved in MCE during 3T3-L1 preadipocyte differentiation by regulating the expression of histone H4 at the transcription level by directly binding to the C/EBP element of histone H4 promoter. We found the following: 1) Knockdown of histone H4 expression repressed the MCE and terminal differentiation of 3T3-L1 preadipocytes, and overexpression of A-C/EBP reduced the induction of histone H4 expression, as well as MCE (Figures 1 and 2). 2) One specific C/EBP-binding site was identified in the region –125 to –117 in the hist4h4 promoter by EMSA and ChIP assays (Figures 3–5), whereas 10 of 12 mouse histone H4 promoters have more than one potential C/EBP-binding site and can be transactivated by C/EBPβ (Figure 6). 3) Knockdown of the expression of C/EBPβ by specific stealth RNAi decreases histone H4 induction and partially blocks MCE as well as adipocyte differentiation of 3T3-L1 preadipocytes (Figure 7), whereas overexpression of C/EBPβ activates histone H4 expression (Figure 8).
The cell cycle is regulated by sequential interactions between cyclins and cyclin-dependent kinases (CDKs) (Tang and Lane, 1999), and cyclin A or E /CDK2 complex at the R-point is the initiator of the G1/S phase boundary (D’Urso et al., 1990; Cardoso et al., 1993; Tang and Lane, 1999). Evidence has been provided that activated CDK2 regulates both DNA replication and histone biosynthesis (Stein et al., 2006). When stimulated by growth factors, activated CDK2 activates the transcription factor E2F by hyperphosphorylation and inactivates pRB, and E2F is a transcription activator for gene expression related to DNA replication; in another way, CDK2 stimulates the function of histone gene expression–related transcription factors, such as SP1 (Birnbaum et al., 1995), IRF-2 (Aziz et al., 1998a; Vaughan et al., 1998), CDP-cut/HiNF-D (Aziz et al., 1998a, 1998b), HiNF-P (Aziz et al., 1998a; Miele et al., 2005, 2007), and HiNF-M (Birnbaum et al., 1995; Aziz et al., 1998b; Vaughan et al., 1998), leading to histone biosynthesis (Aziz et al., 1998a, 1998b; Vaughan et al., 1998; Miele et al., 2005, 2007). Stringent coupling between histone biosynthesis and DNA replication is very important to normal G1/S phase transition. DNA and histone replication should be synchronous, and activated CDK2 is the key regulator (Borun et al., 1972; Dou et al., 1993).
C/EBPβ and C/EBPα play opposite roles in cell proliferation, including liver regeneration, mammary gland development, and MCE during 3T3-L1 preadipocyte differentiation. Whereas C/EBPβ is required for cell proliferation, C/EBPα is a strong inhibitor of cell proliferation (Greenbaum et al., 1998; Robinson et al., 1998; Seagroves et al., 1998; Zhang et al., 2004b). C/EBPα is a direct inhibitor of CDK2 by competing for the interaction between cyclin A and CDK2, and the expression of C/EBPα causes cell growth arrest (Wang et al., 2001). Research from our group and Peter Johnson's group indicated that C/EBPβ is a downstream target gene for CDK2. Findings from Johnson's group indicated that phosphorylation of C/EBPβ by CDK2 is required to promote Ras-induced transformation of NIH 3T3 cells. Our previous study indicated that sequential phosphorylation on Thr-188/Ser-184/Thr-179 of C/EBPβ plays an important role in the acquisition of DNA-binding activity, and phosphorylation on Thr-188 by CDK2 is the priming event in the S phase of MCE (Tang et al., 2005; Li et al., 2007). In this article, we found that C/EBPβ is the transcription activator for the histone H4 gene, and CDK2 can regulate histone H4 expression through regulating the phosphorylation and activation of C/EBPβ. This will help in understanding why C/EBPβ is required for proliferation for many cell types.
MATERIALS AND METHODS
Cell culture and induction of differentiation
3T3-L1 preadipocytes were propagated and maintained in DMEM containing 10% (vol/vol) calf serum. Two-day postconfluent (designated day 0) cells were induced to differentiate with DMEM containing 10% (vol/vol) fetal bovine serum (FBS), 1 μg/ml insulin (I), 1 μM dexamethasone (D), and 0.5 mM 3-isobutyl-1-methyl-xanthine (M) until day 2. Cells were then fed with DMEM supplemented with 10% FBS and 1 μg/ml insulin for 2 d, after which they were fed every other day with DMEM containing 10% FBS. Expression of adipocyte genes and acquisition of adipocyte phenotype begins on day 3 and reaches maximum by day 8.
Electrophoretic mobility shift assay
Nuclei were isolated, and nuclear extracts were prepared by using 1× NUN buffer containing 0.3 M NaCl, 1 M urea, 1% Nonidet P-40, 25 mM HEPES (pH 7.9), and 1 mM dithiothreitol (DTT). Protein concentration was determined by the Bradford method (Bio-Rad, Hercules, CA). EMSA was performed essentially as described previously with the following modification. Reaction mixtures containing ∼0.25 ng of 32P-labeled oligonucleotide, 2 μg of poly(dI-dC), and 10 μg of nuclear extract protein in 30 μl of buffer (10 mM HEPES, 0.1 mM EDTA, 5% glycerol, 100 mM NaCl, 0.3 M urea, 0.3% Nonidet P-40) were incubated on ice for 15 min and at room temperature for another 15 min and were then separated on 5% polyacrylamide gels in 0.5× TBE (44.5 mM Tris, 44.5 mM boric acid, 1 mM EDTA [pH 8.3]). For competition experiments, a 0- to 100-fold excess of unlabeled competitor was added to reaction mixtures before the addition of labeled probe. For supershift experiments, 1 μl of anti-C/EBPβ monoclonal antibody (mAb) (clone H-7, ∼2 μg of IgG protein; Santa Cruz Biotechnology, Santa Cruz, CA,) was added to the reaction mixture before the addition of labeled probe. The sequences for the oligonucleotide probes are as follows:
WT(a): −167TTATGGTTCTTGGGAGACAGCAGAGCACAAAGAGCCAGGGGG TGGAGTCTAGAGCTCCGCCCACTCCTTGCTGTTTTCA −88; WT(b): −135 GAGC CAGGGGGTGGAGTCTAGAGCTCCGCC −106. Mut(a): −167 TTATGGTTCTTGG GAGACAGCAGAGCACAAAGAGCCAGGGGGAAAAGTCTAGAGCTCCGCCC ACTCCTTGCTGTTTTCA −88. Mut(b): −135 GAGCCAGGGGGAAAAGTCT AGAGCTCCGCC −106. The C/EBP-binding site is indicated in italics, and the mutant nucleic acids are underlined.
Transfection analysis of histone H4 promoter
The 12 different histone h4 promoter fragments were amplified with the following primers and cloned into the KpnI and MluI sites of the pGL3-Basic luciferase vector (Promega, Madison, WI):
Hist1h4a (NP_835499)
Upstream: TACCAGAAGTCGACCGAGCTGCTGATC
Downstream: GTCTTAGGAAGAATGGAAAATGGAGTCA
Hist1h4b (NP_835500)
Upstream: AAAGGTGCTGCTTACAGACTTGCTCTAT
Downstream: AGTTAAATTCTTACAAGCTTTCAGTAGG
Hist1h4c (NP_835515)
Upstream: TGCATTCATGGGTGCCATTGAGATCAG
Downstream: GGTTATGACAGCAGGGAGACAGCAAT
Hist1h4d (NP_783585)
Upstream: CAGAAACTCAGAGTCTTTGAATTCTGT
Downstream: GTTAGCAAACTAGGAAGGAAGCTCACT
Hist1h4f (NP_783586)
Upstream: CCTTAAGTGACCTTTGAGTTCCTTGG
Downstream: GGCTATGGAGCACTGAGTTGAAATTC
Hist1h4h (NP_694813)
Upstream: cagagatcgatttacctcacgaactta
Downstream: GGTGTACTTGCTGAAGAAAAAACCTCA
Hist1h4i (NP_783587)
Upstream: GTAGGTTTCCTTTTTAGCAAGTTAGGC
Downstream: GATGAAGACAAGATTGTACTGCTCTAA
Hist1h4j (NP_835582)
Upstream: GGAGACCCAGTGATTCAGATGACGGT
Downstream: GACTGCACTCAGGACAGCTCAGAACA
Hist1h4k (NP_835583)
Upstream: GACCAAGAAGACAACCAGCATTAGAACCT
Downstream: GACTGCACTCAGGACAGCTCAGAACAG
Hist1h4m (NP_783588)
Upstream: AACGACGAGGAGCTCAACAAGCTG
Downstream: GATGAAGACAAAACTGTACTGCTCTAA
Hist2h4 (NP_291074)
Upstream: GCCTAAGGTGACTCAAAACCTACATCT
Downstream: CTGAAACACCAGCTGGTGAGGCATTGGA
Hist4h4 (NP_783583)
Upstream: ACTACAAACTAAACGCATTTATCCTGCT
Downstream: TCCTCGTCCTGACATATTAGTAAACAAC
All 12 promoters have been sequenced and authenticity verified. The hist4h4 promoter and its truncated forms were generated by PCR with following upstream primers and the downstream primer used for hist4h4 as described earlier:
5′- CCATGTACTTCTATCTATGTACATCTT-3′ for 777-Luc
5′- TTATGGTTCTTGGGAGACAGCAGAGCAC -3′ for 167-Luc
5′- TCAGGTCCGCTCCCAGGAAATATAAGCT -3′ for 87-Luc
KpnI and MluI restriction sites were added to the upstream and downstream primers, respectively, when designing the primers. The mutant hist4h4 promoter containing a 3–base pair substitution in the C/EBP-binding element (AAAAGTCTA) was generated with a KOD-Plus-Mutagenesis Kit (Toyobo, Osaka, Japan). Transfection experiments were performed with the transfection kit Lipofectamine 2000 (Invitrogen, Carlsbad, CA) following the manufacturer's instruction; luciferase activities were normalized to internal control Renilla luciferase activity.
Western blotting
Cells were lysed with lysis buffer containing 2% SDS, 10 mM DTT, 50 mM Tris-HCl, pH 6.8, 10% glycerol, 0.002% bromophenol blue, and 1× protease inhibitor mixture. Equal amounts of protein were separated by SDS–PAGE and transferred to polyvinylidene fluoride membranes (Millipore, Billerica, MA), immunoblotted with antibodies (anti-H4 mAb was from Millipore, antiactin mAb from Sigma-Aldrich [St. Louis, MO], anti-BrdU mouse antibody from Sigma-Aldrich), and visualized with horseradish peroxidase–coupled secondary antibodies.
RT-PCR and real-time quantitative PCR
Total RNA was isolated using the TRIzol reagent (Invitrogen) according to the manufacturer's instruction. First-strand cDNA synthesis was performed using a RevertAid First Strand cDNA Synthesis Kit (Fermentas, Glen Burnie, MD) with specific RT primer for all histone h4 genes: CCTGGCGCTTGAGCGCGT. PCR reactions were performed using the synthesized cDNA as the template in a 25-μl reaction mixture containing specific primers for all mouse h4 genes (upstream, 5′-gtgatccgcgacgccgtca-3′; downstream, 5′-CTGGCGCTTGACGCGTAC-3′). Mouse 18S rRNA (NR_003278.1) (upstream, 5z-CGCCGCTAGAGGTGAAATTCT-3′; downstream, 5′-CATTCTTGGCAAATGCTTTCG-3′) was used as control. The PCR was carried out following a cycling protocol: an initial denaturation step at 95°C for 2 min followed by 21 cycles each of a denaturation at 94°C (30 s), annealing at 58°C (20 s), and elongation at 72°C (15 s). The reaction products were resolved by electrophoresis on a 1.5% agarose gel and visualized with ethidium bromide. Real-time quantitative PCRs were performed with 2× PCR Master Mix (Power SYBR Green; Applied Biosystems, Foster City, CA) on a Bio-Rad Q5 instrument (Bio-Rad). The threshold cycles (Ct) for the histone h4 gene and 18S rRNA control signals were determined in triplicate experiments, and the relative RNA quantity was calculated using the comparative Ct method. Primers used for real-time quantitative PCR were the same as that used for RT-PCR.
Knockdown of expression of C/EBPβ and histone H4 with siRNA
Synthetic siRNA oligonucleotide specific for C/EBPβ (NT_039207) (5′ to 3′: CCCUGCGGAACUUGUUCAAGCAGCU) and histone H4 (5′ to 3′: gauccgcg acgccgucaccuacatt) were designed and synthesized by Invitrogen. 3T3-L1 preadipocytes at 30–50% confluence were transfected with the siRNA oligonucleotide by using Lipofectamine RNAiMAX (Invitrogen). Thirty-six hours after cells reached confluence, they were subjected to the standard differentiation protocol as described earlier, and at various times thereafter cells were prepared for the test. Stealth siRNA Negative Control Duplexes (Invitrogen) were used as a negative control.
BrdU labeling and immunofluorescence microscopy
For BrdU labeling, 3T3-L1 preadipocytes plated on glass coverslips were induced to differentiate by using the standard differentiation protocol, and 18 h after induction (during S phase) cells were pulse labeled with 30 μg/ml BrdU for 2 h and then shifted to normal medium. On day 3, coverslips were fixed in 70% ethanol for 30 min and incubated in 100% methanol for 10 min at room temperature, after which they were treated with 1.5 M HCl, blocked with 0.2% Triton in phosphate-buffered saline (PBS) for 5 min, incubated with anti-BrdU primary antibody (1:500) (Sigma-Aldrich) in the same buffer for 2 h at room temperature, and incubated with fluorescein isothiocyanate–conjugated secondary antibody (1:200) with 0.1 μg/ml 4′,6-diamidno-2-phenylindole for 1 h at room temperature. After each step, cells were washed with PBS three times, and the coverslips were mounted on slides and were visualized by fluorescence microscopy.
Oil red O staining
Cells were washed three times with PBS and then fixed for 2 min with 3.7% formaldehyde. Oil red O (0.5% in isopropanol) was diluted with water (3:2), filtered through a 0.45-µm filter, and incubated with the fixed cells for 1h at room temperature. Cells were washed with water, and the stained fat droplets in the cells were visualized by light microscopy.
Fluorescence-activated cell sorting analysis
3T3-L1 preadipocytes were transfected with Stealth siRNA Negative Control Duplexes (Invitrogen) or C/EBPβ siRNA as described earlier. After they were treated with differentiation inducer, 3T3-L1 cells were harvested at every 4 h until 28 h. Cells were further trypsinized, washed with 1× PBS, and fixed with 2% (wt/vol) paraformaldehyde in 1× PBS. They were treated with 0.5 mg/ml RNase A for 1 h at room temperature and incubated with 0.1 mg/ml propidium iodide (Sigma-Aldrich) for 45 min at 37°C. DNA content was determined by flow cytometry (Bio-Rad).
Chromatin immunoprecipitation analysis
Cells were fixed with 1% formaldehyde for 10 min at room temperature with swirling. Glycine was added to a final concentration of 0.125 M, and the incubation was continued for an additional 5 min. Cells were washed twice with ice-cold PBS, harvested by scraping, pelleted, and resuspended in 1 ml of SDS lysis buffer (50 mM Tris-HCl, pH 8.0, 1% SDS, 10 mM EDTA, and protease inhibitors). Samples were sonicated eight times for 30 s each with an interval of 30 s with a Bioruptor sonicator (Diagenode, Denville, NJ). Samples were centrifuged at 14,000 g at 4°C × for 10 min. After removal of an input aliquot (whole-cell extract), supernatants were diluted 10-fold in ChIP dilution buffer (20 mM Tris-HCl, pH 8.0, 150 mM NaCl, 2 mM EDTA, 1% TritonX-100, and complete protease inhibitor tablets). Samples were immunoprecipitated using C/EBPβ antibodies (sc7962; Santa Cruz Biotechnologies) or the nonspecific IgG control (Abcam, Cambridge, MA). Immunoprecipitated samples were eluted and reverse cross linked by incubation overnight at 65°C in elution buffer (50 mM Tris-HCl, pH 8.0, 10 mM EDTA, 1% SDS). Genomic DNA was then extracted with a PCR purification kit (Qiagen, Valencia, CA). Purified DNA was subjected to quantitative PCR amplification using the specific primer for the C/EBP-binding element present in the hist4h4 gene promoter (sense primer, 5-TTATGGTTCTTGGGAGACAG-3; antisense primer, 5-ATTGAAAACAGCAGGAGTG-3). Primers (sense primer, CTTCAGCCC AGTTGACCAAT; antisense primer, AGGGAGGAGGAAAGCAGAAC) in an area of the insulin gene promoter serve as a negative control.
Supplementary Material
Acknowledgments
This research was supported partially by National Key Basic Research Project Grants 2009CB825604 and 2011CB910201, the State Key Program of National Natural Science Foundation 31030048, Shanghai Key Science and Technology Research Project 08dj1400603 and 10JC1401000 (to Q.Q.T.); and National Natural Science Foundation Grant 30870510 and Shanghai Rising Star Program 08QA14012 (to X.L.). The Department of Biochemistry and Molecular Biology is supported by the Shanghai Leading Academic Discipline Project, Project Number B110.
Abbreviations used:
- C/EBPα
CCAAT enhancer binding protein α
- C/EBPβ
CCAAT enhancer binding protein β
- EMSA
electrophoretic mobility shift assay
- MCE
mitotic clonal expansion
- MEF
mouse embryonic fibroblasts
- PPAR γ
peroxisome proliferator–activated receptor γ
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E10-11-0912) on May 11, 2011.
REFERENCES
- Aziz F, van Wijnen AJ, Stein JL, Stein GS. HiNF-D (CDP-cut/CDC2/cyclin A/pRB-complex) influences the timing of IRF-2-dependent cell cycle activation of human histone H4 gene transcription at the G1/S phase transition. J Cell Physiol. 1998a;177:453–464. doi: 10.1002/(SICI)1097-4652(199812)177:3<453::AID-JCP8>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Aziz F, van Wijnen AJ, Vaughan PS, Wu SJ, Shakoori AR, Lian JB, Soprano KJ, Stein JL, Stein GS. The integrated activities of IRF-2 (HiNF-M), CDP/cut (HiNF-D) and H4TF-2 (HiNF-P) regulate transcription of a cell cycle controlled human histone H4 gene: mechanistic differences between distinct H4 genes. Mol Biol Rep. 1998b;25:1–12. doi: 10.1023/a:1006888731301. [DOI] [PubMed] [Google Scholar]
- Birnbaum MJ, et al. Functional role for Sp1 in the transcriptional amplification of a cell cycle regulated histone H4 gene. Biochemistry. 1995;34:7648–7658. doi: 10.1021/bi00023a011. [DOI] [PubMed] [Google Scholar]
- Borun TW, Pearson D, Paik WK. Studies of histone methylation during HELA S-3 cell cycle. J Biol Chem. 1972;247:4288. [PubMed] [Google Scholar]
- Buck M, Poli V, Van Der Geer P, Chojkier M, Hunter T. Phosphorylation of rat serine 105 or mouse threonine 217 in C/EBP beta is required for hepatocyte proliferation induced by TGF alpha. Mol Cell. 1999;4:1087–1092. doi: 10.1016/s1097-2765(00)80237-3. [DOI] [PubMed] [Google Scholar]
- Cardoso MC, Leonhardt H, Nadal-Ginard B. Reversal of terminal differentiation and control of DNA replication: cyclin A and Cdk2 specifically localize at subnuclear sites of DNA replication. Cell. 1993;74:979–992. doi: 10.1016/0092-8674(93)90721-2. [DOI] [PubMed] [Google Scholar]
- Christy RJ, Kaestner KH, Geiman DE, Lane MD. CCAAT/enhancer binding protein gene promoter: binding of nuclear factors during differentiation of 3T3-L1 preadipocytes. Proc Natl Acad Sci USA. 1991;88:2593–2597. doi: 10.1073/pnas.88.6.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dou QP, Levin AH, Zhao SC, Pardee AB. Cyclin-E and cyclin-A as candidates for the restriction point protein. Cancer Res. 1993;53:1493–1497. [PubMed] [Google Scholar]
- D’Urso G, Marraccino RL, Marshak DR, Roberts JM. Cell cycle control of DNA replication by a homologue from human cells of the p34cdc2 protein kinase. Science. 1990;250:786–791. doi: 10.1126/science.2173140. [DOI] [PubMed] [Google Scholar]
- Eaton EM, Hanlon M, Bundy L, Sealy L. Characterization of C/EBP beta isoforms in normal versus neoplastic mammary epithelial cells. J Cell Physiol. 2001;189:91–105. doi: 10.1002/jcp.1139. [DOI] [PubMed] [Google Scholar]
- Fajas L, et al. The organization, promoter analysis, and expression of the human PPAR gamma gene. J Biol Chem. 1997;272:18779–18789. doi: 10.1074/jbc.272.30.18779. [DOI] [PubMed] [Google Scholar]
- Greenbaum LE, Li W, Cressman DE, Peng Y, Ciliberto G, Poli V, Taub R. CCAAT enhancer-binding protein beta is required for normal hepatocyte proliferation in mice after partial hepatectomy. J Clin Invest. 1998;102:996–1007. doi: 10.1172/JCI3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwel P, Tanaka S, Penkov D, Zhang W, Olive M, Moll J, Vinson C, Di Liberto M, Ramirez F. Tumor necrosis factor alpha inhibits type I collagen synthesis through repressive CCAAT/enhancer-binding proteins. Mol Cell Biol. 2000;20:912–918. doi: 10.1128/mcb.20.3.912-918.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heintz N. The regulation of histone gene expression during the cell cycle. Biochim Biophys Acta. 1991;1088:327–339. doi: 10.1016/0167-4781(91)90122-3. [DOI] [PubMed] [Google Scholar]
- Hirata M, Kugimiya F, Ohba S, Kawamura N, Ogasawara T, Kawasaki Y, Ikeda T, Nakamura K, Chung U, Kawaguchi H. C/EBP beta/p57(Kip2) signaling maintains transition from proliferation to hypertrophic differentiation of chondrocytes during skeletal growth. Bone. 2009;44 (suppl 1):S43–S44. [Google Scholar]
- Li X, Kim JW, Gronborg M, Urlaub H, Lane MD, Tang QQ. Role of cdk2 in the sequential phosphorylation/activation of C/EBPbeta during adipocyte differentiation. Proc Natl Acad Sci USA. 2007;104:11597–11602. doi: 10.1073/pnas.0703771104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miele A, et al. HiNF-P directly links the cyclin E/CDK2/p220NPAT pathway to histone H4 gene regulation at the G1/S phase cell cycle transition. Mol Cell Biol. 2005;25:6140–6153. doi: 10.1128/MCB.25.14.6140-6153.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miele A, Medina R, van Wijnen AJ, Stein GS, Stein JL. The interactome of the histone gene regulatory factor HiNF-P suggests novel cell cycle related roles in transcriptional control and RNA processing. J Cell Biochem. 2007;102:136–148. doi: 10.1002/jcb.21284. [DOI] [PubMed] [Google Scholar]
- Pauli U, Chrysogelos S, Stein G, Stein J, Nick H. Protein–DNA interactions in vivo upstream of a cell cycle–regulated human H4 histone gene. Science. 1987;236:1308–1311. doi: 10.1126/science.3035717. [DOI] [PubMed] [Google Scholar]
- Robinson GW, Johnson PF, Hennighausen L, Sterneck E. The C/EBPbeta transcription factor regulates epithelial cell proliferation and differentiation in the mammary gland. Genes Dev. 1998;12:1907–1916. doi: 10.1101/gad.12.12.1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seagroves TN, Krnacik S, Raught B, Gay J, Burgess-Beusse B, Darlington GJ, Rosen JM. C/EBPbeta, but not C/EBPalpha, is essential for ductal morphogenesis, lobuloalveolar proliferation, and functional differentiation in the mouse mammary gland. Genes Dev. 1998;12:1917–1928. doi: 10.1101/gad.12.12.1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sittman DB, Graves RA, Marzluff WF. Histone mRNA concentrations are regulated at the level of transcription and mRNA degradation. Proc Natl Acad Sci USA. 1983;80:1849–1853. doi: 10.1073/pnas.80.7.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein GS, van Wijnen AJ, Stein JL, Lian JB, Montecino M, Zaidi SK, Braastad C. An architectural perspective of cell-cycle control at the G1/S phase cell-cycle transition. J Cell Physiol. 2006;209:706–710. doi: 10.1002/jcp.20843. [DOI] [PubMed] [Google Scholar]
- Tang QQ, Gronborg M, Huang HY, Kim JW, Otto TC, Pandey A, Lane MD. Sequential phosphorylation of CCAAT enhancer-binding protein beta by MAPK and glycogen synthase kinase 3 beta is required for adipogenesis. Proc Natl Acad Sci USA. 2005;102:9766–9771. doi: 10.1073/pnas.0503891102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang QQ, Lane MD. Activation and centromeric localization of CCAAT/enhancer-binding proteins during the mitotic clonal expansion of adipocyte differentiation. Genes Dev. 1999;13:2231–2241. doi: 10.1101/gad.13.17.2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang QQ, Otto TC, Lane MD. Mitotic clonal expansion: a synchronous process required for adipogenesis. Proc Natl Acad Sci USA. 2003a;100:44–49. doi: 10.1073/pnas.0137044100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang QQ, Otto TC, Lane MD. CCAAT/enhancer-binding protein beta is required for mitotic clonal expansion during adipogenesis. Proc Natl Acad Sci USA. 2003b;100:850–855. doi: 10.1073/pnas.0337434100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trautwein C, Rakemann T, Pietrangelo A, Montosi G, Manns MP. C/EBP-beta/LAP controls down-regulation of albumin gene transcription during liver regeneration. J Biol Chem. 1996;27:22262–22270. doi: 10.1074/jbc.271.36.22262. [DOI] [PubMed] [Google Scholar]
- Vaughan PS, Van Der Meijden C, Aziz F, Harada H, Taniguchi T, van Wijnen AJ, Stein JL, Stein GS. Cell cycle regulation of histone H4 gene transcription requires the oncogenic factor IRF-2. J Biol Chem. 1998;273:194–199. doi: 10.1074/jbc.273.1.194. [DOI] [PubMed] [Google Scholar]
- Wang H, Iakova P, Wilde M, Welm A, Goode T, Roesler WJ, Timchenko NA. C/EBPalpha arrests cell proliferation through direct inhibition of Cdk2 and Cdk4. Mol Cell. 2001;8:817–828. doi: 10.1016/s1097-2765(01)00366-5. [DOI] [PubMed] [Google Scholar]
- Zhang JW, Tang QQ, Vinson C, Lane MD. Dominant-negative C/EBP disrupts mitotic clonal expansion and differentiation of 3T3-L1 preadipocytes. Proc Natl Acad Sci USA. 2004a;101:43–47. doi: 10.1073/pnas.0307229101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JW, Tang QQ, Vinson C, Lane MD. Dominant-negative C/EBP disrupts mitotic clonal expansion and differentiation of 3T3-L1 preadipocytes. Proc Natl Acad Sci USA. 2004b;101:43–47. doi: 10.1073/pnas.0307229101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu S, Yoon K, Sterneck E, Johnson PF, Smart RC. CCAAT/enhancer binding protein-beta is a mediator of keratinocyte survival and skin tumorigenesis involving oncogenic Ras signaling. Proc Natl Acad Sci USA. 2002;99:207–212. doi: 10.1073/pnas.012437299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Qi C, Korenberg JR, Chen XN, Noya D, Rao MS, Reddy JK. Structural organization of mouse peroxisome proliferator-activated receptor gamma (mPPAR gamma) gene: alternative promoter use and different splicing yield two mPPAR gamma isoforms. Proc Natl Acad Sci USA. 1995;92:7921–7925. doi: 10.1073/pnas.92.17.7921. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.