Swe1 is a key regulator of mitosis, and its levels are tightly regulated in response to different stress conditions. Budding yeast Dma1 and Dma2 contribute to the control of Swe1 localization, ubiquitylation, and degradation.
Abstract
Timely down-regulation of the evolutionarily conserved protein kinase Swe1 plays an important role in cell cycle control, as Swe1 can block nuclear division through inhibitory phosphorylation of the catalytic subunit of cyclin-dependent kinase. In particular, Swe1 degradation is important for budding yeast cell survival in case of DNA replication stress, whereas it is inhibited by the morphogenesis checkpoint in response to alterations in actin cytoskeleton or septin structure. We show that the lack of the Dma1 and Dma2 ubiquitin ligases, which moderately affects Swe1 localization and degradation during an unperturbed cell cycle with no apparent phenotypic effects, is toxic for cells that are partially defective in Swe1 down-regulation. Moreover, Swe1 is stabilized, restrained at the bud neck, and hyperphosphorylated in dma1Δ dma2Δ cells subjected to DNA replication stress, indicating that the mechanism stabilizing Swe1 under these conditions is different from the one triggered by the morphogenesis checkpoint. Finally, the Dma proteins are required for proper Swe1 ubiquitylation. Taken together, the data highlight a previously unknown role of these proteins in the complex regulation of Swe1 and suggest that they might contribute to control, directly or indirectly, Swe1 ubiquitylation.
INTRODUCTION
Cell cycle progression in eukaryotic cells is driven by cyclin-dependent kinases (Cdks), the activity of which is controlled at various levels. In addition, specific signal transduction pathways called checkpoints delay cell cycle progression in response to different problems in order to preserve genetic stability in proliferating cell populations (Hartwell and Weinert, 1989; Kops et al., 2005). In particular, the morphogenesis checkpoint has been shown to delay the G2/M transition in the budding yeast Saccharomyces cerevisiae by inhibiting degradation of the Swe1 kinase in response to perturbations in bud formation, cell size, actin cytoskeleton, and septin organization (McMillan et al., 1998; Barral et al., 1999; Harvey and Kellogg, 2003; Theesfeld et al., 2003). Cell cycle progression, mitotic spindle elongation, and mitotic entry are also delayed when DNA replication is inhibited, and this response depends on a specific S-phase checkpoint involving the DNA damage checkpoint proteins Mec1 and Rad53 (Weinert et al., 1994). Moreover, a novel pathway controlling yeast cell morphology in response to DNA replication stress has been recently described (Enserink et al., 2006; Smolka et al., 2006), where DNA damage checkpoint proteins appear to promote timely degradation of Swe1, thus restricting bud growth and contributing to maintenance of cell viability.
The budding yeast protein kinase Swe1 inhibits Cdk activity by phosphorylating the conserved Y19 residue of the catalytic subunit of Cdk, Cdc28 (Booher et al., 1993), thus blocking both the switch from polar to isotropic bud growth and nuclear division. This inhibitory phosphorylation is reversed by the Mih1 phosphatase (Russell et al., 1989), leading to Cdc28 activation and entry into mitosis. Cells lacking either Mih1 or Swe1 display normal cell cycle progression in unperturbed conditions, whereas GAL1-SWE1–overexpressing cells are not viable. Indeed, Swe1 overproduction inhibits Cdc28 totally and causes cells to arrest with elongated buds, undivided nuclei (Booher et al., 1993), and monopolar spindles (Lim et al., 1996). Swe1 therefore has a critical role in coordinating cell morphogenesis with nuclear division and is subjected to multiple regulations.
During an unperturbed cell cycle, Swe1 begins to accumulate in the nucleus in S phase. Here it is phosphorylated by the Clb/Cdc28 complexes before being exported to the daughter side of the bud neck (Sia et al., 1998; McMillan et al., 2002; Harvey et al., 2005). Filaments of conserved proteins called septins (Cdc3, Cdc10, Cdc11, Cdc12, and Shs1 in S. cerevisiae) form a dynamic ring structure at the bud neck (for review, see Versele and Thorner, 2005), and this structure is essential for the recruitment of a number of proteins involved in cell cycle progression (Longtine and Bi, 2003; Gladfelter et al., 2005). In particular, the septin ring acts as a platform to recruit Swe1 regulators, such as the Hsl1 protein kinase and its adaptor Hsl7, which are both essential for Swe1 localization to the bud neck and for its phosphorylation (McMillan et al., 1999; Shulewitz et al., 1999; Longtine et al., 2000). In addition, the PAK kinase Cla4 and the polo kinase Cdc5 associate with the septin ring and are involved in Swe1 phosphorylation (Sakchaisri et al., 2004; Asano et al., 2005; Lee et al., 2005). Moreover, the Cdc55 regulatory subunit of protein phosphatase 2A (PP2A) is required for Swe1 degradation, as loss of Cdc55 function causes Swe1 stabilization (Yang et al., 2000). Hyperphosphorylated Swe1 species are probably targeted to ubiquitylation by a still-unidentified ubiquitin ligase and are subsequently degraded via the proteasome to allow mitotic entry (McMillan et al., 2002).
Successful bud formation leads to Swe1 degradation in G2, whereas morphological defects block this degradation, thus delaying entry into mitosis. Swe1 levels are controlled by the morphogenesis checkpoint, whose activation in response to alterations in actin cytoskeleton or septin organization causes Swe1 accumulation and subsequent delay in nuclear division (for review, see Keaton and Lew, 2006). This checkpoint inhibits Swe1 degradation by interfering with the localization of Hsl1 at the bud neck, thus preventing Swe1 recruitment that is mandatory for modifications leading to its degradation (Longtine et al., 2000). Accordingly, the lack of septin localization at the bud neck results in Swe1 stabilization (Barral et al., 1999; Shulewitz et al., 1999; Longtine et al., 2000) and even subtle perturbations in septin structure interfere with Hsl1 and Swe1 localization at the bud neck (Longtine et al., 2000). Moreover, actin depolymerization in budded cells causes both stabilization of Swe1 and Swe1 displacement from the bud neck without altering Hsl1 localization (Longtine et al., 2000), indicating that the activation of the morphogenesis checkpoint prevents Swe1 degradation by interfering with Swe1 localization at the bud neck, thus inhibiting the G2/M transition.
Although our understanding of the complex pathways regulating Swe1 is progressively increasing, relevant molecular details still need to be investigated, such as the involvement of the ubiquitylation pathway and the identity of the related ubiquitin ligase(s). A possible role in this control can be envisaged for the functionally redundant budding yeast proteins Dma1 and Dma2, which belong to the same FHA-RING ubiquitin ligase family as Schizosaccharomyces pombe Dma1 and human Chfr and Rnf8. All of these proteins appear to control different aspects of the mitotic cell cycle, but several molecular details of their functions are still obscure (for review, see Brooks et al., 2008). In particular, the S. cerevisiae Dma proteins are involved in mitotic checkpoints and contribute to control septin ring dynamics and cytokinesis by unknown mechanisms (Fraschini et al., 2004; our unpublished data). Moreover, an in vitro ubiquitin ligase activity of Dma1 and Dma2 has been described (Loring et al., 2008), although their in vivo targets are unknown.
Here we provide genetic and biochemical evidence that the Dma proteins are involved in Swe1 ubiquitylation and contribute to the regulation of Swe1 stability by acting in a step that follows the recruitment of Swe1 to the bud neck and its phosphorylation. This Dma-dependent Swe1 down-regulation, lack of which does not significantly affect unperturbed cell cycle progression when the other Swe1 regulatory pathways are proficient, appears to be crucial for proper response to DNA replication stress.
RESULTS
The Dma proteins control Swe1 protein levels
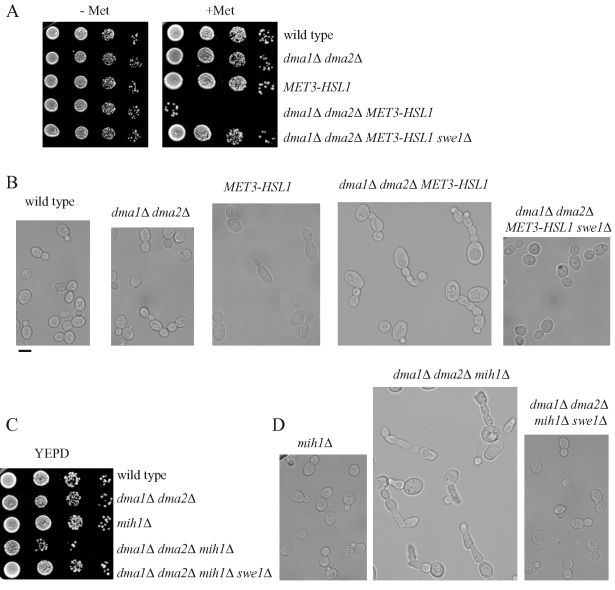
To gain insights into the role of the Dma proteins in cell cycle progression and checkpoints, we looked for synthetic effects between their complete absence (dma1Δ dma2Δ) and available mutations in mitotic genes. Of interest, we found that the lack of Dma proteins was lethal at 25°C for cells that already lacked proteins that negatively regulate Swe1 stability, such as the protein kinase Hsl1 and the Cdc55 regulatory subunit of protein phosphatase 2A. In fact, we could never recover viable dma1Δ dma2Δ hsl1Δ or dma1Δ dma2Δ cdc55Δ spores in meiotic tetrads from diploid strains heterozygous for the deletions under analysis (Table 1). In addition, these lethal effects were suppressed by the deletion of SWE1, indicating that they might be due to Swe1 accumulation (Table 1 and Figure 1A). We then analyzed the terminal phenotype caused by the concomitant absence of the Dma proteins and Hsl1 by using a dma1Δ dma2Δ strain in which the HSL1 gene was replaced by a MET3-HSL1 construct, whose expression can be turned off by addition of methionine that represses the MET3 promoter (Masselot and Surdin-Kerjan, 1977). Methionine addition inhibited dma1Δ dma2Δ MET3-HSL1 cell growth (Figure 1A) and caused cells to arrest with very elongated buds (64% of 204 cells; Figure 1B), a phenotype that resembled the one observed in cells that are unable to degrade Swe1 (Lim et al., 1996). This elongated bud phenotype was completely reversed by deletion of SWE1 (Figure 1B), which also allowed dma1Δ dma2Δ MET3-HSL1 cells to grow normally in the presence of methionine (Figure 1A). Taken together, these genetic interactions suggest that Dma1 and Dma2 might participate, together with Hsl1 and Cdc55, in Swe1 down-regulation.
TABLE 1:
Swe1-dependent effects of the lack of Dma proteins on viability/growth efficiency of hsl1Δ, cdc55Δ, and mih1Δ cells.
| Genotype | Phenotype at 25°C |
|---|---|
| dma1Δ dma2Δ hsl1Δ | Lethal |
| dma1Δ dma2Δ hsl1Δ swe1Δ | Healthy |
| dma1Δ dma2Δ cdc55Δ | Lethal |
| dma1Δ dma2Δ cdc55Δ swe1Δ | Healthy |
| dma1Δ dma2Δ mih1Δ | Sick |
| dma1Δ dma2Δ mih1Δ swe1Δ | Healthy |
Haploid dma1Δ dma2Δ cells were crossed either with hsl1Δ, cdc55Δ, and mih1Δ cells or with hsl1Δ swe1Δ, cdc55Δ swe1Δ, and mih1Δ swe1Δ double-mutant strains. The resulting diploid strains were induced to sporulate, and meiotic segregants of 15 tetrads for each cross were assayed for their ability to form colonies on rich medium at 25°C, followed by genotyping of the viable spores based on the deletion markers. Healthy, spore colony size was undistinguishable from wild type; lethal, no viable spores with the indicated genotypes were found; sick, spores formed very small colonies.
FIGURE 1:
The toxic effects of Dma1/Dma2 lack in cells lacking Hsl1 and Mih1 are counteracted by SWE1 deletion. (A, C) Serial dilutions of stationary-phase cultures of strains with the indicated genotypes were spotted on plates containing either synthetic medium without (−Met) or with (+Met) 2 mM methionine or YEPD (C), which were then incubated for 2 d at 25°C. (B, D) The morphology at 25°C of cells with the indicated genotypes was examined by differential interference contrast (DIC) microscopy. All cell cultures were exponentially growing in YEPD medium, with the exception of those carrying the MET3-HSL1 construct integrated at the HSL1 locus (B), the cellular morphology of which was analyzed after MET3 promoter shutoff for 3 h by 2 mM methionine addition to synthetic growth medium lacking methionine. Bar, 5 μm.
The lack of Dma proteins turned out to be very toxic also for cells lacking the phosphatase Mih1 (Table 1 and Figure 1C), which counteracts Swe1-dependent inihibitory phosphorylation of Cdc28 (Russell et al., 1989). This synthetic effect could be due to the accumulation of the Swe1 kinase together with the loss of Mih1 phosphatase activity in dma1Δ dma2Δ mih1Δ cells, leading to strong Cdc28–cyclin complex inhibition. Accordingly, the low viability and elongated bud phenotypes (83% of 200 cells) of dma1Δ dma2Δ mih1Δ cells were reversed by SWE1 deletion (Figure 1C and D).
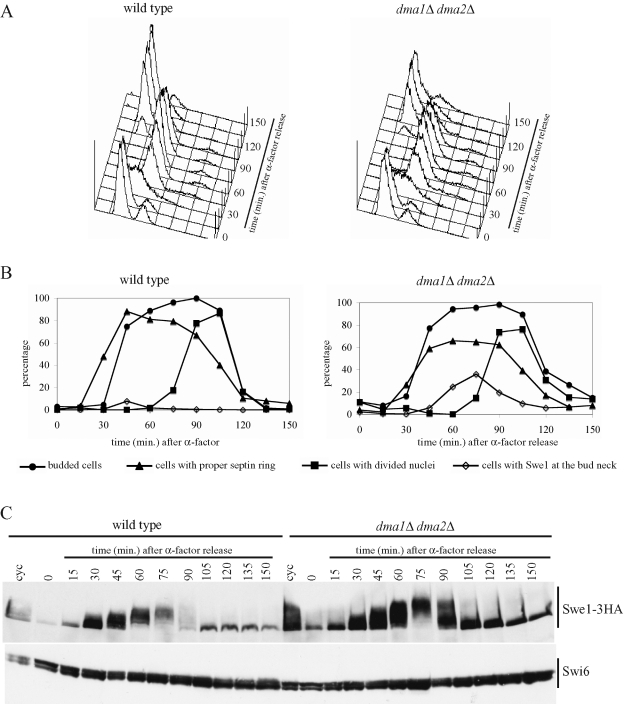
These observations prompted us to compare the levels of the Swe1 protein in wild-type and dma1Δ dma2Δ cells during a single cell cycle. To this purpose, synchronous cultures of cells expressing a functional HA-tagged Swe1 variant (Swe1-3HA) from the SWE1 promoter were released from α-factor–induced G1 arrest, followed by readdition of α-factor 85 min later, when 95% of cells were budded, to arrest cells in the next G1 phase. Samples were taken at different times after the release to monitor the kinetics of DNA replication by fluorescence-activated cell sorting (FACS) analysis (Figure 2A) and budding, nuclear division, and septin ring formation (Figure 2B), as well as Swe1 protein levels (Figure 2C). Wild-type and dma1Δ dma2Δ cells showed very similar profiles of budding, DNA replication, and nuclear division, whereas septin ring deposition appeared to take place with somewhat reduced efficiency, although with similar kinetics, in dma1Δ dma2Δ compared with wild-type cells. Swe1 amount increased in both cultures after release in the cell cycle, concomitantly with cells entering S phase, and decreased as cells started nuclear division. On the basis of the electrophoretic mobility shifts, Swe1 phosphorylation and dephosphorylation (see next paragraph and Figure 3) or other possible posttranslational modifications events seemed to occur on schedule in dma1Δ dma2Δ cells. Nonetheless, these cells were partially defective in targeting Swe1 to degradation, as they showed higher Swe1 levels than wild-type cells throughout the duration of the experiment. Because localization of Swe1 is important for its turnover, we also analyzed this issue in the foregoing experiment (Figure 2B). As expected, Swe1 was found transiently at the bud neck in a very small percentage (8% maximum) of wild-type cells due to the fact that Swe1 is targeted to degradation as soon as it reaches that location (McMillan et al., 2002). Conversely, bud neck localized Swe1 was detected in a significant percentage (up to 38%) of dma1Δ dma2Δ cells. Thus the Dma proteins seem to contribute to proper Swe1 down-regulation under unperturbed conditions, likely by acting after Swe1 recruitment at the bud neck.
FIGURE 2:
The lack of Dma proteins causes Swe1 stabilization. Exponentially growing (cyc) cultures of SWE1-3HA (ySP3427) and dma1Δ dma2Δ SWE1-3HA (yRF661) cells were arrested in G1 by α-factor and released from G1 arrest in YEPD at 25°C (time 0), followed by α-factor readdition after 85 min. At the indicated times after release, cell samples were taken for FACS analysis of DNA contents (A), for scoring budding, nuclear division, septin ring formation, and Swe1-3HA localization (B), and for determining Swe1 levels by Western blot analysis with anti-HA and anti-Swi6 (loading control) antibodies (C).
FIGURE 3:
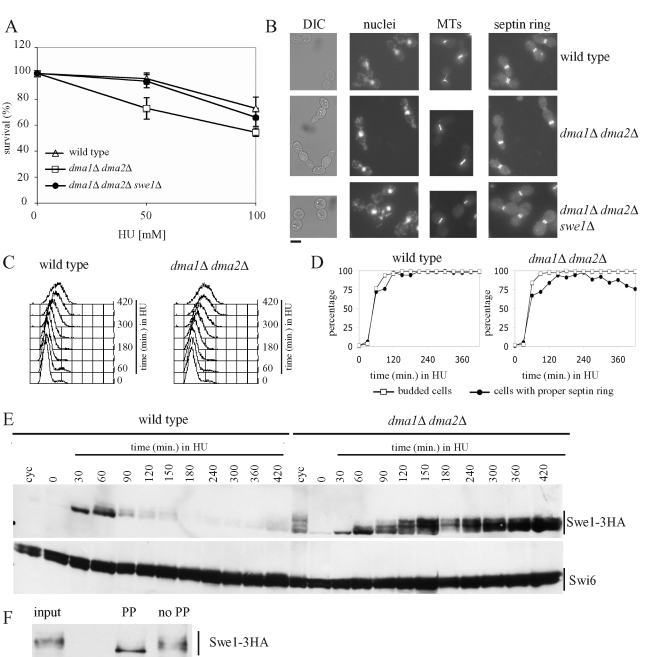
Effect of the lack of Dma proteins on cell viability, cell morphology, and Swe1 stability in HU-treated cells. (A) Serial dilutions of stationary-phase cultures of the indicated strains were spotted on YEPD plates containing the indicated HU concentrations and incubated for 4 d at 25°C. Colony-forming units were then counted to determine cell viability. Plotted values are the mean values ± SD (bars) from three independent experiments. (B) Exponentially growing cultures of wild-type (W303), dma1Δ dma2Δ (ySP1569), and dma1Δ dma2Δ swe1Δ (ySP3164) cells were transferred to YEPD medium containing 200 mM HU at 25°C. After 7 h, cell samples were taken for cell morphology analysis (DIC) and for in situ immunofluorescence analysis of nuclei, mitotic spindles (MTs), and septin ring deposition by using DAPI staining, anti-tubulin antibodies and anti-Cdc11 antibodies, respectively. Bar, 5 μm. (C–E) Exponentially growing (cyc) cultures of SWE1-3HA (ySP3427) and dma1Δ dma2Δ SWE1-3HA (yRF661) cells were arrested in G1 by α-factor and released from G1 arrest at 25°C in YEPD medium containing 200 mM HU (time 0). At the indicated times, cell samples were taken for FACS analysis of DNA contents (C), for scoring budding and septin ring formation (D), and for determining Swe1 levels by Western blot analysis with anti-HA and anti-Swi6 (loading control) antibodies (E). (F) Protein extracts of dma1Δ dma2Δ SWE1-3HA cells grown for 3 h in HU-containing medium (input) were immunoprecipitated with anti-HA antibodies and the immunoprecipitate was incubated for 30 min at 30°C with 20 units/μl lambda phosphatase (PP) or with reaction buffer only (no PP).
The Dma proteins are essential for Swe1 degradation in response to DNA replication stress
Several mutants that are partially impaired in Swe1 inhibition, such as hsl1Δ, hsl7Δ, cla4Δ, and cdc55Δ mutants, were reported to display Swe1-dependent hypersensitivity to the ribonucleotide reductase inhibitor hydroxyurea (HU) (Liu and Wang, 2006), which causes stalling of DNA replication forks due to deoxyribonucleotide depletion (Rosenkranz and Levy, 1965). In addition, dma1Δ dma2Δ cells turned out to be more sensitive to HU than wild-type cells (Figure 3A), although they were as sensitive as wild-type cells to treatments with methyl methanesulfonate, phleomycin, or UV radiation (unpublished data). This HU hypersensitivity was not due to the inability of dma1Δ dma2Δ cells to arrest the cell cycle progression through the S-phase checkpoint, which prevents mitotic entry in response to HU (Weinert et al., 1994). In fact, when cell cultures were released from α-factor into HU-containing medium, neither dma1Δ dma2Δ nor wild-type cells elongated the mitotic spindle (Figure 3B and unpublished data), as expected when the S-phase checkpoint is proficient. The hypersensitivity to HU was not even due to defective recovery of dma1Δ dma2Δ cells from the HU-induced arrest, because dma1Δ dma2Δ cells released from a 120-min treatment with 200 mM HU underwent mitotic spindle elongation after HU removal with kinetics very similar to wild-type cells under the same conditions (Supplemental Figure S1). Thus the Dma proteins are not required for cell recovery from HU, at least after exposure to HU for a short time. As expected, similarly treated hsl1Δ cells, which are partially defective in recovery from S-phase arrest (Liu and Wang, 2006), entered anaphase after HU removal with some delay compared with wild-type cells (Supplementary Figure S1).
Incubation for 8 h in the presence of 200 mM HU caused wild-type cells to accumulate as dumbbells with a big round bud, single nucleus, short mitotic spindle, and normal septin ring (Figure 3B; 91% of 190 cells), as expected. Of interest, a significant percentage of similarly treated dma1Δ dma2Δ cells (35% of 216 cells) accumulated as cells with misshapen, elongated buds, single nucleus, short mitotic spindle, and mispositioned septin rings (Figure 3B). In particular, HU-treated dma1Δ dma2Δ cells first assembled a proper septin ring at the bud neck, but then a new septin ring appeared at the bud tip, also causing a restriction in the growing bud, followed by disassembly of the first assembled ring. This phenotype resembles that of cells with misorganized septins and elevated Swe1 levels (Gladfelter et al., 2005). Therefore both the elongated bud phenotype and the mispositioned septin ring might be caused by high Swe1 levels causing low mitotic Cdc28–cyclin complex activity and thus concomitantly inhibiting isotropic bud growth (Booher et al., 1993) and allowing assembly of a new septin ring at the bud tip (Gladfelter et al., 2005). Indeed, although the deletion of SWE1 only partially suppressed the hypersensitivity to HU of cells lacking the Dma proteins (Figure 3A), it restored both the round bud phenotype and proper septin ring formation in the same cells (Figure 3B; 89% of 205 cells), suggesting that their defects in morphology and septin ring positioning were likely due to Swe1 accumulation.
To further investigate whether the Dma proteins are involved in controlling Swe1 protein levels under DNA replication stress conditions, synchronous cultures of wild-type and dma1Δ dma2Δ cells expressing SWE1-3HA were released from α-factor arrest into HU-containing medium. After both cell cultures entered S phase with similar kinetics, DNA replication was stalled because of the lack of deoxyribonucletides, as shown by FACS analysis of DNA content (Figure 3C). In addition, budding and septin ring deposition after release in the presence of HU took place with similar kinetics in the two strains (Figure 3D), although septin ring deposition seemed to be slightly less efficient in dma1Δ dma2Δ than in wild-type cells. Swe1 levels increased in both cell cultures after transfer to HU (Figure 3E) concomitantly with cells entering S phase, and then Swe1 disappeared in wild-type cells, as previously reported (Enserink et al., 2006), ∼90 min after transfer to HU. It is striking that high levels of the same protein progressively accumulated throughout the duration of the experiment in dma1Δ dma2Δ cells, suggesting that the Dma proteins are required for Swe1 degradation in response to interrupted DNA synthesis.
Swe1 seemed to undergo posttranslational modifications in HU-treated dma1Δ dma2Δ cells, as these cells accumulated Swe1 species with low electrophoretic mobility (Figure 3E), which were transiently observed also during an unperturbed cell cycle (Figure 2C). Because Swe1 must be localized at the bud neck and phosphorylated by multiple kinases to be degraded (Keaton and Lew, 2006), we asked whether the slowly migrating Swe1 species might be due to phosphorylation events. Indeed, such species disappeared after lambda phosphatase treatment of Swe1 immunoprecipitates from HU-treated dma1Δ dma2Δ cells (Figure 3F), indicating that the lack of the Dma proteins allows accumulation of phosphorylated Swe1 in the presence of HU.
The lack of the Dma proteins causes Swe1 accumulation at the bud neck in HU-treated cells
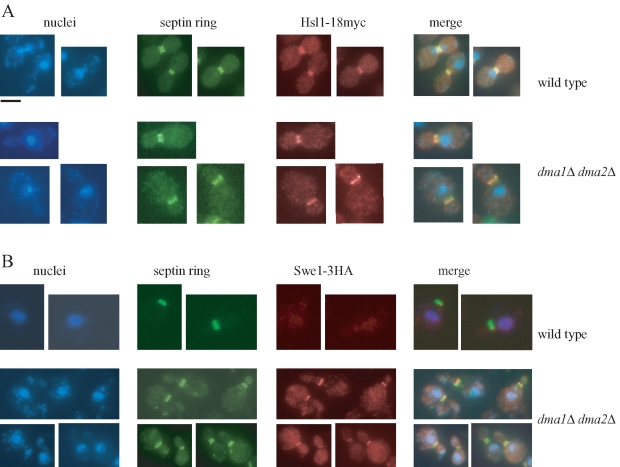
Swe1 must be localized at the bud neck and interact with specific factors to be phosphorylated, and this phosphorylation is essential for subsequent Swe1 degradation (Sakchaisri et al., 2004). To fully rule out the possibility that the Dma proteins might regulate Swe1 degradation in HU-treated cells by controlling Swe1 localization, we initially checked the localization at the bud neck of a functional myc-tagged variant (Hsl1-18myc) of the Hsl1 kinase that is essential for Swe1 recruitment at the septin ring (Shulewitz et al., 1999; Longtine et al., 2000). Hsl1 localization in HU-treated cells was unaffected by the lack of Dma proteins, as 85% of wild-type cells (n = 250) and 86% of dma1Δ dma2Δ cells (n = 260) showed precise colocalization of Hsl1-18myc with the septin ring protein Cdc11 (Figure 4A).
FIGURE 4:
The lack of Dma proteins causes Swe1 retention at the bud neck in HU-treated cells. Exponentially growing cell cultures of HSL1-18MYC (ySP3157) and dma1Δ dma2Δ HSL1-18MYC (yRF672) strains (A) or SWE1-3HA (ySP3427) and dma1Δ dma2Δ SWE1-3HA (yRF661) strains (B) were transferred to YEPD medium containing 200 mM HU at 25°C. After 3 h, nuclei and septin ring deposition were analyzed as in Figure 3, whereas Hsl1-18MYC and Swe1-3HA were visualized by indirect immunofluorescence with anti-Myc and anti-HA antibody, respectively. Bar, 5 μm.
We then analyzed directly Swe1 subcellular localization after incubation for 3 h in the presence of HU of cells expressing SWE1-3HA. As expected, because treatment with HU triggers Swe1 degradation (Enserink et al., 2006) (Figure 3E), Swe1 was undetectable at the bud neck of wild-type cells under these conditions (Figure 4B). The same protein was instead properly localized at the bud side of the mother-bud neck in 50% of dma1Δ dma2Δ cells (n = 170) (Figure 4B), indicating that the lack of the Dma proteins stabilizes Swe1 at the bud neck, in agreement with the previously observed accumulation of Swe1 phosphorylated forms (Figure 3E).
These data are consistent with the accumulation of bud neck–localized Swe1 that we previously observed in dma1Δ dma2Δ cells undergoing an unperturbed cell cycle (Figure 2B), indicating that the Dma proteins are somehow involved in promoting Swe1 release from the bud neck.
Swe1 stabilization in HU-treated dma1Δ dma2Δ cells is not due to morphogenesis checkpoint activation
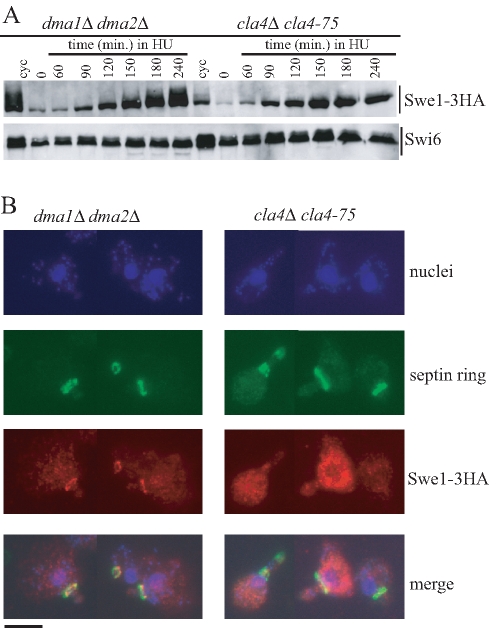
Perturbations of the septin ring architecture and/or actin cytoskeleton cause activation of the morphogenesis checkpoint that prevents nuclear division by inhibiting Swe1 recruitment at the bud neck and therefore Swe1 degradation (Keaton and Lew, 2006). For example, shift of temperature-sensitive cla4-75 mutant cells to the restrictive temperature (37°C) causes septin ring defects (Kadota et al., 2004), which in turn trigger the activation of the morphogenesis checkpoint. As cells lacking the Dma proteins show partial septin ring defects, we compared Swe1 protein levels and localization in HU-treated dma1Δ dma2Δ and cla4Δ cla4-75 cells. Synchronous cultures of cells producing Swe1-3HA were released from α-factor into HU-containing medium at 37°C. As expected, Swe1 appeared to be stabilized in both cell cultures (Figure 5A), but it was striking that it accumulated phosphorylated forms (Figure 5A) and localized at the septin ring (Figure 5B) specifically in dma1Δ dma2Δ cells and not in cla4-75 cells, where its stabilization is known to depend on morphogenesis checkpoint activation (Longtine et al., 2000).
FIGURE 5:
Different patterns of Swe1 stabilization in HU-treated dma1Δ dma2Δ and cla4-75 cells. Exponentially growing (cyc) cell cultures of dma1Δ dma2Δ SWE1-3HA (yRF661) and cla4-75 SWE1-3HA (yRF690) strains were arrested in G1 by α-factor at 25°C (time 0) and released from G1 arrest at 37°C in YEPD medium containing 150 mM HU. Samples were taken at the indicated times after release for determining Swe1 levels (A) as in Figure 2C and in situ immunofluorescence analysis (B) of nuclei, septin ring deposition, and Swe1-3HA localization as in Figure 4. Representative micrographs were taken at t = 180 min. Bar, 5 μm.
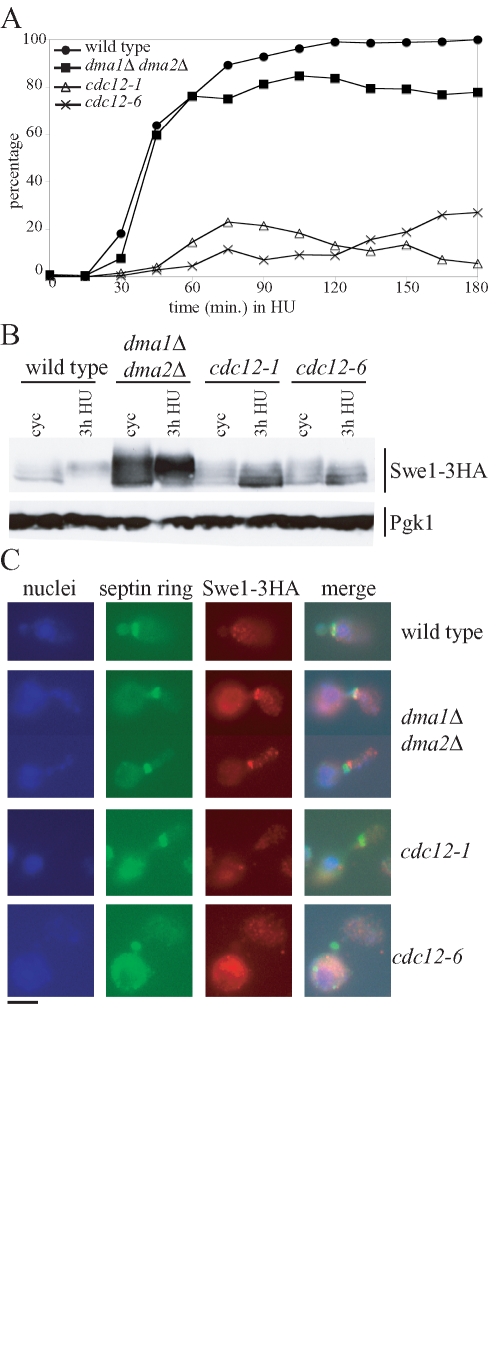
We also analyzed Swe1 regulation in HU-treated cdc12-1 and cdc12-6 conditional mutants, in which the morphogenesis checkpoint is activated by septin ring disassembly (Barral et al., 2000). Synchronous cell cultures producing Swe1-3HA were released from α-factor into HU-containing medium at the permissive temperature for the cdc12 mutants (23°C). As expected, cdc12-1 and cdc12-6 cells were defective in septin ring deposition even at this temperature (Figure 6A), consistent with the Cdc12 role in septin ring architecture (Dobbelaere et al., 2003). Of importance, neither septin mutant accumulated hyperphosphorylated Swe1 within 3 h after release in the presence of HU, whereas high levels of slowly migrating Swe1 species were detectable in similarly treated dma1Δ dma2Δ cells (Figure 6B). Accordingly, these conditions did not allow detection of Swe1 at the bud neck of cdc12 cells, whereas the same protein colocalized with the septin ring in dma1Δ dma2Δ cells (Figure 6C).
FIGURE 6:
Septin mutants do not accumulate hyperphosphorylated Swe1 in the presence of HU. Exponentially growing (cyc) cultures of SWE1-3HA (ySP3427), dma1Δ dma2Δ SWE1-3HA (yRF661), cdc12-1 SWE1-3HA (yRF816) and cdc12-6 SWE1-3HA (yRF828) cells were arrested in G1 by α-factor at 23°C and released in YEPD medium containing 200 mM HU. Samples were taken at the indicated times to determine the kinetics of septin ring deposition (A), as well as Swe1 levels (B) by Western blot analysis with anti-HA (Swe1–3HA) and anti-Pgk1 (loading control) antibodies, and for in situ immunofluorescence analysis (C) of nuclei, septin ring, and Swe1-3HA as in Figure 4. Representative micrographs were taken at t = 180 min. Bar, 5 μm.
Taken together, the foregoing data strengthen the hypothesis that the mechanism leading to Swe1 stabilization in cells lacking the Dma proteins is different from the one triggered by morphogenesis checkpoint activation and does not involve inhibition of Swe1 recruitment at the bud neck.
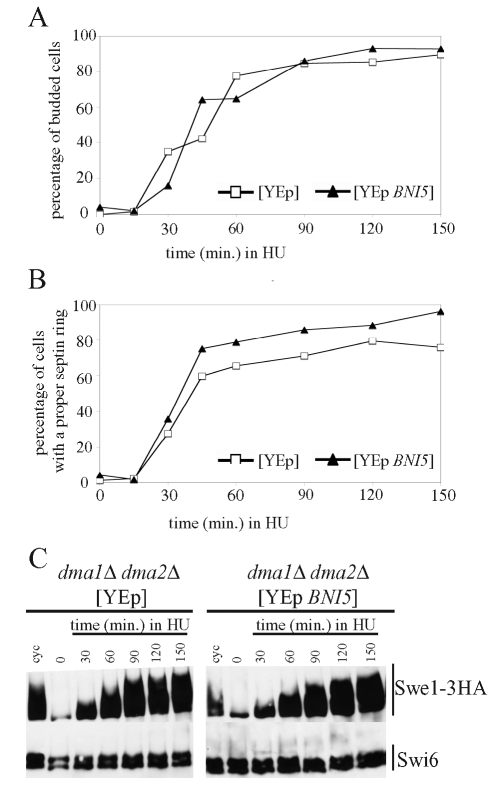
Because HU-treated dma1Δ dma2Δ cells seemed to be slightly impaired in septin ring deposition (Figure 3C), we wanted to fully rule out the possibility that Swe1 stabilization in these cells might merely be a consequence of septin ring problems. To this end, we stabilized the septin ring in dma1Δ dma2Δ cells by overexpressing the BNI5 gene, whose product acts as a septin regulator and has been implicated in septin ring stabilization (Lee et al., 2002). Swe1-3HA–producing cells that carried high–copy number plasmids, either empty or containing BNI5, were grown under selective conditions for plasmid maintenance, arrested in G1 by α-factor, and then released from G1 arrest into HU-containing medium. As shown in Figure 7, budding kinetics after release were similar in the two strains (Figure 7A). Moreover, Swe1 accumulation in dma1Δ dma2Δ cells carrying the empty vector (Figure 7C) was similar to that previously observed in isogenic cells without any plasmid (Figures 3E and 5A). It is striking that high–copy number BNI5 suppressed the slight septin ring deposition defect of dma1Δ dma2Δ cells (Figure 7B), as expected, but did not decrease the accumulation of phosphorylated Swe1 in the same cells (Figure 7C). Thus Swe1 stabilization in HU-treated cells lacking the Dma proteins is independent of septin ring defects.
FIGURE 7:
Septin ring stabilization in dma1Δ dma2Δ cells does not restore Swe1 degradation in the presence of HU. Exponentially growing (cyc) cultures of dma1Δ dma2Δ SWE1-3HA cells containing the 2μ plasmid YEplac181, either empty (YEp) (yRF714) or carrying the BNI5 gene (YEp-BNI5) (yRF710), were grown at 25°C in synthetic medium lacking leucine, arrested in G1 by α-factor, and released from G1 arrest in YEPD medium containing 200 mM HU (time 0). Samples were taken at the indicated times after release for determining the kinetics of budding (A) and septin ring deposition (B) and for analysis of Swe1 levels (C) as in Figure 2C.
The Dma proteins contribute to Swe1 ubiquitylation in vivo
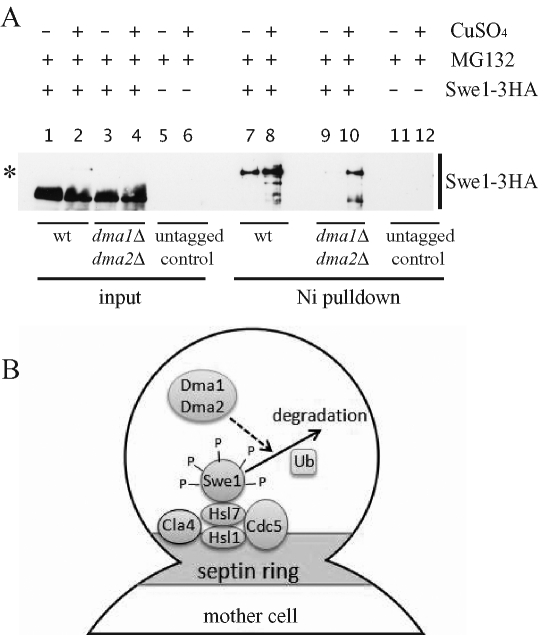
Dma1 and Dma2 act as E3 ubiquitin ligases in vitro (Loring et al., 2008), and Swe1 likely undergoes ubiquitylation in vivo, because incubation of purified Swe1 with a ubiquitylation cocktail in the presence of wild-type cell lysates yields polyubiquitylated Swe1 (Kaiser et al., 1998). Given that polyubiquitylated proteins are targeted to proteasome-mediated degradation (Driscoll and Goldberg, 1990), we wondered whether the Dma proteins might control Swe1 ubiquitylation. To address this question, wild-type SWE1-3HA and dma1Δ dma2Δ SWE1-3HA cells, as well as wild-type cells without the SWE1-3HA fusion, were transformed with a high–copy number plasmid carrying a construct expressing 6xHIS-tagged ubiquitin from the CUP1 inducible promoter (2μ CUP1–6xHIS-UBI4) (Callis and Ling, 2005). Addition of 250 μM CuSO4 to cultures of the transformant clones, exponentially growing under selective conditions (see Materials and Methods), was used to induce the CUP1 promoter, followed by addition, 30 min later, of the proteasome inhibitor MG132 (Gaczynska and Osmulski, 2005) to promote accumulation of ubiquitylated proteins. After 3 h, total protein extracts from CuSO4-induced and uninduced cell cultures were prepared and incubated with a Nickel resin that selectively binds 6xHIS-tagged proteins and therefore purifies the ubiquitylated proteins in our induced cell extracts. The resin eluates were then subjected to SDS–PAGE and Western blot analysis with an anti-HA antibody to detect Swe1. These experimental conditions allowed Swe1 accumulation in both wild-type SWE1-3HA and dma1Δ dma2Δ SWE1-3HA cells (Figure 8A, lanes 1–4), but Swe1 ubiquitylated forms, which clearly accumulated in the wild-type eluates (Figure 8A, lane 8), were significantly less abundant in the eluates deriving from similarly treated dma1Δ dma2Δ cells (Figure 8A, lane 10). No bands were detected either in the inputs or in the eluates of the wild-type strain that did not carry SWE1-3HA (lanes 5, 6, 11, and 12). Thus accumulation of Swe1 ubiquitylated forms in cycling cells under conditions of proteasome inhibition is at least partially dependent on Dma1 and Dma2.
FIGURE 8:
Swe1 ubiquitylation is partially dependent on the Dma proteins. (A) Exponentially growing cultures of wild-type SWE1-3HA (yRF957) and dma1Δ dma2Δ SWE1-3HA (yRF959) cells, as well as of wild-type cells not carrying the SWE1-3HA fusion (yRF1138; untagged control), all containing a high–copy number plasmid with the CUP1–6xHIS-UBI4 construct, were incubated for 3 h in the presence of the proteasome inhibitor MG132 and with (lanes 2, 4, 6, 8, 10, and 12) or without (lanes 1, 3, 5, 7, 9, and 11) 250 μM CuSO4, which induces overproduction of 6xHIS-ubiquitin. Cells were then lysed, and aliquots of the lysates were incubated with Ni-NTA beads to purify the proteins bound by 6xHIS-ubiquitin (Ni pulldown). Bead eluates were subjected to SDS–PAGE electrophoresis and Western blot analysis with anti-HA antibodies. Equivalent aliquots of the lysates were also loaded on the gel (input). The asterisk marks Swe1-ubiquitylated bands. (B) A model for Swe1 down-regulation by the Dma proteins. Swe1 is recruited by the Hsl1/Hsl7 complex to the septin ring, where it interacts with the protein kinases Cla4 and Cdc5 and undergoes multiple phosphorylation events (P). Phosphorylated Swe1 is then likely polyubiquitylated, followed by degradation. The Dma1 and Dma2 proteins partially trigger this degradation by acting after recruitment of Swe1 to the bud neck and its subsequent phosphorylation, possibly by controlling, directly or indirectly, Swe1 ubiquitylation.
We also tried to assess whether Swe1 accumulation in HU-treated dma1Δ dma2Δ cells correlated with defective Swe1 ubiquitylation. Unfortunately, we were unable to obtain significant results, as ubiquitylated Swe1 was undetectable in wild-type cells in the absence of proteasome inhibitors, likely because it was immediately degraded. On the other hand, combining HU with MG132 dramatically affected cell growth under the conditions required for treatment with the proteasome inhibitor (see Materials and Methods) and did not allow recovery of acceptable Swe1 inputs even in wild-type cell extracts. Nonetheless, the foregoing data indicate that the lack of the Dma proteins interferes with Swe1 ubiquitylation in vivo, suggesting that these proteins might be involved in promoting this event.
DISCUSSION
According to the prevailing model for Swe1 inactivation, Swe1 is recruited to the bud neck, where it undergoes stepwise phosphorylations (Park et al., 2004; Sakchaisri et al., 2004; Asano et al., 2005), which likely trigger Swe1 ubiquitylation and subsequent degradation. Swe1 inactivation, which relieves Swe1-dependent inhibition of the Cdk catalytic subunit Cdc28, thus allowing nuclear division, is crucial for coordinating proper bud morphology with cell cycle progression. Localization of the Hsl1/Hsl7 complex at the bud neck provides a platform for Swe1 degradation, which requires also the Cdc55 regulatory subunit of PP2A (Yang et al., 2000). It is worth mentioning that there is no conclusive evidence that Swe1 degradation is essential for mitotic entry, and that phosphorylation might be the key mechanism down-regulating Swe1, as happens for other Wee1 family members (Harvey et al., 2005; Watanabe et al., 2005; Okamoto and Sagata, 2007).
Here we show that also the FHA-RING ubiquitin ligases Dma1 and Dma2 contribute to Swe1 down-regulation. In fact, their absence is lethal for cells lacking either Hsl1 or Cdc55. Moreover, cells that concomitantly lack the Dma proteins and either Hsl1 or the phosphatase Mih1, which counteracts Swe1-mediated Cdc28 phosphorylation, exhibit overelongated buds and growth arrest or very slow growth, respectively. Given that these synthetic effects are relieved by SWE1 deletion, the Dma proteins likely contribute to promote Swe1 turnover. Indeed, their absence partially impairs Swe1 degradation and localization during an unperturbed cell cycle, when dma1Δ dma2Δ cells contain higher amounts than wild-type cells of total and bud neck–localized Swe1, although they show wild-type kinetics of Swe1 accumulation, phosphorylation, and degradation. This larger Swe1 amount does not affect cell cycle progression of unperturbed dma1Δ dma2Δ cells, likely because multiple redundant pathways down-regulate Swe1. Accordingly, partially defective Swe1 degradation in hsl1Δ or hsl7Δ mutants does not cause detectable G2 delay under unperturbed conditions (McMillan et al., 1999).
Dma1 and Dma2 are involved in septin dynamics (Fraschini et al., 2004), and a functional septin ring is required for Swe1 recruitment to the bud neck and subsequent degradation (Theesfeld et al., 2003). Thus septin ring defects in dma1Δ dma2Δ cells might activate the morphogenesis checkpoint, which causes stabilization of Swe1 by interfering with its localization at the bud neck (Longtine et al., 2000). It is striking that Swe1 recruitment to the septin ring is enhanced by the lack of the Dma proteins, which therefore likely control Swe1 degradation by a mechanism that does not involve Swe1 displacement from the bud neck.
Both DNA replication and mitotic spindle elongation are blocked by the S-phase checkpoint that responds to stalled DNA replication forks in wild-type cells treated with the DNA synthesis inhibitor HU (Weinert et al., 1994). Swe1 is properly targeted to the bud neck and degraded under these conditions, and these events seem to be essential for proper cell recovery from HU. In fact, wild-type cells maintain normal cell morphology and high viability during HU-induced S-phase block, whereas mutants defective in Swe1 degradation after HU treatment show abnormally elongated buds and low viability under the same conditions (Enserink et al., 2006). We found that the Dma proteins participate in Swe1 down-regulation in response to DNA replication stress, and this regulation is important for cell survival and morphology. In fact, dma1Δ dma2Δ cells chronically treated with HU are less viable than wild-type cells, form abnormally elongated buds, and accumulate high amounts of Swe1. Moreover, SWE1 deletion fully restores proper cell morphology and partially restores viability of HU-treated dma1Δ dma2Δ cells. The latter partial effect might suggest some specific role of the Dma proteins in the DNA damage response, as shown for their human homologue Rnf8 (Al-Hakim et al., 2010).
Activation of the morphogenesis checkpoint in HU-treated cla4-75, cdc12-1 and cdc12-6 cells leads to accumulation of Swe1 that does not localize at the bud neck and is not phosphorylated, as expected by the current model of morphogenesis checkpoint action (for a review, see Keaton and Lew, 2006). On the contrary, HU-treated dma1Δ dma2Δ cells properly localize Hsl1 and Swe1 at the septin ring and accumulate phosphorylated Swe1. Moreover, the septin ring defects of these cells are likely the consequence, and not the cause, of Swe1 stabilization. In fact, BNI5 overexpression restores a proper septin ring in HU-treated dma1Δ dma2Δ cells but does not change their pattern of Swe1 stabilization. Thus the mechanism by which the lack of Dma proteins causes Swe1 accumulation under DNA replication stress is different from the one promoted by morphogenesis checkpoint activation. This mechanism seems also to be different from that involving the DNA damage checkpoint protein Rad53, which promotes Swe1 degradation in HU-treated cells likely by acting on the septin ring (Enserink et al., 2006; Smolka et al., 2006). In particular, the Dma proteins participate in a step of Swe1 degradation that is downstream of Swe1 recruitment at the division site and Swe1 subsequent phosphorylation (Figure 8B).
Swe1 is likely polyubiquitylated in vivo (Kaiser et al., 1998), suggesting that its degradation occurs via the ubiquitin-proteasome pathway. However, how bud neck–localized Swe1 is targeted to degradation after phosphorylation is unclear. Specific E3 ubiquitin ligases, among which are the key yeast cell cycle regulators Skp1-cullin-F box (SCF) and anaphase-promoting complex APC, dictate the specificity of substrate recognition for ubiquitin conjugation (for review, see Deshaies and Joazeiro, 2009). Intensive analysis of yeast strains bearing F-box gene deletions that abolish SCF substrate specificity failed to identify ubiquitin ligases directly required for Swe1 ubiquitylation (McMillan et al., 2002). On the other hand, whether APC has a role in Swe1 degradation is unclear. In fact, Swe1 degradation was not affected by spindle checkpoint-mediated APC inactivation (Sia et al., 1998), whereas it was suggested to partially involve APC by a later study (Thornton and Toczyski, 2003). In addition, stable Swe1 variants have been described that show proper bud neck localization, phosphorylation, and interaction with known Swe1 regulators (McMillan et al., 2002), suggesting the existence of still unknown Swe1 regulators.
Our data indicate that the Dma1 and Dma2 ubiquitin ligases, whose absence allows accumulation of Swe1 under conditions that normally trigger its degradation, might be involved in Swe1 ubiquitylation. In fact, the amount of ubiquitylated Swe1 species that accumulate under proteasome-inhibiting conditions is strongly reduced in cells lacking these proteins compared with wild-type cells. In spite of our efforts to unravel in vivo physical interactions between Swe1 and Dma1 or Dma2, further experiments will be required to assess whether the Dma proteins directly ubiquitylate Swe1. It is important to point out that both ubiquitylation of Swe1 and its release from the bud neck are inhibited in the absence of the Dma proteins, indicating that these events might be coupled (Figure 8B).
Swe1 down-regulation appears to be essential for cell viability, based on the toxic effects of Swe1 overproduction (Lim et al., 1996) and on the Swe1-dependent synthetic lethality caused by concomitant impairment of different Swe1 down-regulatory pathways (Park et al., 2003; Sakchaisri et al., 2004; this work). Given that dma1Δ dma2Δ cells are viable, other yet unidentified ubiquitin ligases likely ubiquitylate Swe1 under unperturbed conditions. Nonetheless, Dma-dependent control of Swe1 degradation is crucial for Swe1 down-regulation under DNA replication stress, indicating a further important role in cell protection for these evolutionarily conserved proteins.
MATERIALS AND METHODS
Strains, media and reagents, and genetic manipulations
All yeast strains (Table 2) were derivatives of W303 (ade2-1, trp1-1, leu2-3112, his3-11,15, ura3, ssd1) or were backcrossed at least three times to W303. Cells were grown in either synthetic minimal medium supplemented with the appropriate nutrients or 1% yeast extract, 2% bactopeptone, 50 mg/l adenine (YEP) medium supplemented with 2% glucose (YEPD). Unless otherwise stated, α-factor was used at 2 μg/ml and hydroxyurea at 200 mM, and the experimental temperature was 25°C.
TABLE 2:
Strains used in this study.
| Name | Relevant genotype |
|---|---|
| ySP1569 | MATa, dma1::KlTRP1, dma2::KlLEU2 |
| ySP1570 | MATa, dma1::KlTRP1, dma2::KlLEU2 |
| ySP2586 | MATa, mih1::LEU2 |
| ySP3157 | MATa, HSL1-myc18::KlTRP1 |
| ySP3164 | MATa, dma1::KlTRP1, dma2::KlLEU2, swe1::LEU2 |
| ySP3427 | MATa, SWE1-HA3::KlURA3 |
| yRF661 | MATa, dma1::KlTRP1, dma2::KlLEU2, SWE1-HA3::KlURA3. |
| yRF672 | MATa, dma1::LEU2Kl, dma2::HPHMX, HSL1-myc18::KlTRP1 |
| yRF690 | MATa, cla4::kanMX4, SWE1-HA3::klURA3 [CEN-TRP1-cla4–75] |
| yRF710 | MATa, dma1::KlTRP1, dma2::HPHMX, SWE1-HA3::KlURA3 [2μ LEU2-BNI5] |
| yRF714 | MATa, dma1::KlTRP1, dma2::HPHMX, SWE1-HA3::KlURA3 [2μ LEU2] |
| yRF816 | MATa, cdc12-1, SWE1-HA3::KlURA3 |
| yRF828 | MATa, cdc12-6, SWE1-HA3::KlURA3 |
| yRF852 | MATa, dma1::KlTRP1, dma2::HPHMX, mih1::LEU2 |
| yRF862 | MATa, SWE1-HA3::KlURA3, hsl1::MET3-HSL1::TRP1 |
| yRF865 | MATa, SWE1-HA3::KlURA3, dma1::LEU2Kl, dma2::HPHMX, hsl1::MET3-HSL1::TRP1 |
| yRF957 | MATa, SWE1-HA3::KlURA3 [2μ TRP1-CUP1–6xHIS-UBI4] |
| yRF986 | MATa, dma1::LEU2Kl, dma2::HPHMX, hsl1::MET3-HSL1::TRP1, swe1::LEU2 |
| yRF959 | MATa, SWE1-HA3::KlURA3, dma1::LEU2Kl, dma2::HPHMX, [2μ TRP1-CUP1–6xHIS-UBI4] |
| yRF1042 | MATa, dma1::KlTRP1, dma2::HPHMX, mih1::LEU2, swe1::kanMX4 |
| yRF1138 | MATa [2μ TRP1-CUP1–6xHIS-UBI4] |
Standard techniques were used for genetic manipulations (Sherman, 1991; Maniatis et al., 1992). Gene deletions were generated by one-step gene replacement (Wach et al., 1994). One-step tagging techniques were used to create 3HA- and 18MYC-tagged variants of Swe1 and Hsl1 (Janke et al., 2004). All gene replacements and tagging were controlled by PCR-based methods or Southern blot analysis.
Fluorescence microscopy
In situ immunofluorescence was performed on formaldehyde-fixed cells. Nuclei were visualized by staining with 4′,6-diamidino-2-phenylindole (DAPI), 0.05 μg/ml. Visualization of septin rings was performed using anti-Cdc11 polyclonal antibodies (1:200; Santa Cruz Biotechnology, Santa Cruz, CA), followed by indirect immunofluorescence with Alexa Fluor 488–conjugated anti-rabbit antibody (1:100; Invitrogen, Carlsbad, CA). In situ immunofluorescence of Swe1-3HA and Hsl1-18MYC was carried out using 12CA5 and 9E10 monoclonal antibodies, respectively, as previously described (Fraschini et al., 2006), except for the use of tertiary CY3-conjugated antibodies (1:500; Santa Cruz) to visualize Swe1. To detect spindle formation and elongation, α-tubulin immunostaining was performed with the YOL34 monoclonal antibody (1:100; AbD Serotec, Raleigh, NC), followed by indirect immunofluorescence using Pierce rhodamine-conjugated anti-rat antibody (1:500; ThermoFisher Scientific, Waltham, MA). Digital images were taken with a Leica DC350F charge-coupled device camera mounted on a Nikon Eclipse 600 and controlled by the Leica FW4000 software or with the MetaMorph imaging system software on a fluorescent microscope (Eclipse 90i; Nikon Instruments, Melville, NY), equipped with a charge-coupled device camera (CoolSNAP; Photometrics, Tucson, AZ) with an oil 100X 0.5–1.3 PlanFluor oil objective (Nikon).
Protein extracts and analysis
For most experiments total protein extracts were prepared by trichloroacetic acid (TCA) precipitation as previously described (Piatti et al., 1996). For the phosphatase treatment in Figure 2C protein extracts were made in the following breaking buffer: 50 mM HEPES, 150 mM NaCl, 20% glycerol, 5 mM EDTA, 60 mM β-glycerophosphate, and 1 mM Na orthovanadate, supplemented with protease inhibitors (Complete; Boehringer, Manheim, Germany). Swe1-3HA was immunoprecipitated from 1 μg of cleared extracts on incubation with the 12CA5 anti-HA antibody for 1 h at 4°C, followed by incubation with protein A–Sepharose for 1 h at 4°C. The slurry was washed three times in phosphate-buffered saline, resuspended in 60 μl of phosphatase buffer containing 20 U of lambda phosphatase (New England Biolabs, Ipswich, MA) and 2 mM MnCl2, and incubated 30 min at 30°C before loading.
For Western blot analysis, proteins were transferred to Schleicher and Schuell Protran membranes (Whatman, Piscataway, NJ) and probed with Molecular Probes monoclonal antibodies 12CA5 (anti-HA; 1:3000) and 22C5D8 (anti-Pgk1; 1:40,000) (Invitrogen) or with polyclonal anti-Swi6 antibodies (1:100,000), as previously described (Fraschini et al., 2004). Amersham secondary antibodies were purchased from GE Healthcare Bio-Sciences (Piscataway, NJ), and proteins were detected by an enhanced chemiluminescence system according to the manufacturer.
Detection of ubiquitin conjugates in vivo
For the experiments involving the expression of 6xHIS-ubiquitin, cells were grown to log phase at 25°C in selective medium containing 0.17% yeast nitrogen base without ammonium sulfate, 0.1% l-proline, and 2% glucose, followed by dilution to 8 × 106 cells/ml in the same medium containing also 0.003% SDS, as described (Liu et al., 2007). After further incubation for 3 h, 6xHIS-ubiquitin expression was induced by addition of 250 μM CuSO4 to half of each culture, and 75 μM MG132 (C2211, supplied by Sigma-Aldrich, St. Louis, MO) was added 30 min later to all cultures. After 3 h more, yeast cells were then washed in water, and protein extracts were prepared under denaturing conditions as described (Yaffe and Schatz, 1984), TCA precipitates were resuspended in buffer A (6 M guanidium, 100 mM NaPO4, pH 8, 10 mM Tris-HCl, pH 8), and the debris was removed by centrifugation. Ni-pull down was performed as described (Ulrich and Davies, 2009). Briefly, lysates were incubated overnight at room temperature with Ni-NTA agarose beads (Qiagen, Valencia, CA) in the presence of 15 mM imidazole and 0.05% Tween 20. Beads were then washed twice with buffer A plus 0.05% Tween 20 and four times with buffer C (8 M urea, 100 mM NaPO4, pH 6.3, 10 mM Tris-HCl, pH 6.3, 0.05% Tween 20). Bound proteins were eluted by addition of 30 μl of HU buffer (8 M urea, 200 mM Tris-HCl, pH 6.8, 1 mM EDTA, 5% SDS, 0.1% bromophenol blue, 1.5% dithiothreitol) and then subjected to SDS–PAGE followed by Western blot analysis with the 12CA5 anti-HA antibody.
Supplementary Material
Acknowledgments
We are grateful to Erfei Bi and Judy Callis for strains and plasmids; to Simonetta Piatti for reagents, strains and stimulating discussions; and to Maria Pia Longhese, Elena Chiroli, Giulia Rancati, and Nicola Manfrini for critical reading of the manuscript. This work was supported by grants from Programmi di ricerca di Rilevante Interesse Nazionale 2008 to R.F. C.C. is supported by a fellowship from Fondazione Confalonieri.
Abbreviations used:
- APC
anaphase promoting complex
- Cdk
cyclin-dependent kinase
- DAPI
4',6-diamidino-2-phenylindole
- DIC
differential interference contrast
- FACS
fluorescence-activated cell sorting
- FHA
forkhead-associated
- HU
hydroxyurea
- MTs
microtubules
- Ni-NTA
nickel-nitrilotriacetic acid
- PAK
p21 activated kinase
- PP
lambda phosphatase
- PP2A
protein phosphatase 2A
- RING
really interesting new gene
- SCF
Skp1-cullin-F-box containing complex
- TCA
trichloroacetic acid
- YEPD
yeast extract–peptone–dextrose
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E11-02-0127) on May 11, 2011.
REFERENCES
- Al-Hakim A, Escribano-Diaz C, Landry MC, O'Donnell L, Panier S, Szilard RK, Durocher D. The ubiquitous role of ubiquitin in the DNA damage response. DNA Repair. 2010;9:1229–1240. doi: 10.1016/j.dnarep.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asano S, Park JE, Sakchaisri K, Yu LR, Song S, Supavilai P, Veenstra TD, Lee KS. Concerted mechanism of Swe1/Wee1 regulation by multiple kinases in budding yeast. EMBO J. 2005;24:2194–2204. doi: 10.1038/sj.emboj.7600683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barral Y, Parra M, Bidlingmaier S, Snyder M. Nim1-related kinases coordinate cell cycle progression with the organization of the peripheral cytoskeleton in yeast. Genes Dev. 1999;13:176–187. doi: 10.1101/gad.13.2.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barral Y, Mermall V, Mooseker MS, Snyder M. Compartmentalization of the cell cortex by septins is required for maintenance of cell polarity in yeast. Mol Cell. 2000;5:841–851. doi: 10.1016/s1097-2765(00)80324-x. [DOI] [PubMed] [Google Scholar]
- Booher RN, Deshaies RJ, Kirschner MW. Properties of Saccharomyces cerevisiae wee1 and its differential regulation of p34CDC28 in response to G1 and G2 cyclins. EMBO J. 1993;12:3417–3426. doi: 10.1002/j.1460-2075.1993.tb06016.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks L 3rd, Heimsath EG, Jr, Loring GL, Brenner C. FHA-RING ubiquitin ligases in cell division cycle control. Cell Mol Life Sci. 2008;65:3458–3466. doi: 10.1007/s00018-008-8220-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callis J, Ling R. Preparation, characterization, and use of tagged ubiquitins. Methods Enzymol. 2005;399:51–64. doi: 10.1016/S0076-6879(05)99004-6. [DOI] [PubMed] [Google Scholar]
- Deshaies RJ, Joazeiro CA. RING domain E3 ubiquitin ligases. Annu Rev Biochem. 2009;78:399–434. doi: 10.1146/annurev.biochem.78.101807.093809. [DOI] [PubMed] [Google Scholar]
- Dobbelaere J, Gentry MS, Hallberg RL, Barral Y. Phosphorylation-dependent regulation of septin dynamics during the cell cycle. Dev Cell. 2003;4:345–357. doi: 10.1016/s1534-5807(03)00061-3. [DOI] [PubMed] [Google Scholar]
- Driscoll J, Goldberg AL. The proteasome (multicatalytic protease) is a component of the 1500-kDa proteolytic complex which degrades ubiquitin-conjugated proteins. J Biol Chem. 1990;265:4789–4792. [PubMed] [Google Scholar]
- Enserink JM, Smolka MB, Zhou H, Kolodner RD. Checkpoint proteins control morphogenetic events during DNA replication stress in Saccharomyces cerevisiae. J Cell Biol. 2006;175:729–741. doi: 10.1083/jcb.200605080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraschini R, Bilotta D, Lucchini G, Piatti S. Functional characterization of Dma1 and Dma2, the budding yeast homologues of Schizosaccharomyces pombe Dma1 and human Chfr. Mol Biol Cell. 2004;15:3796–3810. doi: 10.1091/mbc.E04-02-0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraschini R, D'Ambrosio C, Venturetti M, Lucchini G, Piatti S. Disappearance of the budding yeast Bub2-Bfa1 complex from the mother-bound spindle pole contributes to mitotic exit. J Cell Biol. 2006;172:335–346. doi: 10.1083/jcb.200507162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaczynska M, Osmulski PA. Small-molecule inhibitors of proteasome activity. Methods Mol Biol. 2005;301:3–22. doi: 10.1385/1-59259-895-1:003. [DOI] [PubMed] [Google Scholar]
- Gladfelter AS, Kozubowski L, Zyla TR, Lew DJ. Interplay between septin organization, cell cycle and cell shape in yeast. J Cell Sci. 2005;118:1617–1628. doi: 10.1242/jcs.02286. [DOI] [PubMed] [Google Scholar]
- Hartwell LH, Weinert TA. Checkpoints: controls that ensure the order of cell cycle events. Science. 1989;246:629–634. doi: 10.1126/science.2683079. [DOI] [PubMed] [Google Scholar]
- Harvey SL, Kellogg DR. Conservation of mechanisms controlling entry into mitosis: budding yeast Wee1 delays entry into mitosis and is required for cell size control. Curr Biol. 2003;13:264–275. doi: 10.1016/s0960-9822(03)00049-6. [DOI] [PubMed] [Google Scholar]
- Harvey SL, Charlet A, Haas W, Gygi SP, Kellogg DR. Cdk1-dependent regulation of the mitotic inhibitor Wee1. Cell. 2005;122:407–420. doi: 10.1016/j.cell.2005.05.029. [DOI] [PubMed] [Google Scholar]
- Janke C, et al. A versatile toolbox for PCR-based tagging of yeast genes: new fluorescent proteins, more markers and promoter substitution cassettes. Yeast. 2004;21:947–962. doi: 10.1002/yea.1142. [DOI] [PubMed] [Google Scholar]
- Kadota J, Yamamoto T, Yoshiuchi S, Bi E, Tanaka K. Septin ring assembly requires concerted action of polarisome components, a PAK kinase Cla4p, and the actin cytoskeleton in Saccharomyces cerevisiae. Mol Biol Cell. 2004;15:5329–5345. doi: 10.1091/mbc.E04-03-0254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser P, Sia RA, Bardes EG, Lew DJ, Reed SI. Cdc34 and the F-box protein Met30 are required for degradation of the Cdk-inhibitory kinase Swe1. Genes Dev. 1998;12:2587–2597. doi: 10.1101/gad.12.16.2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keaton MA, Lew DJ. Eavesdropping on the cytoskeleton: progress and controversy in the yeast morphogenesis checkpoint. Curr Opin Microbiol. 2006;9:540–546. doi: 10.1016/j.mib.2006.10.004. [DOI] [PubMed] [Google Scholar]
- Kops GJ, Weaver BA, Cleveland DW. On the road to cancer: aneuploidy and the mitotic checkpoint. Nat Rev Cancer. 2005;5:773–785. doi: 10.1038/nrc1714. [DOI] [PubMed] [Google Scholar]
- Lee PR, Song S, Ro HS, Park CJ, Lippincott J, Li R, Pringle JR, De Virgilio C, Longtine MS, Lee KS. Bni5p, a septin-interacting protein, is required for normal septin function and cytokinesis in Saccharomyces cerevisiae. Mol Cell Biol. 2002;22:6906–6920. doi: 10.1128/MCB.22.19.6906-6920.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KS, Asano S, Park JE, Sakchaisri K, Erikson RL. Monitoring the cell cycle by multi-kinase-dependent regulation of Swe1/Wee1 in budding yeast. Cell Cycle. 2005;4:1346–1349. doi: 10.4161/cc.4.10.2049. [DOI] [PubMed] [Google Scholar]
- Lim HH, Goh PY, Surana U. Spindle pole body separation in Saccharomyces cerevisiae requires dephosphorylation of the tyrosine 19 residue of Cdc28. Mol Cell Biol. 1996;16:6385–6397. doi: 10.1128/mcb.16.11.6385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Wang Y. The function and regulation of budding yeast Swe1 in response to interrupted DNA synthesis. Mol Biol Cell. 2006;17:2746–2756. doi: 10.1091/mbc.E05-11-1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Apodaca J, Davis LE, Rao H. Proteasome inhibition in wild-type yeast Saccharomyces cerevisiae cells. Biotechniques. 2007;42:158–162. doi: 10.2144/000112389. [DOI] [PubMed] [Google Scholar]
- Longtine MS, Theesfeld CL, McMillan JN, Weaver E, Pringle JR, Lew DJ. Septin-dependent assembly of a cell cycle-regulatory module in Saccharomyces cerevisiae. Mol Cell Biol. 2000;20:4049–4061. doi: 10.1128/mcb.20.11.4049-4061.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longtine MS, Bi E. Regulation of septin organization and function in yeast. Trends Cell Biol. 2003;13:403–409. doi: 10.1016/s0962-8924(03)00151-x. [DOI] [PubMed] [Google Scholar]
- Loring GL, Christensen KC, Gerber SA, Brenner C. Yeast Chfr homologs retard cell cycle at G1 and G2/M via Ubc4 and Ubc13/Mms2-dependent ubiquitination. Cell Cycle. 2008;7:96–105. doi: 10.4161/cc.7.1.5113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniatis T, Fritsch EF, Sambrook J. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor, NY:: Cold Spring Harbor Laboratory Press; 1992. [Google Scholar]
- Masselot M, Surdin-Kerjan Y. Methionine biosynthesis in Saccharomyces cerevisiae. II. Gene-enzyme relationships in the sulfate assimilation pathway. Mol Gen Genet. 1977;154:23–30. doi: 10.1007/BF00265572. [DOI] [PubMed] [Google Scholar]
- McMillan JN., Sia RA, Lew DJ. A morphogenesis checkpoint monitors the actin cytoskeleton in yeast J Cell Biol. 1998;146:1487–1499. doi: 10.1083/jcb.142.6.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillan JN, Longtine MS, Sia RA, Theesfeld CL, Bardes ES, Pringle JR, Lew DJ. The morphogenesis checkpoint in Saccharomyces cerevisiae: cell cycle control of Swe1p degradation by Hsl1p and Hsl7p. Mol Cell Biol. 1999;19:6929–6939. doi: 10.1128/mcb.19.10.6929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillan JN, Theesfeld CL, Harrison JC, Bardes ES, Lew DJ. Determinants of Swe1p degradation in Saccharomyces cerevisiae. Mol Biol Cell. 2002;13:3560–3575. doi: 10.1091/mbc.E02-05-0283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto K, Sagata N. Mechanism for inactivation of the mitotic inhibitory kinase Wee1 at M phase. Proc Natl Acad Sci USA. 2007;104:3753–3758. doi: 10.1073/pnas.0607357104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park CJ, Song S, Lee PR, Shou W, Deshaies RJ, Lee KS. Loss of CDC5 function inSaccharomyces cerevisiae leads to defects in Swe1p regulation and Bfa1p/Bub2p-independent cytokinesis. Genetics. 2003;163:21–33. doi: 10.1093/genetics/163.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JE, Park CJ, Sakchaisri K, Karpova T, Asano S, McNally J, Sunwoo Y, Leem SH, Lee KS. Novel functional dissection of the localization-specific roles of budding yeast polo kinase Cdc5p. Mol Cell Biol. 2004;24:9873–9886. doi: 10.1128/MCB.24.22.9873-9886.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piatti S, Böhm T, Cocker JH, Diffley JF, Nasmyth K. Activation of S-phase-promoting CDKs in late G1 defines a “point of no return” after which Cdc6 synthesis cannot promote DNA replication in yeast. Genes Dev. 1996;10:1516–1531. doi: 10.1101/gad.10.12.1516. [DOI] [PubMed] [Google Scholar]
- Rosenkranz HS, Levy JA. Hydroxyurea: a specific inhibitor of deoxyribonucleic acid synthesis. Biochim Biophys Acta. 1965;95:181–183. doi: 10.1016/0005-2787(65)90225-x. [DOI] [PubMed] [Google Scholar]
- Russell P, Moreno S, Reed SI. Conservation of mitotic controls in fission and budding yeasts. Cell. 1989;57:295–303. doi: 10.1016/0092-8674(89)90967-7. [DOI] [PubMed] [Google Scholar]
- Sakchaisri K, Asano S, Yu LR, Shulewitz MJ, Park CJ, Park JE, Cho YW, Veenstra TD, Thorner J, Lee KS. Coupling morphogenesis to mitotic entry. Proc Natl Acad Sci USA. 2004;101:4124–4129. doi: 10.1073/pnas.0400641101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman F. Getting started with yeast. Methods Enzymol. 1991;194:3–21. doi: 10.1016/0076-6879(91)94004-v. [DOI] [PubMed] [Google Scholar]
- Shulewitz MJ, Inouye CJ, Thorner J. Hsl7 localizes to a septin ring and serves as an adapter in a regulatory pathway that relieves tyrosine phosphorylation of Cdc28 protein kinase in Saccharomyces cerevisiae. Mol Cell Biol. 1999;19:7123–7137. doi: 10.1128/mcb.19.10.7123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sia RA, Bardes ES, Lew DJ. Control of Swe1p degradation by the morphogenesis checkpoint. EMBO J. 1998;17:6678–6688. doi: 10.1093/emboj/17.22.6678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolka MB, Chen SH, Maddox PS, Enserink JM, Albuquerque CP, Wei XX, Desai A, Kolodner RD, Zhou H. An FHA domain-mediated protein interaction network of Rad53 reveals its role in polarized cell growth. J Cell Biol. 2006;175:743–753. doi: 10.1083/jcb.200605081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theesfeld CL, Zyla TR, Bardes EG, Lew DJ. A monitor for bud emergence in the yeast morphogenesis checkpoint. Mol Biol Cell. 2003;14:3280–3291. doi: 10.1091/mbc.E03-03-0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton BR, Toczyski DP. Securin and B-cyclin/CDK are the only essential targets of the APC. Nat Cell Biol. 2003;12:1090–1094. doi: 10.1038/ncb1066. [DOI] [PubMed] [Google Scholar]
- Ulrich HD, Davies AA. In vivo detection and characterization of sumoylation targets in Saccharomyces cerevisiae. Methods Mol Biol. 2009;497:81–103. doi: 10.1007/978-1-59745-566-4_6. [DOI] [PubMed] [Google Scholar]
- Versele M, Thorner J. Some assembly required: yeast septins provide the instruction manual. Trends Cell Biol. 2005;15:414–424. doi: 10.1016/j.tcb.2005.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wach A, Brachat A, Pohlmann R, Philippsen P. New heterologous modules for classical or PCR-based gene disruptions in Saccharomyces cerevisiae. Yeast. 1994;10:1793–1808. doi: 10.1002/yea.320101310. [DOI] [PubMed] [Google Scholar]
- Watanabe N, et al. Cyclin-dependent kinase (CDK) phosphorylation destabilizes somatic Wee1 via multiple pathways. Proc Natl Acad Sci USA. 2005;102:11663–11668. doi: 10.1073/pnas.0500410102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinert TA, Kiser GL, Hartwell LH. Mitotic checkpoint genes in budding yeast and the dependence of mitosis on DNA replication and repair. Genes Dev. 1994;8:652–665. doi: 10.1101/gad.8.6.652. [DOI] [PubMed] [Google Scholar]
- Yaffe MP, Schatz G. Two nuclear mutations that block mitochondrial protein import in yeast. Proc Natl Acad Sci USA. 1984;81:4819–4823. doi: 10.1073/pnas.81.15.4819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Jiang W, Gentry M, Hallberg RL. Loss of a protein phosphatase 2A regulatory subunit (Cdc55p) elicits improper regulation of Swe1p degradation. Mol Cell Biol. 2000;20:8143–8156. doi: 10.1128/mcb.20.21.8143-8156.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.