FAK is linked to aggressive tumors, but its normal function is not clear. FAK knockdown early in Xenopus development anteriorizes the embryo via a loss of Wnt signaling. Wnt3a expression is FAK dependent in both embryos and human breast cancer cells, suggesting that a FAK–Wnt linkage is highly conserved.
Abstract
Focal adhesion kinase (FAK) is a cytoplasmic tyrosine kinase protein localized to regions called focal adhesions, which are contact points between cells and the extracellular matrix. FAK protein acts as a scaffold to transfer adhesion-dependent and growth factor signals into the cell. Increased FAK expression is linked to aggressive metastatic and invasive tumors. However, little is known about its normal embryonic function. FAK protein knockdown during early Xenopus laevis development anteriorizes the embryo. Morphant embryos express increased levels of anterior neural markers, with reciprocally reduced posterior neural marker expression. Posterior neural plate folding and convergence-extension is also inhibited. This anteriorized phenotype resembles that of embryos knocked down zygotically for canonical Wnt signaling. FAK and Wnt3a genes are both expressed in the neural plate, and Wnt3a expression is FAK dependent. Ectopic Wnt expression rescues this FAK morphant anteriorized phenotype. Wnt3a thus acts downstream of FAK to balance anterior–posterior cell fate specification in the developing neural plate. Wnt3a gene expression is also FAK dependent in human breast cancer cells, suggesting that this FAK–Wnt linkage is highly conserved. This unique observation connects the FAK- and Wnt-signaling pathways, both of which act to promote cancer when aberrantly activated in mammalian cells.
INTRODUCTION
Focal adhesion kinase (FAK) is a cytoplasmic protein tyrosine kinase that, when stimulated, localizes to the extracellular matrix at focal adhesions. At these sites, FAK undergoes tyrosine phosphorylation-activation. Multiple signals are responsible for FAK activation, including growth factors, neuropeptides, and mechanical stimuli. The most prevalent mode of FAK regulation is through integrin-dependent adhesion to the extracellular matrix. FAK is an essential component of the integrin signaling pathway (Schaller, 2001, 2010). FAK carries out protein–protein interaction adaptor functions that contribute to focal adhesion “scaffolding,” where it transmits adhesion-dependent and growth factor–dependent signals into the cell. FAK is essential for focal adhesion turnover and for cell locomotion. Establishment of new focal adhesions at the front of the cell and dissociation of the old focal adhesions at the rear are FAK-dependent processes (Mitra et al., 2005). FAK-depleted fibroblast cells round up and migrate poorly (Sieg et al., 1999). Migration, invasion, and resistance to apoptosis are distinct characteristics of metastatic cancers. Numerous reports have linked FAK expression with cancer (Schlaepfer et al., 2004; McLean et al., 2005; Golubovskaya et al., 2009a). Increased FAK mRNA levels were found in invasive metastatic tumors of various origins, especially in breast and colon cancers (Golubovskaya et al., 2009a; Zhao and Guan, 2009).
FAK protein also has nuclear activity, which acts to regulate gene expression. FAK protein promotes cell cycle progression by increasing KLF8 gene expression, which up-regulates CyclinD1 expression (Zhao et al., 2001, 2003; Cox et al., 2006). In addition, FAK nuclear accumulation, but not its kinase activity, was shown to modulate stability of the p53 tumor suppressor protein (Lim et al., 2008). Recent microarray analysis of the FAK protein–depleted breast cancer MCF-7 cell line showed that expression profiles of many genes were altered (Golubovskaya et al., 2009b). Thus both cytoskeletal and nuclear activities of FAK could function in both normal physiological and disease processes (Schaller, 2010).
A few studies have examined a potential role for FAK during vertebrate development. Null FAK mutations in mouse embryos caused a lethal phenotype at day 8.5–9, perhaps as a result of gastrulation morphogenesis defects (Ilic et al., 2004). FAK-null embryos did not develop somites or a notochord and had a rudimentary nonbeating or absent heart (Furuta et al., 1995). Studies using a conditional FAK deletion in different tissues showed that FAK regulates the later developmental processes of vascular, neural, and cardiac tissue development (Beggs et al., 2003; Rico et al., 2004; Grove et al., 2007). Yet, because of early lethality, the exact role of FAK in early vertebrate developmental processes is unknown.
FAK function was studied during somitogenesis and notochord formation in fish and frog embryos. FAK protein expression and phosphorylation-activation were detected in intercalating notochord cells in zebrafish and at the intersomitic boundaries in both zebrafish and frogs (Henry et al., 2001; Kragtorp and Miller, 2006). Although loss of FAK function was not examined in zebrafish, overexpression of the dominant-negative FAK (FRNK) protein in Xenopus showed that FAK was necessary for somite boundary formation (Kragtorp and Miller, 2006). In these studies, FAK's potential role in somite and the notochord structure was examined at relatively late developmental stages. The phenotype of the FRNK-injected embryos was not described, and FRNK's effects on earlier developmental processes were not addressed.
During early Xenopus laevis development, FAK mRNA is expressed maternally in the egg, then through blastula and gastrula stages, with a decrease at neurula stages (Hens and DeSimone, 1995; Zhang et al., 1995). However, FAK protein levels are dynamic in the embryo. FAK protein levels are low before the onset of gastrulation but then increase at the gastrula stage, staying fairly constant at neurula stages, in contrast to the decreased mRNA levels. This temporal pattern of protein expression suggests that FAK could have an important role in early developmental processes. Nevertheless, the role of FAK protein in early neural induction and patterning has not been examined during Xenopus development.
In this study, FAK protein was knocked down during early X. laevis development. FAK is expressed in the neural plate, and in FAK morphant embryos, posterior neural cell fate and morphogenesis are severely perturbed. FAK morphant embryos lose posterior neural cell fates while expanding anterior neural cell fates. The zygotic canonical Wnt-signaling pathway is crucial for posterior neural patterning, and its activity is impaired in FAK morphant embryos. Like FAK, the Wnt3a gene is also expressed in the neural plate. Endogenous Wnt3a expression is strongly reduced in FAK morphants, and ectopic Wnt expression rescues the anteriorized phenotype. Thus, in the developing Xenopus nervous system, Wnt3a acts downstream to FAK in specifying the balance of anterior–posterior (A-P) neural cell fates. We also show that in human breast cancer cells, Wnt3 gene expression is inhibited when FAK protein levels are reduced by RNAi. This study novelly connects FAK protein activity to the Wnt-signaling pathway, both of which have been implicated as promoting cancer when aberrantly activated in mammalian cells.
RESULTS
Expression of FAK mRNA in neurula-stage embryos
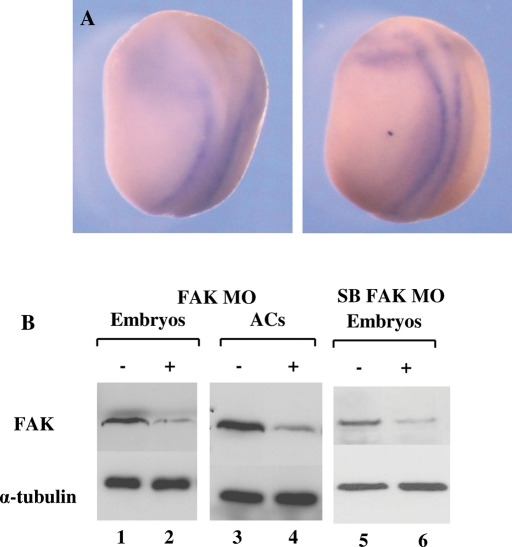
Previous studies examined FAK mRNA levels in tadpole stage embryos, and, in parallel, FAK protein levels were detected at blastula, gastrula, and early tailbud stages by immunostaining (Hens and DeSimone, 1995; Zhang et al., 1995). We examined the FAK gene expression pattern in mid to late neurula-stage embryos since it was not well characterized. FAK mRNA was detected on the neural plate edge but excluded from the more anterior regions, where folds closure is later (Figure 1A). This earlier FAK gene expression in the neural plate is consistent with later expression observed in the tadpole nervous system (Hens and DeSimone, 1995).
FIGURE 1:
Embryonic FAK expression and MO knockdown in Xenopus embryos. (A) In situ hybridization to FAK mRNA in neurula embryos. Embryos are viewed dorsally and oriented anterior (top), posterior (bottom); left, stage 17; right, stage 20. (B) Western analysis shows that FAK MO and SB FAK MO effectively reduce FAK protein levels in morphant embryos (compare lanes lane 1 and 2, and 5 and 6, respectively) and AC explants (compare lanes 3 and 4). Approximately 30 μg of total protein was loaded per lane. Loading per sample is determined by α-tubulin protein.
FAK morpholino oligonucleotides inhibit endogenous FAK protein levels in the embryo
To examine a role for FAK protein during neural plate development, X. laevis FAK protein was knocked down in the early embryo. We designed three antisense morpholino oligonucleotides (MOs) to the FAK gene. The first and primary MO of use was designed to block translation at the 5′-end region of the FAK mRNA, which includes the first codon; the second FAK MO set consisted of a splice blocking (SB) pair of MOs targeting the exon 8–intron junction and the intron–exon 9 junction in the Xenopus FAK gene (Supplemental Figure 1A). Western blot analysis showed that both the translational blocking (Figure 1B, lanes 1–4) and SB (Figure 1B, lanes 5 and 6) MOs strongly reduced endogenous FAK protein levels in both embryos and animal cap (AC) explants. The specificity of the SB MO pair was verified by designing specific RT-PCR primers to both sides of the excised intron (Supplemental Figure 1B). When normal intron excision occurs, a 300–base pair exon fragment is observed by semiquantitative (sq) RT-PCR (Supplemental Figure 1C, lane 2). When splicing is perturbed in vivo by the SB MO pair, there is a strong reduction in this 300–base pair fragment (Supplemental Figure 1C, lane 4). In contrast, the FAK translation blocking MO inhibits protein translation without perturbing endogenous FAK mRNA splicing (Supplemental Figure 1C, lane 3).
FAK protein knockdown anteriorizes embryos
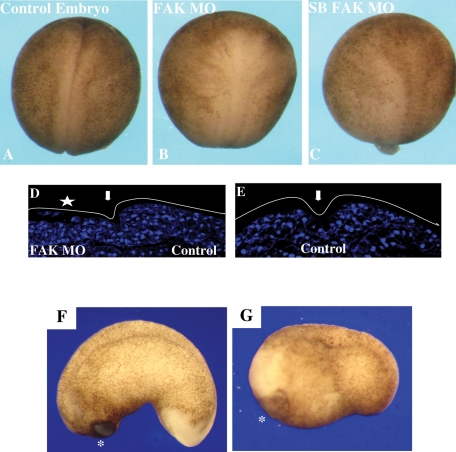
The phenotypes of embryos injected with either type of FAK MO were similar. In neurula-stage FAK morphant embryos, neural plate folding and elongation (convergence-extension) were strongly inhibited, as seen by the open-neural-plate phenotype (compare Figure 2, A to B and C). Gastrulation was not significantly affected in morphant embryos (unpublished data). The effect of the FAK protein knockdown on neural folding was more extensively examined by injecting the FAK MO unilaterally into one blastomere at the two-cell stage. Axial sections of neurula stage embryos were examined. The folding of neural folds on the FAK MO–injected embryo side was severely inhibited, as emphasized by the open neural fold on the FAK MO–injected side (Figure 2D, left) compared with the noninjected side of the same embryo (Figure 2D, right) or an uninjected control embryo (Figure 2E). At later tadpole stages, the embryo body axis was truncated (compare Figure 2, F to G) with a concomitant expansion of anterior ectodermal cement gland tissue (Figure 2G). FAK morphant embryos have a characteristic anteriorized phenotype in which posterior neural plate folding and elongation morphogenesis are highly perturbed and the anterior cement gland tissue is expanded. This phenotype suggests that the reduction of embryonic FAK protein levels triggers an anteriorization of embryonic neuroectoderm.
FIGURE 2:
FAK morphant embryos are anteriorized. (A) Late neurula-stage control embryo. (B) Embryos were injected with FAK MO (60 ng) at the one-cell stage. FAK MO–injected embryos are anteriorized, having a shortened A-P axis and open neural folds in 100% (n = 23) of the embryos. (C) The splice-blocking FAK MO (20 ng)–injected embryos had the same phenotype as in B in 96% (n = 26) of the embryos. (D) TO-PRO-3 iodide nuclear staining of neural tube cross sections in FAK morphant embryos. A transverse view, with dorsal oriented to the top and ventral to the bottom. FAK MO (30 ng) was injected into one blastomere at the two-cell stage. At neurula stages, embryos are transversely sectioned and nuclei stained with TO-PRO-3 iodide. The neural folds are visually analyzed. The arrow indicates the dorsal midline. The injected side is on the left, indicated by a star. The elevation of the neural folds on the injected side (left) is perturbed in comparison to the uninjected control side (right). (E) Control uninjected sibling embryo treated as in D. (F) A lateral view of a control tadpole-stage embryo: oriented anterior to left, posterior to right. Normal phenotypes in 89% (n = 38) of the embryos. (G) In the FAK MO (30 ng)–injected embryos, the A-P axis is truncated, the tail is shortened, and the cement gland is expanded. Anteriorized phenotypes in 97% (n = 31) of the embryos.
FAK protein knockdown modulates expression of posterior and anterior neural markers
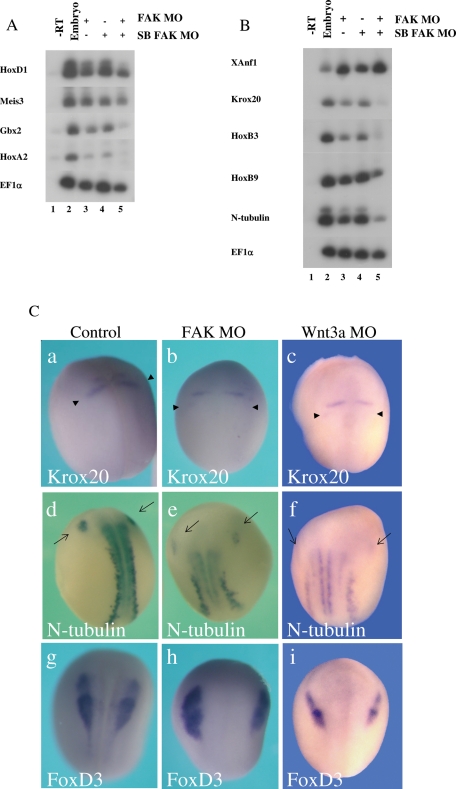
Embryos injected with both FAK MO types were analyzed for posterior neural gene marker expression at early and late neurula stages. At early neurula stages, both MOs strongly inhibited expression of posterior homeobox genes such as HoxA2, Gbx2, and HoxD1 but only slightly inhibited Meis3 expression (Figure 3A). These early-expressed homeobox genes are all required to establish posterior neural cell fates in the Xenopus embryo. When coinjected into the same embryo, both MOs induced a strong additive effect, almost completely eliminating posterior neural marker expression. This observation further supports the high specificity of both MO sets for triggering an identical FAK knockdown phenotype. At late neurula stages, expression of posterior neural markers of the spinal cord, hindbrain, and primary neurons (Krox20, HoxB3, HoxB9, N-tubulin) is typically decreased threefold to sixfold, whereas expression of the anterior forebrain marker XAnf1 is typically increased by threefold to sixfold (Figures 3B and 8B later in the paper). These inhibitory effects are much stronger when both MOs are coinjected (Figure 3B).
FIGURE 3:
The FAK MO and the splice FAK MO perturb posterior neural marker expression in neurula-stage embryos. (A) Embryos (lane 2) were injected at the one-cell stage with the FAK MO (60 ng, lane 3), splice-blocking FAK MOs (18 ng each, lane 4), or both types (lane 5). At early neurula stages, expression of the posterior neural homeobox marker genes HoxD1, Meis3, Gbx2, and HoxA2 was examined by sqRT-PCR. In all experiments, RNA is isolated from pools of 5–10 embryos per group. EF1a serves as a positive control for RNA loading in all shown experiments. (B) RNA from sibling embryos shown in A was isolated at late neurula stages. Expression of the posterior neural homeobox marker genes Krox20, HoxB3, HoxB9, and N-tubulin was examined by sqRT-PCR. Expression of the anterior forebrain marker XAnf1 was also examined. Both MOs inhibited posterior neural marker expression while up-regulating anterior marker expression. (C) By in situ hybridization, FAK and Wnt3a morphant phenotypes are compared. Three different posterior cell types were analyzed by the following probes: Krox20 (a–c, hindbrain), N-tubulin (d–f, primary neuron) and FoxD3 (g–i, neural crest). Embryos were injected at the one-cell stage with the FAK MO (60 ng; b, e, h) or the Wnt3a MO (30 ng; c, f, i). Arrowheads (a–c) mark the r5 Krox20 expression band, and full arrows (d–f) mark the trigeminal (V cranial) nerve expression of N-tubulin. Embryos are at late neurula stages, viewed dorsally; anterior is on top. (a) Krox20 expression is normal in all embryos (n = 10). (b) Krox20 expression is perturbed in 90% of the FAK morphant embryos (n = 10). (c) Krox20 expression is perturbed in all of the Wnt3a morphant embryos (n = 14). (d) N-tubulin expression is normal in all embryos (n = 10). (e) N-tubulin expression is perturbed in all of the FAK morphant embryos (n = 7). (f) N-tubulin expression is perturbed in all of the Wnt3a morphant embryos (n = 17). (g) FoxD3 expression is normal in all embryos (n = 10). (h) FoxD3 expression is at normal levels but morphologically perturbed in 75% of the FAK morphant embryos (n = 12). (i) FoxD3 expression is at normal levels but morphologically perturbed in all of the Wnt3a morphant embryos (n = 16).
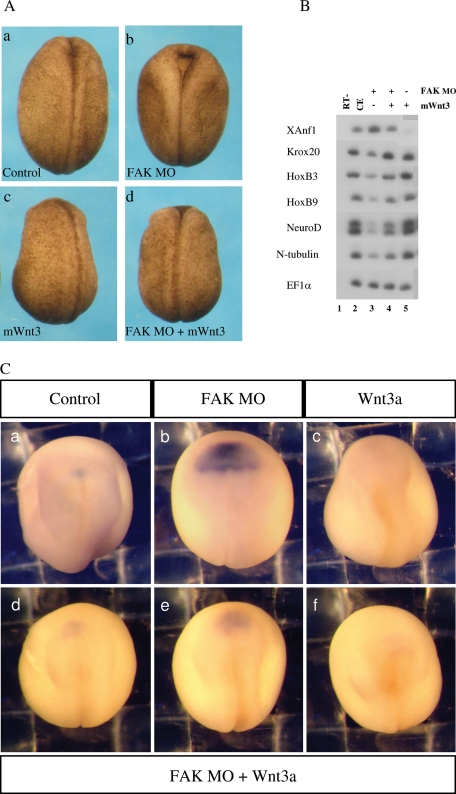
FIGURE 8:
The FAK morphant phenotype is rescued by zygotic mouse Wnt3 ectopic expression. (A) Morphological rescue of neural folding defects in FAK morphant embryos. At the one-cell stage, embryos were separately injected with FAK MO (30 ng) and/or the mWnt3 expression vector driven by the CMV promoter (60 pg). Embryos were analyzed at late neurula stages. (a) All control embryos show normal neural folding (n = 27), (b) all FAK morphant embryos had perturbed neural folding (n = 32), (c) all posteriorized mWnt3-expressing embryos had normal neural folding (b = 16), and (d) FAK MO + mWnt3–expressing embryos had rescued neural folding in 94% of the embryos (n = 16). (B) Posterior neural marker expression is rescued by ectopic mWnt3 in FAK morphant embryos. Embryos were injected at the one-cell stage with FAK MO (30 ng; lane 3), mWnt3 expression vector (50 pg; lane 5), or both (lane 4). As determined by sqRT-PCR, inhibition of hindbrain/spinal cord (Krox20, HoxB3, HoxB9) and primary neuron marker (NeuroD, N-tub) expression, as well as the increase in the anterior XAnf1 marker (forebrain), in FAK morphants is rescued by ectopic mWnt3. C. Wnt3 suppresses expanded XAnf1 expression in FAK morphants. At the one-cell stage, embryos were separately injected with FAK MO (50 ng) and/or the mWnt3 expression vector (60 pg). In situ hybridization to XAnf1 was performed at late neurula stages. (a) Normal XAnf1 expression in control embryos (n = 68). (b) In FAK morphants, XAnf1 expression is strongly expanded in 95% of the embryos (n = 64). (c) In mWnt3-expressing embryos, XAnf1 expression is eliminated in 80% of the embryos (n = 81). In rescued, FAK MO/mWnt3–injected embryos (n = 77), only 26% have expanded XAnf1 expression (unpublished data), 22% have normal XAnf1 expression (d), 25% have slightly expanded expression (e), and 21% have no expression (f).
Neurula-stage embryos injected with the FAK MO at the one-cell stage were examined by in situ hybridization for different posterior neural cell type markers, which included Krox20 (hindbrain; Figure 3, Ca and Cb), N-tubulin (primary neuron; Figure 3, Cd and Ce), and FoxD3 (neural crest; Figure 3, Cg and Ch). In FAK morphant embryos, there was a posterior shift, reduction, and elimination of the rhombomere (r) 5 band of Krox20 expression (compare Figure 3, Ca and Cb, arrowheads). There was a strong posterior shift and down-regulation of N-tubulin expression, seen strikingly in the most anterior trigeminal neuron (compare Figure 3, Cd and Ce, full arrows). There was a significant morphological change in FoxD3 expression, with a widening of the distance between the two sides of the embryo due to poor neural folding, but expression levels were quite normal (compare Figure 3, Cg and Ch; Supplemental Figure 2F). Giving further support to the observed anteriorized phenotype, expression of the forebrain marker XAnf1 is highly expanded in morphant embryos (compare Figure 8, Ca and Cb).
As a control for FAK MO specificity, we also injected a 6–base pair FAK-mismatch (mm) MO. In embryos injected with the FAK-mm MO, we do not observe any abnormal neural phenotypes (Supplemental Figure 2, A–C), and posterior neural markers are expressed like control embryos (Supplemental Figure 2F). For additional specificity, we also show that the FAK-mm MO does not significantly block endogenous FAK protein translation, in contrast to the FAK MO (Supplemental Figure 2G).
Ectopic FAK protein expression rescues the FAK morphant phenotype
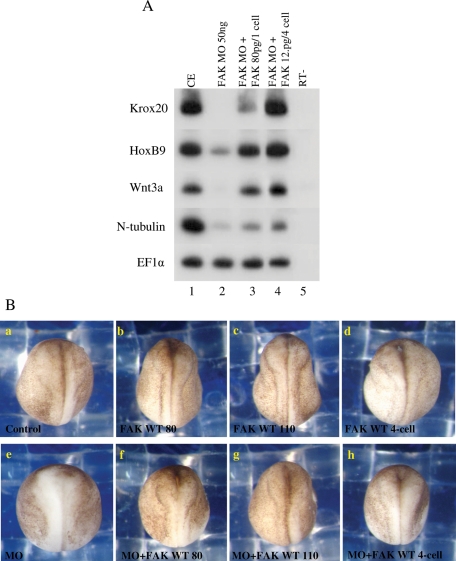
To examine FAK MO specificity, we rescued the morphant phenotype by ectopically expressing FAK protein. Embryos were injected with the FAK MO at the one-cell stage and then full-length X. laevis FAK–encoding mRNA was injected by two strategies. FAK mRNA was injected at either the one-cell stage or the eight-cell stage, where mRNA was injected into the upper animal tier of four blastomeres fated for ectoderm. In both cases, ectopic FAK mRNA expression rescued the FAK morphant anteriorized phenotype. Expression of posterior neural plate markers such as Krox20, HoxB9, N-tubulin, and Wnt3a was strongly inhibited in the morphant embryos (greater than fivefold), and their expression was restored in almost all cases to nearly normal levels after reexpression of FAK (Figure 4A). FAK morphant embryos display a typical anteriorized phenotype with poor neural folding (compare Figure 4, Ba to Be). Ectopic expression of FAK protein rescues this phenotype (compare Figure 4, Be to Bf–h) and in many cases, rescued embryos or normal embryos overexpressing FAK protein display neural-folding phenotypes resembling posteriorized embryos in which the anterior neural folds overthicken and prematurely fold (compare Figure 4, Ba to Bb–d).
FIGURE 4:
The FAK morphant phenotype is rescued by overexpression of FAK mRNA. (A) Posterior neural marker expression is rescued by ectopic FAK in morphant embryos. Embryos were injected with the FAK MO (50 ng) at the one-cell stage, and then full-length X. laevis FAK–encoding mRNA was injected at either the one-cell stage (80 pg) or the eight-cell stage, where RNA was injected into the upper animal tier of four blastomeres (12.5 pg/blastomere) fated for ectoderm. Total RNA was isolated at late neurula stages from pools of six embryos, and sqRT-PCR was performed to the posterior neural Krox20, HoxB9, Wnt3a, and N-tubulin marker genes. (B) Morphological rescue of neural folding defects in FAK morphant embryos. Embryos were separately injected with FAK MO and/or FAK mRNA at the one-cell or eight-cell stage as described for A, except that an additional embryo group was injected with 110 pg of FAK mRNA at the one-cell stage. Embryos were analyzed at late neurula stages: (a) 88% of the control embryos had normal neural folding (n = 34), (b–d) 92% of the FAK-expressing embryos had normal neural folding (n = 77), (e) 67% of the FAK morphant embryos had abnormal neural folding (n = 30), (f) 76% of the FAK MO + FAK (80 pg)–expressing embryos had rescued neural folding (n = 34), (g) 75% of the FAK MO + FAK (110 pg)–expressing embryos had rescued neural folding (n = 36), and (h) 56% of the FAK MO + FAK (12.5 pg/blastomere)–expressing embryos had rescued neural folding (n = 39).
Thus, by four independent experimental approaches, we have shown that the FAK MO acts specifically to inhibit FAK protein expression. We have shown that two different types of FAK MOs (translational and splice blocking) give similar embryonic phenotypes. These two FAK MO types act additively when they are coinjected at limiting concentrations, suggesting a common target. Both FAK MO types block expression of the endogenous Xenopus FAK protein in vivo. Ectopic expression of FAK protein rescues the FAK morphant phenotype, and, finally, FAK-mm MO injection does not significantly alter FAK mRNA translation or cause any severe embryo phenotypes.
FAK protein is required for FoxD1 induction of posterior markers in explants
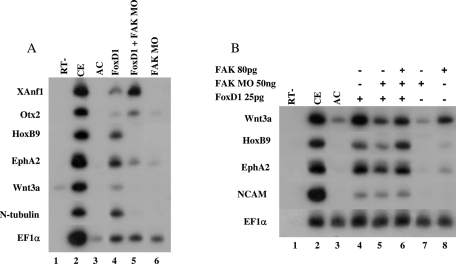
Using a parallel approach in ectodermal AC explants, we determined whether FAK protein activity is required for posterior neural induction by the helix-turn-helix transcription factor FoxD1 (XBF-2) protein. In AC explants, ectopic expression of the FoxD1 protein induces all A-P neural cell fates, and these explants also undergo neural convergence-extension cell movements characteristic of posterior neural cell fates (Mariani and Harland, 1998; Wallingford and Harland, 2002; Borchers et al., 2006). This ex vivo explant system is an excellent way to examine the interaction of factors regulating neural patterning. To elucidate FAK's role in the neural A-P patterning, AC explants expressing FoxD1 protein were coinjected with the FAK MO. FoxD1 alone induced expression of anterior (XAnf1, Otx2) and posterior neural markers (HoxB9, Eph2a, Wnt3a, N-tubulin) in ACs (Figure 5A, compare lanes 3 and 4; Figure 5B, lanes 3 and 4). When coinjected with the FAK MO, anterior neural marker expression increased, with a strong reciprocal reduction of posterior neural marker expression (Figure 5A, compare lanes 4 and 5; Figure 5B, lanes 4 and 5). Neural convergence-extension was also inhibited in these explants (unpublished data). FoxD1 induction of panneural marker NCAM expression was unchanged in morphant explants (Figure 5B, lanes 4 and 5). To further examine MO specificity, coinjection of FAK-encoding mRNA was sufficient to efficiently rescue expression of posterior neural markers (Figure 5B, lane 6) and convergence-extension movements (unpublished data), but FAK alone is not a robust inducer of posterior neural cell fates in AC explants (Figure 5B, lane 8). Thus, recapitulating its embryonic function, FAK protein controls A-P patterning in AC explants induced to neural fates by the FoxD1 protein.
FIGURE 5:
FAK protein knockdown inhibits posterior neural induction by the FoxD1 protein. (A) One-cell-stage embryos were injected separately into the animal hemisphere with the FAK MO (60 ng) and/or 25 pg of FoxD1 mRNA. Animal cap (AC) explants were removed at blastula stages 8 to 9. Explants and embryos were cultured to neurula stages, and total RNA was isolated from pools of eighteen AC explants in each group and five control embryos (CE). sqRT-PCR analysis was performed with the markers XAnf1, otx2, HoxB9, Eph2a, Wnt3a, N-tubulin, and EF1α. sqRT-PCR was performed on total RNA isolated from normal embryos. (B) One-cell-stage embryos were injected separately into the animal hemisphere with the FAK MO (50 ng) and/or of FoxD1 (25 pg) mRNA. For rescue, FAK (80 pg) mRNA was injected. AC explants were cultured and RNA isolated as described in A. sqRT-PCR analysis was performed with the markers Wnt3a, HoxB9, Eph2a, Wnt3a, NCAM, and EF1α.
FAK knockdown resembles the phenotype of embryos knocked down for zygotic canonical Wnt activity
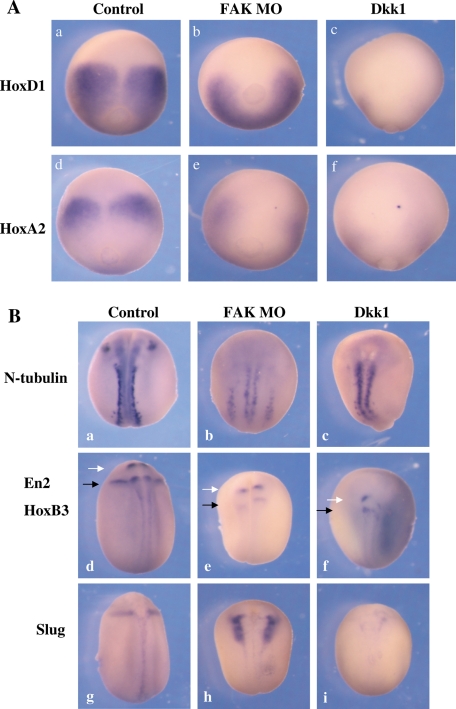
FAK morphant embryos have a phenotype resembling embryos inhibited for a known posteriorizer of the neural plate, zygotic canonical Wnt signaling. A potent inhibitor of embryonic canonical Wnt activity is the Dkk1 protein. Overexpression of Dkk1 protein in Xenopus anteriorizes the embryo, eliminating posterior structures, expanding the anterior cement gland and forebrain structures, and shortening the A-P axis (Glinka et al., 1998), much like the FAK morphant phenotype. We thus compared the FAK morphant and Dkk1 phenotypes. This similarity was confirmed by examining gene expression of two homeobox genes (HoxD1 and HoxA2) expressed in early neurula-stage embryos (Figure 6Aa, d). In FAK morphants, HoxD1 expression was attenuated and shifted posteriorly (Figure 6Ab), whereas HoxA2 expression was eliminated (Figure 6Ae). Ectopic Dkk1 expression showed similar but more potent effects, completely eliminating expression of both genes (Figure 6Ac, f).
FIGURE 6:
Comparison of posterior neural marker gene expression patterns in Dkk1-expressing and FAK morphant embryos. Embryos at the one-cell stage were injected with either FAK MO (60 ng) or Dkk1 (50 pg) mRNA. (A) In situ hybridization was performed at early neurula stages for two Hox genes, HoxD1 and HoxA2. Embryos are viewed dorsally, with anterior at the top: (a) 100% normal HoxD1 expression (n = 15), (b) 28% disrupted HoxD1 expression (n = 18), (c) 100% disrupted HoxD1 expression (n = 20), (d) 100%, normal HoxA2 expression (n = 15), (e) 100% disrupted HoxA2 expression (n = 18), and (f) 100% disrupted HoxA2 expression (n = 15). (B) In situ hybridization was performed at late neurula stages to N-tubulin (primary neuron) En2 (white arrow; midbrain–hindbrain junction), HoxB3 (black arrow; r5/6), and Slug (neural crest). Embryos are viewed dorsally, anterior at the top: (a) 100% normal n-tubulin expression (n = 15), (b) 100% disrupted n-tubulin expression (n = 18), (c) 100% disrupted n-tubulin expression (n = 9), (d) 100% normal En2/Hoxb3 expression (n = 15), (e) 95% disrupted En2/Hoxb3expression (n = 19), (f) 100% disrupted En2/Hoxb3 expression (n = 18), (g) 100% normal Slug expression (n = 15), (h) 83% disrupted but not reduced Slug expression (n = 18), and (i) 100% disrupted and reduced slug expression (n = 17).
At late neurula stages, both FAK MO– and Dkk1-injected embryos had a rounded morphology, with a shortened A-P axis, and wider, less elevated neural folds. Posterior neural marker expression was examined (Figure 6Ba, d, g). The FAK MO and Dkk1 both diminished expression of N-tubulin (primary neurons), eliminating the trigeminal neuron derived from the more anterior r2 of the hindbrain (Figure 6Ba–c). Two posterior brain markers, En2 (midbrain–hindbrain junction; white arrow) and HoxB3 (r5/6 hindbrain; black arrow) were strongly down-regulated and shifted posteriorly by both the FAK MO and Dkk1 (Figure 6Bd–f). Despite strong similarities in inhibiting En2, HoxB3, and N-tubulin gene expression, there was a sharp contrast in Slug (neural crest) expression (Figure 6Bg) in Dkk1- versus FAK MO–injected embryos. Whereas Dkk1 completely eliminated Slug expression (Figure 6Bi), in FAK morphant embryos, the Slug expression pattern (Figure 6Bh) was identical to the pattern seen for FoxD3 expression in morphants (Figure 3Ch), in which inhibition of neural folding prevents fusion of the neural crest at the dorsal midline. These results show that the FAK morphant embryos are similar but not identical to embryos ectopically expressing Dkk1 protein. Dkk1 protein is a more potent anteriorizer and posterior antagonist than the FAK MO, especially with regard to neural crest induction.
Endogenous canonical Wnt activity is inhibited in FAK morphant embryos
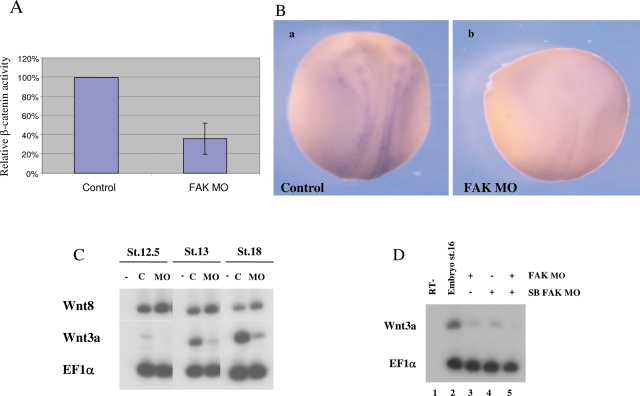
To further determine whether FAK protein depletion is associated with a reduction in canonical Wnt-signaling activity, we measured the endogenous in vivo levels of Wnt transcriptional activity in FAK morphant embryos. Embryos were injected with the β-catenin–responsive luciferase (luc) reporter gene construct (3X-TCF/Luc), containing three copies of the β-catenin/Tcf–binding site consensus sequence. Luc activity is the readout for endogenous β-catenin/Wnt–dependent transcription in the embryo. Coinjection with the FAK MO consistently reduced luc activity about threefold in late gastrula- to early neurula-stage embryos (Figure 7A), thus showing that FAK protein is required to maintain β-catenin–dependent transcription in the embryo. Supporting this observation, by Western blot analysis, we also saw a significant reduction of activated-dephosphorylated β-catenin protein levels (Ossipova et al., 2003) in FAK morphant embryos versus controls (unpublished data).
FIGURE 7:
FAK protein modulates Wnt activity. (A) The FAK MO decreases canonical Wnt signaling transcriptional activity. Embryos were injected with 25 pg of the 3X-TCF-luciferase reporter vector and 60 ng of FAK MO. The graph represents five different experiments at stages 11.5–13. In each experiment, a pool of five embryos was lysed per group. Luciferase activity was normalized to protein levels in control embryo extracts and was set at 100%. (B) The FAK MO (60 ng) down-regulates Wnt3a expression. In situ hybridization to Wnt3a was performed at late neurula stages. (a) Normal Wnt3a expression appears in 86% (n = 14) of the control embryos. (b) Wnt3a expression is eliminated in 95% of the FAK morphant embryos (n = 20). (C) Wnt3a and Wnt8 gene expression was compared from early to late neurula stages by sqRT-PCR in embryos injected with the FAK MO (60 ng). In FAK morphants Wnt3a expression is strongly inhibited at all the stages, whereas Wnt8 expression is normal. (D) Embryos were injected with the FAK MO (60 ng; lane 3), the splice-blocking MO (18 ng; lane 4), or both MOs (lane 5). sqRT-PCR to Wnt3a was performed at the indicated stages.
Wnt3a expression in the neural plate is down-regulated in FAK morphant embryos
We next determined whether the modulation of Wnt/β-catenin activity by FAK is mediated by regulating Wnt ligand expression in the embryo. The Xenopus Wnt3a gene is expressed at the edge of the neural plate, bordering the neural folds region (Figure 7Ba). Wnt3a and FAK share a similar overlapping expression pattern (compare Figure 1A and Figure 7Ba), and thus FAK could regulate Wnt3a expression. Indeed, FAK knockdown causes a strong reduction of zygotically expressed Wnt3a mRNA in the neural plate (Figure 4A, compare lanes 1 and 2; Figure 7Ba, b). Two ligands involved in embryonic canonical Wnt signaling were examined in neurula-stage morphant embryos. Neurally expressed Wnt3a was strongly inhibited from early to late neurula stages (5- to 10-fold), but mesodermal Wnt8 expression was not significantly altered (Figure 7C). The SB FAK MO also inhibited Wnt3a expression (Figure 7D, lane 4), and coinjection of both MOs caused a more severe effect (Figure 7D, lane 5). Ectopic FAK expression experiments in both embryos and explants further support its role in regulating Wnt3a gene expression. In both embryos (unpublished data) and AC explants (Figure 5B, lane 8) ectopic FAK protein levels induced Wnt3a gene expression (fivefold). In rescued FAK morphant embryos and explants, the strongly reduced Wnt3a expression levels were highly increased by ectopic FAK expression (Figure 4A, compare lanes 2, 3, and 4; Figure 5B, compare lanes 4, 5, and 6). Therefore, FAK seems to specifically regulate Wnt3a expression, thus controlling Wnt/β-catenin activity in the developing nervous system.
We also compared the expression levels of posterior neural markers between Wnt3a and FAK morphant embryos. Our previous studies showed that localized injection of the Wnt3a MO to the embryonic marginal zone region fated for mesoderm caused a phenotype in which all posterior cell fates are lost, similar to Dkk1 protein injection (Elkouby et al., 2010). This injection primarily knocks down early mesodermal expression of the Wnt3a gene, which is required to induce posterior neural tissue (Elkouby et al., 2010). Alternatively, when the Wnt3a MO is injected into the embryonic animal pole region fated for ectoderm, a milder phenotype is observed that highly resembles the FAK morphant phenotype (Supplemental Figure 2, compare E to B). In animally injected Wnt3a morphant embryos, as in FAK morphants, we see a strong reduction in Krox20 (compare Figure 3, Cc to Cb) and n-tubulin expression (compare Figure 3, Cf to Ce), with poor neural fold closure but no reduction in the neural crest–specific FoxD3 marker (compare Figure 3, Ci to Ch). The expression profile of posterior neural markers was also examined by sqRT-PCR, and they were similar in both Wnt3a and FAK morphant embryos (unpublished data).
The FAK morphant phenotype can be rescued by zygotic overexpression of Wnt3
If the FAK knockdown phenotype occurs because embryonic Wnt3a expression is eliminated, then Wnt-ligand readdition to FAK morphant embryos should rescue the phenotype. To replenish zygotic Wnt activity, we drove mouse Wnt3 expression via a CMV promoter. In initial experiments, we examined the morphology of delayed neural folds closure in FAK morphant embryos. Morphologically, overexpression of mouse Wnt3 rescued neural folds closure in FAK morphants (Figure 8Aa–d).
In phenotypically rescued embryos, expression of a panel of neural markers was examined. Expression of spinal chord, hindbrain, and primary neuron cell fate markers (Krox20, HoxB3, HoxB9, NeuroD, and N-tubulin) were all reduced twofold to fourfold in FAK morphant embryos (Figure 8B, compare lanes 2 and 3). This effect was reversed by Wnt3 overexpression, where rescued expression levels were close to control levels (Figure 8B, compare lanes 3 and 4). As seen by both sqRT-PCR and in situ hybridization, the forebrain-specific marker XAnf1 is increased and expanded in FAK morphant embryos (Figure 8B, compare lanes 2 and 3; compare Figure 8, Ca and Cb). Ectopic Wnt3a expression alone inhibits XAnf1 expression (Figure 8B, compare lanes 2 and 5; compare Figure 8, Ca and Cc). Ectopic Wnt3a expression in FAK morphants rescues XAnf1 expansion, returning endogenous XAnf1 expression to near control-like levels (Figure 8B, compare lanes 2 and 4; compare Figure 8, Ca and Cd–f).
These results confirm that FAK morphant rescue by Wnt3 occurs at both morphological and gene expression levels. FAK and Wnt3a mRNAs are expressed in similar domains in the neural plate (Figures 1A and 7B). FAK morphant embryos have a strong reduction in embryonic β-catenin activity (Figure 7A) that is likely due to the loss of neural plate–specific Wnt3a expression. These rescue experiments show that the FAK morphant phenotype stems from the loss of zygotic Wnt3a expression in the developing neural plate.
FAK protein regulates Wnt3a/Wnt3 gene expression in human cancer cells
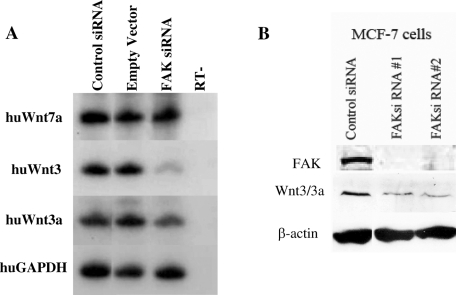
FAK protein levels were depleted by RNAi in the MCF-7 human breast cancer cell line, and microarray analysis showed that the expression of multiple genes was modulated by FAK protein (Golubovskaya et al., 2009b). The Xenopus Wnt3a protein displays equal amino acid sequence similarity and identity (∼85%) to both the human Wnt3 and Wnt3a proteins. In the original human microarray set examined, expression of Wnt3 was significantly reduced by the RNAi depletion of FAK protein, yet expression of other Wnt ligands was not significantly lowered (unpublished data). Wnt3a was not present in that array. By sqRT-PCR, we examined both Wnt3 and Wnt3a expression in these FAK-depleted cells. FAK protein depletion reduced the expression of both Wnt3a and Wnt3 transcripts by threefold to fourfold in the MCF-7 cell line (Figure 9A). As a control, Wnt7a mRNA levels were examined and shown to be unchanged by FAK depletion (Figure 9A). This result was also confirmed by Western blot analysis, in which the FAK-depleted MCF-7 cells also have reduced Wnt3/3a protein levels (Figure 9B). This similarity between frogs and humans suggests that FAK protein regulation of Wnt gene expression could be highly conserved among vertebrates.
FIGURE 9:
Depletion of FAK protein in human MCF-7 breast cancer cells reduces Wnt3 gene expression. (A) sqRT-PCR was performed on total RNA isolated from control siRNA-transfected, empty vector–transfected, and FAK siRNA–transfected human MCF-7 cells. Gene expression analysis was carried out for the human Wnt7a, Wnt3, Wnt3a, and GAPDH (positive control) genes. (B) Western analysis compares Wnt3 protein levels in two separately transfected FAK siRNA cell lines in comparison to a control siRNA. FAK siRNA effectively reduce Wnt3 protein levels vs. the control. Approximately 30 μg of total protein was loaded per lane. Loading per sample is determined by β-actin protein.
DISCUSSION
This work shows that the FAK protein plays an important role during early X. laevis nervous system development. FAK protein knockdown anteriorizes the embryo, with a parallel sharp loss of posterior neural cell fates, such as hindbrain, spinal cord, and primary neurons. Our attempt to find a mechanism explaining this phenotype led us to explore FAK's linkage to a known caudalizer of the vertebrate nervous system, canonical Wnt signaling. FAK knockdown inhibits canonical Wnt signaling in developing embryos by down-regulating Wnt3a gene expression in the neural plate. The readdition of Wnt signaling to FAK morphant embryos is sufficient to rescue the anteriorized phenotype. This work shows, for the first time a surprising connection in which FAK protein is essential for regulating embryonic Wnt3a gene expression.
Endogenous Wnt signaling is strongly impaired in FAK morphant embryos. We show that the expression of the β-catenin–responsive 3X(TCF)/Luc reporter plasmid is highly inhibited in vivo when coexpressed with the FAK MO. We also saw a reduction in the overall amount of activated β-catenin protein in FAK morphant embryos. In FAK morphant embryos, there is a sharp reduction in the early expression of the Gbx2 gene. In Xenopus, the Gbx2 protein is crucial for establishing correct A-P pattern in the neural plate (Tour et al., 2002; Li et al., 2009). This gene is a direct-transcriptional target of the Wnt/β-catenin pathway (Li et al., 2009), suggesting that the earliest-expressed Wnt-target genes in the neural plate require FAK protein.
FAK knockdown causes a similar but not identical phenotype to a known canonical Wnt-pathway inhibitor, the Dkk1 protein. Posterior neural marker expression is inhibited in a similar manner by either FAK knockdown or Dkk1 protein overexpression. Whereas ectopic Dkk1 expression strongly inhibits neural crest induction, FAK knockdown does not. Yet, FAK knockdown does severely perturb cell movements in the neural plate and folds region, which strongly disrupt proper neural crest morphology at neurula stages. FAK protein knockdown inhibits that Wnt pathway weakly and more subtly than the Dkk1 protein. These differing effects could be attributed to the different mechanisms of canonical Wnt-signaling inhibition mediated by either Dkk1 protein or FAK knockdown. Dkk1 protein globally inhibits canonical Wnt-ligand proteins by blocking Wnt–LRP6 coreceptor activity, whereas FAK knockdown only inhibits Wnt3a gene expression in the neural plate. FAK likely regulates a limited temporal and regional range of Wnt3a-dependent β-catenin activity in the embryo.
An alternative but not mutually exclusive explanation is supported by another study (Elkouby et al., 2010). In late gastrula-stage embryos, Wnt3a protein, originating from mesoderm, is essential for early neural Meis3 expression that is responsible for inducing hindbrain, primary neuron, and neural crest fates in the neural plate. Ectopic Dkk1 expression eliminates early Meis3 expression in the neural plate. Consequently, Meis3 activates later Wnt3a expression in the neural plate and Meis3 knockdown inhibits later neural plate specific Wnt3a expression (T.M.E. and D.F., unpublished data). Wnt3a expression partially overlaps Meis3 expression in a narrow region of the neural plate. Possibly, some competence mechanism exists in the neural plate that is responsible for Meis3-dependent activation of Wnt3a expression. Because FAK/Wnt3a expression patterns overlap in the neural plate, FAK could act as this competence factor for Meis3. In early neurula-stage embryos, FAK knockdown robustly inhibited expression of many of the earliest-expressed homeobox genes required for posterior neural patterning but only slightly perturbed Meis3 expression (Figure 6A). This is very different from ectopic Dkk1 protein expression, which efficiently eliminates Meis3 expression at these stages. We suggest that FAK knockdown does not inhibit “early” gastrula-stage Wnt3a expression in the mesoderm, which is responsible for Meis3 expression in the neural plate. However, FAK protein knockdown could inhibit Meis3-dependent activation of “later” Wnt3a expression in the neural plate, and this “later” phenotype is observed in FAK morphant embryos. Supporting this model, the localized injection of Wnt3a MO to the ectoderm caused a phenotype much more similar to that of FAK knockdown than ectopic Dkk1 protein expression. The loss of neural plate–specific Wnt3a expression in FAK morphant embryos is likely recapitulated by ectoderm-specific knockdown of Wnt3a protein by the Wnt3a MO in the neural plate. We suggest that global Wnt antagonism by Dkk1 protein suppresses all zygotic Wnt functions, including both the “early” mesodermal and “late” neural Wnt3a functions. In contrast, we suggest that FAK knockdown inhibits only the “late” Wnt3a expression and function in the neural plate—thus the more moderate phenotype.
FAK morphant embryos resemble Meis3 morphant embryos (Aamar and Frank, 2004) in having poor neural folding and convergence-extension. This raises the question as to whether FAK protein directly regulates some aspect of noncanonical Wnt planar-cell-polarity (PCP) activity that is required for normal neural convergence-extension in the hindbrain and spinal cord (Wallingford and Harland, 2002). None of our experimental results suggest this connection. In previous studies we developed a recombinant AC explant system in which Meis3 protein induces neural convergence-extension that is dependent on downstream Wnt PCP activity (Aamar and Frank, 2004). In this assay, Meis3-expressing AC explants induce strong Wnt PCP-dependent neural convergence-extension when juxtaposed to neuralized AC explants (Aamar and Frank, 2004). In this recombinant explant assay, injection of the FAK MO into the neuralized AC explant side never perturbed Wnt PCP-dependent convergence-extension cell movements induced by Meis3 protein (unpublished data). Thus, in this assay, neural convergence-extension occurs in the absence of FAK protein. Meis3/Wnt3a proteins are required for proper induction of posterior neural cell fates. If proper posterior specification is lost in the neural plate, then these “confused” cells will not undergo proper convergence-extension movements associated with posterior neural cell fates. Wnt-PCP activity regulating neural convergence-extension likely lies way downstream to the FAK protein, whereas FAK protein mediates posterior neural cell specification by regulating more-upstream Wnt3a gene expression in the neural plate. Thus, by directly disrupting posterior cell fate specification, FAK protein depletion indirectly inhibits subsequent neural convergence-extension.
Although our study suggests a specific role for FAK protein in early neural patterning, another study in Xenopus showed that FAK was necessary for later somite-border formation (Kragtorp and Miller, 2006). In that study, the role of FAK was analyzed at later tadpole stages, and loss of FAK function was performed by ectopically expressing FRNK protein (Kragtorp and Miller, 2006). Another recent study in Xenopus has suggested that ectopic FRNK expression perturbs gastrulation cell movements (Stylianou and Skourides, 2009). We never observed any serious gastrulation abnormalities in FAK morphant embryos. FRNK is a naturally occurring truncated dominant-negative form of the FAK protein; FRNK protein's in vivo role as an FAK antagonist in cells is still not entirely clear. FAK interacts with many proteins, so ectopically expressed FRNK protein could form many protein–protein complexes. Thus, unlike the FAK MO that specifically depletes endogenous FAK protein in FAK-expressing cells, FRNK protein could form ectopic protein complexes in cells that do not express the FAK protein but do express FAK interacting proteins. In our study, we have depleted the endogenous Xenopus FAK protein and detected an early phenotype that we think reflects a new relevant function for the FAK protein in early vertebrate development.
Supporting these observations, knockdown of other focal adhesion–associated proteins gives embryonic phenotypes similar to FAK knockdown. Xena is a Xenopus analogue of the Ena/Vasp family of proteins associated with actin regulation that is enriched in focal adhesion regions. The Xena morphant phenotype was analyzed at neurula stages (Kragtorp and Miller, 2006; Roffers-Agarwal et al., 2008). Xena knockdown disrupted neural tube closure by inhibiting elevation and mediolateral movement of neural folds. The Xena morphant phenotype is similar to the FAK morphant phenotype. Despite very detailed morphological analysis of Xena morphant embryos, analysis of A-P patterning defects in the neural plate was not examined. It would be interesting to determine whether Wnt3a gene expression is disrupted in Xena morphant embryos.
Recently, new roles for FAK have emerged that link its action to the nucleus. FAK protein has been shown to localize in the nucleus (Lim et al., 2008), and nuclear FAK protein was shown to interact with the chromatin-modulating methyl CpG–binding protein 2 (MBD2). FAK binding to MBD2 reduces its binding to histone deacetylase 1 protein, thus suggesting a role for FAK in modulating chromatin structure and gene expression (Luo et al., 2009). FAK promotes cell cycle progression through the transcriptional control of CyclinD1 by affecting two transcription factors, increasing the DNA-binding activity of EtsB protein and increasing KLF8 gene expression (Zhao et al., 2001, 2003; Cox et al., 2006). In addition, FAK nuclear accumulation, but not its kinase activity, was also shown to affect the stability of another transcription factor, the p53 tumor suppressor protein (Lim et al., 2008). Thus, like KLF8 gene expression, Wnt3a expression is also dependent on the presence of FAK protein. Recent microarray analysis of the breast cancer MCF-7 cell line depleted for FAK protein showed that the gene expression profile of many genes was altered (Golubovskaya et al., 2009b), including Wnt3 and Wnt3a (Figure 9). Thus our results show that FAK nuclear activity likely plays an important functional role in regulating cell fate specification during normal embryo development.
The human FAK gene promoter region contains transcription factor–binding sites that include two NF-κB– and two p53-binding sites. p53 protein represses FAK gene expression, whereas NF κB activates expression (Golubovskaya et al., 2004). In various estrogen-dependent breast cancer cell lines, the repression of FAK gene expression by p53 is mediated by estradiol (Anaganti et al., 2011). Perhaps not so coincidentally, estradiol was also shown to be involved in suppressing Wnt3/3a gene expression in breast cancer cell lines (Katoh, 2002). In Xenopus, N-acetyl cysteine, an inhibitor of NF-κB activation, gives an anteriorized phenotype similar to FAK knockdown, especially at intermediate concentration levels (Gatherer and Woodland, 1996), hinting that NF-κB could also regulate Xenopus FAK gene expression. Further experiments need to be carried out to determine these connections.
Other intracellular kinases associated with integrin signaling and focal adhesions also influence canonical Wnt signaling. Integrin-linked kinase (ILK) is a cytoplasmic serine-threonine kinase that also has adaptor protein functions in signaling mediated by integrins and different growth factors. ILK overexpression causes nuclear translocation of β-catenin and activation of Wnt-dependent gene expression; phosphorylation and subsequent inactivation of GSK 3β was also observed (Novak et al., 1998; Oloumi et al., 2006). Down-regulation or inhibition of ILK caused a decrease in Wnt3a-dependent stabilization of β-catenin, thus inhibiting the pathway. Of interest, some of the actions of ILK were mediated through activation of PI3 kinase and Akt, both of which are targets of FAK signaling. Although the expression of Wnt genes was not analyzed, the similarities between ILK and FAK protein activities suggest that these two kinases could regulate canonical Wnt signaling by parallel pathways.
This newly uncovered link between FAK and canonical Wnt signaling could be widespread in many biological systems. There are a number of disease and pathological states associated with FAK or Wnt overactivation. Both the canonical Wnt pathway and FAK were independently found to be implicated in idiopathic pulmonary fibrosis (IPF), a common form of lung fibrotic disease of unknown etiology. IPF is a progressive disease characterized by increased fibroblastic proliferation and extracellular matrix remodeling. The result of these processes is the dramatic disruption of the lung's natural architecture. Canonical Wnt signaling was shown to be aberrantly activated in IPF (Chilosi et al., 2003; Konigshoff et al., 2008; Henderson et al., 2010). In addition, FAK phosphorylation and activation were found in models of IPF in mouse, and inhibition of FAK activation was shown to protect against fibrosis (Vittal et al., 2005; Garneau-Tsodikova and Thannickal, 2008; Cai et al., 2010). Perhaps this FAK/Wnt connection is important in regulating disease progression; further investigation of this novel connection could aid to better clarify the pathogenesis of this mysterious disease.
Both canonical Wnt signaling and FAK protein overactivation were independently demonstrated to play a role in numerous types of human cancers, including colon, breast, prostate, and ovary cancers (Smalley and Dale, 1999; McLean et al., 2005; Golubovskaya et al., 2009a). The FAK/Wnt connection described in this work could be further investigated in different models of cancer induction and metastasis. It will be interesting to determine whether there is a causative link connecting both aberrant Wnt signaling and FAK activities in cancer. We have also found this connection in the MCF-7 breast cancer cell line, in which the reduction of FAK protein suppresses Wnt3/3a expression levels. Recent studies in mice also support a FAK/Wnt connection. FAK was required downstream of Wnt to promote intestinal regeneration and tumorigenesis by Akt activation (Ashton et al., 2010). FAK activity is increased during intestinal regeneration in a Wnt/c-myc–dependent manner; in Apc loss-of-function mice that also lack FAK activity, apoptosis was higher and tumor development reduced. In contrast to the frog, Wnt acts upstream to FAK in the mammalian intestine. Another recent study in an osteocyte cell line showed that β-catenin protein levels induced by mechanical stimulation were dependent on FAK protein activity (Santos et al., 2010). In human cell lines, FAK signaling via the Grb2 protein was shown to activate the β-catenin/Wnt pathway (Crampton et al., 2009). In this system, grb2 protein interacts with the disheveled protein to activate the β-catenin/Wnt pathway. Of interest, in FAK inhibitor treated–colon cancer cells, the expression of LRP5 and Frizzled2 Wnt-pathway activating receptors are down-regulated twofold to threefold, whereas expression of the Wnt-pathway inhibitor Dkk1 is up-regulated nearly fourfold (V.M.G., unpublished data). Thus there appear to be multiple interactions between the β-catenin/Wnt and FAK signaling pathways in different cell types. The next step will be to further confirm and elucidate the functional associations between FAK and Wnt activities in these different biological systems. Mutual FAK–Wnt pathway regulation could be a general phenomenon, having many still undetermined roles in either normal physiological or disease processes.
MATERIALS AND METHODS
Xenopus embryos and histology
Ovulation, in vitro fertilization, embryo culture, and dissections were carried out as described (Re'em-Kalma et al., 1995). Embryos were staged according to Nieuwkoop and Faber (1967). Histological sections and nuclear counterstaining detection was performed (Keren et al., 2005) with TO-PRO-3 iodide (Molecular Probes; Invitrogen, Carlsbad, CA).
RNA, DNA, and MO injections
Capped sense in vitro–transcribed FAK, Dkk1 (Glinka et al., 1998), and FoxD1 (Borchers et al., 2006) full-length mRNAs were injected into embryos at the indicated stages. A full-length mouse Wnt3 (pCS105) CMV-promoter plasmid (Elkouby et al., 2010) was injected in zygotic expression assays. Full-length X. laevis FAK cDNA was cloned from total RNA isolated from late neurula-stage embryos using PFU DNA polymerase; the full-length cDNA was subcloned into the pCS107 vector. Embryos were injected with the β-catenin–sensitive luciferase reporter gene construct 3X(TCF)/Luc, and luc activity was detected as previously described (Aamar and Frank, 2004). The Wnt3a MO was used as previously described (Elkouby et al., 2010) but injected animally into embryos. The various FAK MOs (Gene Tools, Philomath, OR) were as follows:
Translational blocking: TTGGGTCCAGGTAAGCCGCAGCCAT
FAK mismatch (mm): TTCGCTCCAGCTAAGCGGCACCGAT
Splice blocking (SB), intron–exon: ATGGGTGGGCTGAAAAACATAACAT
Splice blocking (SB), exon–intron: GAACAATGTACTTACATTAGAGCCC
Whole-mount in situ hybridization
Whole-mount in situ hybridization was carried out with digoxigenin/flourescein–labeled probes (Harland, 1991): FAK, Wnt3a, En2, Krox20, HoxB3, HoxD1, HoxA2, N-tubulin (N-tub), Slug, and FoxD3 (Dibner et al., 2004; Elkouby et al., 2010; Gutkovich et al., 2010).
sqRT-PCR analysis
sqRT-PCR was performed (Snir et al., 2006) on total RNA isolated from Xenopus or human MCF-7 cells. Xenopus primers: EF1a (loading control), NCAM, XANF1, Otx2, Krox20, HoxB9, HoxB3, Eph2a, gbx2, Meis3, HoxD1, HoxA2, Wnt3a, Wnt8, Slug, FoxD3, N-tub, and NeuroD (Elkouby et al., 2010; Gutkovich et al., 2010). Total cellular RNA was isolated from cultured human MCF-7 cells with a NucleoSpin RNA II Purification Kit (Clontech, Mountain View, CA). The human primer sets are as follows:
GAPDH (loading control) A: ACCACAGTCCATGCCATCAC, B: TCCACCACCCTGTTGCTGTA;
Wnt3 A: GGCTGTGACTCGCATCATAA, B: CAGCAGGTCTTCACCTCACA;
Wnt3a A: GCCCCACTCGGATACTTCTT, B: CACTCCTGGATGCCAATCTT; and
Wnt7a A: CCCACCTTCCTGAAGATCAA, B: ACAGCACATGAG-GTCACAGC.
The Xenopus FAK Splice detection primer (300 base pairs) set used for sqRT-PCR is A: GAGTTGGCTATTGGC; B: CTCGCCTTCTTTTTGTGG.
In all sqRT-PCR experiments performed, each sample is routinely assayed a minimum of two times for each marker.
Western blot analysis
Western blot analysis was performed (Dibner et al., 2001; Golubovskaya et al., 2009b). Endogenous Xenopus FAK protein was detected by a FAK (C-903; Santa Cruz Biotechnology, Santa Cruz, CA) rabbit polyclonal antibody (1:1000). Bound antibodies were detected with secondary anti–rabbit antibody (Pierce, 31460), diluted 1:20,000. Human Wnt3 protein was detected by a Wnt3 (ab32249; Abcam, Cambridge, MA) rabbit polyclonal antibody (1:500). Bound antibodies were detected with secondary anti–rabbit antibody (NA934; GE Healthcare Bio-Sciences, Piscataway, NJ), diluted 1:2000.
Supplementary Material
Acknowledgments
We thank C. Niehrs and J. Baker for plasmids and Michal Levy for technical assistance. D.F. was supported by grants from the Israel Science Foundation (197/05, 658/09) and the F.F. Technion Research Fund. D.F. and Y.F. were supported by a grant from the Israel-Niedersachsen Fund (ZN2319). Y.F. was supported by a Regina Leventhal Foundation Prize.
Abbreviations used:
- AC
animal cap
- A-P
anterior–posterior
- CMV
cytomegalovirus
- Dkk1
Dickkopf1
- FAK
focal adhesion kinase
- IPF
idiopathic pulmonary fibrosis
- Luc
luciferase
- mm
mismatch
- MO
morpholino oligonucleotide
- SB
splice blocking
- sqRT-PCR
semiquantitative reverse transcription-PCR
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E10-12-0932) on May 5, 2011.
REFERENCES
- Aamar E, Frank D. Xenopus Meis3 protein forms a hindbrain-inducing center by activating FGF/MAP kinase and PCP pathways. Development. 2004;131:153–163. doi: 10.1242/dev.00905. [DOI] [PubMed] [Google Scholar]
- Anaganti S, Fernandez-Cuesta L, Langerod A, Hainaut P, Olivier M. p53-dependent repression of focal adhesion kinase in response to estradiol in breast cancer cell-lines. Cancer Lett. 2011;300:215–224. doi: 10.1016/j.canlet.2010.10.008. [DOI] [PubMed] [Google Scholar]
- Ashton GH, et al. Focal adhesion kinase is required for intestinal regeneration and tumorigenesis downstream of Wnt/c-Myc signaling. Dev Cell. 2010;19:259–269. doi: 10.1016/j.devcel.2010.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beggs HE, Schahin-Reed D, Zang K, Goebbels S, Nave KA, Gorski J, Jones KR, Sretavan D, Reichardt LF. FAK deficiency in cells contributing to the basal lamina results in cortical abnormalities resembling congenital muscular dystrophies. Neuron. 2003;40:501–514. doi: 10.1016/s0896-6273(03)00666-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borchers A, Fonar Y, Frank D, Baker JC. XNF-ATc3 affects neural convergent extension. Development. 2006;133:1745–1755. doi: 10.1242/dev.02343. [DOI] [PubMed] [Google Scholar]
- Cai GQ, Zheng A, Tang Q, White ES, Chou CF, Gladson CL, Olman MA, Ding Q. Downregulation of FAK-related non-kinase mediates the migratory phenotype of human fibrotic lung fibroblasts. Exp Cell Res. 2010;316:1600–1609. doi: 10.1016/j.yexcr.2010.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chilosi M, et al. Aberrant Wnt/beta-catenin pathway activation in idiopathic pulmonary fibrosis. Am J Pathol. 2003;162:1495–1502. doi: 10.1016/s0002-9440(10)64282-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox BD, Natarajan M, Stettner MR, Gladson CL. New concepts regarding focal adhesion kinase promotion of cell migration and proliferation. J Cell Biochem. 2006;99:35–52. doi: 10.1002/jcb.20956. [DOI] [PubMed] [Google Scholar]
- Crampton SP, Wu B, Park EJ, Kim JH, Solomon C, Waterman ML, Hughes CC. Integration of the beta-catenin-dependent Wnt pathway with integrin signaling through the adaptor molecule Grb2. PLoS One. 2009;4:e7841. doi: 10.1371/journal.pone.0007841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dibner C, Elias S, Frank D. XMeis3 protein activity is required for proper hindbrain patterning in Xenopus laevis embryos. Development. 2001;128:3415–3426. doi: 10.1242/dev.128.18.3415. [DOI] [PubMed] [Google Scholar]
- Dibner C, Elias S, Ofir R, Souopgui J, Kolm PJ, Sive H, Pieler T, Frank D. The Meis3 protein and retinoid signaling interact to pattern the Xenopus hindbrain. Dev Biol. 2004;271:75–86. doi: 10.1016/j.ydbio.2004.02.029. [DOI] [PubMed] [Google Scholar]
- Elkouby YM, Elias S, Casey ES, Blythe SA, Tsabar N, Klein PS, Root H, Liu KJ, Frank D. Mesodermal Wnt signaling organizes the neural plate via Meis3. Development. 2010;137:1531–1541. doi: 10.1242/dev.044750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuta Y, Ilic D, Kanazawa S, Takeda N, Yamamoto T, Aizawa S. Mesodermal defect in late phase of gastrulation by a targeted mutation of focal adhesion kinase, FAK. Oncogene. 1995;11:1989–1995. [PubMed] [Google Scholar]
- Garneau-Tsodikova S, Thannickal VJ. Protein kinase inhibitors in the treatment of pulmonary fibrosis. Curr Med Chem. 2008;15:2632–2640. doi: 10.2174/092986708785908969. [DOI] [PubMed] [Google Scholar]
- Gatherer D, Woodland HR. N-acetyl-cysteine causes a late re-specification of the anteroposterior axis in the Xenopus embryo. Dev Dyn. 1996;205:395–409. doi: 10.1002/(SICI)1097-0177(199604)205:4<395::AID-AJA4>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Glinka A, Wu W, Delius H, Monaghan AP, Blumenstock C, Niehrs C. Dickkopf-1 is a member of a new family of secreted proteins and functions in head induction. Nature. 1998;391:357–362. doi: 10.1038/34848. [DOI] [PubMed] [Google Scholar]
- Golubovskaya V, Kaur A, Cance W. Cloning and characterization of the promoter region of human focal adhesion kinase gene: nuclear factor kappa B and p53 binding sites. Biochim Biophys Acta. 2004;1678:111–125. doi: 10.1016/j.bbaexp.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Golubovskaya VM, Kweh FA, Cance WG. Focal adhesion kinase and cancer. Histol Histopathol. 2009a;24:503–510. doi: 10.14670/HH-24.503. [DOI] [PubMed] [Google Scholar]
- Golubovskaya VM, Zheng M, Zhang L, Li JL, Cance WG. The direct effect of focal adhesion kinase (FAK), dominant-negative FAK, FAK-CD and FAK siRNA on gene expression and human MCF-7 breast cancer cell tumorigenesis. BMC Cancer. 2009b;9:280. doi: 10.1186/1471-2407-9-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grove M, Komiyama NH, Nave KA, Grant SG, Sherman DL, Brophy PJ. FAK is required for axonal sorting by Schwann cells. J Cell Biol. 2007;176:277–282. doi: 10.1083/jcb.200609021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutkovich YE, Ofir R, Elkouby YM, Dibner C, Gefen A, Elias S, Frank D. Xenopus Meis3 protein lies at a nexus downstream to Zic1 and Pax3 proteins, regulating multiple cell-fates during early nervous system development. Dev Biol. 2010;338:50–62. doi: 10.1016/j.ydbio.2009.11.024. [DOI] [PubMed] [Google Scholar]
- Harland RM. In situ hybridization: an improved whole-mount method for Xenopus embryos. Methods Cell Biol. 1991;36:685–695. doi: 10.1016/s0091-679x(08)60307-6. [DOI] [PubMed] [Google Scholar]
- Henderson WR Jr, Chi EY, Ye X, Nguyen C, Tien YT, Zhou B, Borok Z, Knight DA, Kahn M. Inhibition of Wnt/beta-catenin/CREB binding protein (CBP) signaling reverses pulmonary fibrosis. Proc Natl Acad Sci USA. 2010;107:14309–14314. doi: 10.1073/pnas.1001520107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry CA, Crawford BD, Yan YL, Postlethwait J, Cooper MS, Hille MB. Roles for zebrafish focal adhesion kinase in notochord and somite morphogenesis. Dev Biol. 2001;240:474–487. doi: 10.1006/dbio.2001.0467. [DOI] [PubMed] [Google Scholar]
- Hens MD, DeSimone DW. Molecular analysis and developmental expression of the focal adhesion kinase pp125FAK in Xenopus laevis. Dev Biol. 1995;170:274–288. doi: 10.1006/dbio.1995.1214. [DOI] [PubMed] [Google Scholar]
- Ilic D, et al. FAK promotes organization of fibronectin matrix and fibrillar adhesions. J Cell Sci. 2004;117:177–187. doi: 10.1242/jcs.00845. [DOI] [PubMed] [Google Scholar]
- Katoh M. Regulation of WNT3 and WNT3A mRNAs in human cancer cell lines NT2, MCF-7, and MKN45. Int J Oncol. 2002;20:373–377. [PubMed] [Google Scholar]
- Keren A, Bengal E, Frank D. p38 MAP kinase regulates the expression of XMyf5 and affects distinct myogenic programs during Xenopus development. Dev Biol. 2005;288:73–86. doi: 10.1016/j.ydbio.2005.09.020. [DOI] [PubMed] [Google Scholar]
- Konigshoff M, Balsara N, Pfaff EM, Kramer M, Chrobak I, Seeger W, Eickelberg O. Functional Wnt signaling is increased in idiopathic pulmonary fibrosis. PLoS One. 2008;3:e2142. doi: 10.1371/journal.pone.0002142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kragtorp KA, Miller JR. Regulation of somitogenesis by Ena/VASP proteins and FAK during Xenopus development. Development. 2006;133:685–695. doi: 10.1242/dev.02230. [DOI] [PubMed] [Google Scholar]
- Li B, Kuriyama S, Moreno M, Mayor R. The posteriorizing gene Gbx2 is a direct target of Wnt signalling and the earliest factor in neural crest induction. Development. 2009;136:3267–3278. doi: 10.1242/dev.036954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim ST, Chen XL, Lim Y, Hanson DA, Vo TT, Howerton K, Larocque N, Fisher SJ, Schlaepfer DD, Ilic D. Nuclear FAK promotes cell proliferation and survival through FERM-enhanced p53 degradation. Mol Cell. 2008;29:9–22. doi: 10.1016/j.molcel.2007.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo SW, Zhang C, Zhang B, Kim CH, Qiu YZ, Du QS, Mei L, Xiong WC. Regulation of heterochromatin remodelling and myogenin expression during muscle differentiation by FAK interaction with MBD2. EMBO J. 2009;28:2568–2582. doi: 10.1038/emboj.2009.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariani FV, Harland RM. XBF-2 is a transcriptional repressor that converts ectoderm into neural tissue. Development. 1998;125:5019–5031. doi: 10.1242/dev.125.24.5019. [DOI] [PubMed] [Google Scholar]
- McLean GW, Carragher NO, Avizienyte E, Evans J, Brunton VG, Frame MC. The role of focal-adhesion kinase in cancer—a new therapeutic opportunity. Nat Rev Cancer. 2005;5:505–515. doi: 10.1038/nrc1647. [DOI] [PubMed] [Google Scholar]
- Mitra SK, Hanson DA, Schlaepfer DD. Focal adhesion kinase: in command and control of cell motility. Nat Rev Mol Cell Biol. 2005;6:56–68. doi: 10.1038/nrm1549. [DOI] [PubMed] [Google Scholar]
- Nieuwkoop P, Faber J. Normal Table of Xenopus laevis. (Daudin): Amsterdam: North-Holland; 1967. [Google Scholar]
- Novak A, Hsu SC, Leung-Hagesteijn C, Radeva G, Papkoff J, Montesano R, Roskelley C, Grosschedl R, Dedhar S. Cell adhesion and the integrin-linked kinase regulate the LEF-1 and beta-catenin signaling pathways. Proc Natl Acad Sci USA. 1998;95:4374–4379. doi: 10.1073/pnas.95.8.4374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oloumi A, Syam S, Dedhar S. Modulation of Wnt3a-mediated nuclear beta-catenin accumulation and activation by integrin-linked kinase in mammalian cells. Oncogene. 2006;25:7747–7757. doi: 10.1038/sj.onc.1209752. [DOI] [PubMed] [Google Scholar]
- Ossipova O, Bardeesy N, DePinho RA, Green JB. LKB1 (XEEK1) regulates Wnt signalling in vertebrate development. Nat Cell Biol. 2003;5:889–894. doi: 10.1038/ncb1048. [DOI] [PubMed] [Google Scholar]
- Re'em-Kalma Y, Lamb T, Frank D. Competition between noggin and bone morphogenetic protein 4 activities may regulate dorsalization during Xenopus development. Proc Natl Acad Sci USA. 1995;92:12141–12145. doi: 10.1073/pnas.92.26.12141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rico B, Beggs HE, Schahin-Reed D, Kimes N, Schmidt A, Reichardt LF. Control of axonal branching and synapse formation by focal adhesion kinase. Nat Neurosci. 2004;7:1059–1069. doi: 10.1038/nn1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roffers-Agarwal J, Xanthos JB, Kragtorp KA, Miller JR. Enabled (Xena) regulates neural plate morphogenesis, apical constriction, and cellular adhesion required for neural tube closure in Xenopus. Dev Biol. 2008;314:393–403. doi: 10.1016/j.ydbio.2007.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos A, Bakker AD, Zandieh-Doulabi B, de Blieck-Hogervorst JM, Klein-Nulend J. Early activation of the beta-catenin pathway in osteocytes is mediated by nitric oxide, phosphatidyl inositol-3 kinase/Akt, and focal adhesion kinase. Biochem Biophys Res Commun. 2010;391:364–369. doi: 10.1016/j.bbrc.2009.11.064. [DOI] [PubMed] [Google Scholar]
- Schaller MD. Biochemical signals and biological responses elicited by the focal adhesion kinase. Biochim Biophys Acta. 2001;1540:1–21. doi: 10.1016/s0167-4889(01)00123-9. [DOI] [PubMed] [Google Scholar]
- Schaller MD. Cellular functions of FAK kinases: insight into molecular mechanisms and novel functions. J Cell Sci. 2010;123:1007–1013. doi: 10.1242/jcs.045112. [DOI] [PubMed] [Google Scholar]
- Schlaepfer DD, Mitra SK, Ilic D. Control of motile and invasive cell phenotypes by focal adhesion kinase. Biochim Biophys Acta. 2004;1692:77–102. doi: 10.1016/j.bbamcr.2004.04.008. [DOI] [PubMed] [Google Scholar]
- Sieg DJ, Hauck CR, Schlaepfer DD. Required role of focal adhesion kinase (FAK) for integrin-stimulated cell migration. J Cell Sci. 1999;112 (Pt 16):2677–2691. doi: 10.1242/jcs.112.16.2677. [DOI] [PubMed] [Google Scholar]
- Smalley MJ, Dale TC. Wnt signalling in mammalian development and cancer. Cancer Metastasis Rev. 1999;18:215–230. doi: 10.1023/a:1006369223282. [DOI] [PubMed] [Google Scholar]
- Snir M, Ofir R, Elias S, Frank D. Xenopus laevis POU91 protein, an Oct3/4 homologue, regulates competence transitions from mesoderm to neural cell fates. EMBO J. 2006;25:3664–3674. doi: 10.1038/sj.emboj.7601238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stylianou P, Skourides PA. Imaging morphogenesis, in Xenopus with Quantum Dot nanocrystals. Mech Dev. 2009;126:828–841. doi: 10.1016/j.mod.2009.07.008. [DOI] [PubMed] [Google Scholar]
- Tour E, Pillemer G, Gruenbaum Y, Fainsod A. Gbx2 interacts with Otx2 and patterns the anterior-posterior axis during gastrulation in Xenopus. Mech Dev. 2002;112:141–151. doi: 10.1016/s0925-4773(01)00653-0. [DOI] [PubMed] [Google Scholar]
- Vittal R, Horowitz JC, Moore BB, Zhang H, Martinez FJ, Toews GB, Standiford TJ, Thannickal VJ. Modulation of prosurvival signaling in fibroblasts by a protein kinase inhibitor protects against fibrotic tissue injury. Am J Pathol. 2005;166:367–375. doi: 10.1016/S0002-9440(10)62260-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallingford JB, Harland RM. Neural tube closure requires Dishevelled-dependent convergent extension of the midline. Development. 2002;129:5815–5825. doi: 10.1242/dev.00123. [DOI] [PubMed] [Google Scholar]
- Zhang X, Wright CV, Hanks SK. Cloning of a Xenopus laevis cDNA encoding focal adhesion kinase (FAK) and expression during early development. Gene. 1995;160:219–222. doi: 10.1016/0378-1119(95)00153-w. [DOI] [PubMed] [Google Scholar]
- Zhao J, Bian ZC, Yee K, Chen BP, Chien S, Guan JL. Identification of transcription factor KLF8 as a downstream target of focal adhesion kinase in its regulation of cyclin D1 and cell cycle progression. Mol Cell. 2003;11:1503–1515. doi: 10.1016/s1097-2765(03)00179-5. [DOI] [PubMed] [Google Scholar]
- Zhao J, Guan JL. Signal transduction by focal adhesion kinase in cancer. Cancer Metastasis Rev. 2009;28:35–49. doi: 10.1007/s10555-008-9165-4. [DOI] [PubMed] [Google Scholar]
- Zhao J, Pestell R, Guan JL. Transcriptional activation of cyclin D1 promoter by FAK contributes to cell cycle progression. Mol Biol Cell. 2001;12:4066–4077. doi: 10.1091/mbc.12.12.4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.