Abstract
Natural killer (NK) cells can mediate potent antitumor effects, but factors regulating the efficiency of tumor lysis remain unclear. Studies in allogeneic stem cell transplantation highlight an important role for killer cell immunoglobulin-like receptor (KIR) mismatch in overcoming HLA-mediated inhibitory signals. However other activating and inhibitory signals also modulate tumor lysis by NK cells. We used rhIL15 plus artificial antigen presenting cells (APCs) expressing CD137L and IL15Rα to activate and expand peripheral blood NK cells (CD137L/IL15 NK) up to 1000 fold in 3 weeks. Compared to resting NK cells, CD137L/IL15 NK cells demonstrate modest increases in KIR expression and substantial increases in NKG2D, TRAIL and natural cytotoxicity receptors (NCRs: NKp30, NKp44, NKp46). Compared to resting NK cells, CD137L/IL15 NK cells mediate enhanced cytotoxicity against allogeneic and autologous tumors and KIR signaling did not substantially inhibit cytotoxicity. Rather, tumor lysis by CD137L/IL15 activated NK cells was predominantly driven by NCR signaling since blockade of NCRs dramatically diminished lysis of a wide array of tumor targets. Furthermore, tumor lysis by CD137L/IL15 NK cells was tightly linked to NCR expression levels, which peaked on Day 8–10 following NK activation, and cytotoxicity diminished on subsequent days as NCR expression declined. We conclude that KIR mismatch is not a prerequisite for tumor killing by CD137L/IL15 NK cells and that NCR expression provides a biomarker for predicting potency of CD137L/IL15 NK cells in studies of NK cell based immunotherapy.
Introduction
NK cells kill a wide array of tumors and virally-infected cells via natural cytotoxicity and antibody-dependent cellular cytotoxicity[1]. The missing-self model, put forth to explain the capacity for NK cells to respond to foreign or transformed cells while maintaining self tolerance, emphasizes inhibition of NK killing by signaling through inhibitory receptors[2]. Among the most well characterized are the killer cell immunoglobulin-like receptors (KIR), which recognize human leukocyte antigen (HLA) class I allele groups[3–5], CD94/NKG2A which recognizes HLA-E, and LIR-1 which recognizes most HLA Class I molecules[6]. Following T cell depleted allogeneic transplantation for myeloid leukemia, KIR mismatch is an important predictor of leukemia free survival, providing evidence that the “missing self” regulates NK mediated anti-leukemia responses in vivo[7–10]. Several other model systems and clinical scenarios have confirmed an important role for HLA signaling via NK inhibitory receptors in regulating antitumor effects of NK cells[11–14]. Further, numerous inhibitory NK receptors recognize non-HLA molecules (reviewed in [15], including SIGLEC7[6], CEACAM1[16], TIGIT[17], CD66a[17], IRp60[18], and play a role in regulating NK responses in vivo.
NK mediated cytotoxicity also requires engagement with ligands that initiate NK lysis, sometimes termed activating receptors. A substantial proportion of circulating human NK cells lack inhibitory receptors, yet such cells do not attack normal tissues, presumably due to the requirement for activating signals in initiating NK lysis[19]. Similarly, allogeneic stem cell transplant of KIR mismatched grafts have reduced risk of graft-versus-host disease, presumably due to the absence of activating receptor ligands on non-hematopoietic tissues[20]. Activating receptor ligands can represent viral and tumor associated products,[3][21] cell associated and/or released upon cell death.[22][23] Activating NK receptors include CD16, NKG2D[24], and natural cytotoxicity receptors (NCRs): NKp30 (CD337), NKp44 (CD336)[21] and NKp46 (CD335)[25]. NK expression of TRAIL can also induce target cell apoptosis through binding of TR1 and TR2[26]. Several NK activating receptor ligands have not yet been molecularly or structurally defined.
Current paradigms hold that NK mediated tumor killing requires an absence of NK inhibitory signals as well as the presence of NK activating signals, and several studies have concluded that negative signals dominate activating signals in regulating NK lysis[27–30]. However, at least one report demonstrated that activated NK cells lyse KIR ligand expressing targets[31] and over-expression of ligands for NK activating receptors can induce tumor rejection in the presence of KIR ligands[32,33], consistent with a model wherein a threshold level of activating signals can overcome inhibitory NK signals. In this report, we used artificial antigen presenting cells (aAPCs) expressing CD137L and IL15Rα plus rhIL15 to activate and expand human peripheral blood NK cells. CD137L/IL15 NK cells demonstrate increased expression of several activating receptors, including natural cytotoxicity receptors which correlated with cytolytic potency. Cytolytic activity against a wide variety of tumors persisted despite KIR signaling, but was substantially diminished when NCR signaling was blocked. We conclude that NCR expression on CD137L/IL15 NK cells is a biomarker of lytic activity and plays a central role in mediating tumor lysis.
Materials and Methods
Donors and cells
Tumor cells, acute lymphoblastic leukemia (ALL) blasts and peripheral blood mononuclear cells (PBMCs) obtained from patients, or by leukapheresis from healthy donors were acquired following enrollment on NIH or Johns Hopkins Institutional Review Board approved protocols and after informed consent of the patient and/or patient’s parent. Additional ALL blasts were obtained from human-ALL xenografts in immunodeficient mice. Cells were viably cryopreserved and thawed prior to use. The following tumor cell lines were established from biopsies taken from patients with Ewing’s sarcoma at initial diagnosis or tumor recurrence: NCIEWS21, NCIEWS22, NCIEWS24 NCIIEWS61, NCIEWS95[34]. KCNR, AS, BE2, HOS, K562, Daudi, MG63, TC71, KOPN8, REH, MEL1088, SY5Y were obtained through the ATCC. Cell lines were maintained using standard procedures. Where noted, NK cells were enriched by negative selection according to the manufacturer’s instructions (MACS NK cell isolation kit, Miltenyi Biotech), resulting in populations comprising >95% NK cells.
Artificial APCs and NK Cell Expansion Cultures
KT64.41BBL.A2 aAPCs were generated and maintained as described[35]. aAPCs were irradiated (10,000 rads) then cryopreserved in 10% DMSO and 90% FCS. Upon thawing, aAPCs were co-cultured with bead-enriched NK cells at a 10:1 (aAPC:NK) cell ratio in RPMI 1640 with 10% FCS. Where indicated, 20 mcg/ml of anti-IL15Rα (R&D, clone 151307) or anti-CD137L (R&D, clone 282220), rhIL2 (1000 IU/ml, Pepro tech) and/or rhIL15 (10 ng/ml, Pepro tech) was added. Cytokines were refreshed every three days. Lymphokine-activated killer (LAK) cells were generated by culturing peripheral blood NK cells with 1000IU/ml rhIL2 (Pepro tech) for 7d as described[36].
Flow cytometry and FACS sorting
NK cells were washed, centrifuged, coincubated with the appropriate moAb on ice, then resuspended in 100ul of FACS staining solution (PBS containing 0.5% FCS and 2mM EDTA). The following fluorochrome or biotin-conjugated monoclonal antibodies were used from Biolegend: anti-CD3 (clone HIT3a), -CD8 (clone HIT8a), -CD16 (clone 3G8), -CD56 (clone HCD56), -CD69 (clone FN50), -CD158e1 (clone DX9), -NKp30 (clone P30-15), -NKp44 (clone P44-8), -NKp46 (clone 9E2), -CD137 (clone 4B4-1), -TRAIL (clone RIK2), Strepavidin-PE or PerCP/Cy5.5; from BD: anti-CD158a (KIR3DL1) (HP-3E4), -CD158b (KIR2DL3, KIR2DS2) (CH-L); from R&D: anti-IL15Rα (Clone 151303), anti-MICA (Clone 159233), anti-MICB (Clone 236511), anti-NKG2A (Clone 131411), -CD94 (Clone 131412), -CD158d (Clone 181703); from eBioscience: anti-NKG2D (clone 1D11). Flow cytometry was performed using FACS Calibur with CellQuest software or Aria II using FACS diva software (BD) and analyzed by FlowJo 8.8 (Treestar). FACS sorting for CD158a+ and CD158b+ cells was performed using a FACS Aria II (BD). For flow cytometric analysis of NCR ligand expression, NKp30, NKp44, NKp46, NKp80–Fc fusion proteins (R&D) were labeled using Zenon IgG labeling kit using Alexa Fluor 488 (Invitrogen).
Cytolytic Assays by 51Cr release, ADCC Assays and blockade by KAR fusion proteins or by anti-KIR antibodies
Where designated, cultures were harvested, counted and lytic activities measured using conventional 4h Cr51 (PerkinElmer) release assays against tumor targets, using a MicroBeta scintillation counter (Perkin Elmer) supernatants (25ul/well) transferred onto Luma plates (Perkin Elmer). Percent specific lysis was calculated using: 100x(test release–spontaneous release)/(maximal release–spontaneous release). In experiments incorporating activating receptor blockade, 2.5 ug/ml of NKp30, NKp44, NKp46, NKp80 and NKG2D-Fc fusion proteins (R&D) were used to block 51Cr-labeled target tumor cells before co-culture with effector NK cells. For experiments incorporating inhibitory receptor blockade[11], 1 ug/ml of anti-CD158a (HP3E4, BD), anti-CD158b (GL183, Beckman Coulter), anti-CD158e1 (DX9, Biolegend), anti-CD158d (181703, R&D), anti-NKG2A (Z199, Beckman Coulter), plus anti-CD94 (HP3B1, Beckman Coulter) was added before co-culture with 51Cr-labeled target tumor cells. ADCC studies were performed using Rituximab as previously described[11].
Results
Critical Roles for CD137L and rhIL15 in aAPC Induced NK Cell Expansion
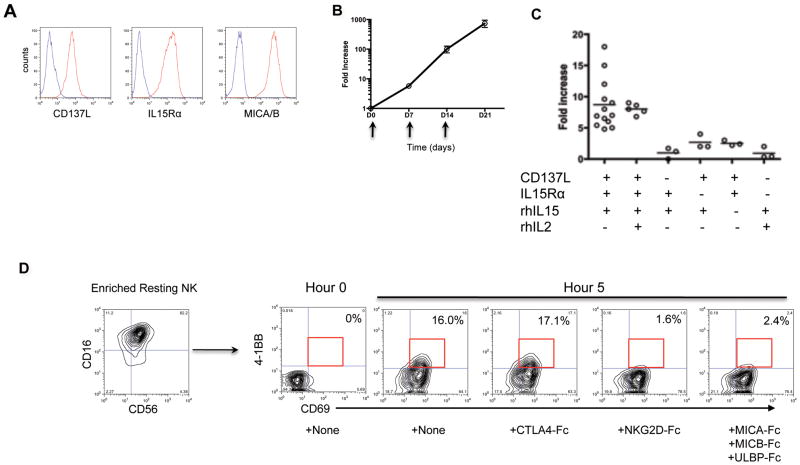
We have previously reported efficient ex vivo expansion of polyclonal and antigen specific CD8+ T cells with enhanced cytotoxicity using CD137L bearing aAPCs[34,35]. In this study, we sought to expand peripheral blood NK cells with a nearly identical aAPC, KT64.41BBL.A2 (hereafter designated as CD137L/aAPC). CD137L/aAPCs stably express with CD137L, and naturally express IL15Rα, and MICA/B (Figure 1A). Stimulation of enriched resting peripheral blood NK cells on days 0, 7 and 14 with CD137L/aAPCs + rhIL15 induced 5–20 fold increases in NK cell number in 7 days and approximately 1000 fold increases in NK cell number over 21 days (Figure 1B). Studies using CD137L/aAPCs ± rhIL15 and/or rhIL2, and ± antibodies to block IL15Rα and/or CD137L demonstrated that CD137L, IL15Rα and rhIL15 were required for efficient 7d NK expansion (Figure 1C), whereas exogenous rhIL2 did not significantly enhance NK expansion in this system. Although the aAPC used here expressed HLA-A2, similar results were observed using nearly identical aAPCs that lacked HLA-A2 expression (data not shown).
Figure 1. CD137L/IL15 NK cell expansion involves NKG2D mediated upregulation of CD137 on NK cells, and requires CD137L, IL15Rα and rhIL15.
(A) CD137L/aAPCs are stably transduced to express CD137L, and naturally express IL15Rα and MICA/MICB. Isotype control antibody in blue, and the designated antibody in red. (B) Peripheral blood NK cells expand 1000 fold in 21d after weekly stimulation by CD137L/aAPCs + rhIL15 (aAPC:NK ratio 10:1). Mean expansion ± SEM of 3 experiments is shown. (C) CD137L, IL15Rα and rhIL15 are required for optimal day 7 NK expansion. Where designated with −, IL15Rα and/or CD137L were blocked with anti-IL15Rα and anti-CD137L moAbs (20ug/ml) respectively. Where indicated with +, rhIL-15 (10 ng/ml) and rhIL-2 (1000IU/ml) were added to the culture. Circles represent results from individual experiments using different donors. (D) NKG2D on resting NK cells interacts with MICA/B on aAPCs to induce NK CD137 expression. Shown is CD69 and CD137 expression on gated CD16+CD56+ NK cells at 0h and 5h co-culture with rhIL15 + CD137L/aAPCs ± addition of the designated fusion proteins. Representative results shown from 3 experiments using NK cells from 3 different donors.
To determine how CD137L co-stimulates enriched resting peripheral blood NK cells, which do not express CD137, we monitored CD137 expression on NK cells during co-cultures with CD137L/aAPCs. Resting peripheral blood NK cells were CD69 negative, but after 5h co-culture with CD137L/aAPCs + rhIL15, most NK cells expressed CD69 and a substantial fraction expressed CD137 (Figure 1D). Induction of CD137 expression was inhibited when NKG2D-Fc or MICA/MICB/ULBP-Fc fusion proteins were added to the co-culture, but not by CTLA4-Fc, a control fusion protein. Thus, interactions between NKG2D on resting NK cells and NKG2D ligands, such as MICA/MICB expressed on CD137L/aAPCs (Figure 1A), upregulate CD137 expression on NK cells, permitting activation and expansion by CD137L/aAPCs.
CD137L/IL-15 NK Cells Mediate Potent Cytotoxicity Regardless of KIR Mismatch
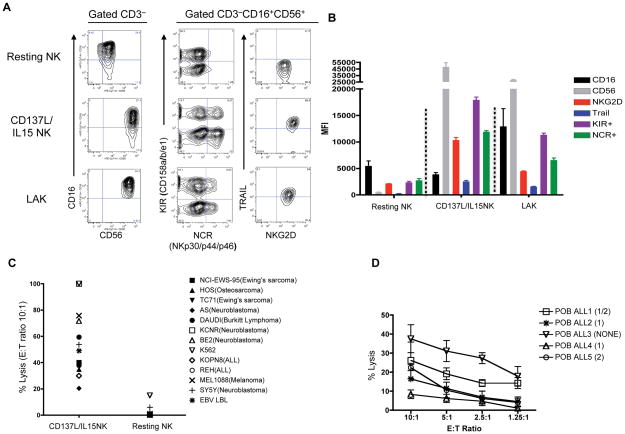
As demonstrated previously[28], resting peripheral blood NK cells are primarily CD56dim with little TRAIL or NCR (NKp30, p44, p46) expression (Figure 2A and 2B). However, 8d following co-culture with CD137L/aAPCs + rhIL15, essentially all NK cells upregulated CD56, NKG2D, and TRAIL, and a sizable fraction expressed NCRs (NKp30, NKp44, or NKp46). These results are consistent with global changes in NK gene expression reported previously using a similar CD137L based aAPC NK activation system[37]. Interestingly, CD137L/IL15 activated NK cells also expressed higher levels of KIR receptors (CD158a, CD158b, or CD158e1) (Figure 2A, 2B) compared to resting NK cells. For comparison, LAK cells generated by culture of resting peripheral blood NK cells with high dose IL2 (1000IU/ml for 7d) showed similar trends, but upregulation of CD56, NCRs, TRAIL and NKG2D was not as robust as that seen on CD137L/IL15 NK cells.
Figure 2. CD137L/IL15 NK cells upregulate activating and inhibitory NK receptors, and mediate enhanced cytotoxicity compared to resting NK cells.
(A) Phenotype of resting peripheral blood NK cells vs. Day 8 CD137L/IL15 NK cells vs. Day 8 LAK cells. NKG2D, KIR (CD158a, CD158b, CD158e1), NCR (NKp30, p44, p46) and TRAIL are upregulated on gated CD3−CD16+CD56+ CD137L/IL15 NK cells and LAK cells compared to resting NK cells. Data is representative of > 20 experiments using NK cells from 20 different individuals. (B) Mean fluorescence intensity (± SEM) of each receptor or a group of receptors on resting vs. Day 8 CD137L/IL15 vs. LAK cells. Results pooled from 3 experiments. Compared to resting NK cells, CD137L/IL15 and LAK NK cells show significant upregulation of CD56, TRAIL, KIR and NCR expression (p<0.0001), but not CD16. CD137L/IL15 NK show significant increases compared to LAK for NKG2D, KIR, NCR (P<0.0001), TRAIL (p<0.001) and CD56 (p<0.01). (C) CD137L/IL15 NK cells mediate enhanced cytolysis of tumor targets. Day 7 CD137L/IL15 NK cells and resting NK cells were tested against designated tumor cell lines (E:T ratio 10:1) in 4h chromium51 release assays. (D) Mean (± SEM) cytolysis of allogeneic ALL blasts from 5 separate patients by Day 7 CD137L/IL15 NK cells from three different healthy donors.
Increased activating receptor expression raised the possibility that CD137L/IL15 expanded NK cells may mediate enhanced cytotoxicity, On the other hand, enhanced KIR expression could limit cytotoxic effects against tumors bearing KIR ligands. To investigate this, we evaluated cytotoxicity of CD137L/IL15 expanded NK cells against a broad panel of tumor cell lines. CD137L/IL15 expanded NK cells from more than twenty healthy donors showed potent cytotoxicity, that was substantially increased compared to resting NK cells (Figure 2C). We also measured lysis of ALL lymphoblasts obtained from patients with differential KIR ligand genotypes. Resting NK cells showed no significant lysis of ALL lymphoblasts, regardless of KIR genotype, even at E:T ratios of 100:1 (data not shown). When co-cultured with CD137L/IL15 NK cells, blasts from ALL3, which lacked both KIR ligands were most efficiently lysed, potentially due to the absence of KIR signals. However, robust lysis was still observed when either or both KIR ligands were present (representative results shown in Figure 2D). Taken together, these results demonstrate that CD137L/IL15 NK cells mediate increased killing of tumor targets compared to resting NK cells, and raise the possibility that CD137L/IL15 NK cells can mediate efficient killing even in the presence of KIR ligands.
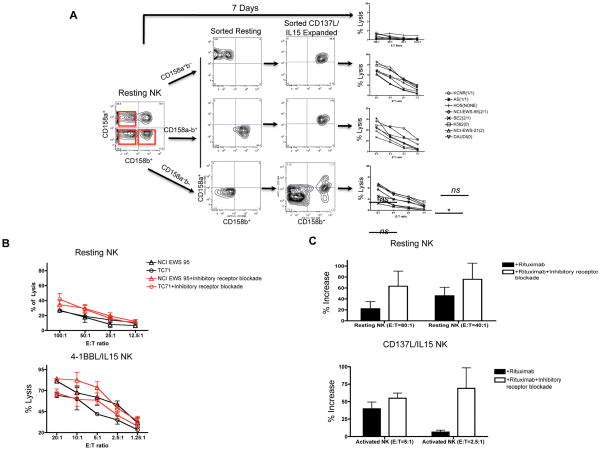
To more directly address the extent to which inhibitory KIR signaling prevented killing by CD158b+ or CD158a+ CD137L/IL15 activated NK cells respectively, we flow sorted CD158a+b−, CD158a−b+ and CD158a−b− NK subsets, then subjected these cells to CD137L/IL15 expansion for 7d. As shown in Figure 3A, post-sorting purity of CD158a+b− and CD158a−b+ and CD158a−b− subsets was 96–100%, but CD137L/IL15 expanded cells upregulated KIR expression, in all sorted subsets, with >95% of the NK cells expanded from the CD158a+b− and CD158a−b+ expressing both CD158a and CD158b. Thus, CD137L/aAPC + rhIL15 activation enhances KIR expression, consistent with CD137L/IL15 inducing NK maturation[28]. Despite substantial KIR expression, CD137L/IL15 NK cells mediated efficient tumor lysis, regardless of whether tumors expressed HLA C Group 1, Group 2, both, or neither (Figure 3A). Interestingly the NK cells expanded from the CD158a−b− subset showed the lowest levels of KIR expression (approximately 10% CD158a+ and 20% CD158b+), but showed no substantial difference in tumor lysis compared to subsets which were >95% CD158a+b+ (Figure 3A). Together, these results demonstrate efficient lysis of tumor targets by KIR expressing CD137L/IL15 NK cells, regardless of the presence of corresponding KIR ligands.
Figure 3. KIR signaling plays a modest role in regulating lysis by CD137L/IL15 NK cells.
(A) Resting peripheral blood NK cells were electronically sorted into CD158a+b−, CD158a−b+ and CD158a−b− subsets, then expanded for 7d with CD137L/aAPCs + rhIL15. Resting NK cells showed minimal tumor killing, while all CD137L/IL15 expanded NK cultures showed substantial tumor killing, regardless of KIR expression or HLA Group C allele. (B) Inhibitory receptor blockade (anti-CD158a, -CD158b, -CD158e1, -CD158d, anti-NKG2A, and anti-CD94 moAbs) modestly augments tumor killing by resting and CD137L/IL15 NK cells. Isotype control antibodies were added to the control cultures. The experiment is representative of 3 experiments yielding similar results. Inhibitory receptor blockade enhanced lysis of CD137L/IL15 NK cells against TC71 (p=0.005 at an E:T of 5:1), whereas resting NK cells showed no difference in lysis. (C) Inhibitory receptor blockade induced non-significant increases in Rituximab mediated ADCC of EBV LBL cells by resting NK cells and a significant increase in ADCC by CD137L/IL15 NK cells at 2.5:1 E:T ratio. The figure includes results summarized from a total of 6 experiments.
Allele specific antibodies are not available to unequivocally demonstrate surface expression of KIR ligands on the cell lines tested, raising the possibility that the lack of KIR mediated inhibition reflected limited class I KIR allele expression on the tumors themselves, rather than diminished potency of KIR signaling on CD137L/IL15 NK cells. We therefore sought to uncover inhibitory signals on resting vs CD137L/IL15 NK cells by comparing tumor killing in the presence or absence of blocking antibodies to inhibitory receptors. Using a combination of antibodies to mediate inhibitory receptor blockade (anti-KIR/anti-NKG2A/anti-CD94) as described by Binyamin et al[11], we observed modest augmentation of tumor cell killing at some E:T ratios (Figure 3B), and increased Rituximab-mediated ADCC (Figure 3C) confirming that inhibitory receptors mediate inhibitory signals to CD137L/IL15 NK cells. Nonetheless, inhibitory signals did not preclude potent killing by these cells, as evidenced in figure 3B, where CD137L/IL-15 NK cells mediated greater than 50% tumor lysis of two separate Ewing’s sarcoma cell lines at low E:T ratios, with or without inhibitory receptor blockade. Thus, inhibitory signals modulate reactivity by CD137L/IL-15 NK cells, but the overall potency of killing is minimally affected by inhibitory signals.
Tumor Lysis by CD137L/IL15 Expanded NK Cells Is Primarily Regulated by Signaling of Natural Cytotoxicity Receptors and Other Activating Receptors
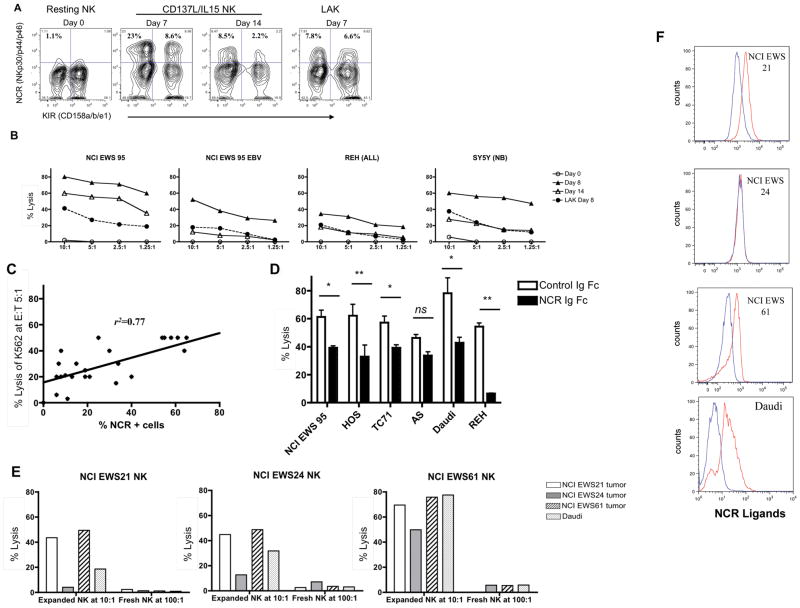
We next investigated the role of activating receptor pathways in regulating tumor lysis by CD137L/IL15 activated NK cells. Figure 4A shows a representative time course of NCR expression. Following stimulation on d0, NCR expression peaks on d7–10 of CD137L/IL15 expansion, then declines (but remains higher than resting levels) by d14. IL-2 activated LAK cells also show increased NCR expression (but to a lesser degree than CD137L/aAPCs), which peaks on approximately d7. Figure 4B demonstrates that killing potency on sequential days of culture against a range of tumor cell lines correlates with NCR expression levels. Interestingly, d8 LAK cells showed killing activity comparable to those of d14 CD137L/IL15 NK cells and similar NCR levels at this time point. This raised the possibility that killing potency of CD137L/IL15 NK cells correlated with NCR receptor expression. To investigate this, CD137L/IL15 NK cells were generated from 3 separate normal donors in multiple experiments and NCR receptor expression and killing potency against K562 cells was evaluated on day 6, 8, 10, 13 or 14 cultures in 3 separate experiments. As shown in Figure 4C, killing potency strongly correlated with NCR receptor expression.
Figure 4. CD137L/IL15 NK cytolysis is highly dependent upon NCR signaling.
(A) NCR (NK p30, p44, p46) expression levels are minimal on d0, peak on d7 and diminish by d14 on CD137L/IL15 NK cells, whereas d7 NCR levels on LAK cells are similar to d14 NCR levels of CD137L/IL15 NK cells. Results are representative of 4 experiments using 4 different NK donors, though peak NCR expression varied from d7-d10. (B) Cytolysis of tumor or EBV LBL cell lines by CD137L/IL15 NK cells (or LAK where indicated) correlates NCR expression on days shown in A. Data representative of three experiments. (C) Percent CD137L/IL15 NK cells expressing NCRs correlates with lysis of K562. Each dot represents a separate assay (including phenotyping and lytic) on day 6, 8, 10, 13 or 14, using 3 donors. (D) Tumor cytolysis (E:T ratio of 2.5:1) is diminished NCR-Ig Fc fusion protein cocktail (NKp30-Fc, p44-Fc, p46-Fc and p80-Fc, closed symbol), compared to a control fusion protein (closed symbol, CTLA4-Ig). Results are summarized from three experiments using NK cells from separate individuals against HOS (osteosarcoma), NCI EWS 95 and TC71 (Ewing’s sarcoma), Daudi (lymphoma), REH (ALL blasts), AS (neuroblastoma). (E) Autologous and allogeneic CD137L/IL15 NK cells potently kill NCIEWS21, and NCIEWS61, but minimally kill NCIEWS24. Resting NK cells do not lyse either autologous or allogeneic tumors despite co-culture at high E:T ratios (100:1). (F) Significant binding of CD137L/IL15 NK sensitive NCIEWS21 and NCIEWS61 to an NCR ligand-Fc cocktail including NK –p30, –p44, –p46, –p80 (red line) labeled with Zenon vs. control CTLA4-Fc (blue line). The NK resistant line, NCIEWS24, does not express NCR ligands.
To probe whether NCR receptors play a functional role in mediating lysis, a cocktail of NKp30-Fc, NKp44-Fc and NKp46-Fc, NKp80-Fc (NCR-Ig) fusion proteins was used to block NCR signaling in tumor lysis assays. As shown in Figure 4D, we observed 40%~85% inhibition of CD137L/IL15 activated NK cell cytotoxicity against a number of tumors following addition of fusion proteins to block NCR signaling (Figure 4D). NCR ligand blockade inhibited essentially all NK mediated lysis of REH, an ALL cell line. Although TRAIL upregulation was consistently observed on CD137L/IL15 NK cells, we found no evidence for TRAIL-mediated lysis in these short-term Cr51 release assays since lysis was not diminished when TR1-Fc and/or TR2-Fc fusion proteins were added to the cultures (data not shown). These results demonstrate that signaling via activating receptors in general, and NCR receptors in particular, play a major role in regulating tumor killing by CD137L/IL15 NK cells.
The demonstration of a central role for NCR signaling and lesser roles for KIR mismatch in regulating antitumor effects of CD137L/IL15 NK cells suggests that autologous and allogeneic CD137L/IL15 NK cells might mediate equipotent antitumor effects. To evaluate this, we generated CD137L/IL15 activated NK cells from three Ewing’s sarcoma patients, and evaluated their capacity to lyse autologous vs. allogeneic tumor lines. As shown in Figure 4E, NCIEWS21 and NCIEWS61 tumors were lysed efficiently and similarly by autologous and allogeneic CD137L/IL15 NK cells, whereas NCIEWS24 showed low level lysis by both autologous and allogeneic CD137L/IL15 NK cells. Surface expression of NCR ligands could be directly observed on NCIEWS21 and NCIEWS61, the two cell lines most susceptible to lysis (Figure 4F), whereas we could not detect surface expression of NCR ligands on NCIEWS24. Results using NCR-Ig cocktails are shown in these experiments, but experiments conducted using singlet NCR-Ig fusion proteins to detect surface expression demonstrated that each NCR studied was expressed on some tumor lines and we could not define a dominant role for one NCR in these studies.
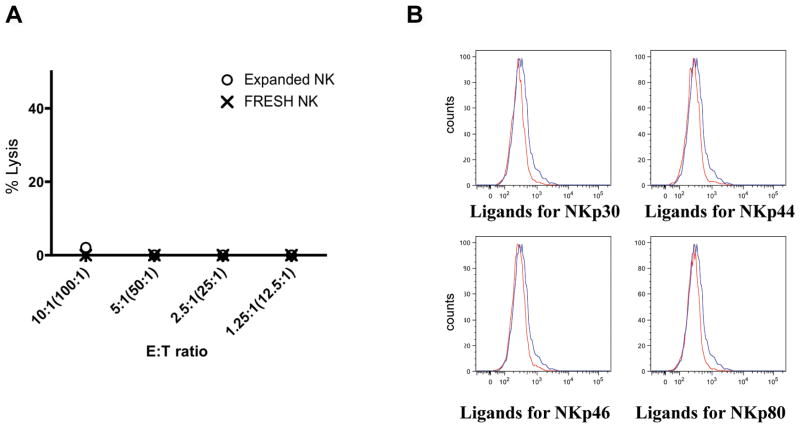
Robust killing activity of CD137L/IL15 activated NK cells on autologous tumor targets and their resistance to KIR mediated inhibition raised the prospect that such cells may mediate autoimmunity if used therapeutically. However, we observed no killing of autologous or allogeneic PHA-blasted PBMCs (Figure 5A), consistent with the findings that PHA-blasts do not express NKp30, p44, p46, or p80 ligands (Figure 5B). Thus, independence from KIR/NKG2A mediated inhibition does not endow CD137L/IL15 NK cells with indiscriminate killing capacity. Together, these data are consistent with a model wherein target cell lysis by CD137L/IL15 NK cells is dictated primarily by expression of target cell expression of activating ligands, with weaker subdominant signals mediated by KIR signaling[38].
Figure 5. CD137L/IL15 NK cells do not lyse PHA blasts.
(A) Neither CD137L/IL15 NK cells nor resting NK cells lyse allogeneic PHA-blasted PBMCs. Shown is one representative of three experiments. (B) PHA-blasted PBMCs do not express NKp30, p44, p46, p80 ligands. Data are representative of three experiments from three separate donors. Binding of PHA-blasted cell lines to the control CTLA4-Fc (blue line) or to the designated NCR ligand-Fc (red line) labeled with Zenon kit.
Discussion
NK cells lyse tumor cells and virus-infected cells without prior sensitization. Interactions between KIRs and other inhibitory receptors play key roles in maintaining NK cell tolerance [28,39,40] and several groups have concluded that inhibitory signals dominate over activating signals[9,10,30]. Indeed, improved survival following KIR mismatched allogeneic transplant for leukemia, and differences in the natural history of infection, inflammatory diseases and cancer in hosts lacking KIR ligands provide evidence for a central role of these inhibitory axes in modulating NK cell activity [41]. At the same time it is well recognized that activating receptors play essential roles in modulating NK activity. Some studies have shown efficient killing by activated NK cells in the presence of KIR ligands[42], raising the prospect that depending upon the status of NK cell activation, the relative dominance of activating vs. inhibitory signals may vary. In this study, we demonstrate enhanced antitumor activity by CD137L/IL15 NK cells and provide the novel insight that killing via CD137L/IL15 activated NK cells is highly dependent upon NCR signaling, but only modestly regulated by KIR signaling. Thus, in CD137L/IL15 NK cells, activating signals dominate inhibitory ones.
Using a variety of approaches, we demonstrate that CD137L/IL15 NK cells mediate robust tumor lysis, which is not significantly inhibited by KIR signaling. This is not due to downregulation of KIRs themselves, as the activation process increases KIR expression. Using a cocktail of blocking antibodies targeting inhibitory receptors (CD158a, CD158b, CD158e1, CD158d, NKG2A), we observed modest increases in CD137L/IL15 NK cell killing and in Rituxmab-mediated ADCC against EBV LBLs, confirming the presence of functional KIR receptors and KIR ligands in these assays. In contrast to weak inhibitory effects mediated by KIR signaling, killing by CD137L/IL15 NK cells were highly dependent upon signaling through activating receptors. We observed upregulation of NCRs in CD137L/IL15 NK cells, a strong correlation between NCR expression and killing potency and substantial decreases in NK cell mediated cytotoxicity when NCR signaling was blocked. Consistent with this, previous studies demonstrated that activated NK cells lyse dendritic cells, which express robust levels of HLA ligands[31] and that some tumors with robust NCR ligand expression are killed by NK cells selected from donors with high NCR expression[38]. Together, these data implicate a central role for NCR signaling in determining the efficiency of tumor lysis and raise the prospect that tumor susceptibility to NK cell therapy could potentially be predicted by measuring NCR expression across tumor types, grades or stages. Indeed, we observed that ALL cells showed diminished sensitivity to CD137L/IL15 NK cells compared to melanoma and Ewing’s sarcoma lines, and NK sensitivity was generally correlated with levels of NCR ligand expression (data not shown). Furthermore, NCR expression on activated NK cells served as a biomarker potency of the NK effectors, which varied widely depending upon the length of culture. While prolonged NK cultures yielded much higher cell numbers, the potency of the NK cell killing was much reduced after 10–12d, associated with diminished expression of NCRs. Across several experiments, we consistently observed peak NCR expression on Days 7–10 following CD137L/IL15 activation.
A recent study concluded that signaling of CD137L on CD137+ human NK cells diminished granule mobilization and cytotoxicity, while signaling of the same axis augments NK killing in mice[43]. Our results and those published by Fujisaki[37] and Cho[44] using a similar CD137L expressing aAPC, are inconsistent with these conclusions. In these three separate studies, artificial APCs engineered to sustain high level expression of CD137L induced activation and expansion of NK cells with enhanced cytolytic capacity compared to resting NK cells. We directly demonstrate that CD137 signaling is critical for NK expansion and we have previously demonstrated a role for CD137 signaling in enhancing cytotoxicity of T cells bearing NK receptors[34]. It remains possible that the effects of CD137 signaling are context dependent, with aAPCs that simultaneously provide IL-15 and possibly other costimulatory signals enhances functionality of CD137 costimulated human NK cells, whereas CD137 signals delivered in other contexts, especially in the presence of tumors, may yield immunosuppressive effects. Similar yin/yang effects of CD137 have been reported in the context of T cell mediated autoimmunity and antitumor immunity (reviewed in [31]).
In summary, our studies demonstrate efficient expansion of highly lytic NK cells with enhanced killing compared to resting NK cells across a wide array of tumors using a combination of CD137 signaling and IL-15, delivered using a cell based aAPC. We provide the novel observation that killing by CD137L/IL15 NK cells is regulated primarily by activating receptor expression on NK cells and that NCR expression on NK cells correlates with lytic potency. KIR signaling, while capable of providing a modest inhibitory signal, does not prevent lysis by these activated NK cells. These studies raise the prospect that tumor susceptibility activated NK cell lysis could be predicted by measuring NCR ligand expression, that NCR expression on NK cells could provide a biomarker of potency and that adoptive cell therapy using NK cells generated in this manner could prove efficacious, both in the autologous and allogeneic setting.
Acknowledgments
This work was supported by the Intramural Research Program of the National Institutes of Health. We would like to thank Dr. Dan Fowler for his careful review of the manuscript. We would like to thank Dr. Louis Weiner and Joe Murray for helpful discussion regarding ADCC assays. We would like to thank Dr. Patrick Brown (Johns Hopkins Hospital) for providing ALL lymphoblasts.
Footnotes
FINANCIAL DISCLOSURE: All authors have declared there are no financial conflicts of interest in regards to this work.
References
- 1.Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nat Immunol. 2008;9:503–510. doi: 10.1038/ni1582. [DOI] [PubMed] [Google Scholar]
- 2.Karre K, Ljunggren HG, Piontek G, Kiessling R. Selective rejection of H-2-deficient lymphoma variants suggests alternative immune defence strategy. Nature. 1986;319:675–678. doi: 10.1038/319675a0. [DOI] [PubMed] [Google Scholar]
- 3.Moretta L, Bottino C, Pende D, Castriconi R, Mingari MC, Moretta A. Surface NK receptors and their ligands on tumor cells. Semin Immunol. 2006;18:151–158. doi: 10.1016/j.smim.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 4.Hamerman JA, Ogasawara K, Lanier LL. NK cells in innate immunity. Curr Opin Immunol. 2005;17:29–35. doi: 10.1016/j.coi.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 5.Lanier LL. NK cell recognition. Annu Rev Immunol. 2005;23:225–274. doi: 10.1146/annurev.immunol.23.021704.115526. [DOI] [PubMed] [Google Scholar]
- 6.Kumar V, McNerney ME. A new self: MHC-class-I-independent natural-killer-cell self-tolerance. Nat Rev Immunol. 2005;5:363–374. doi: 10.1038/nri1603. [DOI] [PubMed] [Google Scholar]
- 7.Ruggeri L, Capanni M, Casucci M, Volpi I, Tosti A, Perruccio K, Urbani E, Negrin RS, Martelli MF, Velardi A. Role of natural killer cell alloreactivity in HLA-mismatched hematopoietic stem cell transplantation. Blood. 1999;94:333–339. [PubMed] [Google Scholar]
- 8.Ruggeri L, Capanni M, Urbani E, Perruccio K, Shlomchik WD, Tosti A, Posati S, Rogaia D, Frassoni F, Aversa F, et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science. 2002;295:2097–2100. doi: 10.1126/science.1068440. [DOI] [PubMed] [Google Scholar]
- 9.Miller JS, Cooley S, Parham P, Farag SS, Verneris MR, McQueen KL, Guethlein LA, Trachtenberg EA, Haagenson M, Horowitz MM, et al. Missing KIR ligands are associated with less relapse and increased graft-versus-host disease (GVHD) following unrelated donor allogeneic HCT. Blood. 2007;109:5058–5061. doi: 10.1182/blood-2007-01-065383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leung W, Iyengar R, Turner V, Lang P, Bader P, Conn P, Niethammer D, Handgretinger R. Determinants of antileukemia effects of allogeneic NK cells. J Immunol. 2004;172:644–650. doi: 10.4049/jimmunol.172.1.644. [DOI] [PubMed] [Google Scholar]
- 11.Binyamin L, Alpaugh RK, Hughes TL, Lutz CT, Campbell KS, Weiner LM. Blocking NK cell inhibitory self-recognition promotes antibody-dependent cellular cytotoxicity in a model of anti-lymphoma therapy. J Immunol. 2008;180:6392–6401. doi: 10.4049/jimmunol.180.9.6392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koh CY, Ortaldo JR, Blazar BR, Bennett M, Murphy WJ. NK-cell purging of leukemia: superior antitumor effects of NK cells H2 allogeneic to the tumor and augmentation with inhibitory receptor blockade. Blood. 2003;102:4067–4075. doi: 10.1182/blood-2003-04-1367. [DOI] [PubMed] [Google Scholar]
- 13.Koh CY, Blazar BR, George T, Welniak LA, Capitini CM, Raziuddin A, Murphy WJ, Bennett M. Augmentation of antitumor effects by NK cell inhibitory receptor blockade in vitro and in vivo. Blood. 2001;97:3132–3137. doi: 10.1182/blood.v97.10.3132. [DOI] [PubMed] [Google Scholar]
- 14.Venstrom JM, Zheng J, Noor N, Danis KE, Yeh AW, Cheung IY, Dupont B, O’Reilly RJ, Cheung NK, Hsu KC. KIR and HLA genotypes are associated with disease progression and survival following autologous hematopoietic stem cell transplantation for high-risk neuroblastoma. Clin Cancer Res. 2009;15:7330–7334. doi: 10.1158/1078-0432.CCR-09-1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ravetch JV, Lanier LL. Immune inhibitory receptors. Science. 2000;290:84–89. doi: 10.1126/science.290.5489.84. [DOI] [PubMed] [Google Scholar]
- 16.Stern N, Markel G, Arnon TI, Gruda R, Wong H, Gray-Owen SD, Mandelboim O. Carcinoembryonic antigen (CEA) inhibits NK killing via interaction with CEA-related cell adhesion molecule 1. J Immunol. 2005;174:6692–6701. doi: 10.4049/jimmunol.174.11.6692. [DOI] [PubMed] [Google Scholar]
- 17.Stanietsky N, Simic H, Arapovic J, Toporik A, Levy O, Novik A, Levine Z, Beiman M, Dassa L, Achdout H, et al. The interaction of TIGIT with PVR and PVRL2 inhibits human NK cell cytotoxicity. Proc Natl Acad Sci U S A. 2009;106:17858–17863. doi: 10.1073/pnas.0903474106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cantoni C, Bottino C, Augugliaro R, Morelli L, Marcenaro E, Castriconi R, Vitale M, Pende D, Sivori S, Millo R, et al. Molecular and functional characterization of IRp60, a member of the immunoglobulin superfamily that functions as an inhibitory receptor in human NK cells. Eur J Immunol. 1999;29:3148–3159. doi: 10.1002/(SICI)1521-4141(199910)29:10<3148::AID-IMMU3148>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 19.Fernandez NC, Treiner E, Vance RE, Jamieson AM, Lemieux S, Raulet DH. A subset of natural killer cells achieves self-tolerance without expressing inhibitory receptors specific for self-MHC molecules. Blood. 2005;105:4416–4423. doi: 10.1182/blood-2004-08-3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shi J, Tricot G, Szmania S, Rosen N, Garg TK, Malaviarachchi PA, Moreno A, Dupont B, Hsu KC, Baxter-Lowe LA, et al. Infusion of haplo-identical killer immunoglobulin-like receptor ligand mismatched NK cells for relapsed myeloma in the setting of autologous stem cell transplantation. Br J Haematol. 2008;143:641–653. doi: 10.1111/j.1365-2141.2008.07340.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cantoni C, Bottino C, Vitale M, Pessino A, Augugliaro R, Malaspina A, Parolini S, Moretta L, Moretta A, Biassoni R. NKp44, a triggering receptor involved in tumor cell lysis by activated human natural killer cells, is a novel member of the immunoglobulin superfamily. J Exp Med. 1999;189:787–796. doi: 10.1084/jem.189.5.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pogge von Strandmann E, Simhadri VR, von Tresckow B, Sasse S, Reiners KS, Hansen HP, Rothe A, Boll B, Simhadri VL, Borchmann P, et al. Human leukocyte antigen-B-associated transcript 3 is released from tumor cells and engages the NKp30 receptor on natural killer cells. Immunity. 2007;27:965–974. doi: 10.1016/j.immuni.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 23.Brandt CS, Baratin M, Yi EC, Kennedy J, Gao Z, Fox B, Haldeman B, Ostrander CD, Kaifu T, Chabannon C, et al. The B7 family member B7-H6 is a tumor cell ligand for the activating natural killer cell receptor NKp30 in humans. J Exp Med. 2009;206:1495–1503. doi: 10.1084/jem.20090681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gonzalez S, Lopez-Soto A, Suarez-Alvarez B, Lopez-Vazquez A, Lopez-Larrea C. NKG2D ligands: key targets of the immune response. Trends Immunol. 2008;29:397–403. doi: 10.1016/j.it.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 25.Pessino A, Sivori S, Bottino C, Malaspina A, Morelli L, Moretta L, Biassoni R, Moretta A. Molecular cloning of NKp46: a novel member of the immunoglobulin superfamily involved in triggering of natural cytotoxicity. J Exp Med. 1998;188:953–960. doi: 10.1084/jem.188.5.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lundqvist A, Yokoyama H, Smith A, Berg M, Childs R. Bortezomib treatment and regulatory T-cell depletion enhance the antitumor effects of adoptively infused NK cells. Blood. 2009;113:6120–6127. doi: 10.1182/blood-2008-11-190421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Anfossi N, Andre P, Guia S, Falk CS, Roetynck S, Stewart CA, Breso V, Frassati C, Reviron D, Middleton D, et al. Human NK cell education by inhibitory receptors for MHC class I. Immunity. 2006;25:331–342. doi: 10.1016/j.immuni.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 28.Caligiuri MA. Human natural killer cells. Blood. 2008;112:461–469. doi: 10.1182/blood-2007-09-077438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Imai C, Iwamoto S, Campana D. Genetic modification of primary natural killer cells overcomes inhibitory signals and induces specific killing of leukemic cells. Blood. 2005;106:376–383. doi: 10.1182/blood-2004-12-4797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yu J, Heller G, Chewning J, Kim S, Yokoyama WM, Hsu KC. Hierarchy of the human natural killer cell response is determined by class and quantity of inhibitory receptors for self-HLA-B and HLA-C ligands. J Immunol. 2007;179:5977–5989. doi: 10.4049/jimmunol.179.9.5977. [DOI] [PubMed] [Google Scholar]
- 31.Ferlazzo G, Tsang ML, Moretta L, Melioli G, Steinman RM, Munz C. Human dendritic cells activate resting natural killer (NK) cells and are recognized via the NKp30 receptor by activated NK cells. J Exp Med. 2002;195:343–351. doi: 10.1084/jem.20011149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cerwenka A, Baron JL, Lanier LL. Ectopic expression of retinoic acid early inducible-1 gene (RAE-1) permits natural killer cell-mediated rejection of a MHC class I-bearing tumor in vivo. Proc Natl Acad Sci U S A. 2001;98:11521–11526. doi: 10.1073/pnas.201238598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Diefenbach A, Jensen ER, Jamieson AM, Raulet DH. Rae1 and H60 ligands of the NKG2D receptor stimulate tumour immunity. Nature. 2001;413:165–171. doi: 10.1038/35093109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang H, Snyder KM, Suhoski MM, Maus MV, Kapoor V, June CH, Mackall CL. 4-1BB is superior to CD28 costimulation for generating CD8+ cytotoxic lymphocytes for adoptive immunotherapy. J Immunol. 2007;179:4910–4918. doi: 10.4049/jimmunol.179.7.4910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Suhoski MM, Golovina TN, Aqui NA, Tai VC, Varela-Rohena A, Milone MC, Carroll RG, Riley JL, June CH. Engineering artificial antigen-presenting cells to express a diverse array of co-stimulatory molecules. Mol Ther. 2007;15:981–988. doi: 10.1038/mt.sj.6300134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grimm EA, Mazumder A, Zhang HZ, Rosenberg SA. Lymphokine-activated killer cell phenomenon. Lysis of natural killer-resistant fresh solid tumor cells by interleukin 2-activated autologous human peripheral blood lymphocytes. J Exp Med. 1982;155:1823–1841. doi: 10.1084/jem.155.6.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fujisaki H, Kakuda H, Shimasaki N, Imai C, Ma J, Lockey T, Eldridge P, Leung WH, Campana D. Expansion of highly cytotoxic human natural killer cells for cancer cell therapy. Cancer Res. 2009;69:4010–4017. doi: 10.1158/0008-5472.CAN-08-3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Markel G, Seidman R, Besser MJ, Zabari N, Ortenberg R, Shapira R, Treves AJ, Loewenthal R, Orenstein A, Nagler A, et al. Natural killer lysis receptor (NKLR)/NKLR-ligand matching as a novel approach for enhancing anti-tumor activity of allogeneic NK cells. PLoS One. 2009;4:e5597. doi: 10.1371/journal.pone.0005597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vales-Gomez M, Reyburn HT, Erskine RA, Lopez-Botet M, Strominger JL. Kinetics and peptide dependency of the binding of the inhibitory NK receptor CD94/NKG2-A and the activating receptor CD94/NKG2-C to HLA-E. EMBO J. 1999;18:4250–4260. doi: 10.1093/emboj/18.15.4250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vales-Gomez M, Reyburn HT, Erskine RA, Strominger J. Differential binding to HLA-C of p50-activating and p58-inhibitory natural killer cell receptors. Proc Natl Acad Sci U S A. 1998;95:14326–14331. doi: 10.1073/pnas.95.24.14326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kulkarni S, Martin MP, Carrington M. The Yin and Yang of HLA and KIR in human disease. Semin Immunol. 2008;20:343–352. doi: 10.1016/j.smim.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bakker AB, Wu J, Phillips JH, Lanier LL. NK cell activation: distinct stimulatory pathways counterbalancing inhibitory signals. Hum Immunol. 2000;61:18–27. doi: 10.1016/s0198-8859(99)00160-3. [DOI] [PubMed] [Google Scholar]
- 43.Baessler T, Charton JE, Schmiedel BJ, Grunebach F, Krusch M, Wacker A, Rammensee HG, Salih HR. CD137 ligand mediates opposite effects in human and mouse NK cells and impairs NK-cell reactivity against human acute myeloid leukemia cells. Blood. 2009;115:3058–3069. doi: 10.1182/blood-2009-06-227934. [DOI] [PubMed] [Google Scholar]
- 44.Cho D, Shook DR, Shimasaki N, Chang YH, Fujisaki H, Campana D. Cytotoxicity of Activated Natural Killer Cells Against Pediatric Solid Tumors. Clin Cancer Res. 2010 doi: 10.1158/1078-0432.CCR-10-0735. [DOI] [PMC free article] [PubMed] [Google Scholar]