Abstract
S-nitrosoglutathione reductase (GSNOR), a ubiquitously expressed protein central to the control of protein S-nitrosylation, plays critical roles in many biological systems. We showed recently that GSNOR is often deficient in human hepatocellular carcinoma and that germ line deletion of the GSNOR gene in mice causes hepatocellular carcinoma through S-nitrosylation and proteasomal degradation of the key DNA repair protein O6-alkylguanine-DNA alkyltransferase (AGT). We report here the generation of mice with targeted deletion of GSNOR in hepatocytes or in cells of the hematopoietic lineage. We found that during inflammatory responses induced by intraperitoneal injection of diethylnitrosamine (DEN) or lipopolysaccharide, the amount of liver AGT was not changed in mice with GSNOR deletion in hematopoietic cells but was almost completely depleted in mice with GSNOR deletion in hepatocytes. In livers of DEN-challenged mice, GSNOR deletion in hepatocytes but not hematopoietic cells resulted in an increase in phosphorylated histone H2AX, a well-established marker of DNA double-strand breaks. Hepatocyte deletion of GSNOR increased DEN-induced mortality, which was abolished in mice deficient in both GSNOR and inducible nitric oxide synthase. Thus, protection of AGT and resistance to nitrosamine-induced genotoxicity critically depends on GSNOR in hepatocytes. In addition, our findings suggest that nitrosative inactivation of AGT from GSNOR deficiency might sensitize cancerous cells to alkylating drugs in cancer treatment.
Introduction
Protein S-nitrosylation, the covalent modification of cysteine residues by nitric oxide, may affect functions of a wide range of proteins and is important to the ubiquitous influence of nitric oxide in biological systems (1). Protein S-nitrosylation is not only influenced by nitric oxide synthases but also prominently regulated by S-nitrosoglutathione reductase (GSNOR), a major denitrosylase in cells (2–4). GSNOR is expressed ubiquitously in all the cells (2,5) and serves many important functions (2–4,6–8). Studies using GSNOR-null (GSNOR−/−) mice showed that GSNOR is critical for protecting mice from endotoxic and septic shock by preventing hazardous increase of protein S-nitrosylation and extensive cell death in liver and lymphoid tissues (3). GSNOR deficiency impairs DNA repair and promotes hepatocarcinogenesis (8). GSNOR also regulates protein S-nitrosylation and cell apoptosis in the thymus and is important for normal development of the immune system (4). In addition, GSNOR deficiency protects mice from experimental myocardial infarction (7) and prevents airway hyperresponsiveness in experimental asthma (6). The diverse roles of GSNOR in various systems, as revealed by the studies of GSNOR−/− mice, suggest that the ubiquitously expressed GSNOR may affect functions of a wide range of cells. Cell type-specific functions of GSNOR in vivo, however, remain to be firmly established.
The key DNA repair protein O6-alkylguanine-DNA alkyltransferase (AGT) repairs highly mutagenic and cytotoxic O6-alkylguanines by transferring the alkyl group from DNA to the enzyme active site cysteine, resulting in irreversible inactivation of AGT and the restoration of guanine (9). O6-alkylguanines are produced by alkylating N-nitroso compounds, including dialkylnitrosamines, which are present widely in the environment and can be formed endogenously (10–12). O6-alkylguanines are mispaired by DNA polymerases to thymine during DNA replication and the O6-alkylguanine:T mispairs, through a further round of DNA replication, can result in G:C to A:T mutations and DNA double-strand breaks, a potent trigger of cell death (13). DNA double-strand breaks and cell death may result from futile repair of O6-alkylguanine:T by the mismatch repair system and require at least two rounds of DNA replication (14). O6-alkylguanine:T mispairs also activate DNA damage responses, which might contribute to cell death (15). Mice deficient in AGT are more susceptible not only to tumorigenesis but also to acute mortality from alkylating N-nitroso compounds (16–19).
Nitric oxide is involved in carcinogenesis, in part through its influence on DNA repair proteins (20). AGT can be inactivated by nitric oxide and S-nitrosoglutathione (GSNO) through S-nitrosylation of the cysteine in the enzyme active site in vitro (21,22). The studies of GSNOR−/− mice showed that during inflammatory responses after intraperitoneal injection of diethylnitrosamine (DEN) and lipopolysaccharide (LPS), lack of GSNOR in entire animals causes S-nitrosylation, proteasomal degradation and depletion of AGT in the liver (8). Consequently, repair of O6-alkylguanines in the liver was impaired and hepatocarcinogenesis was increased. S-nitrosylation and depletion of AGT, accumulation of O6-alkylguanines and increased hepatocarcinogenesis were all abolished by further deletion in GSNOR−/− mice of the inducible nitric oxide synthase (iNOS) gene, providing additional support that AGT inactivation in GSNOR−/− mice results from iNOS-derived S-nitrosylation of AGT. Expression of iNOS, a major inflammatory mediator, can be induced in both hepatocytes and inflammatory cells including Kupffer cells (23). Both hepatocytes and non-parenchymal cells in liver express AGT (24). Because the ubiquitously expressed GSNOR regulates protein S-nitrosylation, the modification affecting functions of a wide range of cells, it is unknown whether protection of liver AGT by GSNOR in vivo critically depends on its expression in hepatocytes, non-parenchymal liver cells or both.
To study cell type-specific functions of GSNOR, we have generated mice with floxed GSNOR alleles (the alleles flanked by loxP sites) and then mice with targeted deletions of GSNOR in hepatocytes or in cells of the hematopoietic lineage. We found that protection of liver AGT from nitrosative inactivation in inflammatory responses critically depends on expression of GSNOR in hepatocytes. In addition, we found that GSNOR-deficient mice are highly susceptible to cytotoxic DNA damage and acute mortality from DEN treatment.
Materials and methods
Generation of GSNORf/f mice
The DNA fragment from nucleotide 1801 to 10809 of the mouse GSNOR gene (Accession number, NC_000069; region 138106128-138118463) was subcloned from bacterial artificial chromosome clone 91m09 (Invitrogen, Carlsbad, CA) into plasmid pL253 through recombineering (25). A LoxP sequence with addition of an SspI restriction site was inserted into intron 4 (after nt 7369), and an FRT-Neo-FRT-loxP cassette (25) was introduced into intron 6 (before nt 8824). The resulting GSNOR-targeting vector was linearized by NotI and introduced into embryonic stem (ES) cells from 129sv mice for homologous recombination (UCSF transgenic mouse facility). Neomycin-resistant ES clones were screened for homologous recombination first by polymerase chain reaction (PCR) using a neo-derived primer (Neo3′se, 5′-GCTTCTGAGGCGGAAAGAACC-3′) and a GSNOR primer (GSNOR3′as, 5′-AATGGCTCCCCAGTTCCAGCA-3′) external to the homologous region in the targeting vector. This PCR reaction detects a 2.2 kb DNA fragment only in the cells with the targeted disruption. Further screens to identify ES clones with correctly disrupted allele was conducted by Southern analyses of SspI-digested genomic DNA, using the DIG Easy Hyb system (Roche, Basel, Switzerland) with digoxigenin-labeled 5′ (nt 848-1027) and 3′ (10863-11293) probes that are external to the homologous region in the targeting vector.
Correctly targeted ES clones with normal karyotype were used to generate chimeric mice, which were subsequently bred with C57BL/6 mice to produce F1 heterozygotes with germ line transmission of the disrupted GSNOR allele. These F1 mice were mated with FLPeR mice (Jackson Laboratory, Bar Harbor, Maine) to remove the FRT-flanked neo marker, and the resulting heterozygous line with floxed GSNOR allele was referred to as GSNORf/+. The wild-type and floxed GSNOR alleles were detected by the absence and presence of the LoxP1 site, respectively through PCR using 5′-GATAGGTCCTTCTCTCAGAGA-3′ and 5′-CTGGACGTTGTGTCTTCTCTT-3′ primers.
Generation of mice with targeted deletion of GSNOR in hepatocytes and hematopoietic cells
Following consecutive backcrossing to C57BL/6 mice a total of 10 times, GSNORf/+ mice, congenic to C57BL/6, were crossed with Alb-cre mice (Jackson Laboratory). The F1 progeny, Alb-creGSNORf/+ mice, were backcrossed to GSNORf/f mice to produce Alb-creGSNORf/f mice, which were crossed to GSNORf/f mice to produce Alb-creGSNORf/f and GSNORf/f littermates for the present study. The Alb-Cre transgene was detected by PCR genotyping with the primers 5′-ACCTGAAGATGTTCGCGATTATCT-3′ and 5′-ACCGTCAGTACGTGAGATATCTT-3′, which amplify a 370 bp fragment (26). Similarly, GSNORf/+ mice were crossed with Vav-cre mice (Jackson Laboratory) to produce Vav-creGSNORf/f and GSNORf/f mice. The Vav-cre transgene was detected in genotyping by PCR with the primers 5′-AGATGCCAGGACATCAGGAACCTG-3′ and 5′-ATCAGCCACACCAGACACAGAGATC-3′.
DEN acute toxicity
DEN (Sigma, St. Louis, MO) was prepared in phosphate-buffered saline without calcium or magnesium. Male pups were given at postnatal day 15 a single intraperitoneal injection of DEN (37.5 or 50 μg/g body wt when indicated) to study acute toxicity. Mice were monitored for defined periods after DEN injection and survivors were scored. Kaplan–Meier survival analysis was done using the GraphPad Prism software.
LPS treatment
LPS (Escherichia coli, serotype 026:B6; Sigma) at dosages of 7.5 and 10 μg/g was injected intraperitoneally into adult female GSNORf/f, Alb-creGSNORf/f and Vav-creGSNORf/f mice. The LPS used (lot number 119K4044) contains 3 million endotoxin U/mg.
Mice thymocyte lysates
Thymocytes were obtained by grinding mice thymus through a 70 μm filter insert in six-well plates (BD Biosciences, Franklin Lakes, NJ). Thymocytes were collected by centrifugation and lysed in ice-cold lysis buffer [50 mM Tris–HCl (pH = 8.0), 1 mM ethylenediaminetetraacetic acid, 150 mM NaCl, 0.1% NP-40 and 1 mM phenylmethylsulfonyl fluoride, supplemented with 1× Complete protease inhibitor cocktail (Roche)] by sonication on a Virtis 600 Ultrasonic Disruptor (SP Industries, Warminster, PA). Protein lysates were transferred to a clean microfuge tube and cleared at 14 000 r.p.m. in a bench-top Eppendorf centrifuge.
Mice liver lysates
Protein lysates from mice liver samples were prepared in ice-cold lysis buffer [50 mM Tris–HCl (pH = 8.0), 1.0 mM ethylenediaminetetraacetic acid, 150 mM NaCl, 0.1% NP-40 and 1 mM phenylmethylsulfonyl fluoride, supplemented with 1× Complete protease inhibitor cocktail (Roche)]. Liver samples were ground on ice for 2 min, using a ceramic pestle on a Glas–Col Homogenizer, with speed setting at 200 r.p.m. Protein lysates were transferred to a clean microfuge and cleared at 14 000 r.p.m. in a bench-top Eppendorf centrifuge.
GSNOR enzymatic activity
The GSNOR activity was measured by GSNO-dependent consumption of reduced form of nicotinamide adenine dinucleotide (NADH) (8). Briefly, 50 μg/ml liver lysate or 250 μg/ml thymocyte lysate was incubated with 75 μM NADH in reaction buffer [20 mM Tris–HCl (pH 8.0) and 0.5 mM ethylenediaminetetraacetic acid] containing 100 μM GSNO at room temperature, and NADH fluorescence (absorption at 340 nm and emission at 455 nm) was measured over time to determine the initial rate of GSNO-dependent NADH consumption.
Immunoblot
Proteins in liver homogenates were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis, transferred to nitrocellulose membranes, and probed with rabbit antiserum to GSNOR, β-actin mouse monoclonal antibody (Sigma A-5441), goat antiserum to AGT (R&D Systems, Minneapolis, MN) or phosphorylated histone H2AX (γ-H2AX) mouse monoclonal antibody (JBW301; Millipore, Billerica, MA). GSNOR, β-actin and AGT were detected and quantified with infrared fluorescent secondary antibodies—a goat antibody to rabbit coupled to Alexa Fluor 680 (Invitrogen), a goat antibody to mouse coupled to IRDye 800 (Rockland Immunochemicals, Gilbertsville, PA) and a donkey antibody to goat coupled to Alexa Fluor 680 (Invitrogen)—with an infrared fluorescence imaging system (Odyssey; LI-COR Biosciences, Lincoln, NE). AGT was also detected with a donkey secondary antibody to goat coupled to horseradish peroxidase and SuperSignal West Femto Chemiluminescent Substrate (Thermo Fisher Scientific, Rockford, IL). γ-H2AX was detected with a goat secondary antibody to mouse coupled to horseradish peroxidase and SuperSignal West Pico Chemiluminescent Substrate (Thermo Fisher Scientific).
Statistical analysis
Kaplan–Meier survival curves were analyzed by the log-rank test. Survival rates were analyzed by the Fisher’s exact test of contingency tables. All the other data were analyzed with the Student’s t-test.
Results
Increased sensitivity of GSNOR−/− mice to acute DEN toxicity
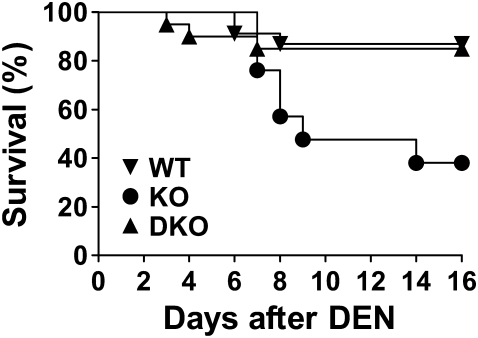
During the study of DEN-induced hepatocarcinogenesis in GSNOR−/− mice, we noticed that when challenged with a relatively high dose of DEN (25 μg/g), many GSNOR−/− mice died unexpectedly in a few days following the DEN challenge (supplementary Figure S1 is available at Carcinogenesis Online). To confirm and further investigate the hypersensitivity to acute DEN toxicity from GSNOR deficiency, we studied the survival patterns following DEN challenge in wild-type, GSNOR−/− and iNOS−/−GSNOR−/− mice (Figure 1). We found that most wild-type mice survived well but ∼60% of GSNOR−/− mice died within 2 weeks following DEN challenge. Most death of the mice in this experiment occurred between 7 and 9 days after DEN injection, indicating delayed death that probably resulted from a secondary response to DEN toxicity. The increased mortality of GSNOR−/− mice after DEN injection was abolished in iNOS−/−GSNOR−/− mice (Figure 1). Thus, GSNOR−/− mice are highly susceptible to acute DEN toxicity and the increased sensitivity of GSNOR−/− mice to DEN is due to iNOS activity. Our data therefore suggest that GSNOR, through metabolizing iNOS-derived GSNO, protects mice against acute DEN toxicity.
Fig. 1.
Increased sensitivity of GSNOR−/− mice to acute DEN toxicity. Kaplan–Meier survival curves of wild-type (WT, n = 23), GSNOR−/− (KO, n = 22), and iNOS−/−GSNOR−/− (DKO, n = 20) mice following intraperitoneal injection of DEN (37.5 μg/g). Survival of GSNOR−/− mice was significantly lower than that of wild-type (P < 0.002, log-rank test) or iNOS−/−GSNOR−/− (P < 0.006) mice.
Generation of mice with targeted deletion of GSNOR in hepatocytes and hematopoietic cells
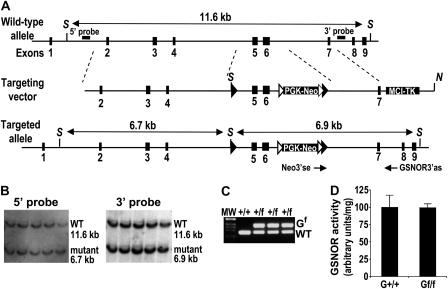
To generate mice with a floxed GSNOR allele, a GSNOR-targeting construct (Figure 2A), in which exons 5 and 6 of the GSNOR gene were flanked by a loxP sequence and an FRT-Neo-FRT-loxP cassette, was introduced into ES cells for homologous recombination. ES cells with correctly targeted GSNOR allele, as indicated by Southern analyses using both 5′ and 3′ probes external to the homologous region in the vector (Figure 2B), were used to generate chimeric mice. By breeding the chimeras with C57BL/6 mice, we obtained F1 heterozygotes with germ line transmission of the disrupted GSNOR allele. These F1 mice were bred with FLPeR mice to remove the FRT-flanked neo marker, and the resulting heterozygous line with floxed GSNOR allele was referred to as GSNORf/+ (Figure 2C). The GSNORf/+ mice were backcrossed consecutively to C57BL/6 mice a total of 10 times to make the transgenic mice congenic to C57BL/6. Analysis of GSNOR activity in tail, liver and thymocytes indicates that insertion of the loxP sequences in the GSNOR allele has little effect on the expression and activity of GSNOR (Figure 2D and data not shown).
Fig. 2.
Generation of GSNORf/f mice. (A) Strategy for conditional targeting of the GSNOR gene. The structures of the targeting vector, wild-type and targeted GSNOR alleles are shown. The restriction sites used for construction of the targeting vector and Southern analysis are: S, SspI and N, NotI. Cassettes PGK-Neo and MCI-TK are the selectable genes neo and tk under the control of PGK and MCI promoters, respectively. Double-headed arrows represent expected fragments of the wild-type (wt) and disrupted (mutant) GSNOR alleles in Southern analyses with SspI restriction and the indicated 5′ or 3′ probe. Neo3′se and GSNOR3′as are the PCR primers used to detect the targeted allele. Filled triangles represent loxP sites and empty triangles represent FRT sites. (B) Southern blot of Ssp I-digested genomic DNA with the 5′ (left) and 3′ (right) probes identified and confirmed five ES cell clones that carry the correctly targeted GSNOR allele (mutant). (C) Genotyping by PCR to detect floxed (Gf) and wild-type GSNOR alleles in transgenic mice. (D) GSNOR activity in livers of wild-type (G+/+) and homozygous GSNORf/f (Gf/f) mice. Data (mean ± standard deviation) are from three wild-type and three GSNORf/f mice.
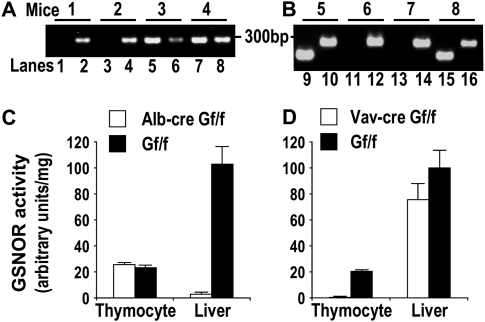
To delete GSNOR selectively in hepatocytes in mice, we generated Alb-creGSNORf/f mice by crossing GSNORf/+ mice with Alb-cre transgenic mice (26) (Figure 3A). Alb-cre transgene expresses the Cre recombinase from a rat albumin promoter and drives deletion of floxed DNA fragments in hepatocytes (26). Whereas GSNOR activity in thymocytes of Alb-creGSNORf/f mice was not changed compared with GSNORf/f control, GSNOR activity and protein level were greatly reduced in livers of Alb-creGSNORf/f mice, indicating efficient and selective deletion of GSNOR in hepatocytes in the mice (Figure 3C and Figure 4).
Fig. 3.
Targeted deletion of GSNOR in hepatocytes and hematopoeitic cells in mice. (A) Genotyping GSNORf/f (Gf/f) and Alb-creGSNORf/f littermates by PCR using primers specific to the floxed allele of GSNOR (even lanes) and the Alb-cre transgene (odd lanes). (B) Genotyping GSNORf/f and Vav-creGSNORf/f littermates by PCR using primers specific to the floxed allele of GSNOR (even lanes) and the Vav-cre transgene (odd lanes). (C) GSNOR activity in liver and isolated thymocytes from Alb-creGSNORf/f and GSNORf/f mice. Data (means ± standard errors) are from 3 to 5 mice. (D) GSNOR activity in liver and isolated thymocytes from Vav-creGSNORf/f and GSNORf/f mice. Data (means ± standard errors) are from 3 to 5 mice.
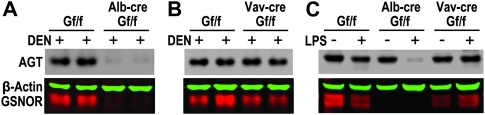
Fig. 4.
AGT protein is depleted in livers of Alb-CreGSNORf/f mice after DEN or LPS challenge. (A and B) Immunoblot of AGT, β-actin and GSNOR in livers of Alb-creGSNORf/f (A), Vav-creGSNORf/f (B) and GSNORf/f littermates 6 days after DEN (50 μg/g) injection. (C) Immunoblot of AGT, β-actin and GSNOR in livers of the mice before or 24 h after a single intraperitoneal injection of LPS (10 μg/g).
To generate mice deficient of GSNOR only in cells of the hematopoietic lineage, we crossed GSNORf/f mice with Vav-cre transgenic mice (Figure 3B), which expresses the Cre recombinase mostly in the hematopoietic cells including inflammatory cells (27). We found that GSNOR activity was absent in thymocytes of Vav-creGSNORf/f mice, indicating efficient deletion of GSNOR in the hematopoietic cells in the mice (Figure 3D). GSNOR activity was slightly reduced in liver of Vav-creGSNORf/f mice, probably from deletion of GSNOR in Kupffer cells, the resident macrophages in liver (Figure 3D).
Depletion of AGT in livers of DEN- and LPS-challenged Alb-creGSNORf/f mice
GSNOR deficiency, in the model of DEN challenge, results in nitrosative inactivation of liver AGT in GSNOR−/− mice (8). We found by immunoblot analysis that after DEN challenge, the abundance of AGT protein in the liver of Alb-creGSNORf/f mice was much lower than that in GSNORf/f littermates (Figure 4A). In contrast, the amount of liver AGT was comparable between DEN-challenged Vav-creGSNORf/f and GSNORf/f mice (Figure 4B). Thus, protection of liver AGT largely depends on expression of GSNOR in hepatocytes. GSNOR deficiency in GSNOR−/− mice also results in nitrosative inactivation of liver AGT following LPS challenge, another model of nitrosative stress from inflammatory response (8). We found that mouse survival was reduced from hepatocyte deletion of GSNOR two days after an intraperitoneal injection of LPS (supplementary Figure S2 is available at Carcinogenesis Online). Importantly, in the LPS model, AGT abundance was greatly reduced in the liver of Alb-creGSNORf/f mice compared with GSNORf/f and Vav-creGSNORf/f mice (Figure 4C). Our data thus suggest that hepatocyte GSNOR critically protects liver AGT from nitrosative inactivation in inflammatory responses induced in various biological processes.
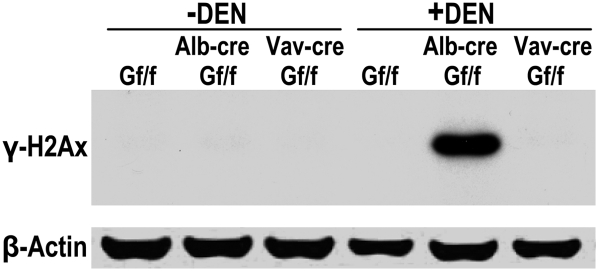
Increase of γ-H2AX in livers of DEN-challenged Alb-creGSNORf/f mice
AGT deficiency is expected to impair repair of O6-alkylguanines and persistent O6-alkylguanine lesions can result in stalled DNA replication and DNA double-strand breaks (13). We therefore probed the induction of γ-H2AX, a well-established marker of DNA double-strand breaks. We found that 6 days after DEN injection, γ-H2AX was absent in the livers of GSNORf/f and Vav-creGSNORf/f mice but substantially induced in the livers of Alb-creGSNORf/f mice (Figure 5). Thus, the data suggest that GSNOR deficiency in hepatocytes, but not in inflammatory cells, may increase DEN-induced DNA double-strand breaks in the liver.
Fig. 5.
Increase in γ-H2AX in livers of Alb-CreGSNORf/f mice after DEN challenge. Immunoblot of γ-H2AX and β-actin in livers of GSNORf/f, Alb-creGSNORf/f and Vav-creGSNORf/f mice before or 6 days after DEN injection.
Increased mortality in DEN-challenged Alb-creGSNORf/f mice
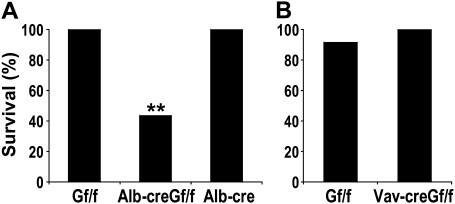
Although DEN is predominantly a hepatotoxin, it also targets other organs (28). We found that following DEN challenge, GSNORf/f and Alb-cre mice survived well but ∼60% of Alb-creGSNORf/f mice died (Figure 6A). In contrast, Vav-creGSNORf/f mice survived as well as GSNORf/f littermates after DEN treatment (Figure 6B). Thus, protection against acute mortality from DEN depends on GSNOR in hepatocytes.
Fig. 6.
Targeted deletion of GSNOR in hepatocytes increases mice sensitivity to acute DEN toxicity. (A) Survival of Alb-creGSNORf/f mice (n = 17) in 6 days after DEN injection was significantly lower than that of GSNORf/f mice (n = 10; P < 0.004) and Alb-cre mice (n = 10; P < 0.004). **P < 0.004, Fisher's exact test. (B) Survival of Vav-creGSNORf/f mice (n = 12) in 6 days after DEN injection was comparable with that of GSNORf/f mice (n = 11).
Discussion
Our results suggest that protection of AGT from nitrosative inactivation critically depends on GSNOR, likely through its cell-autonomous function in hepatocytes. We showed previously that during inflammatory and immune responses, liver AGT is highly susceptible to nitrosative inactivation in mice completely lacking GSNOR (8). The ubiquitously expressed GSNOR affects multiple cellular processes in hepatocytes, immune cells and other cells (2–4,6–8), raising the question as to whether the protection of liver AGT in vivo critically depends on GSNOR in hepatocytes. AGT activity in the liver, which is much higher in hepatocytes than in non-parenchymal cells, is mostly in hepatocytes (24). Because most AGT in livers of DEN- or LPS-treated Alb-creGSNORf/f mice was depleted, AGT activity in hepatocytes is most likely depleted in the mice. This notion is supported by the fact that DEN treatment of GSNOR−/− mice resulted in a significant increase in O6-alkylguanines in the liver (8). Thus, hepatocyte GSNOR appears to be critical for protection of AGT in hepatocytes. In contrast, liver AGT was not depleted in DEN-challenged Vav-creGSNORf/f mice, indicating that protection of hepatocyte AGT does not critically depend on the function of GSNOR in Kupffer or other immune cells. Increased DNA double-strand breaks in the livers of DEN-treated Alb-creGSNORf/f mice further support the important role on DNA repair by GSNOR in hepatocytes. GSNOR is often deficient in cells of hepatocellular carcinomas through somatic mutations in human (8,29,30). Our current findings thus provide further support for the hypothesis that GSNOR deficiency may result in nitrosative inactivation of AGT and contribute significantly to hepatocarcinogenesis in human.
Our findings of increased mortality from DEN challenge by GSNOR deficiency are consistent with its prominent effect on nitrosative inactivation of AGT. Alkylating N-nitroso compounds, including dialkylnitrosamines, cause cytotoxic O6-alkylguanines and increase mortality when repair of O6-alkylguanines is impaired from AGT deficiency (16,18,19). The temporal pattern of death in DEN-treated GSNOR−/− mice is comparable with that in methylnitrosourea-treated AGT-null mice and is indicative of a secondary response to persistent O6-alkylguanines (18). Whereas methylnitrosourea is a direct alkylating agent that does not require metabolic activation, DEN requires activation by P450 enzymes (28). Whereas methylnitrosourea-induced death results largely from the cytotoxicity on cells of hematopoeitic lineage (17), the mechanism of DEN-induced mortality is less clear. DEN targets mainly hepatocytes but also other cells including Kupffer cells (31,32). Our findings of increased mortality from DEN challenge in Alb-creGSNORf/f but not Vav-creGSNORf/f mice suggest that death induced by DEN may well result from its effect on hepatocytes. Our results thus show that GSNOR deficiency in hepatocytes increases sensitivity of the cells to the genotoxic and cytotoxic effects of DEN, a representative of alkylating carcinogens.
The findings of the cell-autonomous effects of GSNOR deficiency on AGT and cell sensitivity to an alkylating agent might have implications in cancer treatment using chemotherapeutic alkylating agents. Sensitivity of cancer cells to alkylating drugs is affected by AGT activity of cancerous cells in glioma and other cancers (11). AGT activity can be reduced at the level of transcription of AGT through the methylation of its promoter (11) and as shown by our results, also at the level of protein stability through nitrosative inactivation. The human GSNOR gene is in chromosome 4q, which is frequently lost in glioma and lung and other cancers (33). Thus, nitrosative inactivation of AGT from GSNOR deficiency might play a role in cellular responses to alkylating drugs in cancer treatment.
In summary, we found that protection of AGT and resistance to genotoxicity from an alkylating agent critically depends on GSNOR expressed in hepatocytes. Our findings further define the role of GSNOR in a mechanism potentially important to carcinogenesis and in addition, might have implications in chemotherapeutic treatment of cancer. GSNORf/f mice and related conditional knockout mice would provide a valuable means to study cell type-specific functions of GSNOR, a ubiquitous denitrosylase playing important roles in many biological systems.
Supplementary material
Supplementary Figures S1 and S2 can be found at http://carcin.oxfordjournals.org/
Funding
National Institutes of Health (R01CA55578, R01CA122359 and P01CA123328 to L.L. and P30 DK026743); and Sandler Family Supporting Foundation (to L.L.).
Supplementary Material
Acknowledgments
Conflict of Interest Statement: None declared.
Glossary
Abbreviations
- AGT
O6-alkylguanine-DNA alkyltransferase
- DEN
diethylnitrosamine
- ES
embryonic stem
- GSNOR
S-nitrosoglutathione reductase
- γ-H2AX
phosphorylated histone H2AX
- iNOS
inducible nitric oxide synthase
- LPS
lipopolysaccharide
- NADH
reduced form of nicotinamide adenine dinucleotide
- PCR
polymerase chain reaction
References
- 1.Hess DT, et al. Protein S-nitrosylation: purview and parameters. Nat. Rev. Mol. Cell Biol. 2005;6:150–166. doi: 10.1038/nrm1569. [DOI] [PubMed] [Google Scholar]
- 2.Liu L, et al. A metabolic enzyme for S-nitrosothiol conserved from bacteria to humans. Nature. 2001;410:490–494. doi: 10.1038/35068596. [DOI] [PubMed] [Google Scholar]
- 3.Liu L, et al. Essential roles of S-nitrosothiols in vascular homeostasis and endotoxic shock. Cell. 2004;116:617–628. doi: 10.1016/s0092-8674(04)00131-x. [DOI] [PubMed] [Google Scholar]
- 4.Yang Z, et al. Lymphocyte development requires S-nitrosoglutathione reductase. J. Immunol. 2010;185:6664–6669. doi: 10.4049/jimmunol.1000080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Uotila L, et al. Expression of formaldehyde dehydrogenase and S-formylglutathione hydrolase activities in different rat tissues. Adv. Exp. Med. Biol. 1997;414:365–371. doi: 10.1007/978-1-4615-5871-2_42. [DOI] [PubMed] [Google Scholar]
- 6.Que LG, et al. Protection from experimental asthma by an endogenous bronchodilator. Science. 2005;308:1618–1621. doi: 10.1126/science.1108228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lima B, et al. Endogenous S-nitrosothiols protect against myocardial injury. Proc. Natl Acad. Sci. USA. 2009;106:6297–6302. doi: 10.1073/pnas.0901043106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wei W, et al. S-nitrosylation from GSNOR deficiency impairs DNA repair and promotes hepatocarcinogenesis. Sci. Transl. Med. 2010;2:19ra13. doi: 10.1126/scitranslmed.3000328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pegg AE. Repair of O(6)-alkylguanine by alkyltransferases. Mutat. Res. 2000;462:83–100. doi: 10.1016/s1383-5742(00)00017-x. [DOI] [PubMed] [Google Scholar]
- 10.Liu RH, et al. Elevated formation of nitrate and N-nitrosodimethylamine in woodchucks (Marmota monax) associated with chronic woodchuck hepatitis virus infection. Cancer Res. 1991;51:3925–3929. [PubMed] [Google Scholar]
- 11.Gerson SL. MGMT: its role in cancer aetiology and cancer therapeutics. Nat. Rev. Cancer. 2004;4:296–307. doi: 10.1038/nrc1319. [DOI] [PubMed] [Google Scholar]
- 12.Lundberg JO, et al. Nitrate, bacteria and human health. Nat. Rev. Microbiol. 2004;2:593–602. doi: 10.1038/nrmicro929. [DOI] [PubMed] [Google Scholar]
- 13.Kaina B, et al. MGMT: key node in the battle against genotoxicity, carcinogenicity and apoptosis induced by alkylating agents. DNA Repair (Amst.) 2007;6:1079–1099. doi: 10.1016/j.dnarep.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 14.Quiros S, et al. Processing of O6-methylguanine into DNA double-strand breaks requires two rounds of replication whereas apoptosis is also induced in subsequent cell cycles. Cell Cycle. 2010;9:168–178. doi: 10.4161/cc.9.1.10363. [DOI] [PubMed] [Google Scholar]
- 15.Yoshioka K, et al. ATR kinase activation mediated by MutSalpha and MutLalpha in response to cytotoxic O6-methylguanine adducts. Mol. Cell. 2006;22:501–510. doi: 10.1016/j.molcel.2006.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iwakuma T, et al. High incidence of nitrosamine-induced tumorigenesis in mice lacking DNA repair methyltransferase. Carcinogenesis. 1997;18:1631–1635. doi: 10.1093/carcin/18.8.1631. [DOI] [PubMed] [Google Scholar]
- 17.Tsuzuki T, et al. Targeted disruption of the DNA repair methyltransferase gene renders mice hypersensitive to alkylating agent. Carcinogenesis. 1996;17:1215–1220. doi: 10.1093/carcin/17.6.1215. [DOI] [PubMed] [Google Scholar]
- 18.Kawate H, et al. Separation of killing and tumorigenic effects of an alkylating agent in mice defective in two of the DNA repair genes. Proc. Natl Acad. Sci. USA. 1998;95:5116–5120. doi: 10.1073/pnas.95.9.5116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Glassner BJ, et al. DNA repair methyltransferase (Mgmt) knockout mice are sensitive to the lethal effects of chemotherapeutic alkylating agents. Mutagenesis. 1999;14:339–347. doi: 10.1093/mutage/14.3.339. [DOI] [PubMed] [Google Scholar]
- 20.Hussain SP, et al. Radical causes of cancer. Nat. Rev. Cancer. 2003;3:276–285. doi: 10.1038/nrc1046. [DOI] [PubMed] [Google Scholar]
- 21.Laval F, et al. Inhibition by nitric oxide of the repair protein, O6-methylguanine-DNA-methyltransferase. Carcinogenesis. 1994;15:443–447. doi: 10.1093/carcin/15.3.443. [DOI] [PubMed] [Google Scholar]
- 22.Liu L, et al. Inactivation and degradation of O(6)-alkylguanine-DNA alkyltransferase after reaction with nitric oxide. Cancer Res. 2002;62:3037–3043. [PubMed] [Google Scholar]
- 23.Moncada S, et al. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol. Rev. 1991;43:109–142. [PubMed] [Google Scholar]
- 24.Swenberg JA, et al. Cell-specific differences in O6-alkylguanine DNA repair activity during continuous exposure to carcinogen. Proc. Natl Acad. Sci. USA. 1982;79:5499–5502. doi: 10.1073/pnas.79.18.5499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu P, et al. A highly efficient recombineering-based method for generating conditional knockout mutations. Genome Res. 2003;13:476–484. doi: 10.1101/gr.749203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Postic C, et al. Dual roles for glucokinase in glucose homeostasis as determined by liver and pancreatic beta cell-specific gene knock-outs using Cre recombinase. J. Biol. Chem. 1999;274:305–315. doi: 10.1074/jbc.274.1.305. [DOI] [PubMed] [Google Scholar]
- 27.de Boer J, et al. Transgenic mice with hematopoietic and lymphoid specific expression of Cre. Eur. J. Immunol. 2003;33:314–325. doi: 10.1002/immu.200310005. [DOI] [PubMed] [Google Scholar]
- 28.Verna L, et al. N-nitrosodiethylamine mechanistic data and risk assessment: bioactivation, DNA-adduct formation, mutagenicity, and tumor initiation. Pharmacol. Ther. 1996;71:57–81. doi: 10.1016/0163-7258(96)00062-9. [DOI] [PubMed] [Google Scholar]
- 29.Nagai H, et al. Comprehensive allelotyping of human hepatocellular carcinoma. Oncogene. 1997;14:2927–2933. doi: 10.1038/sj.onc.1201136. [DOI] [PubMed] [Google Scholar]
- 30.Yeh SH, et al. Chromosomal allelic imbalance evolving from liver cirrhosis to hepatocellular carcinoma. Gastroenterology. 2001;121:699–709. doi: 10.1053/gast.2001.27211. [DOI] [PubMed] [Google Scholar]
- 31.Gray R, et al. Chronic nitrosamine ingestion in 1040 rodents: the effect of the choice of nitrosamine, the species studied, and the age of starting exposure. Cancer Res. 1991;51:6470–6491. [PubMed] [Google Scholar]
- 32.Scherer E, et al. Immunocytochemical analysis of O6-alkylguanine shows tissue specific formation in and removal from esophageal and liver DNA in rats treated with methylbenzylnitrosamine, dimethylnitrosamine, diethylnitrosamine and ethylnitrosourea. Cancer Lett. 1989;46:21–29. doi: 10.1016/0304-3835(89)90210-3. [DOI] [PubMed] [Google Scholar]
- 33.Struski S, et al. Compilation of published comparative genomic hybridization studies. Cancer Genet. Cytogenet. 2002;135:63–90. doi: 10.1016/s0165-4608(01)00624-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.