Abstract
Our previous studies reported that caffeine or voluntary exercise decreased skin tumor multiplicity, in part, by decreasing fat levels in the dermis. These data suggest that tissue fat may play an important role in regulating ultraviolet light (UV) B-induced skin tumor development. In the present study, we explored the effects of high-fat diets rich in either omega-3 or omega-6 fatty acids on UVB-induced skin carcinogenesis. SKH-1 mice were irradiated with 30 mJ/cm2 of UVB once a day, two times per week for 39 weeks. During UVB treatment, one group of mice was given a high-fat fish oil (HFFO) diet rich in omega-3 fatty acids and the other group of mice was given a high-fat mixed-lipids (HFMLs) diet rich in omega-6 fatty acids. The results showed that, compared with HFML diet, HFFO treatment (i) increased latency for the development of UVB-induced skin tumors; (ii) decreased the formation of papilloma, keratoacanthoma and carcinoma by 64, 52 and 46%, respectively and (iii) decreased the size of papilloma, keratoacanthoma and carcinoma by 98, 80 and 83%, respectively. Mechanistic studies with antibody array revealed that compared with HFML diet, administration of HFFO to the mice significantly decreased the UVB-induced increases in the levels of TIMP-1, LIX and sTNF R1 as well as other several proinflammatory cytokines and stimulated the UVB-induced apoptosis in the epidermis. Our results indicate that omega-3 fatty acids in HFFO diet have beneficial effects against UVB-induced skin carcinogenesis, and these effects may be associated with an inhibition on UVB-induced inflammatory response.
Introduction
Our earlier studies reported that oral administration of caffeine or voluntary running wheel exercise decreased the number of ultraviolet light (UV) B-induced skin tumors per mouse, decreased the weight of the parametrial fat pads and decreased the thickness of the dermal fat layer away from tumors and directly under tumors (1,2). Using data from individual mice and linear regression and correlation analysis, we found a highly significant positive correlation between the thickness of the dermal fat layer away from tumors and the number of tumors per mouse (1,2). The results suggested that oral administration of caffeine or voluntary exercise may have decreased skin tumor multiplicity, in part, by decreasing fat levels in the dermis and the size of parametrial fat pads. Our previous studies also showed that surgical removal of the parametrial fat pads (partial lipectomy) enhanced UVB-induced apoptosis in the epidermis (3). These data suggest that tissue fat may play an important role in regulating UVB-induced apoptosis and skin carcinogenesis. Accordingly, in the present study, we explored the effects of two different types of high-fat diets rich in either omega-3 or omega-6 fatty acids on UVB-induced skin carcinogenesis in SKH-1 hairless mice. It was already known that rats fed the high-fat diet rich in omega-3 fatty acids showed significantly lower colon tumor incidence and multiplicity compared with rats fed the high-fat diet rich in omega-6 fatty acids (4). Although both types of fatty acids are considered essential, their functions in inflammation are opposite. Omega-3 fatty acids encourage the production of body chemicals that help to control inflammation in the tissues, whereas omega-6 fatty acids shift the physiologic state to a proinflammatory response (5). Since many studies indicate the importance of inflammation during carcinogenesis (6,7), we determined whether high-fat diets rich in either omega-3 or omega-6 fatty acids affect UVB-induced skin carcinogenesis and if these effects are associated with altered levels of inflammatory proteins.
Materials and methods
Animals
Female SKH-1 hairless mice (6–7 weeks old) were purchased from Charles River Breeding Laboratories, Wilmington, MA and kept in our animal facility for 1 week before use. Mice were maintained on a 12 h light/12 h dark cycle and provided food and water ad libitum with fresh food replenished every day.
Composition of high-fat diets rich in omega-3 or omega-6 fatty acids
Two different types of high-fat diets rich in either omega-3 or omega-6 fatty acids were purchased from Dyets (Bethlehem, PA) and stored at −20°C until use. The composition of the experimental diets was based on a modified American Institute of Nutrition-76A diet and was adjusted so that all diets would provide the same amount of calories, protein, vitamins, minerals and fiber (Table I). The high-fat diet rich in omega-3 fatty acids contained 10% fish oil [high-fat fish oil (HFFO)] and 10% mixed lipids premix. The high-fat diet rich in omega-6 fatty acids contained 20% mixed lipids premix [high-fat mixed lipids (HFMLs)]. The mixed lipids premix was formulated to simulate the fat content of the American diet derived from hydrogenated soybean oil (30%), corn oil (27%), beef tallow (16%), butter fat (12%), lard (10%) and peanut oil (5%). The mixed lipids diet contained 28% polyunsaturated omega-6, 24% monounsaturated omega-7/9 and 45% saturated fatty acids. The fish oil contained 32% polyunsaturated omega-3 (docosahexaenoic acid and eicosapentaenoic acid), 8% polyunsaturated omega-6, 16% monounsaturated omega-9 (oleic acid) and 29% saturated fatty acids as described by Reddy et al. 2005 (4).
Table I.
Composition of high-fat diets rich in omega-3 (HFFO) or omega-6 fatty acids (HFML)
| Ingredient | HFML (%) | HFFO (%) | kcal/kg |
| Casein | 23.50 | 23.50 | 841 |
| Corn starch | 35.70 | 35.70 | 1285 |
| Dextrose | 9.02 | 9.02 | 328 |
| DL-Methionine | 0.35 | 0.35 | 14 |
| Alpha-cellulose | 5.90 | 5.90 | 0 |
| Fish oil (Menhaden oil) | — | 10.00 | 900 |
| Mixed lipids | — | 10.00 | 900 |
| Mixed lipids | 20.00 | — | 1800 |
| Mineral mix | 4.11 | 4.11 | 19 |
| Vitamin mix | 1.18 | 1.18 | 46 |
| Choline bitartrate | 0.24 | 0.24 | 0 |
All diets were formulated on the basis of the AIN (American Institute of Nutrition) standard reference diet with the modification of varying sources of carbohydrate. Mixed lipid diet containing American blend fat developed by the Institute of Shortening and Edible Oils was formulated to simulate the lipid content of the average American diet. The composition of mixed lipids is described in detail in ‘Materials and Methods’ (4).
Exposure of mice to UVB and the preparation of skin sections
The UV lamps (FS72T12-UVB-HO; National Biological Corp., Twinsburg, OH) emitted UVB (280–320 nm; 75–80% of total energy) and UVA (320–375 nm; 20–25% of total energy). The dose of UVB was quantified with a UVB Spectra 305 dosimeter (Daevlin Co., Byran, OH).
Eighty female SKH-1 mice were equally divided into two groups (40 mice per group, 10 mice per cage). They were irradiated with 30 mJ/cm2 of UVB once a day, two times per week for 39 weeks. From the beginning of UVB treatment, one group of mice was given a high-fat diet rich in polyunsaturated omega-3 fatty acids (HFFO), and the other group of mice was given a high-fat diet rich in polyunsaturated omega-6 fatty acids (HFML).
The mice were killed, and the two parametrial fat pads were removed and weighed. The dorsal skin surrounding each grossly observed mass was taken, stapled flat to a plastic sheet and placed in 10% phosphate-buffered formalin at 4°C for 18–24 h. Parts of tumors from HFFO and HFML groups were cut and used for measurement of inflammatory cytokines with an antibody array (see below). The skin samples were then dehydrated in ascending concentrations of ethanol, cleared in xylene and embedded in Paraplast (Oxford Labware, St Louis, MO). Four micrometers skin sections were made, deparaffinized, rehydrated with water and used for regular hematoxylin and eosin staining.
Characterization of skin tumors and measurement of tumor size
Body weight and skin tumor growth (number of tumors per mouse, tumor volume per mouse) were measured every 2 weeks. The counting and characterization of all tumors were performed blind with respect to treatment group as described previously (8). Tumor volume was determined by measuring the three-dimensional size (height, length and width) of each mass. The average of the three measurements was used as the diameter. The radius (r) was determined, and the tumor volume was calculated by: volume = 4πr3/3.
Mouse inflammation antibody array
Mechanistic study was conducted to determine the effects of two types of high-fat diets on inflammatory cytokine levels in different types of tumors induced by chronic UVB exposure for 39 weeks utilizing a RayBio® Mouse Inflammation Antibody array kit (detection of 40 mouse inflammation factors, purchased from RayBiotech, Norcross, GA).
In a separate experiment, the same antibody array was used to evaluate the early effects of these two types of high-fat diets on inflammatory cytokine levels in the epidermis of mice before the tumor formation. In this study, female SKH-1 mice (6–7 weeks old, five mice per group) were irradiated with 30 mJ/cm2 of UVB once a day, two times per week for 10 or 20 weeks. From the beginning of UVB treatment, one group of mice was fed the HFFO diet and the other group was fed the HFML diet. The reasons for choosing these two time points is based on our previous studies where we found that multiple mutant p53-positive epidermal patches appeared after 10 weeks of UVB treatment (9), and skin tumors started to develop after 20 weeks of UVB treatment (our high-risk mouse model) (8).
The epidermal tissue lysate was prepared as described previously (10). Briefly, dorsal skin samples were removed and immediately placed in a buffer solution containing 50 mM potassium phosphate (pH 7.7) at 52°C for 20 s. The skin samples were then immediately submerged in an ice bath containing the same buffer for 40 s and the epidermis was scraped from the dermis. The epidermis or tumors were cut into small pieces and placed in 1 ml of tissue lysis buffer (catalog no: 9803 from Cell Signaling Technology Danvers, MA) containing 1 mM phenylmethylsulfonyl fluoride for 30 min and then sonicated five times (each for 5 s at 4°C at 5 s intervals). Samples were centrifuged at 17 800g for 10 min at 4°C.
The antibody array was performed following the instructions provided by the manufacturer. Briefly, membranes were treated with 2 ml of blocking buffer and incubated overnight with 1 ml of 150 μg of tissue lysates at 4°C. After washing, 1 ml of biotin-conjugated antibodies were added for 1 h and incubated with 2 ml of horseradish peroxidase-conjugated streptavidin at room temperature for 2 h. The membranes were then treated with 500 μl of detection buffer for 2 min and, finally, exposed to X-ray film using film developer.
By comparing the signal intensities, relative expression levels of cytokines were determined and quantified by densitometry. Positive controls were used to normalize the results from the different membranes being compared.
Measurement of apoptotic sunburn cells and caspase 3-positive cells in the epidermis after a single dose of UVB—a short-term mechanistic study
Mechanistic study with an immunohistochemical method was conducted to evaluate the effects of two types of high-fat diets on UVB-induced apoptosis in the epidermis. In this study, female SKH-1 mice (6–7 weeks old, 10 mice per group) were given the HFFO diet or HFML diet for 2 or 4 weeks. The reasons for choosing these two time points are based on our previous studies where we found an enhancement of UVB-induced apoptosis in the mice pretreated with oral administered green tea or caffeine for 2 weeks (11). These mice were then treated with a single dose of UVB (30 mJ/cm2). Another two groups of mice without UVB exposure served as controls. All of the mice were killed 6 h after UVB. Our previous studies showed that 6 h after UVB is the peak time point for UVB-induced apoptosis (12).
Identification of apoptotic sunburn cells was based morphologically on cell shrinkage and nuclear condensation as we have done previously (12). Apoptotic sunburn cells were identified by their intensely eosinophilic cytoplasm and small dense nuclei, which were observed in hematoxylin- and eosin-stained histological sections of the skin using light microscopy.
Affinity-purified polyclonal rabbit antibody that reacts with the mouse p20 subunit of caspase 3 was purchased from Cell Signaling Technology(catalog no: 9661). Endogenous peroxidase was blocked with 3% hydrogen peroxide in methanol. The sections were incubated with a protein block for 1 h at room temperature. The sections were incubated with caspase 3 primary antibody (1:300 dilution) overnight at 4°C followed by incubation with a biotinylated anti-rabbit secondary antibody for 30 min, followed by incubation with conjugated avidin solution (ABC elite kit purchased from Vector Laboratory, Burlingame, CA) for 30 min. Color development was achieved by incubation with 0.02% 3,3′-diaminobenzidine tetrahydrochloride containing 0.02% hydrogen peroxide for 10 min at room temperature. The slides were then counterstained with hematoxylin and dehydrated. A positive reaction was shown as a brown precipitate in the cytoplasm and/or perinuclei of the cells (12).
The percentage of apoptotic sunburn cells and caspase 3-positive cells were calculated per 100 epidermal cells counted from the entire 20 mm length of epidermis for each skin section.
Statistical analysis
For the analyses of tumor-free distributions between the two treatments, the Kaplan–Meier method was used for estimations, and the log-rank test was used to test homogeneity of the distributions between the two treatments (13). The repeated measurement models (14) were used for the analyses of the treatment effects on body weight, tumor number per mouse and tumor volume per mouse between the two groups. To stabilize the variation, transformed responses were used in the analyses. More specifically, the square root of tumor number per mouse and cubic root tumor volume per mouse were used in the analyses. Covariates included time (week), time squared and treatment together with the interactions. The treatment effects were assessed based on the comparisons of slope (linear and quadratic trends over time) of the regression lines. Heterogenous autoregressive covariance structures were used to account for the within-mouse correlation. Different covariance structure parameters were used for different treatment groups to account for the heterogeneity of the covariance between the two treatment groups. The comparisons were also performed at the last time point (week 39). A 5% significance level was used for all the tests.
Results
Effects of high-fat diets rich in either omega-3 or omega-6 fatty acids on the formation of skin tumors
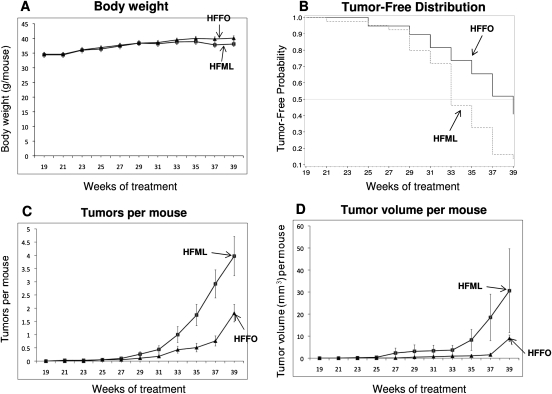
Our results showed that mice fed a high-fat diet rich in either omega-3 or omega-6 fatty acids had no significant differences in body weight over the 39 weeks of UVB treatment (P ≥ 0.2962) (Figure 1A).
Fig. 1.
Effects of high-fat diets rich in either omega-3 or omega-6 fatty acids on UVB-induced skin carcinogenesis in SKH-1 mice. Female SKH-1 mice (6–7 weeks old) were equally divided into two groups (40 mice per group). They were irradiated with 30 mJ/cm2 of UVB once a day, two times per week for 39 weeks. From the beginning of UVB treatment, one group of mice was given a high-fat diet rich in omega-3 fatty acids (HFFO) and the other group of mice was given a high-fat diet rich in omega-6 fatty acids (HFML). Body weight (A), tumor-free distribution (B), number of tumors per mouse (C) and tumor volume per mouse (D) were measured every 2 weeks. All the tumors were taken and characterized by histological examination. Each value is the mean ± SE.
Animals fed the HFFO diet had an increased latency for the development of skin tumors compared with animals fed the HFML diet. In the HFML group, mice started developing tumors during the 21st week after the start of UVB exposure, whereas animals in the HFFO group started developing tumors during the 25th week after the start of UVB exposure (Figure 1B). Thereafter, until the end of the 39th week of UVB exposure, the tumor incidence in the HFFO group was always lower than the HFML group. Kaplan–Meier estimates of the median tumor-free time for the HFML group was 33 weeks of UVB exposure with a 95% confidence interval from week 33 to week 35. The median tumor-free time for the HFFO group was 39 weeks of UVB exposure with a 95% confidence interval from week 37. The log-rank test of the homogeneity of the tumor-free distribution function showed that the difference between the two treatment groups was statistically significant (P = 0.0025) (Figure 1B).
In both the HFML and HFFO groups, the number of tumors per mouse increased with time (Figure 1C). The interaction between treatment and the squared of time was significant (P < 0.0001), indicating that the rate of increase (quadratic trend) in tumor numbers per mouse for the HFML group was significantly greater than that for the HFFO group (Figure 1C). On average, at week 39, the square root of tumor number per mouse for the HFML group was ∼2.4 times more than that for the HFFO group (Figure 1C) (P < 0.0001). These results indicate that HFFO treatment decreased tumor number per mouse.
The tumor volume per mouse increased over time in both groups (Figure 1D), as did the variation of the tumor volume per mouse. The quadratic time trend between treatments was significant (P = 0.012). On average, at week 39, the cubic root of tumor volume per mouse for the HFML group was ∼2.8 times more than that for the HFFO group (P < 0.0001) (Figure 1D). These results indicate that HFFO treatment decreased tumor size.
Histopathological examinations revealed that administration of the HFFO diet significantly decreased the percentage of mice with squamous cell papillomas, keratoacanthomas and squamous cell carcinomas by 38, 20 and 41%, respectively (Table I). The HFFO diet decreased the number of squamous cell papillomas, keratoacanthomas and squamous cell carcinomas by 64, 52 and 46%, respectively (Table II). Interestingly, the HFFO diet significantly decreased the size of non-malignant tumors and malignant tumors. The HFFO diet decreased the total volume of squamous cell papillomas and keratoacanthomas per mouse by 98 and 80%, respectively. The total volume of squamous cell carcinomas per mouse was decreased by 83% (Table III).
Table II.
Effects of high-fat diets rich in either omega-3 or omega-6 fatty acids on the incidence and multiplicity of histologically characterized skin tumors in UVB-treated SKH-1 mice
| Diets | No. of mice | Squamous cell papillomas |
Keratoacanthomas |
Squamous cell carcinomas |
Total tumors |
||||
| % of mice with tumors | Tumors per mouse | % of mice with tumors | Tumors per mouse | % of mice with tumors | Tumors per mouse | % of mice with tumors | Tumors per mouse | ||
| HFML | 37/40 | 8 | 0.14 ± 0.09 | 95 | 14.35 ± 1.76 | 51 | 1.00 ± 0.21 | 95 | 15.49 ± 1.90 |
| HFFO | 37/40 | 5 | 0.05 ± 0.04 | 76 | 6.92 ± 1.11a | 30b | 0.54 ± 0.16b | 76 | 7.51 ± 1.20a |
| Percent decrease | 38 | 64 | 20 | 52 | 41 | 46 | 20 | 52 | |
Female SKH-1 mice (6–7 weeks old) were equally divided into two groups (40 mice per group). All mice were irradiated with 30 mJ/cm2 of UVB once each day, two times per week for 39 weeks. From the beginning of UVB treatment, one group of mice was given a high-fat diet rich in polyunsaturated omega-3 fatty acids (HFFO) and the other group of mice was given a high-fat diet rich in polyunsaturated omega-6 fatty acids (HFML) as described in ‘Materials and Methods’. All tumors were characterized by histopathology studies. Each value is the mean ± SE.
P < 0.01.
P < 0.05 (statistically different from the HFML-treated group).
Table III.
Effects of high-fat diets rich in either omega-3 or omega-6 fatty acids on the size of histologically characterized skin tumors in UVB-treated SKH-1 mice
| Diets | No. of mice | Tumor volume per mouse (mm3) |
|||
| Squamous cell papillomas | Keratoacanthomas | Squamous cell carcinomas | Total tumors | ||
| HFML | 37/40 | 21.24 ± 20.57 | 26.55 ± 5.25 | 70.69 ± 30.85 | 118.49 ± 37.42 |
| HFFO | 37/40 | 0.40 ± 0.28 | 5.31 ± 1.27a | 12.26 ± 4.97b | 17.97 ± 5.38a |
| Percent decrease | 98 | 80 | 83 | 85 | |
Female SKH-1 mice (6–7 weeks old) were equally divided into two groups (40 mice per group). All mice were irradiated with 30 mJ/cm2 of UVB once each day, two times per week for 39 weeks. From the beginning of UVB treatment, one group of mice was given a high-fat diet rich in polyunsaturated omega-3 fatty acids (HFFO) and the other group of mice was given a high-fat diet rich in polyunsaturated omega-6 fatty acids (HFML) as described in ‘Materials and Methods’. All tumors were characterized by histopathology studies and the size of each tumor was determined. Each value is the mean ± SE.
P < 0.01.
P < 0.05 (statistically different from the HFML-treated group).
There was no effect on the weight of the parametrial fat pads and the thickness of the dermal fat layer between mice fed the HFFO or HFML diets (data not shown). However, the mice in the HFFO group had 22% lower blood levels of triglycerides than animals in the HFML group (P < 0.01), which are consistent with others (15,16).
Effects of high-fat diets rich in either omega-3 or omega-6 fatty acids on the levels of inflammatory cytokines
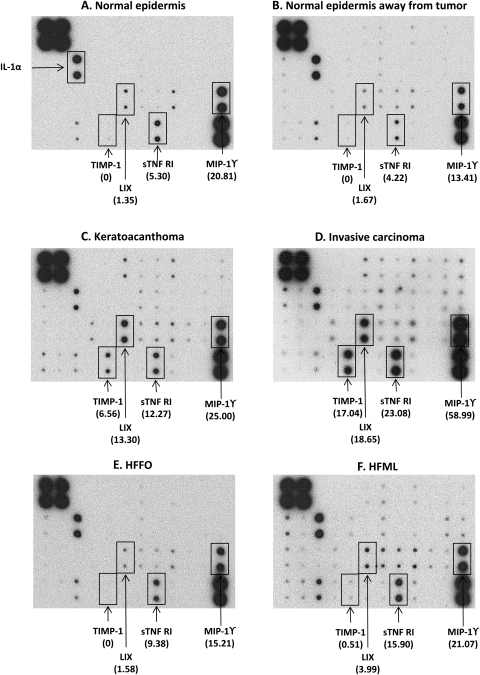
An antibody array was first conducted to compare the levels of inflammatory cytokines in normal epidermis (without UVB exposure), normal epidermis at least 0.5 cm away from the tumors, keratoacanthomas and invasive squamous cell carcinomas between the HFFO and HFML groups. The results revealed that there was no obvious difference in the cytokine levels in tumors between these two diets (data not shown). However, the inflammatory cytokine levels changed significantly with the tumor type. The representative blots are present in Figure 2. In the normal epidermis (Figure 2A) or in the normal epidermis away from the tumors (Figure 2B), only a small number of cytokines was observed, and most of these dots were lightly stained except interleukin-1α (IL-1α), macrophage inflammatory protein-1γ (MIP-1γ) and soluble tumor necrosis factor-alpha receptor 1 (sTNF RI). The number and levels of inflammatory cytokines were significantly increased in keratoacanthomas (Figure 2C) and reached the highest level in invasive carcinomas (Figure 2D). Among 40 cytokines examined, three cytokines were strikingly changed. They were: tissue inhibitor of metalloproteinases-1 (TIMP-1), lipopolysaccharide-induced CXC chemokine (LIX) and sTNF RI.
Fig. 2.
Effects of high-fat diets rich in either omega-3 or omega-6 fatty acids on the levels of inflammatory cytokines in the epidermis. Normal epidermis (A), normal epidermis away from tumors (B), keratoacanthomas (C) and invasive squamous cell carcinomas (D) were taken from mice on the HFFO diet treated with UVB for 39 weeks. The epidermis of HFFO (E) or HFML (F) were from the mice (five mice per group) treated with UVB (30 mJ/cm2 once a day, two times per week) for 20 weeks. Tumor tissue and epidermal lysates were prepared and subjected to antibody array as described in ‘Materials and Methods’. Each value in parenthesis is the average intensity of two parallel signals quantified by densitometry. Positive controls were used to normalize the results from different membranes being compared.
There was an undetectable level of TIMP-1 in the normal epidermis and in the normal epidermis away from the tumors, whereas a high level of TIMP-1 and a very high level of TIMP-1 were observed in keratoacanthoma and carcinoma. The level of TIMP-1 in carcinoma was 160% higher than that in keratoacanthoma. In addition to TIMP-1, the levels of LIX and sTNF RI were also significantly increased in tumors. The LIX and sTNF R1 in keratoacanthomas were increased by 885 and 132%, and the LIX and sTNF R1 in carcinomas were increased by 1281 and 335%, respectively, when compared with the normal epidermis. Interestingly, a high level of MIP-1γ was observed in carcinomas and not in keratoacanthomas. Besides these four cytokines, many other cytokines were lightly detected in tumors. Nearby, half of these were also found in the normal epidermis away from tumors in the mice treated with chronic UVB for 39 weeks, but most of them were not detected in the normal epidermis without UVB exposure. These results suggest that TIMP-1, LIX and sTNF RI may play an important role in UVB-induced skin tumor formation and MIP-1γ may be involved in tumor progression.
In a separate study, the same antibody array was employed to explore the early effects of two types of high-fat diets in the epidermis of mice treated with UVB for 10 or 20 weeks (before tumor formation). Our results indicated that there was a small difference in cytokine detection between the two groups after 10 weeks of UVB treatment (data not shown). After 20 weeks, HFFO-treated mice (Figure 2E) continued to remain low levels of inflammatory cytokines. As compared with HFML-treated mice (Figure 2F), the level of LIX in HFFO-treated mice was lower by 60%, sTNF RI by 41% and MIP-1γ by 28%, respectively. TIMP-1 was not detected in the epidermis of HFFO-treated mice, whereas a pair of faint dots of TIMP-1 was observed in HFML-treated mice. In addition, 18 other cytokines, such as CD30L, Eotaxin-2, GM-CSF, IFN-γ, IL-4, IL-12p40p70, IL-12p70, IL-13, IL-17, lymphotactin, MCP-1, M-CSF, MIG, MIP-1α, RANTES, SDF-1, trichloroacetic acid-3 and sTNF RII, were lightly detected in HFML-treated epidermis, but only six of them were seen in HFFO-treated mice.
Effects of high-fat diets rich in either omega-3 or omega-6 fatty acids on epidermal apoptosis in mice treated with a single dose of UVB
An immunohistochemical method was conducted to evaluate the effects of two types of high-fat diets on UVB-induced apoptosis in the epidermis. Our results indicated that mice were given the HFFO diet for 4 weeks and then treated with a single dose of UVB (30 mJ/cm2) enhanced the UVB-induced increase in apoptotic sunburn cells by 96% (P < 0.01) and caspase 3 (active form)-positive cells by 116% (P < 0.01) compared with the HFML diet. HFFO-treated mice had no effects on the number of apoptotic sunburn cells or caspase 3-positive cells in non-UVB-treated mouse epidermis (Table IV). Mice given the HFFO diet for 2 weeks also exhibited an enhanced increase in UVB-induced apoptotic sunburn cells, and the effect was smaller than that for 4 weeks (data not shown).
Table IV.
Effects of high-fat diets rich in either omega-3 (HFFO) or omega-6 fatty acids (HFML) on UVB-induced apoptosis in the epidermis of SKH-1 mice
| Diet | No. of mice per group | No-UVB treatment |
UVB treatment |
||
| Percent apoptotic sunburn cell | Percent caspase 3-positive cell | Percent apoptotic sunburn cell | Percent caspase 3-positive cell | ||
| HFML | 10 | 0.015 ± 0.004 | 0.005 ± 0.001 | 0.289 ± 0.095 | 0.311 ± 0.115 |
| HFFO | 10 | 0.020 ± 0.008 | 0.006 ± 0.002 | 0.566 ± 0.038a | 0.671 ± 0.048 a |
| Percent increase | 33 | 20 | 96 | 116 | |
Female SKH-1 mice (6–7 weeks old, 10 mice per group) were given a high-fat diet rich in polyunsaturated omega-3 fatty acids (HFFO) or high-fat diet rich in polyunsaturated omega-6 fatty acids (HFML) for 4 weeks. They were then treated with a single dose of UVB (30 mJ/cm2). Another two groups of mice without UVB exposure served as controls. All mice were killed at 6 h after UVB. Percent apoptotic sunburn cells and percent caspase 3 (active form)-positive cells in the epidermis were determined as described in ‘Materials and Methods’. Each value is the mean ± SE.
P < 0.01 (statistically different from the HFML-treated group).
Discussion
In the present study, we report that mice given a high-fat diet rich in omega-3 fatty acid (HFFO) during UVB exposure had decreased tumor incidence, decreased the number of tumors per mouse and decreased tumor volume per mouse when compared with mice given the same amount of calories, protein, vitamins, minerals and fiber in a high-fat diet rich in omega-6 fatty acid (HFML). As we expected, there were no differences in body weight between these two groups of mice from the beginning to the end of the studies and there was also no effect on the weight of the parametrial fat pads and the thickness of the dermal fat layer between these two groups. These results suggest that in addition to a high amount of tissue fat, tissue fat composition may play an important role in UVB-induced skin carcinogenesis. Although our present study did not support this hypothesis, research data reported that dietary omega-3 and omega-6 fatty acid sources result in marked differences in epidermal cell membrane composition (17). In future studies, we will analyze the tissue fat composition in the animals fed these two different high-fat diets. We will determine the effects of oral administration of caffeine or voluntary exercise on UVB-induced skin carcinogenesis in animals fed the HFFO diet. We expect these combination treatments would further modulate tissue fat content and synergistically decrease the tumor formation.
The first indication that dietary fat could influence ultraviolet radiation-induced skin carcinogenesis was demonstrated by Baumann and Rusch early in 1939 when they observed that animals fed high levels of fat formed UV-induced tumors more rapidly than animals fed low-fat diets (18). Since then, a series of studies conducted mainly by Black et al. (5) were begun. They observed that the degree of saturation of dietary lipids markedly influenced the UV-carcinogenic response (19). This group and others reported an approximate linear relationship between omega-6 fatty acids intake and UV-carcinogenic expression (19–21). Black et al. (22) found that diets containing low-fat levels of 4% menhaden oil resulted in significantly longer tumor latent periods and lower tumor multiplicities when compared with 4% corn oil-fed animals. Our present studies with two types of high-fat diets containing 20% mixed lipids rich in omega-6 fatty acids or 10% mixed lipids plus 10% menhaden oil rich in omega-3 fatty acids provide additional data for a relationship between omega-3 or omega-6 fatty acid consumption and skin carcinogenesis in animals.
Although it has been reported that dietary omega-3 fatty acids show promise as photoprotective agents, the mechanism, especially in animal models, is not fully understood. A number of studies have focused on the effects of dietary omega-3 fatty acids to reduce the inflammatory response when compared with an equivalent dietary level of omega-6 fatty acids. It was reported that dietary omega-3 fatty acid content has a pronounced effect on prostaglandin (PGE2) levels. After 2 weeks on the respective diets, plasma PGE2 levels of 4 or 12% corn oil-fed animals were ∼6-fold greater than those of 4 or 12% menhaden oil-fed groups (23). In the animals fed 12% menhaden oil, both the UV-induced ornithine decarboxylase activity and the inflammatory response (erythema and edema) were dramatically inhibited when compared with a 12% corn oil diet (23). A similar response was found in the dermis (23). However, little information has been known about the effects of dietary omega-3 fatty acids to modulate the expressions of inflammatory cytokines by protein array. Our results demonstrate that after 20 weeks of UVB treatment, the HFFO diet significantly decreased the UVB-induced increases in the numbers and levels of inflammatory cytokines compared with HFML diet, especially decreased the levels of TIMP-1, LIX, sTNF RI and MIP-1γ in the epidermis. Our in vivo study showed that high levels of TIMP-1, LIX, sTNF RI and MIP-1γ were found in tumors but not in the normal epidermis or in the normal epidermis away from the tumors. These facts suggest that these cytokines may be related to UVB-induced skin tumor formation. The present results suggest that omega-3 fatty acids present in HFFO diet may protect against UVB-induced skin carcinogenesis by decreasing proinflammatory cytokines production. It should be excluded that other ingredients in this diet may also play a role. These findings provide biomarker candidates for future mechanistic studies with these diets. We plan to confirm these findings by western blot analysis and immunohistochemical methods with the appropriate antibodies.
It was reported that TIMP-1, LIX, sTNF RI and MIP-1γ are all proinflammatory cytokines. The TIMP-1 protein is able to promote cell proliferation in a wide range of cell types and may have an antiapoptotic function. TIMP-1 plays a significant role in regulation of extracellular matrix remodeling and angiogenesis in cancers (24,25). TIMP-1 concentrations were significantly increased in plasma obtained from patients with colon or rectal cancer (26). TIMP-1 and TIMP-2 may play an important role in the pathogenesis of non-melanoma skin cancer and their expression levels are useful indicators of cutaneous cancer invasion and progression (27). LIX has potent chemoattractant activity for neutrophils in vitro and in vivo and amplifies a proinflammatory cytokine response via a phosphatidylinositol 3-kinase–nuclear factor-kappaB pathway (28). Tumor necrosis factor -α is one of the most potent proinflammatory cytokines produced by activated macrophages in response to tissue injury or chronic inflammation. Its production leads to the shedding of soluble tumor necrosis factor receptors (sTNF RI and sTNF RII) from cell membranes into the circulation. Through its proinflammatory actions, TNF-α may play a role in cancer growth and metastasis by inducing reactive oxygen species, which can cause DNA damage and inhibit DNA repair (29). An increase in the serum level of sTNF R1 was significantly associated with a higher risk of endometrial cancer and the severity of development of multiple inflammatory-related symptoms in patients for colorectal and esophageal cancer (29). MIP-1γ activates human granulocytes and lead to acute neutrophilic inflammation. MIP-1γ also induces the synthesis and release of other proinflammatory cytokines such as IL-1, IL-6 and TNF-α from fibroblast and macrophages. Our present studies provided evidences suggesting that TIMP-1, LIX, sTNF RI and MIP-1γ play an important role in UVB-induced skin carcinogenesis.
Although it has been demonstrated that dietary omega-3 fatty acids reduced an inflammatory response, there is little information about the effects of high-fat diets rich in omega-3 or omega-6 fatty acids on UVB-induced apoptosis in the epidermis of mice. Our previous studies showed that the mechanism of caffeine or voluntary exercise to inhibit UVB-induced skin carcinogenesis may be caused by stimulation of UVB-induced epidermal apoptosis. Our present study reported that mice fed HFFO diet rich in omega-3 fatty acid for 4 weeks also stimulated UVB-induced increases in apoptosis in the epidermis of mice compared with the HFML diet rich in omega-6 fatty acid. It is probably that these effects may contribute, at least in part, to the inhibitory effect of HFFO diet on UVB-induced carcinogenesis. The results of our studies are important since the relationship between skin carcinogenesis and increased apoptosis by HFFO diet has not been studied previously. In many situations, omega-3 fatty acids act as competitive antagonists for omega-6 fatty acids. Omega-3 fatty acids reportedly modulate oxidative stress and the highly unsaturated omega-3 fatty acids may become targets for free radical attack, resulting in the production of oxidation products and protection against UVB-induced oxidative injury (30,31). In addition, dietary omega-3 and omega-6 fatty acid sources result in marked differences in epidermal cells membrane composition (17). These altered membrane composition might be a possible mechanism through which dietary omega-3 fatty acids increases UVB-induced apoptosis in the epidermis. Furthermore, mechanistic studies are needed to confirm these hypotheses.
In summary, our results suggest that a HFFO diet rich in omega-3 fatty acids inhibits UVB-induced skin carcinogenesis in mice continually exposed to UVB. The HFFO diet decreased tumor incidence, decreased tumor number and decreased tumor size compared with animals fed an HFML premix diet rich in omega-6 fatty acids. The HFFO diet decreased the UVB-induced increases in the levels of TIMP-1, LIX and sTNF R1 as well as other several proinflammatory cytokines and stimulated UVB-induced apoptosis in the epidermis. Our results indicate that omega-3 fatty acids in an HFFO diet have beneficial effects against UVB-induced skin carcinogenesis in mice and these effects may be associated with an inhibition on UVB-induced inflammatory response.
Funding
National Cancer Institute (RO1CA128997).
Acknowledgments
The content is solely the responsibility of the authors and does not necessary represent the official views of the National Cancer Institute or the National Institutes of Health.
Conflict of Interest Statement: None declared.
Glossary
Abbreviations
- HFFO
high-fat fish oil
- HFML
high-fat mixed-lipid
- MIP-1γ
macrophage inflammatory protein-1γ
- TIMP-1
tissue inhibitor of metalloproteinases-1
- sTNF RI
soluble tumor necrosis factor-alpha receptor 1
- UV
ultraviolet light
References
- 1.Lu Y-P, et al. Inhibitory effects of orally administered green tea, black tea, and caffeine on skin carcinogenesis in mice previously treated with ultraviolet B light (high-risk mice): relationship to decreased tissue fat. Cancer Res. 2001;61:5002–5009. [PubMed] [Google Scholar]
- 2.Michna L, et al. Inhibitory effects of voluntary running wheel exercise on UVB-induced skin carcinogenesis in SKH-1 mice. Carcinogenesis. 2006;27:2108–2115. doi: 10.1093/carcin/bgl057. [DOI] [PubMed] [Google Scholar]
- 3.Lu YP, et al. Stimulatory effect of voluntary exercise or fat removal (partial lipectomy) on apoptosis in the skin of UVB light-irradiated mice. Proc. Natl Acad. Sci. USA. 2006;103:16301–16306. doi: 10.1073/pnas.0607789103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reddy BS, et al. Prevention of colon cancer by low doses of celecoxib, a cyclooxygenase inhibitor, administered in diet rich in omega-3 polyunsaturated fatty acids. Cancer Res. 2005;65:8022–8027. doi: 10.1158/0008-5472.CAN-05-0212. [DOI] [PubMed] [Google Scholar]
- 5.Black HS, et al. The potential of omega-3 fatty acids in the prevention of non-melanoma skin cancer. Cancer Detect. Prev. 2006;30:224–232. doi: 10.1016/j.cdp.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 6.Mantovani A, et al. Molecular pathways and targets in cancer-related inflammation. Ann. Med. 2010;42:161–170. doi: 10.3109/07853890903405753. [DOI] [PubMed] [Google Scholar]
- 7.Mantovani A, et al. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 8.Lou YR, et al. Effects of oral administration of tea, decaffeinated tea, and caffeine on the formation and growth of tumors in high-risk SKH-1 mice previously treated with ultraviolet B light. Nutr. Cancer. 1999;33:146–153. doi: 10.1207/S15327914NC330205. [DOI] [PubMed] [Google Scholar]
- 9.Lu YP, et al. Administration of green tea or caffeine enhances the disappearance of UVB-induced patches of mutant p53 positive epidermal cells in SKH-1 mice. Carcinogenesis. 2005;26:1465–1472. doi: 10.1093/carcin/bgi086. [DOI] [PubMed] [Google Scholar]
- 10.Lu YP, et al. Effect of caffeine on the ATR/Chk1 pathway in the epidermis of UVB-irradiated mice. Cancer Res. 2008;68:2523–2529. doi: 10.1158/0008-5472.CAN-07-5955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lu YP, et al. Stimulatory effect of oral administration of green tea or caffeine on ultraviolet light-induced increases in epidermal wild-type p53, p21(WAF1/CIP1), and apoptotic sunburn cells in SKH-1 mice. Cancer Res. 2000;60:4785–4791. [PubMed] [Google Scholar]
- 12.Lu YP, et al. Stimulatory effect of topical application of caffeine on UVB-induced apoptosis in mouse skin. Oncol. Res. 2002;13:61–70. [PubMed] [Google Scholar]
- 13.Lawless JF. Statistical Models and Methods for Lifetime Data. New York, NY: John Wiley & Sons, Inc.; 1982. [Google Scholar]
- 14.Lindey JK. Models for Repeated Measurements. Oxford, UK: Clarendon Press; 1993. [Google Scholar]
- 15.Qi K, et al. Omega-3 fatty acid containing diets decrease plasma triglyceride concentrations in mice by reducing endogenous triglyceride synthesis and enhancing the blood clearance of triglyceride-rich particles. Clin. Nutr. 2008;27:424–430. doi: 10.1016/j.clnu.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 16.Leaf DA, et al. The effect of lean fish consumption on triglyceride levels. Phys. Sportsmed. 2009;37:37–43. doi: 10.3810/psm.2009.04.1681. [DOI] [PubMed] [Google Scholar]
- 17.Fischer MA, et al. Modification of membrane composition, eicosanoid metabolism, and immunoresponsiveness by dietary omega-3 and omega-6 fatty acid sources, modulators of ultraviolet-carcinogenesis. Photochem. Photobiol. 1991;54:381–387. doi: 10.1111/j.1751-1097.1991.tb02031.x. [DOI] [PubMed] [Google Scholar]
- 18.Baumann CA, et al. Effect of diet on tumors induced by ultraviolet light. Am. J. Cancer. 1939;35:213–221. [Google Scholar]
- 19.Black HS, et al. Influence of dietary lipid upon ultraviolet-light carcinogenesis. Nutr. Cancer. 1983;5:59–68. doi: 10.1080/01635588309513780. [DOI] [PubMed] [Google Scholar]
- 20.Black HS, et al. Relation of antioxidants and level of dietary lipid to epidermal lipid peroxidation and ultraviolet carcinogenesis. Cancer Res. 1985;45:6254–6259. [PubMed] [Google Scholar]
- 21.Reeve VE, et al. Effect of dietary lipid on UV light carcinogenesis in the hairless mouse. Photochem. Photobiol. 1988;48:689–696. doi: 10.1111/j.1751-1097.1988.tb02882.x. [DOI] [PubMed] [Google Scholar]
- 22.Orengo IF, et al. Influence of dietary menhaden oil upon carcinogenesis and various cutaneous responses to ultraviolet radiation. Photochem. Photobiol. 1989;49:71–77. doi: 10.1111/j.1751-1097.1989.tb04080.x. [DOI] [PubMed] [Google Scholar]
- 23.Henderson CD, et al. Influence of omega-3 and omega-6 fatty acid sources on prostaglandin levels in mice. Lipids. 1989;24:502–505. doi: 10.1007/BF02535129. [DOI] [PubMed] [Google Scholar]
- 24.Chirco R, et al. Novel functions of TIMPs in cell signaling. Cancer Metastasis Rev. 2006;25:99–113. doi: 10.1007/s10555-006-7893-x. [DOI] [PubMed] [Google Scholar]
- 25.Stamenkovic I. Extracellular matrix remodelling: the role of matrix metalloproteinases. J. Pathol. 2003;200:448–464. doi: 10.1002/path.1400. [DOI] [PubMed] [Google Scholar]
- 26.Holten-Andersen MN, et al. High preoperative plasma tissue inhibitor of metalloproteinase-1 levels are associated with short survival of patients with colorectal cancer. Clin. Cancer Res. 2000;6:4292–4299. [PubMed] [Google Scholar]
- 27.O’Grady A, et al. Differential expression of matrix metalloproteinase (MMP)-2, MMP-9 and tissue inhibitor of metalloproteinase (TIMP)-1 and TIMP-2 in non-melanoma skin cancer: implications for tumour progression. Histopathology. 2007;51:793–804. doi: 10.1111/j.1365-2559.2007.02885.x. [DOI] [PubMed] [Google Scholar]
- 28.Chandrasekar B, et al. Ischemia-reperfusion of rat myocardium activates nuclear factor-KappaB and induces neutrophil infiltration via lipopolysaccharide-induced CXC chemokine. Circulation. 2001;103:2296–2302. doi: 10.1161/01.cir.103.18.2296. [DOI] [PubMed] [Google Scholar]
- 29.Balkwill F. Tumor necrosis factor or tumor promoting factor? Cytokine Growth Factor Rev. 2002;13:135–141. doi: 10.1016/s1359-6101(01)00020-x. [DOI] [PubMed] [Google Scholar]
- 30.Rhodes LE, et al. Dietary fish-oil supplementation in humans reduces UVB-erythemal sensitivity but increases epidermal lipid peroxidation. J. Invest. Dermatol. 1994;103:151–154. doi: 10.1111/1523-1747.ep12392604. [DOI] [PubMed] [Google Scholar]
- 31.Liu G, et al. Omega 3 but not omega 6 fatty acids inhibit AP-1 activity and cell transformation in JB6 cells. Proc. Natl Acad. Sci. USA. 2001;98:7510–7515. doi: 10.1073/pnas.131195198. [DOI] [PMC free article] [PubMed] [Google Scholar]