Abstract
During certain months of the year, viral respiratory infections lead to a dramatic increase in pediatric emergency room visits and hospital admissions. Rapid identification of the infectious organism results in timely treatment and reductions in hospital cost and length of stay. Before the introduction of molecular testing to the virology laboratory, diagnosis relied on the standard methods of immunofluorescence and culture. These tests can be labor-intensive and costly. Recent studies have demonstrated the higher sensitivity, faster turnaround, and broader diagnostic spectrum provided by multiplexed RT-PCR assays. Data comparing the laboratory cost and labor efficiency of the tests are lacking. To address this issue, we chose to implement the principles of operational workflow analysis using lean methodology to critically evaluate the potential advantages of a multiplexed RT-PCR assay both in terms of workflow and cost effectiveness. Our results indicated that the implementation of the Luminex xTAG Respiratory Viral Panel (RVP) resulted in a standardized workflow with decreased requirements in laboratory cost as well as improvement in efficiency. In summary, we demonstrate that, in our laboratory, the Luminex xTAG RVP is more operationally streamlined and cost-effective than standard viral direct fluorescent antibody and culture. Further studies are needed to highlight additional benefits of the test, including shortened hospital stay and improved patient outcome.
In today's environment of increased demand and decreased supply of medical technologists, hospital laboratories are scrambling to find ways to reduce labor time and cost while maintaining and improving the quality of diagnostic medicine. As a result, a wide variety of laboratory management strategies to reduce cost while improving quality and efficiency have been proposed. One of the most successful strategies is that of lean methodology, a set of production principles developed and implemented by the Toyota company. In an attempt to simply define the Lean worldview, James P. Womack and Daniel T. Jones1 defined five basic principles of Lean thinking in 1996:
-
1
Clear definition of the customer's perception of product value;
-
2
Identification of the components in production that add to product value with elimination of all other non-valuable (“waste”) components;
-
3
Streamlining of the sequence of the remaining steps to allow for a smoother work flow;
-
4
Building a system that is driven by the pull of the customer's requirements rather than the push of the manufacturer;
-
5
Pursuit of perfection through continuous re-evaluation and improvement.
Since their conception, Lean principles have been applied successfully to various industries and processes, increasingly within the health care community.2–5 Although hindered by challenges including staunch adherence to traditional laboratory practices, complexity of workflow, and marked variability in sample numbers, hospital laboratories can still adapt the basic Lean principles to maximize productivity, reduce cost, and ensure quality results. The processes involved in creating a Lean laboratory environment vary greatly due to individual laboratory differences in testing menus and sample numbers, but the basic tools remain the same and include observation and documentation of the current environment and workflow, identification of waste in both materials and activity, redesign of the process to best facilitate a continuous flow of work that involves the least waste, implementation of the new design, and, finally, ongoing re-assessment and improvement.
The xTAG Respiratory Viral Panel (RVP) (Luminex Corporation, Austin, TX), which includes the use of real-time RT-PCR methodology, has the potential to improve detection rates, increase laboratory efficiency, and reduce the length and cost of hospital stay due to respiratory infections.6,7 The first RVP approved by the US Food and Drug Administration (FDA), the Luminex xTAG RVP system has been shown to improve viral detection compared to more traditional methods including direct fluorescent antibody (DFA) and culture methodology.6,8 The platform uses multiplex RT-PCR to detect and amplify 12 viral targets that are then identified using xMAP Technology (Luminex Corporation).9 Some centers report a turnaround time (TAT) of 3 hours,7 although batch testing and individual laboratory cut-off times result in TAT variation. TAT at our institution, from time of receipt to time of result, ranges from 22 to 47 hours. If a specimen is received in our laboratory by 1 PM (the official cut-off time), processing will begin that same day and results will be finalized the following day between 11 AM and noon (total TAT of about 22 to 23 hours). However, if a specimen comes in after the stated cut-off time, it will be processed at 1 PM the following day and finalized the subsequent day between 11 AM and noon, thus resulting in a TAT of 46 to 47 hours.
Although others have demonstrated the improved sensitivity and broader diagnostic coverage of the Luminex xTAG RVP,6 comparison of its operational efficiency and impact on laboratory workflow against standard DFA and culture has not been studied. These factors play a role in the decision to integrate new technology to the clinical laboratory; thus, the two-fold aim of our study was to implement Lean analysis techniques to evaluate the operational efficiency of the Luminex xTAG RVP and to assess the cost-effectiveness of the test compared with the standard methods of DFA and culture.
Materials and Methods
Study Population
The Children's Medical Center of Dallas (CMC) is a free-standing pediatric hospital with almost 500 inpatient beds and one of the busiest pediatric emergency departments in the United States, with more than 100,000 visits each year. The virology laboratory at CMC performs viral testing on approximately 7000 respiratory samples annually. The process complexity and TAT for these tests varies, ranging from the point-of-care direct antigen test to the DFA assay with its TAT of 2 hours and the more complex set-up and analysis of tube cultures with their maximum TAT of 10 days. The latter method is labor intensive–large testing volumes during peak respiratory viral season, therefore, can considerably impact the virology laboratory's workflow and personnel requirements. The following study was performed as part of a broader evaluation of the Luminex xTAG RVP, approved by the University of Texas Southwestern Medical Center Institutional Review Board.
Materials
From January to March, nasopharyngeal samples for detection of respiratory viruses in pediatric patients seen at CMC were collected on one sterile flexible flocked swab, placed in M4 MicroTest Transport Media (Remel, Lenexa, KS) and sent within 30 minutes to the microbiology laboratory. Rapid antigen tests for influenza A and B and respiratory syncytial virus (Meridian Biosciences, Inc., Cincinnati, OH) were performed as requested. Positive samples were not processed further and were excluded from the study. Negative samples were reflexed for both DFA (Diagnostic Hybrids, Inc., Athens, OH) and Luminex xTAG RVP testing.
Two-well screening cytospin slides were prepared and incubated with DFA screening reagent, which includes a mixture of antibodies directed against influenza A and B; respiratory syncytial virus; adenovirus and parainfluenza viruses 1, 2, and 3 (Diagnostic Hybrids, Inc.). If necessary, 8-well cytospin slides were subsequently prepared and hybridized with monoclonal reagents directed against the same viruses (Diagnostic Hybrids, Inc.). If the DFA was positive, culture was not performed; if the DFA was negative, shell vial and tube cultures (Diagnostic Hybrids, Inc.) were both performed according to standard laboratory procedures. Shell vials were monitored at 24 and 48 hours with fluorescent antibody stains performed on the cells growing on the coverslip in the bottom of each vial. Positive cultures, as evidenced by viral cytopathic effects, were subjected to virus-specific DFA to confirm the infecting pathogen. Shell vials and tube cultures were held for 3 and 10 days, respectively, before negative results were reported (Figure 1).
Figure 1.
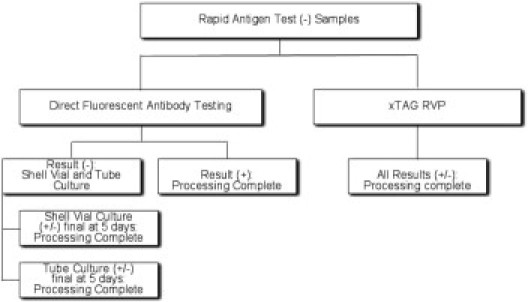
Respiratory viral testing procedure. Workflow of respiratory viral testing samples with negative rapid antigen results.
Samples for the Luminex xTAG RVP were extracted using the easyMAG automated extractor (BioMerieux, Durham, NC). The extracted nucleic acid was then reverse transcribed in an overnight RT-PCR reaction. The next morning, the amplicons were treated with shrimp alkaline phosphatase (SAP) and exonuclease I to degrade remaining nucleotides and primers. This step was followed by target specific primer extension (TSPE). Viral amplicons present in the sample bind to primers and are elongated using biotinylated deoxycytidine triphosphate. The virus-specific primers contain unique “tag” identifier sequences that correspond to specific complimentary “antitags” attached to Luminex beads. Thus, TSPE resulted in products that contained both biotin markers and virus-specific tags. After TSPE, the amplicons were incubated with Luminex beads, allowing hybridization between primer tags and bead antitags. Because each bead is spectrally distinct, its type can be detected by the platform. Hybridization was followed by the addition of the reporter, streptavidin phycoerythrin and then placed on the Luminex xMAP, which reads the amount of fluorescence emitted by each bead and interprets it using the Luminex xTAG RVP software.
Lean Analysis
To evaluate the efficiency of each method, Lean operational analysis was performed between April and May. Although Lean analysis is most easily applied to a single-piece workflow, assumptions can be made to adapt the analysis techniques to the multipart workflow that is used in both the classic and molecular viral testing procedures. Operational analysis began with videotaping of each procedure performed during testing including DFA 2- and 8-well slide preparation and reading, tube culture and shell vial inoculation, shell vial and tube culture reading, culture media change, acid lability testing of rhinovirus-positive cultures, easyMAG extraction, and RVP amplification and detection. The operational analysis was performed using batch sizes comparable to the average number of samples seen in the virology laboratory during the videotaping time period. For example, DFA samples were processed in batch sizes of 14, whereas samples for RVP, which can accommodate a greater number of samples per technologist, were processed in batch sizes of 60. Points of interest captured during the videotaping of each procedure included total hands on time and the total number of operator steps necessary to complete the process. Total hands-on time was defined as the amount of time spent by the technologist to process the sample and did not include time involved in activities such as sample incubation or centrifugation. The total number of operator steps was determined by using detailed lists of each individual action performed by the operator during the process. These steps include activities such as loading tubes, adding reagents, and pipetting. To determine the hands-on time and operator steps for each sample, the totals for each were divided by batch size.
Once the operational analysis was complete, the results were applied to the study population. After initial DFA, the subsequent sample workflow varies widely in terms of operator steps and hands-on time. If a DFA is positive, culture is not performed. If negative, the specimen is automatically reflexed to culture. To facilitate comparison with Luminex xTAG RVP workflow, the hands-on time required for each sample was calculated by reviewing the historical data for each sample, determining the exact testing performed on each and calculating the required hands-on time and operator steps. All individual results were then totaled to yield an overall result for the DFA/culture study arm.
Cost Analysis
A cost analysis was performed between the two arms of the study. All hospital and laboratory costs associated with testing including reagents, supplies, technician time, and laboratory overhead were calculated for each test. The results were then applied to the study population to determine the cost associated with a single sample. Cost of the xMAP platform was incorporated into the cost of the RVP and spread across the total annual test volume for that platform. The instrument was depreciated over 2 years and the cost of a 1-year service agreement was also included in the overall cost of the RVP. Due to the variability in costs associated with DFA and culture results, we calculated the costs of a DFA-positive sample, a DFA-negative sample with culture results requiring the least amount of technician hands-on time, and a DFA-negative sample with culture results requiring the greatest technician hand-on time. These calculations provided a range of possible costs incurred by samples processed by standard testing.
Results
Clinical Results
During the study period, 1032 specimens submitted to the virology laboratory were negative for rapid antigen testing and were submitted for follow-up DFA/culture and Luminex xTAG RVP. Of these, 233 were positive on screening DFA. The remaining 799 were submitted for both shell vial and tube viral culture. Seventeen of these samples were eliminated because culture results yielded pathogens not included in the RVP testing assay. These include cytomegalovirus and herpes simplex virus. Of the remaining samples, respiratory viruses were identified in 29 by shell vial only, 46 by tube culture only, 15 by both methods.
Operational Analysis Results
Operational data obtained for each component of the procedures are shown in Table 1. These data were then used to calculate the operator steps and hands-on time for each individual sample (Table 2). All samples subjected to RVP required 52 operator steps, including 35 extraction steps and 17 amplification/detection steps. The calculated hands-on time for extraction and amplification/detection were 2.43 and 2.31 minutes per sample, respectively. Every DFA-positive sample required 34 operator steps and 4.26 minutes of hands-on time. DFA-negative samples ranged from 191 steps and 16:58 minutes for an influenza B-positive shell vial culture to 383 steps and 32:11 minutes for a rhinovirus-positive culture that showed results in 10 days. Combining the individual scores for each of the 1015 samples revealed that the Luminex xTAG RVP required a total of 52,780 steps and 80 hours, compared with 342,775 steps and 503 hours for DFA and culture (Table 3).
Table 1.
Operational Analysis of RVP, DFA, and Culture Procedures
| Component | Batch size | Hands-on time/batch (minutes) | Hands-on time/sample (minutes) | Operator steps/batch | Operator steps/sample |
|---|---|---|---|---|---|
| EasyMAG extraction | 8 | 19.44 | 2.43 | 280 | 35 |
| RVP amplification and detection | 60 | 138.6 | 2.31 | 1020 | 17 |
| DFA 2-well slide | 14 | 59.64 | 4.26 | 476 | 34 |
| DFA 8-well slide | 3 | 24.33 | 8.11 | 129 | 43 |
| Culture inoculation | 7 | 14.14 | 2.02 | 147 | 21 |
| Shell vial reading | 30 | 30.00 | 1.00 | 570 | 19 |
| Tube culture reading | 25 | 5.75 | 0.23 | 100 | 4 |
| Culture media change | 25 | 17.25 | 0.69 | 225 | 9 |
| Culture acid lability testing | 3 | 20.97 | 6.99 | 210 | 70 |
Table 2.
Operational Analysis Applied to Study Population
| n | Per sample (minutes) | Total (hours) | Per sample | Total | |
|---|---|---|---|---|---|
| RVP (+/−) | 1015 | 4.74 | 80.2 | 52 | 52,780 |
| DFA (+) | 234 | 12.4 | 48.4 | 77 | 18,018 |
| DFA (−)/culture (+) | |||||
| Rhinovirus | 28 | 33.6 | 15.7 | 427 | 11,969 |
| Influenza A | 16 | 33.9 | 9.0 | 396 | 6333 |
| Influenza B | 2 | 33.9 | 1.1 | 397 | 794 |
| Parainfluenza 3 | 6 | 34.9 | 3.5 | 416 | 2496 |
| Adenovirus | 17 | 33.3 | 9.4 | 389 | 6618 |
| RSV | 3 | 34.1 | 1.7 | 402 | 1206 |
| Parainfluenza 2 | 1 | 33.9 | 0.6 | 397 | 397 |
| DFA (−)/culture (−) | 709 | 35.0 | 413.6 | 416 | 294,944 |
Table 3.
Cumulative Operational Analysis Results Traditional versus Molecular Methodology
| Component | Hands-on time (hours) | Operator steps |
|---|---|---|
| RVP | 80 | 52,780 |
| Traditional | 503 | 342,775 |
Cost Analysis Results
Calculation of testing costs and subsequent analysis (Table 4) established that the lowest costs were required by DFA-positive samples ($99.75 per sample), whereas the greatest costs were incurred by all DFA-negative samples, whether they were associated with the shortest time to culture ($329.68 per sample) or the longest time to culture ($429.07 per sample.) The cost per sample of the Luminex xTAG RVP was $135.03.
Table 4.
Cumulative Cost Analysis Results, Traditional versus Molecular Methodology
| Test | Hands-on time per sample | Technician and overhead cost per sample | Batch size (n) | Reagent cost per batch | Reagent cost per sample | Total cost per sample |
|---|---|---|---|---|---|---|
| RVP ancillary | ||||||
| Reagents/supplies | 1032 | $20,640.28 | $20.00 | $20.00 | ||
| ID-tag RVP (list price) | 96 | $10,800.00 | $112.50 | $112.50 | ||
| Total RVP costs | 4.74 | $2.53 | $135.03 | |||
| DFA positive | 4.26 | $2.27 | 233 | $97.48 | $97.48 | $99.75 |
| DFA negative with shortest culture time | 25.86 | $13.80 | $315.88 | $315.88 | $329.68 | |
| DFA negative with longest culture time | 40.73 | $21.73 | $407.34 | $407.34 | $429.07 |
Discussion
Prior studies have already demonstrated the improved ability of the Luminex xTAG RVP to detect respiratory viral pathogens when compared with the more traditional methods of DFA and culture.6 Our study builds on this prior evidence, suggesting that the Luminex xTAG RVP has the ability to increase laboratory efficiency by reducing technologist hands-on time and operational steps while standardizing workflow for all respiratory viral specimens. It is important to note that 23% of our study population was DFA positive. DFA-positive specimens require the shortest TAT; omission of the DFA screen would increase the time to diagnosis for these patients. However, although the Luminex xTAG RVP requires more time and steps than screening DFA, we believe that the percentage of DFA-negative specimens requiring follow-up culture was large enough to overshadow this drawback. Future studies focusing on the clinical impact of this delay are warranted.
In addition to the benefits of workflow simplification and increased laboratory efficiency, our findings show the potential of Luminex xTAG RVP implementation to reduce laboratory costs. These findings are consistent with those found by Mahony10 in a prior, broader cost analysis that included overall cost of hospitalization. Although the Luminex xTAG RVP is associated with a higher cost per sample than screening DFA, the costs associated with any sample requiring follow-up culture were even greater. In addition, it is not unreasonable to posit that the faster TAT of the Luminex xTAG RVP would be associated with shorter hospital stays and, therefore, lower overall hospital costs, resulting in savings benefit not only for the laboratory but for the institution as a whole.
In summary, the Luminex xTAG RVP provides not only increased diagnostic capability, but also maximizes efficiency and productivity when compared to viral DFA with culture. We acknowledge that these findings are limited to our own unique laboratory environment and may be greatly affected by the extreme variability in batch size during the different seasons of the year. Future multicenter studies with statistical analysis may strengthen the findings. Keeping in mind the variability in workload and laboratory environment, we suggest that, in this age of financial instability and workforce shortage, the Luminex xTAG RVP may be able to foster the efforts of a pediatric virology laboratory to maintain and improve diagnostic capability while maximizing the efficiency and productivity of its limited financial and personnel resources.
Acknowledgments
We thank Tm Bioscience Corporation (Toronto, Ontario, Canada) for providing the reagents for the RVP and the funds for performance of extractions.
Footnotes
Tm Bioscience Corporation provided funds for performing the extractions as well as the reagents for the RVP.
Research performed at the Children's Medical Center of Dallas, Department of Pathology, Dallas, Texas.
References
- 1.Womack J.P., Jones D.T. Beyond Toyota: how to root out waste and pursue perfection. Harv Bus Rev. 1996;74:140–158. [Google Scholar]
- 2.Bahensky J.A., Roe J., Bolton R. Lean sigma: will it work for healthcare. J Healthc Inf Manag. 2005;19:39–44. [PubMed] [Google Scholar]
- 3.Persoon T.J., Zaleski S., Frerichs J. Improving preanalytic processes using the principles of lean production (Toyota production system) Am J Clin Pathol. 2006;125:16–25. [PubMed] [Google Scholar]
- 4.Raab S.S., Grzybicki D.M., Sudilovsky D., Balassanian R., Janosky J.E., Vrbin C.M. Effectiveness of Toyota process redesign in reducing thyroid gland fine-needle aspiration error. Am J Clin Pathol. 2006;126:585–592. doi: 10.1309/NJQ1L7KA10UKV93Q. [DOI] [PubMed] [Google Scholar]
- 5.Graban M. Productivity Press; New York, NY: 2009. Lean Hospitals: Improving Quality, Patient Safety, and Employee Satisfaction. [Google Scholar]
- 6.Mahony J., Chong S., Merante F., Yaghoubian S., Sinha T., Lisle C., Janeczko R. Development of a respiratory virus panel test for detection of twenty human respiratory viruses by use of multiplex PCR and a fluid microbead-based assay. J Clin Microbiol. 2007;45:2965–2970. doi: 10.1128/JCM.02436-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Letant S.E., Ortiz J.I., Tammero L.F.B. Multiplexed reverse transcriptase PCR assay for identification of viral respiratory pathogens at the point of care. J Clin Microbiol. 2007;45:3498–3505. doi: 10.1128/JCM.01712-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grondahl B., Puppe W., Weigl J., Schmitt H.J. Comparison of the BD directigen flu a+b kit and the Abbott test pack RSV with a multiplex RT-PCR ELISA for rapid detection of influenza viruses and respiratory syncytial virus. Clin Microbiol Infect. 2005;11:848–850. doi: 10.1111/j.1469-0691.2005.01223.x. [DOI] [PubMed] [Google Scholar]
- 9.Merante F., Yaghoubian S., Janeczko R. Principles of the xTAG respiratory viral panel assay (RVP assay) J Clin Virol. 2007;40:S31–S35. doi: 10.1016/S1386-6532(07)70007-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mahony J.B., Blackhouse G., Babwah J., Smieja M., Buracond S., Chong S., Ciccotelli W., O'Shea T., Alnakhli D., Griffiths-Turner M., Goeree R. Cost analysis of multiplex PCR testing for diagnosing respiratory virus infections. J Clin Microbiol. 2009;47:2812–2817. doi: 10.1128/JCM.00556-09. [DOI] [PMC free article] [PubMed] [Google Scholar]


