Abstract
Background and Purpose
Stroke causes brain injury with activation of an inflammatory response which can contribute to injury. We tested the hypothesis that the anti-inflammatory cytokine IL-4 reduces injury following stroke using IL-4 knockout (KO) adult male mice.
Methods
IL-4 KO and wild type (WT) mice were subjected to transient middle cerebral artery occlusion (MCAO). Outcome was assessed by triphenyletrazolium chloride staining for infarct volume, neuroscore and spontaneous activity for behavioral outcome, and immunostaining and stereological counting for cellular response.
Results
Infarction volume at 24 hrs was significantly larger in IL-4 KO mice, neurological score was significantly worse, and spontaneous activity was reduced compared to WT mice. Increased macrophage/microglial infiltration, increased numbers of myeloperoxidase-positive cells and increased Th1/Th2 ratio were observed in the infarct core in IL-4 KO mice. Reduced astrocyte activation was observed in the cortical penumbra in IL-4 KO mice. Recombinant IL-4 administered intracerebroventricularly before MCAO significantly reduced infarct volume, improved neurological score, reduced macrophages/microglia, and lowered the Th1/Th2 ratio in IL-4 KO mice, but not in WT.
Conclusion
Loss of IL-4 signaling in KO mice was associated with worse outcome, and this was reversed by giving exogenous IL-4. Worsened outcome was associated with increased inflammation in the core, which was reversed in IL-4 KO but not significantly changed in WT mice by exogenous IL-4. This is consistent with IL-4 signaling leading to reduced inflammation in the core, and a possible beneficial role for activated astrocytes in the penumbra.
Keywords: astrocyte, inflammation, microglia, stroke, Th1
Introduction
Stroke causes brain injury and infarction of tissue with activation of an inflammatory response that includes activation of microglia and astrocytes.1–4 Ischemia induces transformation of resting microglia into reactive, proliferating, hypertrophied microglia. Activated microglia express major histocompatibility complex (MHC) class II and adhesion/costimulation molecules in response to neuronal damage, suggesting a role in T cell activation.5 Initially, reactive microglia accumulate in the boundary zone or penumbra,1, 5 subsequently accumulating in the ischemic core. While activated microglia are often considered harmful, microglia can also release beneficial neurotrophic factors.6 Astrocytes also respond to and produce inflammatory signals,7 and via their interaction with microglia and neurons help determine the outcome from injury. T lymphocytes enter the brain parenchyma and accumulate in the ischemic core within 24 hr after stroke.8, 9 They can produce damage by releasing proinflammatory mediators.10, 11 IL-4 plays a central role in the differentiation of antigen-stimulated naïve T cells into Th2 cells that produce anti-inflammatory cytokines IL-4, IL-10, and IL-13 while suppressing generation of Th1 cells which produce pro-inflammation cytokines IL-1 and IFN-γ.12 The anti-inflammatory cytokine IL-10 was shown to be protective in stroke,13 but IL-4 has not yet been studied. Serum levels of IL-4 from Th2 cells were elevated significantly in patients with cerebral infarction.14 In vitro, astrocytes express IL-4 receptors, which can regulate astrocyte activation. IL-4 induces astrocytes to secrete nerve growth factor,15 suggesting a protective function of IL-4–stimulated astrocytes. Although IL-4 can induce microglial proliferation, the cells display more of a resting phenotype with down-regulated phagocytic and antigen-presenting functions.16 Despite the likely importance of IL-4 signaling in stroke, the effects of endogenous IL-4 are not clear. We used IL-4 knockout (KO) mice to assess the role of IL-4 on infarct volume, neurological outcome, and glial activation 24 hr after middle cerebral artery occlusion (MCAO).
Material and methods
Animals
BALB/cJ and IL-4 KO mice (BALB/c-IL-4tm2Nnt/J) were purchased from Jackson Laboratory (Bar Harbor, ME) then bred in our animal facility as homozygotes. Mice were housed in the Stanford Medical School Animal Care Facilities and all use of animals was according to protocols approved by the Stanford Institutional Animal Care and Use Committee and were conducted according to the National Institutes of Health (NIH) Guide for Care and Use of Laboratory Animals.
Model of focal cerebral ischemia
Male mice, 10–12 weeks old, were anesthetized with 2% isoflurane in 70% N2O and balance O2 by facemask. Cerebral ischemia was 1 hr of MCAO with a silicone-coated 6-0 monofilament (Doccol Corp., Redlands, CA) followed by reperfusion.17 Sham-operated mice underwent an identical procedure, without inserting the suture. Rectal temperature was maintained at 37 ± 0.5°C with a heating pad (Harvard Apparatus, Holliston, MA). Heart rate, oxygen saturation, and respiratory rate were monitored continuously (STARR Life Sciences Corp., Allison Park, PA). Animals with no observable deficits immediately after ischemia, those that died before 24 hr, and those with subarachnoid hemorrhage at the time of death were excluded from analysis; the mortality rate in WT mice was 12.5% and in IL-4KO mice was 19.2%.
Measurement of cerebral infarction area
Twenty-four hr after MCAO and assessment of neurological score, mice were anesthetized with isoflurane and decapitated. Brains were removed and sectioned coronally with a rodent brain slicer matrix (Zivic Instruments, Pittsburgh, PA). Sections were incubated in 2% 2, 3,5-triphenyletrazolium chloride (TTC) and infarction volume determined using four slices per mouse analyzed by a blinded observer and corrected for edema using the NIH Image program (Image J 1.37v, Wayne Rasband, available through NIH) as described previously.17 Infarction volume is the sum of infarction areas multiplied by slice thickness.
Evaluation of neurological status
Neurological status was evaluated at 24 hr according to a neurological grading score,17 from 0, no observable neurological deficit to 4, unable to walk spontaneously and a depressed level of consciousness. The evaluator was blinded to experimental treatment.
Treatment with recombinant human IL-4 (RhIL-4)
RhIL-4 (R&D Systems, Minneapolis, MN) was injected intracerebroventricularly (icv). IL-4 KO mice and WT mice (25–30g) were anesthetized with 2% isoflurane in 70% N2O balance O2 by facemask and placed in a stereotaxic frame with mouse head holder. Either vehicle (0.1% bovine serum albumin (BSA) in 0.9% phosphate-buffered saline (PBS) or 1 μg of rhIL-4 dissolved in vehicle was infused in 5 μl (over 10 min) into the right lateral ventricle via a burr hole once 30 min before MCAO based on a prior study.13 In pilot experiments we tested two injections of IL-4 but observed almost 50% mortality. Due to this unexplained toxicity we chose a single injection protocol. After that the bone wound was closed with bone wax and the mouse prepared for MCAO. After 24 hr of reperfusion the animals were sacrificed and the brains removed for TTC staining.
Assessment of spontaneous activity
The SmartCage™ system (AfaSci, Inc., Burlingame, CA) was used for automated analysis of spontaneous animal activity. Data sampled at 4 Hz in each sensor is gathered by two rows of infrared photobeams mounted on a plexiglass box into which a standard mouse cage fits. Automated data analysis used CageScore™ software (AfaSci, Inc.). The home cage activity variables assessed were beam breaks (x, y, and z photo-beam break counts), and locomotion by distance traveled and velocity. Distance traveled in centimeters was obtained from the lower horizontal sensors (x and y) taking into account the path taken. Average velocity was distance traveled/second in the forward direction, averaged over the monitoring period. All mice were assessed continuously for 24 hr beginning after 3 hr reperfusion.
Immunohistochemistry
Ischemic or sham-operated mice were anesthetized and perfused with 0.9% saline, followed by paraformaldehyde in PBS (pH 7.4) as previously described.18 After 48 hr in 4% paraformaldehyde in PBS (pH 7.4) 50μm sections were cut. Immunohistochemistry was carried out on free-floating sections. Washes and incubations were in 0.1M PBS (pH 7.4) containing 0.3% Triton X-100. Sections were incubated for 1 hr with blocking solution (0.1M PBS, 0.3% Triton X-100 and 5% equine serum), washed several times, then incubated overnight at 4°C with an anti-glial fibrillary acidic protein antibody (GFAP, diluted 1:5, #22522, Immunostar, Hudson, WI), for reactive and resting astrocytes, antibody to CD68 (diluted 1:200; MCA1957GA, Serotec, Kidlington, UK), for reactive macrophages/microglia, or anti-myeloperoxidase (MPO, diluted 1:50, #A0398, DAKO, Glostrup, Denmark) for leukocytes. Sections were rinsed, incubated for 2 hr at room temperature with Alexa 594-conjugated secondary antibody (for GFAP+ cells or MPO+ cells) or Alexa 488-conjugated secondary (for CD68+ cells, diluted 1:200, Invitrogen), washed and mounted on glass slides using Vectashield mounting medium with 4′, 6-diamidino-2-phenylindole (DAPI; Vector Laboratories, Burlingame, CA). Immunostaining was absent when the first antibody was omitted.
For stereological evaluation of Th1 and Th2 cells diaminobenzidine staining was used. All washes and incubations were 0.1M PBS, 0.3% BSA and 0.3% Triton X-100 buffer. After 1 hr in blocking solution endogenous peroxidase activity was quenched for 10 min in 3% hydrogen peroxide in 30% methanol. After several washes, sections were incubated for 1 day at 4°C with an antibody for T-bet (diluted 1:100, rabbit anti-mouse T-bet, clone Ch-210, Santa Cruz Biotechnology Inc, Santa Cruz, CA), or for GATA-3 (diluted 1:100, mouse anti-GATA-3, clone L50-823, BD Pharmingen™, San Diego, CA). After incubating with primary antibody, sections were rinsed and incubated with biotinylated goat anti-rabbit immunoglobulin G (BA-1000 Vector Laboratories, Burlingame, CA; diluted 1:300 in washing buffer) or biotinylated horse anti-mouse immunoglobulin G (BA-2000 Vector Laboratories diluted 1:300 in buffer). After several washes sections were incubated for 90 min with avidin–biotin-peroxidase complex (ImmunoPure ABC peroxidase staining kit, Pierce, Rockford, IL; diluted1:250), then incubated with 2 μg/ml 3,3′-diaminobenzidine (Sigma-Aldrich, St. Louis, MO) and 0.01% hydrogen peroxide in 0.1 M phosphate buffer. Sections were dehydrated and mounted on gelatinized slides.
Morphometric analysis
The expression of GFAP, CD68 or MPO was analyzed with the optical fractionator method on epifluorescence photomicrographs taken with a Zeiss Axiovert inverted epifluorescence microscope (Zeiss, Jena, Germany) covering a total of 0.16 mm2. The expression of T-bet or GATA3 was observed with a Nikon Eclipse TE300 microscope (Nikon, Tokyo, Japan) covering a total of 0.78mm2. For each animal the number of GFAP or CD68 immunoreactive cells in the cortical penumbra or ischemic core (for CD68 and MPO, −1.70 to −2.18 mm relative to Bregma) was counted, and a total of 48 counting frames of 100 × 100 um were assessed per animal using Image J software. Representative micrographs showing glial response to injury were photographed at 20x or 40x magnification using a digital camera attached to a Zeiss LSM 510 META inverted laser scanning confocal microscope (Zeiss).
Western Blotting
The ipsilateral hemisphere was harvested 24 hr after ischemia, homogenized in cold lysis buffer (10mmol/L 4-2-hydroxyethyl-1-piperazine-ethanesulfonic acid (pH 7.9), 1.5 mmol/L MgCl2, 10 mmol/L KCl, 1mmol/L dithiothreitol) plus protease inhibitor cocktail (Roche Diagnostic, Mannheim, Germany). Protein concentrations were determined using bichinoninic acid (Pierce, Rockford, IL). Equal amounts (50 μg) of protein were separated on 4–15% polyacrylamide gels (Bio-Rad, Hercules, CA), and electrotransferred to Immobilon polyvinylidene fluoride membranes (Millipore Corp., Bedford, MA) as previously described.17 Membranes were blocked with 5% nonfat dry milk in PBS with 0.1% Tween 20 for 1 hr, incubated overnight with rat anti-CD68 antibody (1:1000 dilution, Serotec), or anti-GFAP (1:1500, Cell Signaling, Danvers, MA), washed three times with 0.1% Tween in PBS, and incubated with 1:2000 secondary antibody (Cell Signaling), in 5% milk, 0.1% Tween in PBS for 90 min. ECL reagent (Amersham, Piscataway, NJ) and film were used for detection. Equal protein loading was confirmed by blotting with anti-β-actin antibody (1:1000, Santa Cruz Biotechnology). Densitometric analysis of bands was performed using Image J software.
Statistical analysis
Data is expressed as mean ± SEM. Differences were considered statistically significance for p-value <0.05. Student t-test was used when only two groups were compared. Two-way ANOVA was used when both genotype and treatment were taken into account, followed by Bonferroni post-tests using Prism 4 (GraphPAD Software for Science, San Diego, CA).
Results
Infarction volume and neurological deficit are increased in IL-4 KO mice
Physiological variables were not significantly different between WT and IL-4 KO mice before MCAO, during MCAO, or at 24 hr reperfusion for respiratory rate or O2 saturation (Table 1). Heart rate was lower in IL-4 KO mice than in WT (Table 1) at 24 hr reperfusion. Infarction volume at 24 hr was significantly larger in IL-4 KO mice (48.8 ± 4.3%), compared to WT (36 ± 3.2%; n=10/group, p = 0.032, Figure 1A, B) and the neurological score was significantly worse (Figure 1C).
Table 1.
Physiological measurements
| WT | IL-4 KO | |
|---|---|---|
| (n=40) | (n=40) | |
| Before MCAO | ||
| Heart rate (bpm) | 450.80 ± 7.30 | 465.90 ± 9.91 |
| SpO2 (%) | 95.80 ± 0.20 | 94.65 ± 0.18 |
| Temperature (°C) | 37.03 ± 0.02 | 36.94 ± 0.06 |
| During MCAO | ||
| Heart rate (bpm) | 456.40 ± 9.02 | 438.70 ± 9.47 |
| SpO2 (%) | 95.70 ± 0.16 | 96.10 ± 0.18 |
| Temperature (°C) | 36.61 ± 0.09 | 36.83 ± 0.11 |
| After 24 hr reperfusion | ||
| Heart rate (bpm) | 439.15 ± 7.60 | 405.45 ± 10.26* |
| SpO2 (%) | 94.50 ± 0.22 | 94.90 ± 0.16 |
| Temperature (°C) | 36.09 ± 0.42 | 35.52 ± 0.37 |
Values are mean ± SEM. Before MCAO = 5 min before MCAO; During MCAO = at the end of 60 min MCAO; SpO2 = oxygen saturation.
p=0.01.
Figure 1.
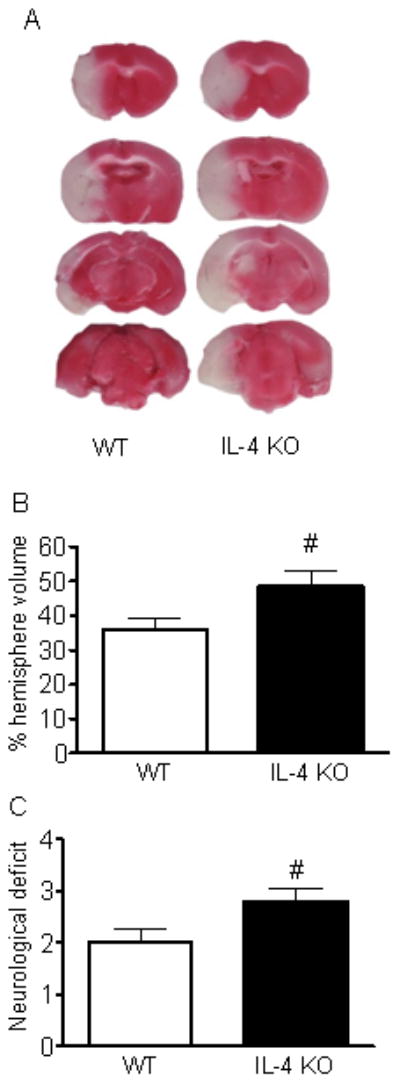
Ischemic infarct volume and neurological score were increased in IL-4 KO mice. (A) Representative TTC-stained coronal sections. (B) Quantification of infarct volume expressed as a percent of hemispheric volume (P=0.032, n=10). (C) The neurological deficit assessed at 24 hr was greater in IL-4 KO (P=0.039, n=10).
Spontaneous motor activity is reduced more in IL-4 KO mice after MCAO
After surgery animals were allowed to recover for 3 hr on a heating pad, and then while still in their home cage placed within the SmartCage to analyze spontaneous motor activity for 24 hr. Overall motor activity indicated by photobeam breaks and total travel distance were not significantly different between IL-4 KO mice and WT mice in either the naive or sham surgery groups. Following MCAO beam breaks and travel distance, but not velocity, were significantly decreased in IL-4 KO mice compared to WT and sham controls (Figure 2A–D). These results are consistent with the difference in neurological scores and infarct volume.
Figure 2.
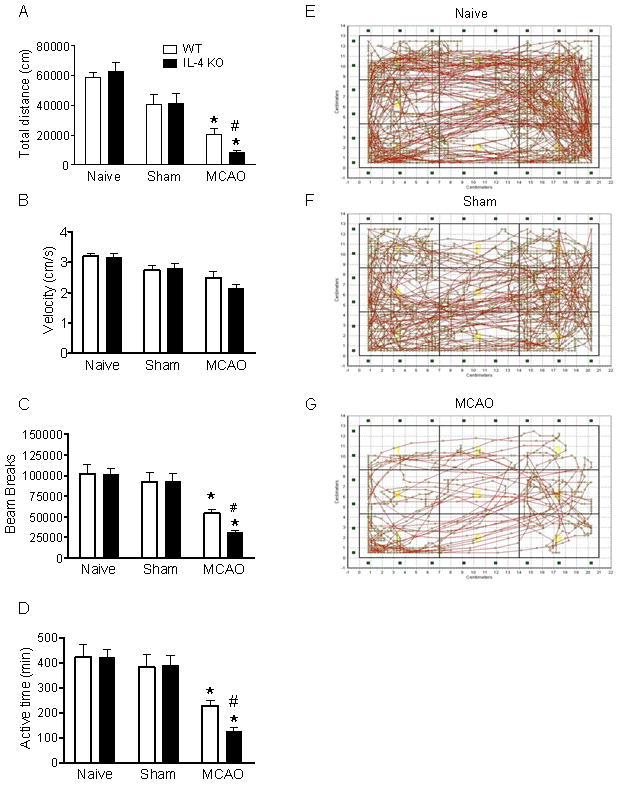
Spontaneous activity is reduced more in IL-4 KO mice following MCAO assessed for 24 hr starting 2 hr after MCAO. (A) Total travel distance was calculated based on x, y position and accumulated path length. Total distance was significantly reduced after MCAO compared to naïve or sham surgery. Total travel distance for IL-4 KO MCAO was significant shorter than WT MCAO (n=12, p<0.05). (B) Average velocity did not differ between groups. (C) Beam breaks in IL-4 KO MCAO were significantly fewer than WT (n=12, p<0.05). (D) Active time is the total time spent moving/24 hr. (E–G) Individual representative travel patterns demonstrate decreased spontaneous activity after MCAO. For all panels * P<0.05 vs sham and naive of the same genotype, # P <0.05 vs WT MCAO.
Increased macrophage/microglial infiltration, reduced astrocyte activation in IL-4 KO
Glial activation was prominent in animals after MCAO. A panoramic low power view is shown (Figure 3A). Morphometric analysis (Figure 3B, C) shows a greater increase in the number of GFAP positive cells in the cortical penumbra (CP) in WT animals compared to IL-4 KO animals at 24 hr reperfusion. Almost no GFAP+ astrocytes are observed in the ischemic core in either genotype. No significant differences in astrocyte activation were observed in sham-operated animals. The total number of activated macrophages/microglia increased to a significantly greater extent in the ischemic core of IL-4 KO compared to WT animals (Figure 3C). This contrasts with very low numbers of GFAP positive cells in the IC (data not shown). This increased number of activated macrophages/microglia in the IC is associated with significantly increased infiltration of leukocytes, detected as MPO positive cells in the IC (Figure 3C). Consistent with the increased numbers of activated microglia/macrophages, CD68 protein levels were significantly higher in IL-4 KO mice compared with WT mice 24 hr after MCAO in the ischemic hemisphere (Figure 4A). In contrast GFAP expression did not differ by genotype (Figure 4B).
Figure 3.
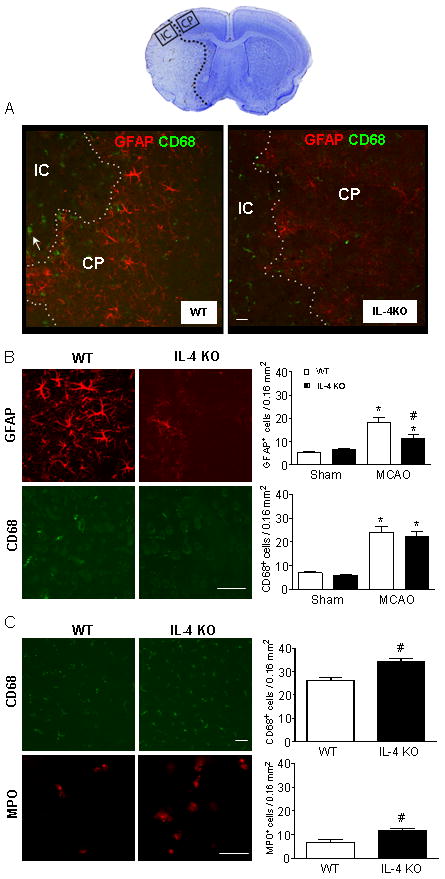
GFAP, CD68 and MPO immunoreactive cells in the cortical penumbra and ischemic core were assessed at 24 hr reperfusion. The upper picture shows a whole brain section in which the cortical penumbra (CP) and ischemic core (IC) areas are indicated. Panel (A) shows glial activation in the CP and IC from WT and IL-4 KO animals (20x). (B) Images and quantitation of GFAP+ astrocytes or CD68+ microglia/macrophages at 40x magnification in CP. Increase in GFAP+ cells is greater in WT, with no statistical difference in CD68+ between genotypes. (C) CD68+ cells and MPO+ leukocytes (20x) were counted in the IC after MCAO. CD68+ and MPO+ cells were greater in the IL-4 KO group. Scale bars, 50 μm. * P <0.05 vs sham of the same genotype, # P<0.05 vs WT MCAO. n=4 for MCAO IL-4 KO, n=5 MCAO WT, n=3 both sham groups.
Figure 4.
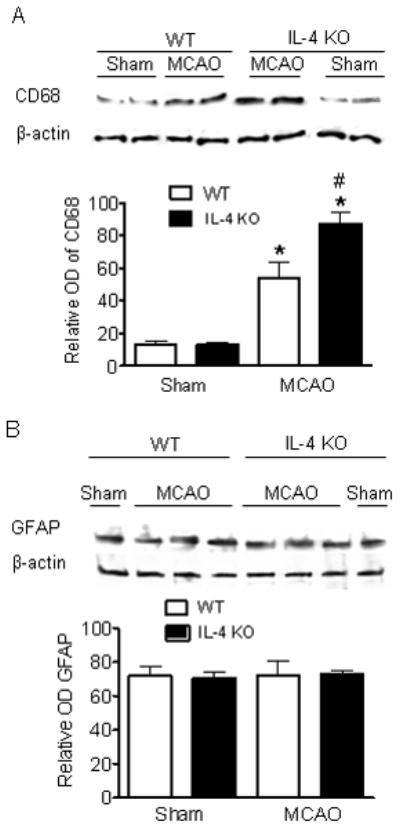
CD68 (A) and GFAP (B) expression in the ischemic hemisphere assessed by Western. (A) CD68 protein level increases after MCAO are greater in IL-4 KO, p=0.037. n=3 sham, n=6 WT MCAO, n=7 IL-4 KO MCAO. (B) GFAP protein levels increased similarly in IL-4 KO and WT after MCAO. * P <0.05 vs sham same genotype, # P<0.05 vs WT MCAO, n=3 all groups.
RhIL-4 reduces infarction volume, neurological score, and inflammation in IL-4 KO mice
RhIL-4 administered icv before MCAO significantly reduced infarct volume from 50.6 ± 1.9 (n=9) to 33.4 ± 3.3 (n=8, p=0.0006) (Figure 5A) and reduced neurological score from (2.80 ± 0.20 n=5) to 1.78 ± 0.22 (n=9, p=0.01; Figure 5B). RhIL-4 administration decreased CD68+ cells in the core from 35.75 ± 2.25 to 22.00 ± 1.09 (p=0.001, n=4/group; Figure 5C) and penumbra from 24.25 ± 1.11 to 18.00 ± 2.58 (p=0.068, n=4/group; Figure 5D). Staining for the Th1 marker T-bet and the Th2 marker GATA3 (Figure 5E–H) showed decreased Th1/Th2 ratio from 1.67 ± 0.21 to 0.79 ± 0.10 (p=0.008, n=4/group) (Figure 5F) in IL-4 KO mice, but no change in WT mice.
Figure 5.
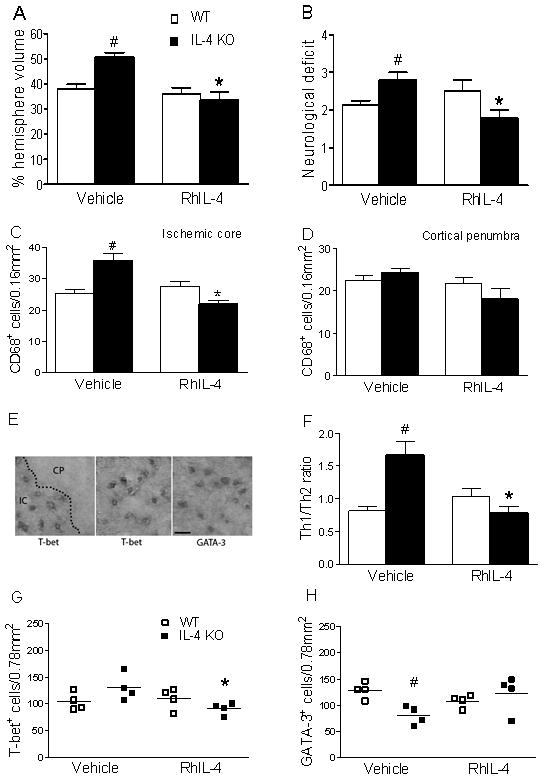
Administration of RhIL-4 protects only in IL-4 KO mice. (A) Infarct volume in WT was unchanged with RhIL-4 (n=7) but significantly decreased in IL-4 KO-RhIL-4 (n=9) compared to IL-4KO-vehicle (p=0.0006, n=8). (B) Neurological score was unchanged in WT-RhIL-4 (p=0.28, n=4) but significantly decreased in IL-4 KO-RhIL-4 (n=9) compared to vehicle treated (n=5, p=0.01); (C) RhIL-4 did not change CD68+ cells in the ischemia core in WT but significantly decreased CD68+ cells in IL-4 KO-RhIL-4 compared to IL-4KO-vehicle (p=0.002, n=4/group). (D) IL-4 treatment did not change CD68+ cells in penumbra of WT (n=4) or IL-4 KO (p=0.068, n=4/each group). (E) Images of T-bet+ cells (Th1) or GATA-3+ (Th2) at 20x magnification in IC. Scale bar, 50 μm (F) Th1/Th2 ratio was greater in IL-4 KO-vehicle compared to WT-vehicle (p=0.008) or IL-4KO-RhIL-4 (p=0.008, n=4/group). Th1/Th2 ratio was unchanged in WT with/without RhIL-4 (n=4/group). (G) RhIL-4 significantly decreased T-bet+ cells in the core of IL-4 KO compared to IL-4KO-vehicle, p=0.029 (H) Fewer GATA3+ cells were in the ischemic core in IL-4 KO-vehicle compared to WT-vehicle (p=0.007, n= 4/group). * P <0.05 vs vehicle injected same genotype, # P<0.05 vs WT-vehicle.
Discussion
The main finding of the present study is that IL-4 reduces ischemic injury, microglial activation and Th1/Th2 ratio following stroke. The studies were conducted with mice bred homozygously. While the effect of replacing IL-4 suggests that the loss of IL-4 accounts for the main effects observed, to rule out another genetic contribution the studies should be confirmed using mice bred from heterozygous crosses. We observed greater reduction of spontaneous locomotion following MCAO in IL-4 KO mice. Clinical studies have shown that early neurological functional deficit is a major predictor of stroke outcome.19, 20 In rodents, reduction of infarct volume and neuroscore are widely employed as primary outcomes; neuroscore correlates well with infarct volume in acute stroke,21, 22 but less well with mild neurological deficits.23 The assessment of spontaneous motor activity with the SmartCage™ system provides detailed quantitative assessment, which here was consistent with infarct and neuroscore. Additional studies are needed to test this correlation over a range of injuries, but this may be a useful addition to outcome assessment. Additional studies are also needed for long term outcome.
The absence of IL-4 was associated with decreased numbers of GFAP immunoreactive astrocytes in the penumbra. Astrocyte activation encompasses a range of changes in gene expression and activity, so it is not clear whether this indicates fewer activated astrocytes, or an alteration in activation pattern with reduced induction of GFAP. It is currently unknown whether the observed change in astrocyte activation in the penumbra in the absence of IL-4 contributes to increased inflammation. Prior work demonstrated a protective role for activated astrocytes following stroke and brain injury,2, 3 so reduced astrocyte activation acutely may contribute to injury. Prior work has shown that astrocytes are as efficient as microglia at inducing IL-4 secretion from Th2 cells, but less efficient at stimulating Th1 responses.24 Future studies should examine interactions between astrocytes, microglia, and T lymphocytes.
Activated microglia not only synthesize numerous soluble and membrane-bound inflammatory mediators including pro-inflammatory cytokines IL-1β, TNF-α, chemokines MIP-1α, MCP-1, MIP-2, reactive oxygen, and nitrogen species, but can also present antigen to activate T cells. We observed an increased Th1/Th2 ratio and increased numbers of macrophages/microglia in IL-4 KO mice, both of which reversed with exogenous IL-4. This suggests that activated microglia/macrophages and increased Th1/Th2 ratio may contribute to ischemic damage in IL-4 KO mice.
T and B-cell deficient mice have smaller infarcts.11 Both CD4+ and CD8+ T cells increase stroke injury.10 The increased Th1/Th2 ratio in IL-4 KO mice is consistent with a detrimental role for Th1CD4+ cells. Prior work suggests that the balance between Th1 and Th2 polarization may critically contribute to stroke25 with Th2 cells being protective, but potentially also contributing to infectious complications. T cells express NADPH oxidase 2 in stroke, and recruitment is greater in males9 suggesting that greater oxidative stress may also contribute to worsened injury.
IL-4 is a major negative regulator of pro-inflammatory cytokine production by both brain cells and T lymphocytes and appears to play a key role in controlling neuroinflammation. On the other hand, microglia are normal brain constituents, defend against infection and toxic substances released from dying cells, and are beneficial in some settings.26 Therefore, fine tuning the inflammatory response by the integrated signaling of IL-4 and other factors is likely critical under both physiological and pathological conditions. Here, absence of IL-4 was associated with increased infiltration of MPO positive cells into the core. Prior studies have shown that inhibiting adhesion molecules reduced stroke volume,27 consistent with the idea that excessive infiltration of leukocytes worsens injury.
In conclusion increased injury following MCAO in IL-4 KO mice was associated with increased CD68+ cells, MPO+ cells, and Th1/Th2 ratio in the core, and decreased GFAP+ cells in the penumbra. The greater inflammation observed in the IL-4 KO mice suggests increased Th1 polarization is associated with greater injury, and a possible beneficial role for activated astrocytes. Interestingly, intracerebroventricular injection of IL-4 provided no additional benefit in WT, suggesting that the beneficial effects in WT are already optimal.
Acknowledgments
Sources of Funding
This work was supported in part by NIH grant RO1 GM49831 and NS014543 to RGG and R01 MH078194-02 and R43MH076309 to X. Xie
Footnotes
Conflict of interest
The authors declare no conflict of interest
References
- 1.del Zoppo GJ, Milner R, Mabuchi T, Hung S, Wang X, Berg GI, Koziol JA. Microglial activation and matrix protease generation during focal cerebral ischemia. Stroke. 2007;38:646–651. doi: 10.1161/01.STR.0000254477.34231.cb. [DOI] [PubMed] [Google Scholar]
- 2.Pekny M, Nilsson M. Astrocyte activation and reactive gliosis. Glia. 2005;50:427–434. doi: 10.1002/glia.20207. [DOI] [PubMed] [Google Scholar]
- 3.Sofroniew MV. Molecular dissection of reactive astrogliosis and glial scar formation. Trends Neurosci. 2009;32:638–647. doi: 10.1016/j.tins.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang Q, Tang XN, Yenari MA. The inflammatory response in stroke. J Neuroimmunol. 2007;184:53–68. doi: 10.1016/j.jneuroim.2006.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marks L, Carswell HV, Peters EE, Graham DI, Patterson J, Dominiczak AF, Macrae IM. Characterization of the microglial response to cerebral ischemia in the stroke-prone spontaneously hypertensive rat. Hypertension. 2001;38:116–122. doi: 10.1161/01.hyp.38.1.116. [DOI] [PubMed] [Google Scholar]
- 6.Liang J, Takeuchi H, Jin S, Noda M, Li H, Doi Y, Kawanokuchi J, Sonobe Y, Mizuno T, Suzumura A. Glutamate induces neurotrophic factor production from microglia via protein kinase c pathway. Brain Res. 2010;1322:8–23. doi: 10.1016/j.brainres.2010.01.083. [DOI] [PubMed] [Google Scholar]
- 7.Li L, Lundkvist A, Andersson D, Wilhelmsson U, Nagai N, Pardo AC, Nodin C, Stahlberg A, Aprico K, Larsson K, Yabe T, Moons L, Fotheringham A, Davies I, Carmeliet P, Schwartz JP, Pekna M, Kubista M, Blomstrand F, Maragakis N, Nilsson M, Pekny M. Protective role of reactive astrocytes in brain ischemia. J Cereb Blood Flow Metab. 2008;28:468–481. doi: 10.1038/sj.jcbfm.9600546. [DOI] [PubMed] [Google Scholar]
- 8.Liesz A, Hagmann S, Zschoche C, Adamek J, Zhou W, Sun L, Hug A, Zorn M, Dalpke A, Nawroth P, Veltkamp R. The spectrum of systemic immune alterations after murine focal ischemia: Immunodepression versus immunomodulation. Stroke. 2009;40:2849–2858. doi: 10.1161/STROKEAHA.109.549618. [DOI] [PubMed] [Google Scholar]
- 9.Brait VH, Jackman KA, Walduck AK, Selemidis S, Diep H, Mast AE, Guida E, Broughton BR, Drummond GR, Sobey CG. Mechanisms contributing to cerebral infarct size after stroke: Gender, reperfusion, t lymphocytes, and nox2-derived superoxide. J Cereb Blood Flow Metab. 2010;30:1306–1317. doi: 10.1038/jcbfm.2010.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yilmaz G, Arumugam TV, Stokes KY, Granger DN. Role of t lymphocytes and interferon-gamma in ischemic stroke. Circulation. 2006;113:2105–2112. doi: 10.1161/CIRCULATIONAHA.105.593046. [DOI] [PubMed] [Google Scholar]
- 11.Hurn PD, Subramanian S, Parker SM, Afentoulis ME, Kaler LJ, Vandenbark AA, Offner H. T- and b-cell-deficient mice with experimental stroke have reduced lesion size and inflammation. J Cereb Blood Flow Metab. 2007;27:1798–1805. doi: 10.1038/sj.jcbfm.9600482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abbas AK, Murphy KM, Sher A. Functional diversity of helper t lymphocytes. Nature. 1996;383:787–793. doi: 10.1038/383787a0. [DOI] [PubMed] [Google Scholar]
- 13.Spera PA, Ellison JA, Feuerstein GZ, Barone FC. Il-10 reduces rat brain injury following focal stroke. Neurosci Lett. 1998;251:189–192. doi: 10.1016/s0304-3940(98)00537-0. [DOI] [PubMed] [Google Scholar]
- 14.Kim HM, Shin HY, Jeong HJ, An HJ, Kim NS, Chae HJ, Kim HR, Song HJ, Kim KY, Baek SH, Cho KH, Moon BS, Lee YM. Reduced il-2 but elevated il-4, il-6, and ige serum levels in patients with cerebral infarction during the acute stage. J Mol Neurosci. 2000;14:191–196. doi: 10.1385/JMN:14:3:191. [DOI] [PubMed] [Google Scholar]
- 15.Brodie C, Goldreich N, Haiman T, Kazimirsky G. Functional il-4 receptors on mouse astrocytes: Il-4 inhibits astrocyte activation and induces ngf secretion. J Neuroimmunol. 1998;81:20–30. doi: 10.1016/s0165-5728(97)00154-9. [DOI] [PubMed] [Google Scholar]
- 16.Wirjatijasa F, Dehghani F, Blaheta RA, Korf HW, Hailer NP. Interleukin-4, interleukin-10, and interleukin-1-receptor antagonist but not transforming growth factor-beta induce ramification and reduce adhesion molecule expression of rat microglial cells. J Neurosci Res. 2002;68:579–587. doi: 10.1002/jnr.10254. [DOI] [PubMed] [Google Scholar]
- 17.Han RQ, Ouyang YB, Xu L, Agrawal R, Patterson AJ, Giffard RG. Postischemic brain injury is attenuated in mice lacking the beta2-adrenergic receptor. Anesth Analg. 2009;108:280–287. doi: 10.1213/ane.0b013e318187ba6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu L, Voloboueva LA, Ouyang Y, Emery JF, Giffard RG. Overexpression of mitochondrial hsp70/hsp75 in rat brain protects mitochondria, reduces oxidative stress, and protects from focal ischemia. J Cereb Blood Flow Metab. 2009;29:365–374. doi: 10.1038/jcbfm.2008.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Adams HP, Jr, Davis PH, Leira EC, Chang KC, Bendixen BH, Clarke WR, Woolson RF, Hansen MD. Baseline nih stroke scale score strongly predicts outcome after stroke: A report of the trial of org 10172 in acute stroke treatment (toast) Neurology. 1999;53:126–131. doi: 10.1212/wnl.53.1.126. [DOI] [PubMed] [Google Scholar]
- 20.Jongbloed L. Prediction of function after stroke: A critical review. Stroke. 1986;17:765–776. doi: 10.1161/01.str.17.4.765. [DOI] [PubMed] [Google Scholar]
- 21.Bederson JB, Pitts LH, Germano SM, Nishimura MC, Davis RL, Bartkowski HM. Evaluation of 2,3,5-triphenyltetrazolium chloride as a stain for detection and quantification of experimental cerebral infarction in rats. Stroke. 1986;17:1304–1308. doi: 10.1161/01.str.17.6.1304. [DOI] [PubMed] [Google Scholar]
- 22.Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20:84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- 23.Nedelmann M, Wilhelm-Schwenkmezger T, Alessandri B, Heimann A, Schneider F, Eicke BM, Dieterich M, Kempski O. Cerebral embolic ischemia in rats: Correlation of stroke severity and functional deficit as important outcome parameter. Brain Res. 2007;1130:188–196. doi: 10.1016/j.brainres.2006.10.087. [DOI] [PubMed] [Google Scholar]
- 24.Aloisi F, Ria F, Penna G, Adorini L. Microglia are more efficient than astrocytes in antigen processing and in th1 but not th2 cell activation. J Immunol. 1998;160:4671–4680. [PubMed] [Google Scholar]
- 25.Arumugam TV, Granger DN, Mattson MP. Stroke and t-cells. Neuromolecular Med. 2005;7:229–242. doi: 10.1385/NMM:7:3:229. [DOI] [PubMed] [Google Scholar]
- 26.Streit WJ, Conde JR, Fendrick SE, Flanary BE, Mariani CL. Role of microglia in the central nervous system’s immune response. Neurol Res. 2005;27:685–691. doi: 10.1179/016164105X49463a. [DOI] [PubMed] [Google Scholar]
- 27.Yenari MA, Kunis D, Sun GH, Onley D, Watson L, Turner S, Whitaker S, Steinberg GK. Hu23f2g, an antibody recognizing the leukocyte cd11/cd18 integrin, reduces injury in arabbit model of transient focal cerebral ischemia. Exp Neurol. 1998;153:223–233. doi: 10.1006/exnr.1998.6876. [DOI] [PubMed] [Google Scholar]


